Abstract
Background/Objective:
Human exposure to per- and polyfluoroalkyl substances (PFAS) has changed since the early 2000s, in part, because of the phase-out and replacement of some long-chain PFAS. Studies of PFAS exposure and its temporal changes have been limited to date mostly to adults and pregnant women. We examined temporal trends and determinants of PFAS serum concentrations among mothers with a young child who participated in the CHARGE (CHildhood Autism Risk from Genetics and Environment) case-control study.
Methods:
We quantified nine PFAS in serum samples collected from 2009 to 2016 in 450 Northern California mothers when their child was 2 to 5 years old. Except for four compounds that were detected in less than 50% of the samples, we used multiple regression to estimate least square geometric means (LSGMs) of PFAS concentrations with adjustment for sampling year and other characteristics that may affect maternal concentrations (e.g., breastfeeding duration). We used time-related regression coefficients to calculate percent changes over the study period.
Results:
LSGM concentrations of perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), and perfluorohexane sulfonate (PFHxS) decreased over the study period [percent change (95% confidence interval): −10.7% (−12.7%, −8.7%); −10.8% (−12.9%, −8.5%); −8.0% (−10.5%, −5.5%), respectively]. On the other hand, perfluorononanoate (PFNA) and perfluorodecanoate (PFDA) showed mixed time trends. Among the selected covariates, longer breastfeeding duration was associated with decreased maternal serum concentrations of PFOA, PFOS, PFHxS, PFNA and PFDA.
Conclusions:
Our study demonstrated that body burden of some common long-chain PFAS among California mothers with a young child decreased over the study period and that breastfeeding appears to contribute to the elimination of PFAS in lactating mothers.
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of fluorinated compounds, widely used for industrial and commercial applications including food packaging, paints, cookware, waterproof clothing, stain-resistant fabrics and firefighting foams (Buck et al., 2011; Lindstrom et al., 2011). PFAS migrate from food-contact paper or food packaging into foods (Begley et al., 2008; Begley et al., 2005) and are likely to be transferred from consumer products to indoor air and household dust (Eriksson and Karrman, 2015; Winkens et al., 2017; Yao et al., 2018). Thus, PFAS have been detected in most of the serum samples of the general population worldwide (Eriksson et al., 2017; Haug et al., 2009; Hurley et al., 2018; Jain, 2018; Kato et al., 2011; Nost et al., 2014). Based on laboratory animal toxicity testing, some of the common long-chain PFAS including perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) are known to have neurotoxicity (Austin et al., 2003; Harada et al., 2005), reproductive toxicity (Butenhoff et al., 2004; Vandenheuvel et al., 1992), hepatotoxicity (Kudo et al., 1999; Liu et al., 1996; Seacat et al., 2002; Vandenheuvel et al., 1992), and developmental toxicity (Lau et al., 2004; Lau et al., 2006; Lau et al., 2003; Thibodeaux et al., 2003).
Increasing public health concern over toxicity from PFAS exposure in the early 2000s led to regulatory and voluntary efforts in restricting the production, use, and emissions of the common long-chain PFAS. One of the largest manufacturers of PFOS worldwide, 3M, voluntarily ceased the production of PFOS and its precursors in 2002 (EPA, 2017). The European Union (EU) restricted use of PFOS in finished and semi-finished products in 2006 (EU, 2007). The U.S. Environmental Protection Agency (EPA) established a 2005/2010 PFOA Steward Program for reducing emissions from factories and product content of PFOA and its precursors (EPA, 2017). Consequently, the eight largest companies in the PFOA industry achieved complete elimination of PFOA from their production in 2015. In addition to PFOS and PFOA, national and/or regional regulations were enacted in several countries against the use of other long-chain PFAS in consumer products (Wang et al., 2017), including perfluorohexane sulfonate (PFHxS), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUA), and 2-(N-methyl-perfluorooctanesulfonamido) acetate (Me-FOSAA). As a result of the efforts for reducing exposures to some long-chain PFAS and their precursors since the early 2000s, serum concentrations of PFOS, PFOA, PFHxS, and Me-FOSAA (a derivative of PFOS) declined whereas those of PFNA, PFDA, and PFUA showed mixed time trends in the United States and some European countries (Bjerregaard-Olesen et al., 2016; Gribble et al., 2015; Hurley et al., 2018; Okada et al., 2013; Shu et al., 2018).
Children’s early life exposure to PFAS is of public health concern. However, studies of PFAS exposure and its temporal changes have been limited to date mostly to adults and pregnant women. For young children (e.g., 1 to 5 years old), their blood samples are not easy to obtain because of parents’ limited consent on sampling their child’s blood and child’s intentional refusal of blood sampling. As young children likely share a large portion of PFAS exposure sources (e.g., drinking water, food, house dust) with their mothers (Koppen et al., 2019; Song et al., 2013), studying PFAS exposure for mothers with a young child may help understand early childhood exposure to PFAS. In this study, we used 450 serum samples collected from 450 mothers when the child was 2 to 5 years old in a case-control study to (1) assess temporal trends of PFAS maternal serum concentrations and (2) identify determinants of PFAS maternal serum concentrations.
2. Methods
2.1. Study population and sample collection
This study included mothers participating in CHARGE (CHildhood Autism Risk from Genetics and Environment), a population-based case-control study designed to identify causes and contributing factors for autism (Hertz-Picciotto et al., 2006). Children with autism were primarily recruited through the California Department of Developmental Services, as well as from other studies and various clinics, by self- or provider referrals. The general population group (controls) were identified from state birth files and were frequency-matched to the age, sex, and catchment area distribution of the autism cases. Details of study design, recruitment, eligibility for cases and controls, sample size, exposure data, and developmental diagnosis are available elsewhere (Hertz-Picciotto et al., 2006).
