Abstract
During human immunodeficiency virus (HIV-1) entry into target cells, binding of the virus to host receptors, CD4 and CCR5/CXCR4, triggers serial conformational changes in the envelope glycoprotein (Env) trimer that result in the fusion of the viral and cell membranes. Recent discoveries have refined our knowledge of Env conformational states, allowing characterization of the targets of small-molecule HIV-1 entry inhibitors and neutralizing antibodies, and identifying a novel off-pathway conformation (State 2A). Here, we provide an overview of the current understanding of these conformational states, focusing on: 1) the events during HIV-1 entry; 2) conformational preferences of HIV-1 Env ligands; 3) evasion of the host antibody response; 4) and potential implications for therapy and prevention of HIV-1 infection.
Keywords: HIV-1 Env, conformational states, bNAbs, entry inhibitors, ADCC, vaccine design
I. General overview of Env
Discovered in 1981, human immunodeficiency virus (HIV-1) represents a major threat to human health, infecting ~75 million people and causing ~32 million deaths since the start of the epidemic [1]. HIV-1 is a member of the lentivirus subfamily of retroviruses; lentiviruses can establish persistent infections in immunocompetent hosts. In contrast to the unprecedented pace of development of antiretroviral therapies that can control HIV-1 infection and delay disease progression, prophylaxis of HIV-1 transmission has been less successful. Despite decades of effort, a protective vaccine has not been developed. As the only HIV-1 protein on the surface of infected cells and virions, the envelope glycoprotein (Env) trimer is the sole target of host antibodies that mediate virus neutralization or antibody-dependent cellular cytotoxicity (ADCC) [2, 3]. In monkey models of HIV-1 infection, protection strongly correlates with the levels of serum antibodies that can neutralize the challenge virus [4–9].
In the infected cell, Env is produced and processed in the secretory pathway. The env gene encodes an ~850-residue precursor that is cotranslationally inserted into the endoplasmic reticulum (ER) membrane, trimerizes and is modified by high-mannose glycans [10]. After transport from the ER to the Golgi, the gp160 precursor is incompletely modified by complex glycans and proteolytically cleaved by a cellular furin protease into the surface unit (gp120) and the transmembrane unit (gp41) [11, 12]. Three gp120-gp41 heterodimers form a functional trimer spike on the surface of the infected cell; the non-covalent association of gp120 with the Env trimer results in shedding of gp120 from a subset of Envs. Some of the mature Env trimers are incorporated into budding viruses. A fully infectious virion displays approximately 10–14 spikes per particle [13]. This relatively low level of Envs may help the virus evade the immune response.
HIV-1 naturally infects T lymphocytes, as well as monocytes, macrophages, dendritic cells and, in the central nervous system, microglia. These cells express receptors for HIV-1, CD4 and a coreceptor, either CCR5 or CXCR4. Upon binding these receptors, HIV-1 Env mediates the fusion of the viral and target cell membranes, resulting in the delivery of the viral core into the cytoplasm of the cell [14, 15]. Both cell-free virus spread and the highly efficient cell-cell transmission of HIV-1 across virological synapses require Envs that are competent for receptor binding and membrane fusion [16, 17].
Untreated HIV-1-infected individuals experience a progressive decrease in their CD4+ T lymphocytes, which ultimately leads to life-threatening acquired immunodeficiency syndrome (AIDS). Direct viral cytopathic effects, which include syncytium formation and the lysis of single cells, are mediated by Env and contribute to T-lymphocyte depletion [18, 19]. Surface-displayed HIV-1 Envs can fuse the infected cell with uninfected, receptor-bearing cells, resulting in lethal multinucleated syncytia [20, 21]. Intracellularly, Envs and receptors in the secretory pathway can interact, leading to membrane-damaging fusion events and single-cell lysis [22, 23]. Host immune effectors, including cytotoxic T lymphocytes and ADCC-mediating natural killer cells, could potentially destroy infected cells and innocent bystander CD4+ cells that have captured shed gp120, respectively [18, 24].
Lentiviruses like HIV-1 establish persistent infections in their hosts, continuing to replicate in the face of the antiviral immune response. As the sole virus-specific protein on the surface of HIV-1 virions, Env has evolved features that diminish the elicitation and impact of antibodies that could potentially neutralize HIV-1 or destroy infected cells by ADCC. These Env features include: 1) a high degree of glycosylation, with more than half of the molecular mass of gp120 composed of N-linked carbohydrates [25]; 2) extensive variation among HIV-1 strains, with five hypervariable gp120 regions (V1-V5) interspersed among more constant regions (C1-C5); 3) conformational masking of antibody epitopes (described below). As a consequence of these Env features, syncytium formation and infection of most primary, clinical HIV-1 variants (“Tier 2” or “Tier 3” viruses) are not effectively inhibited by the high-titer anti-Env antibodies elicited during natural infection [20, 25]. Antibody binding to any Env surface can lead to neutralization, so this resistance to a polyclonal, diverse set of antibodies is a remarkable testament to the effectiveness of Env’s defenses. Some of the easily elicited, poorly neutralizing antibodies can mediate potent ADCC against HIV-1-infected cells [28, 29]; as will be discussed below, HIV-1 has evolved strategies to minimize the antiviral impact of these antibodies. Here, we review current understanding of HIV-1 Env conformations and discuss the implications for HIV-1 therapy and prophylaxis.