CHARGE started collecting serum aliquots in 2009. For the current study, we selected 450 mothers with sufficient volume of available blood serum for quantification of PFAS at the time of enrollment. Among the mothers who enrolled since 2009, approximately 36% of them were not included in the current study because they provided insufficient volume of available serum or had a child who had no diagnosis or incomplete diagnosis, was pending for diagnosis, or received a final diagnosis other than autism spectrum disorder or typical development by February 2017. We included samples collected between 2009 and 2016 in the current study. Two mothers who provided samples in January 2017 were also included in a batch of 2016. The mean age of the child at the time of blood collection from mothers was just under four years (average ± Std. Dev.: 46.5 ± 9.5 months); the youngest and oldest children were 25 months and 61 months old, respectively. Serum was separated from the blood and placed in a −80°C freezer within 24 hours until analysis.
2.2. Quantification of PFAS in serum
We shipped 0.5 mL serum aliquots to the Centers for Disease Control and Prevention (CDC) for analysis. At CDC, we quantified serum concentrations of nine PFAS using solid-phase extraction linked with reversed-phase high-performance liquid chromatography-isotope dilution tandem mass spectrometry (Kato et al., 2011). We applied strict quality control/quality assurance protocols to the analytical measurements. In addition to study samples, we analyzed 38 duplicates for quality assurance. Depending on the analyte and concentration, median relative standard deviations for 19 pairs of blind duplicates ranged from 0 to 6%, except for PFHxS (17%) and PFDA (20%).
The nine PFAS quantified in this study were: PFOA, PFOS, PFHxS, PFNA, PFDA, PFUA, perfluorododecanoate (PFDoDA), Me-FOSAA, and 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-FOSAA). For all PFAS, the limit of detection (LOD) was 0.1 ng/mL (nanograms per milliliter = ppb).
2.3. Statistical analysis
We conducted all statistical analyses using STATA/IC 15.1 (StataCorp LLC, College Station, TX, USA). For concentrations below the LOD, we assigned a value of LOD divided by the square root of 2 (Hornung, 1990). We used ln-transformed PFAS serum concentrations in the regression to account for skewed distributions. To test for significant changes in PFAS serum concentrations over time, we used multiple regression with adjustment for maternal race/ethnicity (white, Hispanic, others), maternal age (in years), breastfeeding duration (in months), maternal education (less than college, bachelor, graduate or professional), pre- pregnancy body mass index (BMI) (in kg/m2), homeownership (yes, no), and parity (1 to 7). After checking crude temporal trends from plots of the sampling time (years) versus yearly average PFAS serum concentrations, we centered our sampling years (2009–2016) at 2013 to robustly account for nonlinear time trends of PFAS serum concentrations.
To estimate the least square mean (LSM) which is the mean of ln[C] for each sampling year, we used the estimated regression coefficients and the computed yearly-specific fractions and averages of the selected covariates. We also computed the least square geometric mean (LSGM) of PFAS serum concentrations for each sampling year, as exp(LSM) with 95% confidence intervals (CIs) as exp(LSM ± 1.97·SELSM), where SELSM is the standard error of the LSM. To construct CIs, we used a critical value 1.97 from the t-distribution, based on the degree of freedom given the numbers of both the study participants and the selected covariates. To examine the relative concentration changes over our study period, we computed average annual percent changes of PFAS serum concentrations using an equation [exp(aRGR) − 1] × 100% with 95% CIs as [exp(aRGR ± 1.97·SEβ) − 1], where aRGR is the average relative growth rate and SEβ is the standard error of time-related regression coefficients (refer to Supplemental Material for derivation). For PFUA, PFDoDA, Me-FOSAA, and Et-FOSAA, which were detected less than 50% in our serum samples, we did not perform the statistical analysis.
We also used multiple regression to examine the association between maternal PFAS serum concentrations and the covariates adjusted in the above regression (refer to Supplemental Material for details). To compare PFAS serum concentrations between CHARGE mothers and the general female population in the U.S. National Health and Nutrition Examination Survey (NHANES) (CDC, 2019), we computed geometric means (GMs) and 95% CIs of PFAS serum concentrations in CHARGE and NHANES by restricting our comparison to the same age range (i.e., 16 to 47 years of age in the two populations). Because NHANES documents biannual biomonitoring data, we grouped our 8-yearly data (2009–2016) every two years, resulting in the four cycles (i.e., 2009–2010, 2011–2012, 2013–2014, 2015–2016), which are matched with NHANES survey cycles. Five PFAS detected in more than 60% of the samples (i.e., PFOA, PFOS, PFHxS, PFNA, PFDA) were included in the comparison, while the remaining four PFAS (i.e., PFUA, PFDoDA, Me-FOSAA, Et-FOSAA) were not included because of their relatively low detection frequencies (<50%) in the CHARGE subjects (Table 1).
Table 1.
Distributions of PFAS maternal serum concentrations (ng/mL) collected from 450 CHARGE mothers.
| % detect | LOD | Min. | 25th | 50th | Mean | GM | 75th | Max. | Std. Dev. | |
|---|---|---|---|---|---|---|---|---|---|---|
| PFOA | 100 | 0.1 | 0.22 | 0.67 | 1.07 | 1.35 | 1.10 | 1.67 | 8.07 | 0.98 |
| PFOS | 100 | 0.1 | 0.37 | 2.20 | 3.20 | 4.07 | 3.29 | 4.90 | 23.80 | 3.05 |
| PFHxS | 98 | 0.1 | <LOD | 0.30 | 0.50 | 0.58 | 0.46 | 0.70 | 4.70 | 0.49 |
| PFNA | 95 | 0.1 | <LOD | 0.40 | 0.50 | 0.60 | 0.49 | 0.70 | 3.40 | 0.42 |
| PFDA | 68 | 0.1 | <LOD | <LOD | 0.20 | 0.21 | 0.16 | 0.30 | 2.10 | 0.20 |
| PFUA | 35 | 0.1 | <LOD | <LOD | <LOD | * | * | 0.10 | 1.70 | * |
| PFDoDA | 3 | 0.1 | <LOD | <LOD | <LOD | * | * | <LOD | 0.60 | * |
| Me-FOSAA | 46 | 0.1 | <LOD | <LOD | <LOD | * | * | 0.20 | 2.40 | * |
| Et-FOSAA | 1 | 0.1 | <LOD | <LOD | <LOD | * | * | <LOD | 0.30 | * |
Detection frequency was calculated based on 450 mothers recruited by CHARGE during 2009–2016.
Not calculated: The proportion of results above the limit of detection was too low to provide a valid result.
Abbreviation: limit of detection (LOD), geometric mean (GM), perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorohexane sulfonate (PFHxS), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUA), perfluorododecanoate (PFDoDA), 2-(N-methyl-perfluorooctanesulfonamido) acetate (Me-FOSAA), 2-(N-ethylperfluorooctanesulfonamido) acetate (Et-FOSAA)
3. Results
3.1. Characteristics of CHARGE mothers
The average age of the mothers at the time of blood serum collection was 30.3 years old, ranging from 16 to 47 years old. The average breastfeeding duration between the delivery of the participating child (or index child) and blood collection was 8.0 months. The mothers included in the study were 59.3% white, 20.2% Hispanic, and 20.4% others (28% black, 48% Asian, and 24% multiracial). Summary statistics of other maternal characteristics are available in Table S1.
3.2. PFAS maternal serum concentrations
Four PFAS were detected in more than 90% of the samples: PFOA (100%), PFOS (100%), PFHxS (98%), and PFNA (95%) (see Table 1). PFDA, PFUA, and Me-FOSAA were detected in 68%, 35%, and 46% of the samples, respectively. The remaining two PFAS were detected in less than 5% of the samples: PFDoDA (3%) and Et-FOSAA (1%). The highest GM was observed in PFOS (3.29 ng/mL), followed by PFOA, PFNA, PFHxS, and PFDA (1.10, 0.49, 0.46, and 0.16 ng/mL, respectively). For PFOA, PFOS, PFHxS, and PFNA, the GMs of CHARGE mothers were lower than those of the general female population reported in NHANES during most of survey cycles (see Figure S1).
3.3. Temporal trends of PFAS maternal serum concentrations
After adjusting for the selected covariates, the LSGMs of PFOA, PFOS, and PFHxS linearly decreased during the study period [percent change (95% confidence interval): −10.7% (12.7%, −8.7%); −10.8% (−12.9%, −8.5%); −8.0% (−10.5%, −5.5%), respectively] (Figure 1). The LSGMs of PFNA increased in early study years and decreased in later study years. On the other hand, the LSGMs of PFDA decreased in early study years and increased in later study years (Table S2).