II. Conformational changes associated with HIV-1 entry
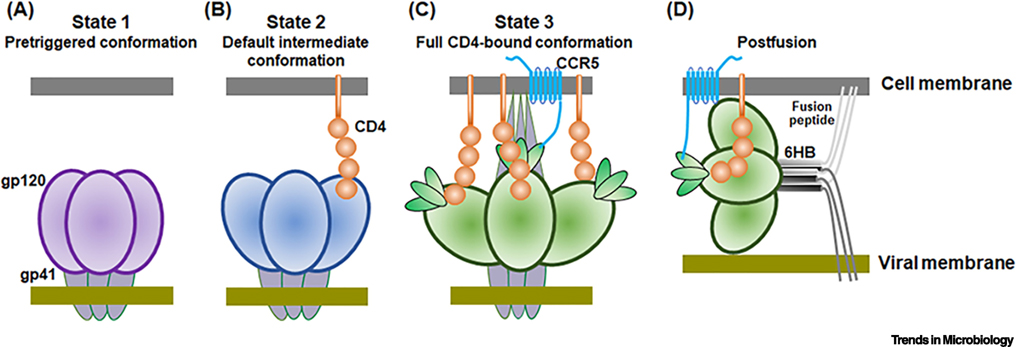
The HIV-1 Env trimer is a receptor-activated, membrane-fusing molecular machine [2, 14]. Unlike many proteins that fold into a low-energy ground state, the Env trimer is trapped during its folding in a local energy well. The potential energy stored in the pretriggered (State-1) conformation of the Env trimer is used to drive the fusion of the viral and target cell membranes. The implied tension and compression in the metastable State-1 Env trimer is relaxed in a graduated manner by receptor binding. The conformational transitions in the HIV-1 Env resulting from CD4 binding have been studied by single-molecule fluorescence resonance energy transfer (smFRET) [30, 31] (Figure 1). CD4 engagement triggers the transition of Env from State 1 to an intermediate conformation, State 2. When one protomer of the Env trimer binds CD4, the other two protomers assume State-2 conformations [31]. Additional CD4 binding transforms Env into the full CD4-bound (State-3) conformation. In State 3, gp120 assumes a conformation that is competent for CCR5/CXCR4 interaction, with the V3 variable region exposed and the bridging sheet formed. The heptad repeat 1 (HR1) region in gp41 forms a trimeric helical coiled coil in the State-3 Env. The binding of gp120 to the coreceptor, either CCR5 or CXCR4, leads to additional Env conformational transitions [2, 14, 15]. During the process of receptor binding, the hydrophobic fusion peptide at the N terminus of gp41 inserts into the target cell membrane. Subsequently, the heptad repeat 2 (HR2) region of gp41, which is located near the viral membrane, binds in an anti-parallel manner to the hydrophobic groove on the HR1 trimeric coiled coil. This results in the formation of an energetically stable six-helix bundle (6HB), driving the viral and target cell membranes into proximity. Some studies have suggested that a small number of Env spikes are needed for HIV-1 entry, a model consistent with the low spike density on HIV-1 virion particles [13, 32].
Figure 1. Env conformational states during HIV-1 entry into cells.
The figure schematically depicts the HIV-1 Env conformational states relevant to the virus entry process. (A) The pretriggered (State-1) conformation resists the binding of all antibodies, except for most bNAbs and autologous (strain-restricted) neutralizing antibodies. (B) Binding to a single CD4 receptor triggers a transformation of the State-1 Env to a default intermediate, partially “open” conformation (State 2). (C) In the full CD4-bound conformation (State 3), the gp41 heptad repeat (HR1) region coiled coil is formed and exposed. This pre-hairpin intermediate is competent for interaction with the CCR5 coreceptor, which promotes additional conformational changes in Env. (D) As a result, the hydrophobic fusion peptide at the N-terminus of gp41 is inserted into the target cell membrane and the energetically stable six-helix bundle (6HB) is formed, driving the fusion of the viral and cell membranes.
CD4-induced transitions of HIV-1 Env from State-1 to State-2 conformations have been targeted by small-molecule entry inhibitors. Conformational blockers, the prototype of which is BMS-806, inhibit CD4-induced formation/exposure of the gp41 HR1 coiled coil; at higher concentrations, BMS-806 can interfere with gp120-CD4 interaction [33–37]. CD4-mimetic compounds (CD4mcs) bind near the CD4-binding site of gp120, competitively inhibit CD4 binding, and prematurely trigger conformational changes in Env similar to those induced by CD4 [38–40]. Envs bound to two or three CD4mcs are inactivated, with a functional half-life of approximately 5 minutes at 37°C [41, 42]. At lower stoichiometry, CD4mcs facilitate the exposure of CD4-induced epitopes on the Env spike, sensitizing virus particles to neutralization and infected cells to ADCC by otherwise ineffectual antibodies [43, 44].