Figure 1.
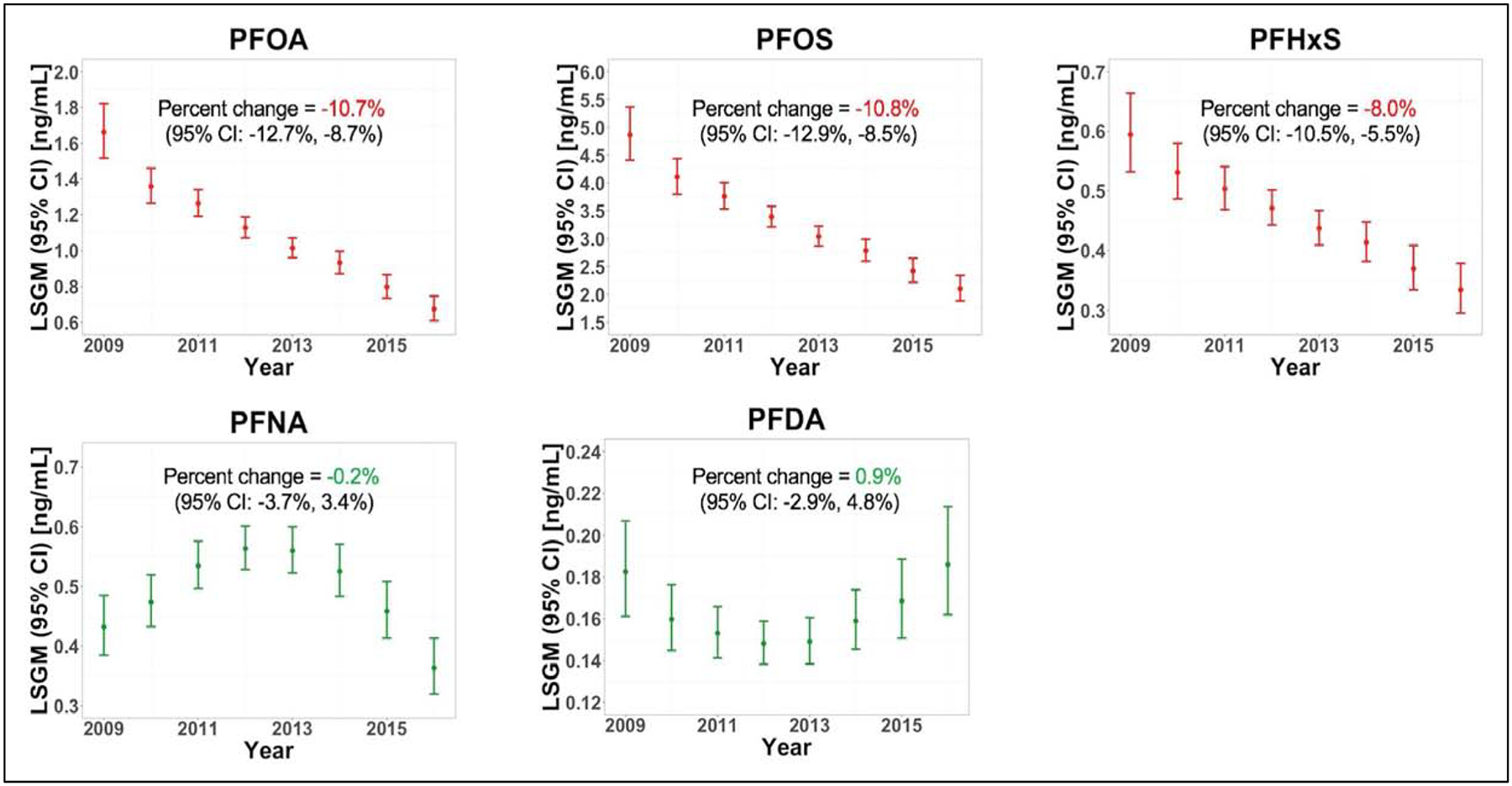
Least square geometric means (LSGMs) of PFAS maternal serum concentrations during our study period and annual average percent change (95% confidence interval). The LSGMs in this figure were estimated from our regression models after adjusting for selected covariates.
3.4. PFAS maternal serum concentrations by maternal characteristics
When analyses were stratified by race/ethnicity, we found that concentration patterns were different among different race/ethnicity groups. For example, for three PFAS showing linear decreasing trends among all mothers (i.e., PFOA, PFOS, PFHxS), LSGMs did not change or relatively slowly decreased over our study period for mothers who are neither white nor Hispanic, compared to white or Hispanic mothers (Figure S2, Table 2). When stratified by maternal education, we found that LSGMs of PFOS, PFHxS, and PFDA were the highest and declined relatively rapidly among mothers who had graduate or professional degrees (Figure S3), compared to mothers with less education (Table 2). For PFDA, LSGMs varied little in the ‘less than college’ subgroup. When stratified by homeownership, we observed that mothers who owned a home tended to have higher LSGMs of PFOA, PFOS, and PFDA than those who did not own a home (Results were not shown because of similar patterns with Figure S3). For PFDA, LSGMs were not different among mothers who did not own a home during our study period (Table 2).
Table 2.
Average annual percent change in serum concentrations and 95% confidence interval among subgroups of each categorical covariate
| Subgroups | Average annual percent change with 95% confidence interval | |||||
|---|---|---|---|---|---|---|
| PFOA | PFOS | PFHxS | PFNA | PFDA | ||
| Race/Ethnicity | white | −11.4* (−14.1, −8.7) | −10.5* (−13.4, −7.5) | −8.6* (−11.9, −5.2) | 1.2 (−3.5, 6.1) | 2.0 (−2.8, 7.1) |
| Hispanic | −11.9 (−16.6, −6.9) | −14.2* (−18.8, −9.3) | −9.8* (−14.9, −4.3) | −6.5 (−14.5, 2.1) | −1.7 (−10.5, 8.0) | |
| Othersa | −7.7* (−13.0, −2.2) | −9.3* (−14.6, −3.7) | −3.3 (−10.0, 4.0) | 2.4 (−6.1, 11.5) | 0.6 (−8.5, 10.5) | |
| Education | Less than college | −12.4* (−15.4, −9.4) | −11.4* (−14.5, −8.2) | −7.7* (−11.3, −4.0) | −3.2 (−8.4, 2.2) | 0.7 (−4.8, 6.5) |
| Bachelor | −9.2* (−12.9, −5.4) | −9.9* (−13.6, −5.9) | −6.3* (−10.6, −1.7) | 2.4 (−3.2, 8.2) | 2.0 (−4.6, 9.0) | |
| Graduate or professional | −10.8* (−15.9, −5.3) | −12.9* (−18.8, −6.7) | −12.8* (−19.7, −5.2) | 1.9 (−8.6, 13.6) | −2.7 (−11.5, 7.0) | |
| Homeownership | No | −9.2* (−12.9, −5.3) | −10.1* (−13.7, −6.4) | −4.3 (−9.0, 0.7) | −3.7 (−9.7, 2.5) | 1.6 (−5.1, 8.7) |
| Yes | −11.6* (−14.1, −9.1) | −11.3* (−14.0, −8.4) | −9.8* (−12.7, −6.8) | 1.3 (−3.2, 6.0) | 0.1 (−4.5, 4.9) | |
For PFOA, PFOS, and PFHxS, we provided significance (* or **) of p-value of the null hypothesis that the regression coefficient (β) of ‘year’ equals to zero.
For PFNA and PFDA, we provided significance (*) of p-value of the null hypothesis that the regression coefficient (β) of ‘year2’ equals to zero.
p-value < 0.05
Includes 28% black, 48% Asian, and 28% multiracial.
For PFOA and PFDA, LSGMs increased with mother’s age [percent change (95% CIs) per one year of age: 1.2% (0.1%, 2.3%); 1.7% (0.3%, 3.2%), respectively] (Table 3). LSGMs of PFOA, PFOS, PFHxS, PFNA, and PFDA decreased with breastfeeding duration [percent change (95% CIs) per one month of breastfeeding: −2.8% (−3.5%, −2.2%); −2.0% (−2.6%, −1.3%); −1.8% (−2.6%, −1.1%); −1.5% (−2.3%, −0.7%); −1.3% (−2.1%, −0.5%), respectively]. We also found that LSGMs of PFNA and PFDA decreased with pre-pregnancy BMI [percent change (95% CIs) per one unit increase in the pre-pregnancy BMI (in kg/m2): −1.5% (−2.5%, −0.4%); −1.9% (−3.0%, 0.8%), respectively]. In addition, LSGMs of PFOS and PFHxS decreased with increasing parity [percent change (95% CIs) per each additional pregnancy: −8.6% (−14.1%, −2.8%); −14.5% (−20.2%, −8.3%), respectively].
Table 3.
Percent changes of PFAS maternal serum concentrations per one-unit increase of each covariate
| Covariate | Compound | βa | SEβb | p-valuec | % change per one- unit increase | 95% confidence interval |
|---|---|---|---|---|---|---|
| Maternal age (in year) | PFOA | 0.012 | 0.006 | 0.04 | 1.2 | (0.1%, 2.3%) |
| PFOS | 0.010 | 0.006 | 0.09 | 1.0 | (−0.2%, 2.2%) | |
| PFHxS | 0.006 | 0.007 | 0.41 | 0.6 | (−0.8%, 1.9%) | |
| PFNA | 0.011 | 0.007 | 0.11 | 1.1 | (−0.2%, 2.5%) | |
| PFDA | 0.017 | 0.007 | 0.02 | 1.7 | (0.3%, 3.2%) | |
| Breastfeeding duration (in month) | PFOA | −0.029 | 0.003 | < 0.01 | −2.8 | (−3.5%, −2.2%) |
| PFOS | −0.020 | 0.003 | < 0.01 | −2.0 | (−2.6%, −1.3%) | |
| PFHxS | −0.018 | 0.004 | < 0.01 | −1.8 | (−2.6%, −1.1%) | |
| PFNA | −0.015 | 0.004 | < 0.01 | −1.5 | (−2.3%, −0.7%) | |
| PFDA | −0.013 | 0.004 | < 0.01 | −1.3 | (−2.1%, −0.5%) | |
| Pre-pregnancy BMI (in kg/m2) | PFOA | −0.005 | 0.004 | 0.26 | −0.5 | (−1.3%, 0.4%) |
| PFOS | −0.006 | 0.005 | 0.17 | −0.6 | (−1.5%, 0.3%) | |
| PFHxS | −0.001 | 0.005 | 0.88 | −0.1 | (−1.1%, 0.9%) | |
| PFNA | −0.015 | 0.005 | < 0.01 | −1.5 | (−2.5%, −0.4%) | |
| PFDA | −0.019 | 0.006 | < 0.01 | −1.9 | (−3.0%, −0.8%) | |
| Parity | PFOA | −0.026 | 0.030 | 0.39 | −2.5 | (−8.1%, 3.4%) |
| PFOS | −0.090 | 0.032 | < 0.01 | −8.6 | (−14.1%, −2.8%) | |
| PFHxS | −0.156 | 0.036 | < 0.01 | −14.5 | (−20.2%, −8.3%) | |
| PFNA | −0.028 | 0.037 | 0.45 | −2.8 | (−9.6%, 4.6%) | |
| PFDA | −0.007 | 0.039 | 0.86 | −0.7 | (−8.0%, 7.2%) |
β represents the regression coefficient of each covariate in the regression models.
SEβ represents the standard error of the regression coefficient (β).
p-value of the null hypothesis that the regression coefficient (β) of each covariate equals to zero.
4. Discussion
In this study, we used samples from mothers with a young child to examine temporal changes of PFAS maternal serum concentrations and to identify determinants of PFAS maternal serum concentrations. Our results showed that PFOA, PFOS, and PFHxS serum concentrations of mothers with a young child decreased during the study period. On the other hand, PFNA and PFDA maternal serum concentrations exhibited mixed time trends over the same period. Longer breastfeeding duration was associated with decreased maternal serum concentrations of PFOA, PFOS, PFHxS, PFNA, and PFDA, which were detected most frequently in our study subjects. Older mothers tended to have higher maternal serum concentrations of PFOA and PFDA. We also observed that pre-pregnancy BMI, parity, maternal education, race/ethnicity, and homeownership affected maternal serum concentrations of some PFAS.
The decreasing trends of PFOA, PFOS, and PFHxS observed in our study are consistent with those of other studies reported for other U.S. populations (Table S3) (Gribble et al., 2015; Hurley et al., 2018; Olsen et al., 2012). The results of these studies and our current study suggest that serum concentrations of PFOA, PFOS, and PFHxS are decreasing in the U.S. general population. Furthermore, the decreasing trends of PFOA, PFOS, and PFHxS in this study are also consistent with results of other studies outside the USA (Bjerregaard-Olesen et al., 2016; Eriksson et al., 2017; Okada et al., 2013; Shu et al., 2018) (Table S3). Serum concentrations of PFNA and PFDA did not show decreasing time trends in the present study. Three studies reported downward trends of PFNA and PFDA serum concentrations within the U.S. (Gribble et al., 2015; Hurley et al., 2018; Jain, 2018), whereas one study reported increasing trends (Olsen et al., 2012) (Table S3). The inconsistent results of PFNA and PFDA among studies may partially relate to the differences in study populations, study periods, and concentrations.