The unliganded, pretriggered Env of most primary HIV-1 strains is in a State-1 conformation [30, 31]. However, changes in specific gp120 and gp41 amino acid residues can destabilize State 1, disrupting its closed, pretriggered conformation; these amino acids have been termed “restraining residues” because they are postulated to help maintain the Env trimer in a State-1 conformation [45–50]. Their conservation among HIV-1 strains suggests that the State-1 Env conformations of most HIV-1 variants share common features. Intriguingly, many HIV-1 Envs with alterations in restraining residues spontaneously assume downstream conformations on the virus entry pathway. Some Env mutants become completely independent of CD4 and can support HIV-1 entry into CD4-negative cells expressing CCR5 or CXCR4; these mutants are thought to sample State-3-like conformations spontaneously [50–52]. More often, viruses with altered Env restraining residues remain CD4-dependent, but can infect cells with lower levels of CD4, compared with the parental wild-type virus. These mutants exhibit greater sensitivity to inhibition by soluble CD4, CD4mcs and many poorly neutralizing antibodies [47–49]. In some cases, these viruses are less readily neutralized by State-1-preferring ligands, such as broadly neutralizing antibodies and conformational blockers like BMS-806 [45, 46]. The identification of the State-2 intermediate and the documentation that Env mutants with altered restraining residues exhibit greater occupancy of State 2 provided a satisfying explanation of the observed viral phenotypes [45, 46]. Because multiple diverse amino acid changes in Env result in viruses with similar phenotypes, we suggest that State 2 represents a partially open default Env trimer conformation readily derived when the metastable State-1 conformation is disrupted. T cell line-adapted (TCLA) HIV-1 variants exhibit Tier-1 neutralization sensitivity phenotypes similar to those of HIV-1 mutants with altered restraining residues; indeed, Envs from some TCLA viruses have been shown to sample State-2 conformations more frequently than Envs from most primary HIV-1 strains [30]. The propensity of HIV-1 Envs to leave State 1 and move into downstream conformations (States 2 and 3) is dictated by the activation barrier separating these states; the “intrinsic reactivity” or “triggerability” of Env is inversely proportionate to the height of this activation barrier [50, 51]. Destabilization of State 1 results in Envs that are readily triggered by, and thus globally sensitive to, multiple State 2/3-preferring ligands such as soluble CD4, CD4mcs, and many antibodies; such Envs are also inactivated by exposure to the cold. Although most naturally occurring HIV-1 strains, including transmitted/founder viruses, are relatively resistant to antibody neutralization, they can exhibit a range of sensitivities to soluble CD4 and cold; thus, the activation barriers for each of these stimuli can be regulated independently by changes in Env [49, 53–55]. Alteration of the particular activation barriers between State-1 and State-2 Env conformations can affect HIV-1 requirements for target cell levels of CD4, susceptibility to small-molecule virus entry inhibitors, and sensitivity to neutralization or ADCC by antibodies. Thus, the existence of an intermediate (State-2) conformation between the pretriggered (State-1) and full CD4-bound (State-3) Env conformations equips HIV-1 with the flexibility to adjust to the specific requirements imposed by the local environment.
III. Interactions of HIV-1 Env ligands with particular conformational states
The host antibody response imposes major selective pressure on the HIV-1 Env (Figure 2). Any antibody that binds the functional Env trimer can potentially neutralize the virus [56], so avoiding the binding of antibodies is a requirement for HIV-1 to survive in the host. High titers of non-neutralizing or poorly neutralizing antibodies directed against Env are elicited early during the course of natural HIV-1 infection. Many of these antibodies recognize epitopes that are exposed on the Env trimer only after CD4 binding. These epitopes include structures in the gp120 V2 and V3 variable loops at the trimer apex, the highly conserved gp120 binding site for the CCR5 or CXCR4 coreceptor, the Cluster A gp120 element, and the gp41 ectodomain. Once the viral Env spike engages CD4 on the target cell membrane, most of these epitopes are sterically inaccessible to antibodies [57]. Of interest, Fab fragments of these non-neutralizing antibodies can inhibit HIV-1 infection, implicating steric hindrance in the lack of efficacy of the full antibody.
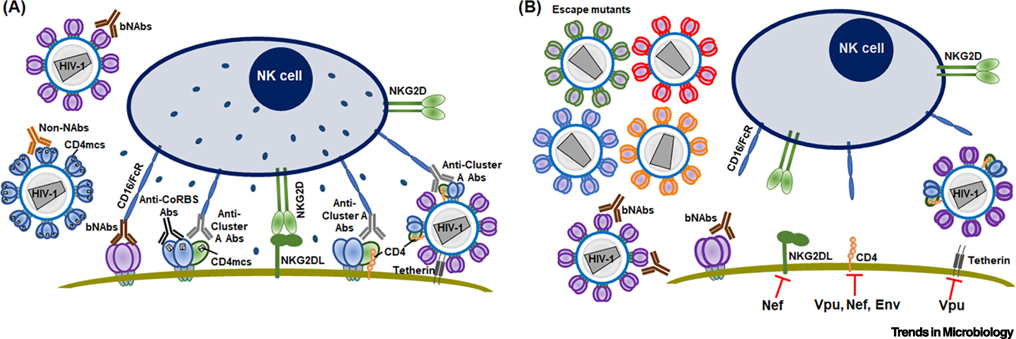
Figure 2. Antibody-mediated antiviral mechanisms and HIV-1 evasion strategies.
(A) Antiviral mechanisms involving antibodies against Env are depicted. State-1 Envs on the surface of primary HIV-1 can be bound by bNAbs, resulting in virus neutralization. Non-neutralizing antibodies (Non-NAbs) can bind and neutralize HIV-1 only if Env samples downstream conformations. Tier 1 Envs spontaneously sample these conformations. Alternatively, membrane-bound CD4 or CD4mc can stabilize these more “open” conformations. Various scenarios leading to the elimination of infected cells by ADCC are depicted. The binding of some bNAbs to cell-surface Env can result in ADCC. The Cluster A epitope on the gp120 inner domain is a target for potent ADCC-mediating antibodies and is displayed in an asymmetric, “partially open” Env conformation (State 2A). State 2A can be induced by a combination of a CD4mc and a CD4i (anti-CoRBS) antibody, or by the interaction of Env and CD4 on the same membrane. (B) Strategies used by HIV-1 to evade inhibition by antibodies are depicted. Viruses accumulate changes in Env sequence, allowing escape from bNAbs. HIV-1 accessory proteins decrease the effectiveness of ADCC. Nef prevents the expression of NKG2D ligands (NKG2DL), thus inhibiting NK cell activation. CD4 expression is reduced by Vpu, Nef and Env; as a result, the CD4i epitopes of Env are no longer triggered by membrane-bound CD4 and the infected cells remain resistant to ADCC mediated by CD4i Abs. Vpu also protects infected cells from ADCC responses by allowing efficient viral release, thus diminishing the number of Env antigens on the cell surface.