Different annual percent changes may reflect physiological differences of individual PFAS. For example, for PFOA and PFOS that were relatively strictly regulated during similar time periods and have relatively similar biological elimination half-lives, we observed that the rates of decrease were almost identical for PFOA (−10.7% per year) and PFOS (−10.8% per year). However, PFHxS serum concentrations were decreasing rather slowly (8.0% per year) compared to PFOA and PFOS in the same population, although PFHxS was phased out in the early 2000s with PFOS (Calafat et al., 2007). The slower rate of decrease in PFHxS serum concentrations observed in this study is also consistent with results of previous studies (Bjerregaard-Olesen et al., 2016; Gribble et al., 2015; Hurley et al., 2018). One possible reason for the slower decrease of PFHxS serum concentrations is its longer biological elimination half-life (5.3 – 15.5 years) (Li et al., 2018; Olsen et al., 2007; Worley et al., 2017) than those of PFOA (2.4 – 3.9 years) (Bartell et al., 2010; Gomis et al., 2016; Li et al., 2018; Olsen et al., 2007; Russell et al., 2015; Worley et al., 2017) and PFOS (3.3 – 5.4 years) (Li et al., 2018; Olsen et al., 2007; Worley et al., 2017). Moreover, because PFHxS was used in some Scotchgard formulation to treat carpets and furniture (Beesoon et al., 2012) and these consumer items tend to have a long product life, it is likely that PFHxS exposure may continue for years after PFHxS was phased out for use.
Our study identified several determinants of maternal PFAS serum concentrations (Tables 2 and 3, Figures S2, S3, and S4). For example, breastfeeding duration was found to be negatively associated with PFAS maternal serum concentrations for the five most frequently detected compounds (i.e., PFOA, PFOS, PFHxS, PFNA, PFDA) in the current study (Table 2). This is additional evidence that lactation is a major excretion route of PFAS for nursing mothers, as presented in previous studies (Mogensen et al., 2015; Papadopoulou et al., 2016). We observed differences in the PFAS maternal serum concentrations and rates of decrease with different maternal race/ethnicity. This differences might be, in part, due to differences in dietary habits or metabolism by different race/ethnicity (Jain, 2014; Johnson, 1997). Higher maternal serum concentrations of PFOA and PFDA were associated with increased maternal age. This is consistent with a previous study reporting higher serum concentrations in older people (Bjermo et al., 2013). Older mothers are likely to have greater chances of PFAS exposure than younger mothers before the common long-chain PFAS were phased out. In addition, mothers with high parity had relatively low serum concentrations of PFOS and PFHxS. Multiple placental transfers of PFAS and subsequent lactational transfers may contribute to the low serum concentrations of the CHARGE mothers with high parity (Bjermo et al., 2013; Brantsaeter et al., 2013; Park et al., 2019). Mothers who received higher education tended to have high serum concentrations of PFOS, PFHxS, and PFDA in early study years, which were consistent with results of a previous study (Bjermo et al., 2013). The fact that women in the United States with higher education were known to consume more fast foods that likely contain PFAS in packaging materials may explain this finding (Hidaka et al., 2018). Mothers who had high pre-pregnancy BMI also had high maternal serum concentrations of PFNA and PFDA, which were consistent with results of previous studies (Fei et al., 2007; Olsen et al., 1998).
There are limitations and strengths of this study. We cannot ascertain that our study results (e.g., decreasing trends in maternal PFAS serum concentrations, determinants of maternal PFAS serum concentrations) are applicable to the U.S. general female population, because our study population was limited to a select group of mothers with a young child, living in California. For PFOA, PFOS, PFHxS, and PFNA, serum concentrations of the CHARGE mothers were overall lower than those of the NHANES participants. The differences in PFAS serum concentrations between our study and NHANES (Figure S1) may relate to breastfeeding practices and child birth within our study population. For PFNA and PFDA showing a mixed trend or no significant trend in our study, their concentrations were decreasing in NHANES. We did not directly determine the degree to which concentrations decreased because of those factors individually. Furthermore, temporal trends of PFAS serum concentrations within same participants over time were not examined in the current study because our samples were from a case-control study with a single sample for each individual woman. In spite of these limitations, results in our study can help us understand exposure to common long-chain PFAS and their time trends among mothers with a young child. From the investigation of the relationship between breastfeeding duration and maternal serum concentrations, which has not been thoroughly evaluated by previous studies on PFAS temporal trend, we found that breastfeeding duration was inversely associated (p-values <0.01) with maternal serum concentrations of the five most dominant compounds (i.e., PFOA, PFOS, PFHxS, PFNA, PFDA) in the current study.
While there is a public health need to estimate PFAS body burden levels in young children, PFAS concentrations in cord blood are problematic, as evidenced by the weak to moderate correlations between PFAS concentrations in cord blood and those in 3-year-old child’s serum: 0.45 for PFOA, 0.21 for PFOS, and 0.46 for PFHxS (Kingsley et al., 2018). Among infants who exclusively breastfed without consuming other foods, their PFAS serum concentrations increased 27.8% for PFOA, 29.2% for PFOS, 20.8% for PFNA, and 18.1% for PFDA per month of breastfeeding (Mogensen et al., 2015), suggesting that breastfeeding duration would be another important predictor of young children’s PFAS exposure. PFAS serum concentrations of mothers with a young child were positively associated with those of their young child (Wu et al. 2015). Our results further support the importance of accounting for breastfeeding histories in a pharmacokinetic model with PFAS serum concentrations collected from mothers with a young child to help estimate or reconstruct early childhood exposure.