Although more reactive (triggerable) Envs might be advantageous with respect to the efficiency of HIV-1 entry, they are counterselected by the poorly neutralizing antibodies. Therefore, most primary viruses maintain a “closed” State-1 Env conformation that resists the binding of readily elicted anti-Env antibodies. smFRET studies indicate that at least one protomer of the Env trimer spontaneously samples downstream conformations beyond State 1 [30, 31]. However, primary HIV-1 can resist prolonged exposure to poorly neutralizing antibodies, suggesting that the availability of the cognate epitopes is at best transient [58]. Presumably, an antibody that binds the Env trimer and subsequently departs will fail to neutralize the virus. Such an event can result in the mild enhancement of primary HIV-1 infection that has been seen for some poorly neutralizing antibodies [59]. Of course, for the more reactive/triggerable HIV-1 Envs like those found in the Tier-1 T cell line-adapted viruses, poorly neutralizing antibodies may access their epitopes and inhibit virus infection [53].
Some readily elicited antibodies that recognize the coreceptor-binding site (anti-CoRBS) or Cluster A epitopes on gp120 can mediate ADCC against HIV-1-infected cells [60, 61]. The CD4-induced epitopes that overlap the CCR5/CXCR4-binding site encompass the bridging sheet and the base of the V3 loop [62–64]. Cluster A epitopes are located in the gp120 inner domain (seven-stranded β-sandwich, Layers 1 and 2, and N- and C-termini) [65, 66]. The binding of anti-CoRBS antibodies, anti-Cluster A antibodies and CD4mc stabilizes a novel “open” Env conformation designated State 2A [61]. Interestingly, membrane-bound CD4 can engage Env in the infected cell, inducing a State-2A conformation [61]. Although both anti-CoRBS antibodies and anti-Cluster A antibodies recognize State-2A Envs, only the anti-Cluster A antibodies mediate potent ADCC [61]. It has been suggested that differences in ADCC activity among antibodies belonging to the same family are due to the angle of approach; this angle can affect the orientation of the Fc portion recognized by FcƔR on the effector cells [60]. Another factor that might contribute to variable antibody effectiveness at mediating ADCC is the differential rate of antibody-induced internalization of Env bound to antibodies targeting State-1 versus State-2/3 conformations [67]. Antibodies that recognize the highly conserved Cluster A epitopes on the State 2A Env conformation meet the multiple requirements for efficient ADCC and are the most potent mediators of this adaptive immune response.
After several years of infection, about 10–15% of HIV-1-infected individuals produce antibodies that can neutralize a large percentage (40–99%) of naturally occurring HIV-1 Group M strains [68, 69]. Broadly neutralizing antibodies (bNAbs) typically exhibit extensive somatic hypermutation compared with their germline precursors; long complementarity-determining region (CDR) loops often allow these antibodies to access recessed structures or to bind glycans on the HIV-1 Env trimer [70–73]. Several HIV-1 Env bNAb epitopes have been characterized (see Table 1) [70–74]. All bNAbs have the ability to bind the State-1 Env conformation represented by the mature HIV-1 Envs on the surface of expressing cells and on virions [30]. Most bNAbs also retain the ability to recognize more reactive Envs that sample State 2 conformations. This promiscuity with respect to Env conformation contributes to the breadth of the bNAbs: thus, most bNAbs neutralize Tier 1, laboratory-adapted viruses that exhibit propensities to occupy State 2/3 Env conformations.
Table 1.
Conformational preferences of HIV-1 Env ligands
| HIV-1 Env ligands | Epitope/Binding site | Conformational preference | |
|---|---|---|---|
| Human bNAbs | PG9 PGT145 PG16 |
gp120 V2 quaternary | State 1 |
| PGT128 10-1074 |
gp120 V3 glycan | State 1 | |
| VRC01 3BNC117 VRC03 |
gp120 CD4-binding site (CD4BS) | State 1 | |
| PGT151 | gp120-gp41 interface | State 2 | |
| 35O22 | No state preference | ||
| SF12 VRC-PG05 |
“Silent face” of gp120 outer domain | No state preference? | |
| VRC34 | gp41 fusion peptide/gp120 glycan | State 2 | |
| 4E10 | gp41 MPER | State 2 | |
| 10E8 | No state preference | ||
| Cow bNAb | NC-Cow9 | gp120 CD4-binding site | State 2 |
| Conformational blockers | BMS-806 BMS-529 484 18A |
A hydrophobic gp120 pocket between the alpha-1 helix and β20-β21 loop | State 1 |
| CD4mc | BNM-IM-170 | gp120 Phe 43 cavity | State 2/3 |
Nonetheless, some bNAbs have demonstrated a preference for particular HIV-1 Env conformations [Table 1]. The V2 quaternary antibodies and VRC01, VRC03 and 3BNC117 CD4-binding site antibodies exhibit lower neutralization potency against HIV-1 Env mutants with changes that destabilize State 1 [45]. PGT151, which targets the gp120-gp41 interface, initially recognizes State-1 Env, but induces an asymmetric, State-2-like conformation [75, 76]. Most bNAbs against the gp41 MPER recognize CD4-bound conformations more efficiently than the pretriggered Env [77, 78]. Thus, although achieving neutralization breadth requires that bNAbs tolerate Env conformational changes to an extent, the affinity of some bNAbs can be influenced by the conformational state of the Env trimer.
Small-molecule and peptide inhibitors of HIV-1 entry also exhibit preferences for specific Env conformations. One group of small-molecule conformational blockers includes BMS-806, BMS-806 analogues, 484 and 18A [33–35, 46]. These conformational blockers bind State-1 Envs and inhibit transitions to downstream conformations. These compounds target a hydrophobic pocket between the α1 helix and the β20-β21 loop of gp120, near the CD4-binding site [35, 79]. At an effective antiviral concentration, the conformational blockers interfere with CD4-induced changes such as exposure of the gp41 HR1 coiled coil; at higher concentrations, these compounds decrease CD4 binding [36, 37, 46]. Incubation of some membrane Envs that spontaneously occupy States 2 or 3 with BMS-806 analogues results in a shift back to a State-1-like conformation, underscoring the State-1 preference of these compounds and suggesting their potential utility for structural studies of State 1 [75]. This State-1 preference means that BMS-806 analogues can bind the functional Env trimer with only minimal induction of conformational change, allowing high binding affinities and significant antiviral potency to be achieved in many cases.