In addition to the compounds detected in our serum samples, other 8 PFAS were detected in residential indoor dust (Shin et al., 2019). It is known that thousands of PFAS are, or have been, on the global market (Wang et al., 2017). Since the common long-chain PFAS, including PFOA, PFOS, and PFHxS, were phased out, there must be increasing trends in the production of replacement to these long-chain PFAS or novel PFAS. In addition, there are many overlooked compounds that are structurally similar to PFOS, PFOA, or their precursors, and are produced in high volumes (e.g., >10 tones per year, see references 6 to 9 in Wang et al. 2017). Because of structural similarity among many PFAS, future biomonitoring studies may need to include a large number of PFAS that have been detected in environmental samples, food, or consumer products.
5. Conclusions
We reported time trends in PFAS serum concentrations among 450 Northern California women with a young child between 2009 and 2016. Maternal serum concentrations of PFOA, PFOS, and PFHxS monotonically decreased during the study period, while those of PFNA and PFDA exhibited mixed trends. The current study also found that breastfeeding duration was negatively associated with maternal serum concentrations of PFOA, PFOS, PFHxS, PFNA, and PFDA, adding to the existing evidence that lactation is a major excretion route of the common long-chain PFAS for nursing mothers. To improve our understanding of PFAS exposure trends, further biomonitoring studies are recommended to include substitutes of the common long-chain PFAS and other PFAS frequently detected in indoor dust and other exposure matrix.
Supplementary Material
PFOA, PFOS, and PFHxS monotonically decreased during the study period.
PFNA and PFUA exhibited mixed trends.
Breastfeeding contributed to the elimination of the common PFAS in lactating mothers
Our study participants had lower PFAS serum concentrations than NHANES participants
Acknowledgments
Authors would like to acknowledge the CHARGE participants for allowing us to make this research possible. The authors also wish to thank Kayoko Kato and the late Xiaoyun Ye for the quantification of the PFAS serum concentrations. This research was supported by grants from the National Institute of Environmental Health Sciences (R21ES028131, R01ES015359, R01ES031701, P30ES023513); NIH Office of the Director (UH3OD023365); and the U.S. EPA STAR #R829388, R833292, and R835432.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC (Centers for Disease Control and Prevention). Use of trade name is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Ethics approval and consent to participate
The CHARGE study protocol and this study were approved by the institutional review boards for the State of California, the University of California-Davis (UC-Davis), and the University of Texas-Arlington (UT-Arlington). Participants provided written informed consent before collection of any data. The analysis of coded specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined by CDC not to constitute engagement in human subject research.
Availability of data and material
Portions of the datasets generated and analyzed during this study are publicly available in the National Institute of Mental Health (NIMH) Data Archive. The entire non-identifiable dataset is available from the authors upon reasonable request and with permission from the IRBs at UT-Arlington and UC-Davis.
Competing interests
Authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- Austin ME, et al. , 2003. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 111, 1485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, et al. , 2010. Rate of Decline in Serum PFOA Concentrations after Granular Activated Carbon Filtration at Two Public Water Systems in Ohio and West Virginia. Environmental Health Perspectives. 118, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, et al. , 2012. Exceptionally High Serum Concentrations of Perfluorohexanesulfonate in a Canadian Family are Linked to Home Carpet Treatment Applications. Environmental Science & Technology. 46, 12960–12967. [DOI] [PubMed] [Google Scholar]
- Begley TH, et al. , 2008. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Additives and Contaminants. 25, 384–390. [DOI] [PubMed] [Google Scholar]
- Begley TH, et al. , 2005. Perfluorochemicals: Potential sources of and migration from food packaging. Food Additives and Contaminants. 22, 1023–1031. [DOI] [PubMed] [Google Scholar]
- Bjermo H, et al. , 2013. Serum concentrations of perfluorinated alkyl acids and their associations with diet and personal characteristics among Swedish adults. Molecular Nutrition & Food Research. 57, 2206–2215. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, et al. , 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environment International. 91, 14–21. [DOI] [PubMed] [Google Scholar]
- Brantsaeter AL, et al. , 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environment International. 54, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, et al. , 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 7, 513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, et al. , 2004. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 196, 95–116. [DOI] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2007. Polyfluoroalkyl chemicals in the US population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environmental Health Perspectives. 115, 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Fourth National Report on Human Exposure to Environmental Chemicals. 1, 1–866. [Google Scholar]
- EPA, Technical Fact Sheet-Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA), EPA (505-F-17–001). 2017.