Another class of HIV-1 entry inhibitors includes the CD4-mimetic compounds (CD4mcs), exemplified by BNM-III-170 or MCG-IV-210 [38–40, 80]. CD4mcs target the gp120 Phe 43 cavity, which is formed in State-3 Envs as CD4 binds [40, 62, 81]. The Phe 43 cavity is a highly conserved pocket between the gp120 inner and outer domains, the entrance to which is plugged by phenylalanine 43 of CD4 as Env engages its receptor [62]. CD4mcs penetrate deeper into the Phe 43 cavity than CD4, competitively blocking CD4 binding and prematurely inducing short-lived State 2/3 Env conformations [40–42]. The requirement to induce significant conformational changes upon binding Env lowers the affinity and antiviral potency of CD4mcs, compared with the conformational blockers. HIV-1 Env changes that raise or lower the activation barrier separating State 1 and States 2/3 can decrease or increase, respectively, HIV-1 sensitivity to CD4mcs [41, 45–47]. The consequences of CD4mc binding depend upon the stoichiometry of Env trimer occupancy: trimers with all three sites occupied by CD4mcs can be irreversibly inactivated, whereas trimers with only one occupied protomer may remain functional but are “opened,” sensitized to the binding of otherwise poorly neutralizing antibodies [41, 43]. Likewise, CD4mcs can render HIV-1-infected cells susceptible to ADCC mediated by CD4-induced antibodies [44, 61, 82]. Finally, CD4-mimetic proteins like eCD4-Ig, which mimics both CD4 and the CCR5 N-terminus, can also induce State 2/3 Env conformations, inactivate virus, and enhance the susceptibility of infected cells to ADCC [83–85].
Enfuvirtide (T20, Fuzeon) is a peptide corresponding to the HR2 region of gp41 that inhibits HIV-1 Env function by acting as a dominant-negative inhibitor of six-helix bundle formation [86, 87]. Enfuvirtide binds the gp41 HR1 coiled coil, which only becomes available after CD4 binding, in a State-3 Env [37]. Several studies have suggested that the kinetics with which State 3 proceeds to the six-helix bundle can influence HIV-1 sensitivity to enfuvirtide [88, 89].
IV. Evasion of the host antibody response
Preclinical studies in animal models have demonstrated the abilities of passively infused bNAbs to prevent HIV-1 or SHIV infection or to suppress viremia in a therapeutic setting [4–9, 90–93]. Clinical trials using similar bNAbs for immunotherapy of HIV-1-infected individuals have shown transient reduction in viral loads or delay in the rebound of the virus after analytical treatment interruption [94–100]. In a humanized mouse model, a combination of up to five bNAbs was found to suppress HIV-1 viremia in a greater percentage of animals than a 3-bNAb regimen [92]. In HIV-1-infected humans who make bNAbs, the virus has been shown to be resistant to the bNAb; often the Env epitope is intact, but Env changes distant from the antibody-binding site can determine escape [101–103]. Presumably, modulating Env conformations provides another means to evade host antibodies. For example, an E658K change in the gp41 HR2 region altered the neutralization sensitivity of the parental SHIV to PGT121, a bNAb that targets a V3-glycan epitope in the gp120 outer domain [104]. Another study examined a large panel of HIV-1 Env variants for resistance to the PGT151 bNAb, which recognizes a quaternary gp120-gp41 epitope [76, 105]. Changes in the epitope identified previously were confirmed to allow virus escape, but additional novel changes in amino acid residues distant from the defined epitope also led to PGT151 resistance [76, 105].
Antibody-dependent cell cytotoxicity (ADCC) has been implicated in slowing the rate of AIDS disease progression in HIV-1-infected individuals [106–108], and in the modest level of protection observed in the RV144 HIV-1 vaccine trial in Thailand [109, 110]. Both bNAbs and non-nAbs have been reported to mediate ADCC functions [111–115]. HIV-1 has evolved several mechanisms to limit ADCC responses (Figure 2). One strategy consists of downregulating CD4 from the cell surface, an activity mediated by the Nef and Vpu accessory proteins [66, 116–118]. Nef downregulates CD4 from the plasma membrane by directing the receptor to the lysosome for degradation [116]. Vpu, on the other hand, interacts with newly-synthesized CD4 through endoplasmic-reticulum-associated protein degradation (ERAD) [117]. The action of Vpu liberates Env from CD4-dependent retention in the ER, allowing trafficking of Env in its unliganded form to the plasma membrane [118]. Limited CD4 expression at the cell surface precludes interaction with Env, thereby avoiding the exposure of Env in the “open” State-2A conformation [61].
A significant effort is being made to devise vaccine immunogens that will consistently elicit bNAbs against HIV-1 in humans (Figure 3). Many current immunization strategies employ stabilized versions of soluble HIV-1 Env trimers [119, 120]. Soluble gp140 SOSIP.664 Env trimers are stabilized by an I559P change in gp41, by an artificial disulfide bond linking gp120 and gp41, and by a truncation of the gp41 ectodomain at residue 664 [119]. Soluble gp140 SOSIP.664 trimers are recognized by most bNAbs and are inefficiently recognized by most poorly neutralizing antibodies [9, 119]. One exception to the latter point is the efficient binding of soluble gp140 SOSIP.664 trimers by non-neutralizing antibodies directed against the gp120 V3 loop [119, 120]. Soluble gp140 SOSIP.664 trimers also bind peptides such as enfuvirtide (T20) corresponding to the gp41 HR2 region [121]. Antibodies against the V3 loops and enfuvirtide (T20) typically bind native membrane Envs only after CD4 binding, so the binding of these ligands suggest that not all elements of soluble gp140 SOSIP.664 trimers are in a State-1 conformation. Nonetheless, these soluble Env trimers have been extremely valuable in mapping the epitopes recognized by bNAbs [73, 74]. In immunized animals, soluble gp140 SOSIP.664 trimers raised only autologous (strain-restricted) neutralizing antibodies, and failed to elicit heterologous bNAbs against primary Tier-2 viruses [122–128]. Some heterologous neutralizing antibodies have been raised in rabbits by repeated immunization with soluble Env trimers stabilized in a slightly different fashion from the soluble gp140 SOSIP.664 trimers [130]. Occasional monkeys immunized with gp41 fusion peptides and soluble Env trimers generate neutralizing antibodies that cross-react with a small number of heterologous HIV-1 strains [131]. The results of animal immunizations with stabilized soluble gp140 SOSIP.664 Env trimers indicate that the elicitation of bNAbs in humans will likely be very inefficient.