- Eriksson U, Karrman A, 2015. World-Wide Indoor Exposure to Polyfluoroalkyl Phosphate Esters (PAPs) and other PFASs in Household Dust. Environmental Science & Technology. 49, 14503–14511. [DOI] [PubMed] [Google Scholar]
- Eriksson U, et al. , 2017. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environmental Pollution. 220, 168–177. [DOI] [PubMed] [Google Scholar]
- EU, 2007. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH). Offical journal of the European Union. L396. [Google Scholar]
- Fei CY, et al. , 2007. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environmental Health Perspectives. 115, 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, et al. , 2016. Contribution of Direct and Indirect Exposure to Human Serum Concentrations of Perfluorooctanoic Acid in an Occupationally Exposed Group of Ski Waxers. Environmental Science & Technology. 50, 7037–7046. [DOI] [PubMed] [Google Scholar]
- Gribble MO, et al. , 2015. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environmental Research. 143, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, et al. , 2005. Effects of PFOS and PFOA on L-type Ca2+ currents in guinea-pig ventricular myocytes. Biochemical and Biophysical Research Communications. 329, 487–494. [DOI] [PubMed] [Google Scholar]
- Haug LS, et al. , 2009. Time Trends and the Influence of Age and Gender on Serum Concentrations of Perfluorinated Compounds in Archived Human Samples. Environmental Science & Technology. 43, 2131–2136. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, et al. , 2006. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 114, 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka BH, et al. , 2018. Fast food consumption is associated with higher education in women, but not men, among older adults in urban safety-net clinics: A cross-sectional survey. Prev Med Rep. 12, 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW et al. , 1990. Estimation of average concentrations in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 5, 46–51. [Google Scholar]
- Hurley S, et al. , 2018. Time Trends in Per- and Polyfluoroalkyl Substances (PFASs) in California Women: Declining Serum Levels, 2011–2015. Environmental Science & Technology. 52, 277–287. [DOI] [PubMed] [Google Scholar]
- Jain RB, 2014. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. International Journal of Hygiene and Environmental Health. 217, 52–61. [DOI] [PubMed] [Google Scholar]
- Jain RB, 2018. Time trends over 2003–2014 in the concentrations of selected perfluoroalkyl substances among US adults aged >= 20 years: Interpretational issues. Science of the Total Environment. 645, 946–957. [DOI] [PubMed] [Google Scholar]
- Johnson JA, 1997. Influence of race or ethnicity on pharmacokinetics of drugs. Journal of Pharmaceutical Sciences. 86, 1328–1333. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. , 2011. Trends in Exposure to Polyfluoroalkyl Chemicals in the US Population: 1999–2008. Environmental Science & Technology. 45, 8037–8045. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, et al. , 2018. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environmental Research. 165, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen G, et al. , 2019. Mothers and children are related, even in exposure to chemicals present in common consumer products. Environmental Research. 175, 297–307. [DOI] [PubMed] [Google Scholar]
- Kudo N, et al. , 1999. Alterations by perfluorooctanoic acid of glycerolipid metabolism in rat liver. Chem Biol Interact. 118, 69–83. [DOI] [PubMed] [Google Scholar]
- Lau C, et al. , 2004. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 198, 231–41. [DOI] [PubMed] [Google Scholar]
- Lau C, et al. , 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicological Sciences. 90, 510–518. [DOI] [PubMed] [Google Scholar]
- Lau C, et al. , 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 74, 382–92. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. , 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and Environmental Medicine. 75, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, et al. , 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 45, 7954–61. [DOI] [PubMed] [Google Scholar]
- Liu RC, et al. , 1996. Effect of the peroxisome proliferator, ammonium perfluorooctanoate (C8), on hepatic aromatase activity in adult male Crl:CD BR (CD) rats. Fundam Appl Toxicol. 30, 220–8. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, et al. , 2015. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environmental Science & Technology. 49, 10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nost TH, et al. , 2014. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: Assessing time trends, compound correlations and relations to age/birth cohort. Environment International. 67, 43–53. [DOI] [PubMed] [Google Scholar]
- Okada E, et al. , 2013. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environment International. 60, 89–96. [DOI] [PubMed] [Google Scholar]
- Olsen GW, et al. , 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental Health Perspectives. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, et al. , 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. Journal of Occupational and Environmental Medicine. 40, 614–622. [DOI] [PubMed] [Google Scholar]
- Olsen GW, et al. , 2012. Temporal Trends of Perfluoroalkyl Concentrations in American Red Cross Adult Blood Donors, 2000–2010. Environmental Science & Technology. 46, 6330–6338. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, et al. , 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environment International. 94, 687–694. [DOI] [PubMed] [Google Scholar]
- Park SK, et al. , 2019. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environmental Research. 175, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, et al. , 2015. Calculation of chemical elimination half-life from blood with an ongoing exposure source: The example of perfluorooctanoic acid (PFOA). Chemosphere. 129, 210–216. [DOI] [PubMed] [Google Scholar]
- Seacat AM, et al. , 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicological Sciences. 68, 249–264. [DOI] [PubMed] [Google Scholar]
- Shin HM, et al. , 2019. Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, et al. , 2018. Temporal trends and predictors of perfluoroalkyl substances serum levels in Swedish pregnant women in the SELMA study. Plos One. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NR, et al. , 2013. Biomonitoring of urinary di(2-ethylhexyl) phthalate metabolites of mother and child pairs in South Korea. Environment International. 54, 65–73. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, et al. , 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 74, 369–81. [DOI] [PubMed] [Google Scholar]
- Vandenheuvel JP, et al. , 1992. Renal Excretion of Perfluorooctanoic Acid in Male-Rats - Inhibitory Effect of Testosterone. Journal of Biochemical Toxicology. 7, 31–36. [DOI] [PubMed] [Google Scholar]
- Wang ZY, et al. , 2017. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science & Technology. 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- Winkens K, et al. , 2017. Perfluoroalkyl acids and their precursors in indoor air sampled in children’s bedrooms. Environmental Pollution. 222, 423–432. [DOI] [PubMed] [Google Scholar]
- Worley RR, et al. , 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environment International. 106, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, et al. , 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res. 136, 264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YM, et al. , 2018. Per- and Polyfluoroalkyl Substances (PFASs) in Indoor Air and Dust from Homes and Various Microenvironments in China: Implications for Human Exposure. Environmental Science & Technology. 52, 3156–3166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