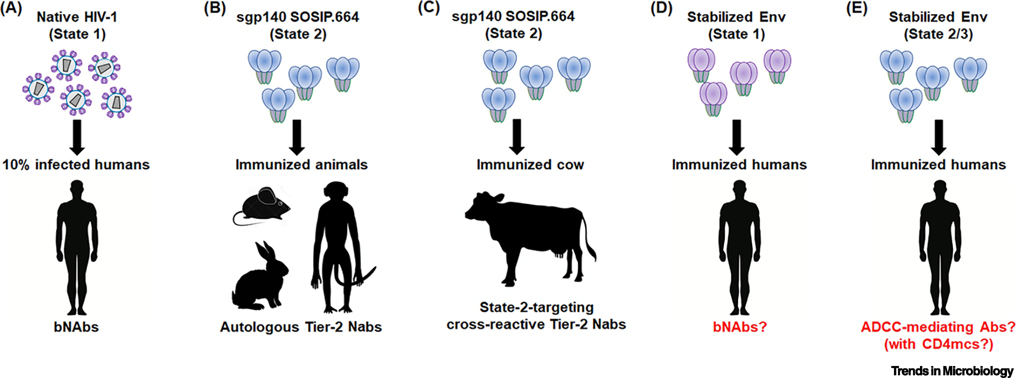
Figure 3. Env conformation: implications for HIV-1 prophylaxis.
(A) Envs on primary HIV-1 viral particles are predominantly in a State-1 conformation, which presumably elicits bNAbs in approximately 10% of HIV-1-infected humans. (B,C) Soluble gp140 SOSIP.664 trimers are stabilized in a State-2 conformation, and induce autologous Tier-2 neutralizing Abs in guinea pigs, rabbits and monkeys. They also elicit cross-reactive Tier-2 neutralizing Abs in cows. (D) Methods to stabilize Env in State-1 may potentially increase the efficiency of bNAb generation in humans. One such method of stabilizing State-1 conformations is the use of small-molecule conformational blockers like BMS-806. (E) CD4mcs, such as BNM-III-170, induce State 2/3 Env conformations. State 2/3 Env immunogens could raise antibodies that neutralize viral particles exposed to CD4mc and mediate ADCC against CD4mc-treated, HIV-1-infected cells in the course of treatment or prophylaxis.
Recent studies shed light on the difficulties of eliciting bNAbs with currently available Env formulations and suggest ways to improve immunogens to achieve this elusive goal. Analyses of membrane HIV-1 Envs indicate that some of the changes that have been used to stabilize soluble gp140 trimers disrupt the antigenicity and function of native Envs [120, 132–134]. Moreover, some of the predictions of Env structural models based on sgp140 SOSIP.664 trimers were not borne out by mutagenic studies of functional Envs [41, 47]. Single-molecule FRET (smFRET) studies revealed that soluble gp140 SOSIP.664 trimers predominantly sample a State-2-like conformation [75]. Although the soluble gp140 SOSIP.664 trimers could be driven into State 3 by soluble CD4, State-1-preferring ligands were unable to return these soluble trimers to pretriggered, State-1 conformations [75]. A detergent-solubilized membrane Env trimer purified in a complex with the Fabs of a bNAb, PGT151, was also found to be in a State-2 conformation [75, 135]. This is consistent with the similarity of the structures of these Env trimers [135, 138]. Crosslinking/mass spectrometry (XL/MS) studies of gp140 SOSIP.664 and mature Env trimers on cell surfaces indicate that differences in the gp120 trimer association domain (V1, V2 and V3 variable regions) and in the gp41 ectodomain exist between these Envs [121]. Collectively, these studies indicate that the detailed structure of the State-1 Env conformation that is targeted by most bNAbs is currently unknown. Recent attempts to use V2 quaternary antibody Fabs to purify HIV-1 Envs from membranes yielded structures similar to those of the soluble gp140 SOSIP.664 trimers [139, 140]. Presumably, all of these detailed structures represent State-2-like conformations, helping us to understand the nature of this intermediate.
The consistency with which soluble or detergent-solubilized Env trimers occupy a State-2-like conformation suggests that this state represents a preferred, default conformation readily sampled when State-1 Env is destabilized. This model explains the observation that changes in multiple, distantly situated residues of a primary HIV-1 Env result in a similar State-2-associated set of phenotypes, i.e., increased sensitivity to soluble CD4, CD4mcs, cold exposure and poorly neutralizing antibodies [45, 46]. These properties are also associated with laboratory-adapted (TCLA) HIV-1, which have been shown to sample State 2 more frequently than their primary HIV-1 counterparts [30]. The structures of soluble or detergent-solubilized Env trimers may provide insights into laboratory adaptation of HIV-1, which typically allows the virus to replicate efficiently on target cells with lower levels of CD4 [141, 142].
Having a functional, State-1 Env that is highly unstable and easily devolves into a State-2 conformation represents a useful adaptation for a persistent virus like HIV-1. The high titers of non-neutralizing, State 2/3-directed antibodies that arise during natural HIV-1 infection or following vaccination may be a consequence of this strategy. The antibodies raised in the natural primate hosts for the simian and human immunodeficiency viruses against State 2/3 Env conformations encounter steric barriers to binding these intermediates on the virus entry pathway [57]. Of interest, more broadly neutralizing antibodies can be elicited in cows by sgp140 SOSIP.664 immunization [143]. These cow bNAbs have been shown to induce a State-2 conformation in the viral Env [75]. As one might expect, the State-2-like gp140 SOSIP.664 trimers elicit antibodies that prefer this conformation. Presumably because of the extremely long complementarity-determining region of bovine immunoglobulins [144], these antibodies can access functional HIV-1 Envs to some extent. Such a fortuitous outcome is much less likely in primates, which lack this unusual antibody feature. Generating State 1-directed antibodies with breadth in humans may benefit from Env immunogens stabilized in State 1, allowing this normally immunorecessive conformation to be presented to the immune system. An understanding of State 1 and the ligands and Env changes that stabilize this conformation should assist these efforts.
In addition to attempts to raise bNAbs, might the high titers of largely ineffectual anti-Env antibodies that are directed against States 2/3 be utilized for prevention or treatment of HIV-1 infections? For these purposes, we can take advantage of the ability of CD4mcs to trigger conformational changes in State-1 Envs, opening them to State-2/3 conformations and sensitizing HIV-1 to antibodies that target downstream states [43, 44, 61]. Non-neutralizing antibodies elicited by HIV-1 gp120 protected monkeys from a stringent mucosal challenge with a heterologous Tier-2 SHIV, provided the virus encountered a CD4mc at the time of challenge [82]. The ADCC activity of HIV-1 Env-specific antibodies can also contribute to protection against infection or, in a therapeutic setting, to a reduction in the burden of infected cells [106–110]. ADCC-mediating antibodies preferentially target open Envs, particularly those in a State-2A conformation [28, 29, 44, 66, 111–114, 145]. In the presence of a CD4mc, the CD4i antibody 17b is able to bind Env, rendering infected cells susceptible to ADCC by State 2A-recognizing antibodies like A32 [29, 44, 61]. Thus, by binding Env and modifying its conformation, CD4mc could unleash the virus-neutralizing and ADCC-mediating potential of the abundant antibodies targeting State 2 and State 3.
V. Concluding Remarks
To date, five conformational states of HIV-1 Env have been identified by smFRET and structural analyses. During virus entry, HIV-1 Env undergoes a series of conformational transitions triggered by its interaction with CD4 and the CCR5/CXCR4 coreceptor. The pretriggered State 1 readily transitions into the default intermediate State 2, and CD4 further pushes the Env into the CD4-bound (State-3) conformation. Coreceptor interaction drives the formation of the six-helix bundle conformation, a stable, low-energy form. Off-pathway asymmetric conformations like State 2A can be induced in Env by CD4mc and antibodies, potentially rendering infected cells vulnerable to ADCC. HIV-1 has evolved strategies based on Env conformation to evade host antibodies. An understanding of these strategies could lead to novel therapeutic and prophylactic approaches to HIV-1 infection.
Outstanding Questions.
What does the functional, pretriggered State-1 conformation of HIV-1 Env look like? How similar are the State-1 conformations of natural HIV-1 variants? How do these State-1 Env conformations differ from State-2 Env?
How many sub-states are encompassed by each of the major conformations of HIV-1 Env?
What are the energetic relationships among the states?
Can individual Env conformations be stabilized? Do particular Env conformations (e.g., State 1) have an advantage as an immunogen?
How do small-molecule entry inhibitors interact with specific Env conformations to stabilize or destabilize these states? Can this information be used to improve the potency and breadth of the inhibitors?
Highlights.
In primary HIV-1, Env exists in a metastable pretriggered state (State 1). Broadly neutralizing antibodies predominantly target this conformation. Receptor binding drives Env from State 1 to downstream conformations on the virus entry pathway.
A recently discovered off-pathway Env conformation (State 2A) is recognized by antibodies against Env epitopes induced by CD4 binding that efficiently mediate antibody-dependent killing of HIV-1-infected cells.
Current soluble Env trimers are stabilized in State-2 default conformations. Characterizing the structure of State-1 Envs and testing immunogens stabilized in State 1 are worthy goals.
The efficacy of CD4-mimetic compounds (CD4mcs) in protecting gp120-vaccinated monkeys from simian-human immunodeficiency virus (SHIV) infection highlights the potential of modulating Env conformation for prevention approaches.
Acknowledgments
We thank Ms. Elizabeth Carpelan for manuscript preparation. J.S. and A.F. are supported by grants from the National Institutes of Health and Gilead Sciences. J.S. is also supported by a gift from the late William F. McCarty-Cooper. A.F. is also supported by the Canadian Institutes of Health Research and the Canada Research Chair program.
Glossary
- Retrovirus
enveloped viruses that reverse transcribe their RNA genomes into a DNA copy, which becomes integrated into the host cellular DNA, allowing stable infection of the cell.
- Lentivirus
a subfamily of retroviruses that establishes persistent infections in their hosts; HIV-1 is a member of the lentivirus subfamily.
- Envelope
the membrane, viral envelope glycoproteins, and any incorporated host membrane proteins.
- Envelope glycoproteins
for HIV-1, a trimeric spike on the viral membrane that consists of three gp120 exterior envelope glycoproteins and three gp41 transmembrane envelope glycoproteins; the mature Env results from the proteolytic cleavage of a trimeric gp160 precursor in the Golgi.
- Conformation or conformational state
the shape of a protein that is determined by its amino acid sequence, its secondary and tertiary (domain) structures, and its quaternary interactions with other subunits on the oligomeric complex.
- Metastable
describes a molecule not in its lowest-energy (most stable) conformation.
- Glycoprotein
a protein that is modified by carbohydrate moieties (glycans); HIV-1 Env is extensively glycosylated, which is thought to facilitate its evasion of the host immune response.
- Receptor
a molecule on the host cell membrane that binds the viral Env spikes and thereby facilitates the entry of the virus; HIV-1 uses two receptors sequentially, first CD4 and then one of two chemokine receptors, either CCR5 or CXCR4.
- Coreceptor binding site (CoRBS)
a highly conserved gp120 region involved in binding the coreceptors, CCR5 or CXCR4. The CoRBS becomes exposed upon the interaction of gp120 with CD4.
- Epitope
the region on a molecule recognized by an antibody. Some epitopes on the envelope glycoproteins are conserved among HIV-1 strains, whereas others are extremely variable.
- Broadly neutralizing antibodies (bNAbs)
antibodies that target conserved epitopes on the Env trimer and thereby inhibit multiple HIV-1 strains.
- CD4-induced epitope (CD4i epitope)
an epitope that is usually occluded in the unbound Env trimer but becomes exposed upon CD4 binding.
- Small CD4-mimetic compounds (CD4mcs)
small molecules that, like CD4, interact with the conserved gp120 Phe 43 cavity and induce conformational transitions to more “open” Env conformations.
- Single-molecule fluorescence resonance energy transfer (smFRET)
an advancement of the biophysical technique of fluorescence resonance energy transfer (FRET) that allows measurement of dynamic conformational transitions within single molecules.
- Soluble gp140 (sgp140) SOSIP.664
a soluble and stable gp140 Env trimer widely used for structural studies and as a vaccine immunogen. Soluble gp140 (sgp140) glycoproteins were produced by truncation of the transmembrane region and cytoplasmic tail of the gp41 envelope glycoprotein. Stabilized sgp140 trimers were obtained by incorporating a disulfide link between the gp120 (A501C) and the gp41 (T605C), by introducing a proline at isoleucine 559 (I559P), and truncating the gp41 ectodomain at residue 664.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed).
- 2.Wyatt R and Sodroski J (1998) The human immunodeficiency virus (HIV-1) envelope glycoproteins: fusogens, antigens and immunogens. Science 280 (5371), 1884–8. [DOI] [PubMed] [Google Scholar]
- 3.Freed EO and Martin MA (1995) The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J Biol Chem 270 (41), 23883–6. [DOI] [PubMed] [Google Scholar]
- 4.Hessell AJ et al. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15 (8), 951–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascola JR et al. (1999) Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73 (5), 4009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascola JR et al. (2000) Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6 (2), 207–10. [DOI] [PubMed] [Google Scholar]
- 7.Moldt B et al. (2012) Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109 (46), 18921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parren PW et al. (2001) Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75 (17), 8340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauthner MG et al. (2019) Vaccine-induced protection from homologous Tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50 (1), 241–252 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Checkley MA et al. (2011) HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol 410 (4), 582–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCune JM et al. (1988) Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53 (1), 55–67. [DOI] [PubMed] [Google Scholar]
- 12.Cao L et al. (2017) Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat Commun 8, 14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu P et al. (2003) Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A 100 (26), 15812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B (2019) Molecular mechanism of HIV-1 entry. Trends Microbiol 27 (10), 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaik MM et al. (2019) Structural basis of coreceptor recognition by HIV-1 envelope spike. Nature 565 (7739), 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agosto LM et al. (2015) HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy. Trends Microbiol 23 (5), 289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen P et al. (2007) Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81 (22), 12582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garry RF (1989) Potential mechanisms for the cytopathic properties of HIV. AIDS 3 (11), 683–94. [DOI] [PubMed] [Google Scholar]
- 19.Etemad-Moghadam B et al. (2001) Membrane-fusing capacity of the human immunodeficiency virus (HIV-1) envelope proteins determines the efficiency of CD4+ T-cell depletion in macaques infected by a simian-human immunodeficiency virus. J Virol 75 (12), 5646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sodroski J et al. (1986) Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature 322 (6078), 470–4. [DOI] [PubMed] [Google Scholar]
- 21.Lifson JD et al. (1986) AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science 232 (4754), 1123–7. [DOI] [PubMed] [Google Scholar]
- 22.Cao J et al. (1996) Molecular determinants of acute single-cell lysis by human immunodeficiency virus Type 1. J Virol 70 (3), 1340–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Bonte J et al. (2000) Importance of membrane fusion mediated by the human immunodeficiency virus envleope glycoproteins for lysis of primary CD4-positive T cells. J Virol 74 (22), 10690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richard J et al. (2016) Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 3, 122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaman MS et al. (2010) Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84 (3), 1439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go EP et al. (2017) Glycosylation benchmark profile for HIV-1 envelope glycoprotein production based on eleven Env trimers. J Virol 91 (9), e02428–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doria-Rose NA et al. (2010) Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84 (3), 1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard J et al. (2017) Unlocking HIV-1 Env: implications for antibody attack. AIDS Res Ther 14 (1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard J et al. (2018) Impact of HIV-1 envelope conformation on ADCC responses. Trends Microbiol 26 (4), 253–265. [DOI] [PubMed] [Google Scholar]
- 30.Munro JB et al. (2014) Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346 (6210), 759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X et al. (2018) HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. Elife 7, e34271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X et al. (2005) Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J Virol 79 (19), 12132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T et al. (2003) Discovery of 4-benzoyl-1-[(4-methoxy-1H- pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2- (R)-methylpiperazine (BMS-378806): a novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. J Med Chem 46 (20), 4236–9. [DOI] [PubMed] [Google Scholar]
- 34.Lin PF et al. (2003) A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci U S A 100 (19), 11013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herschhorn A et al. (2014) A broad HIV-1 inhibitor blocks envelope glycoprotein transitions critical for entry. Nat Chem Biol 10 (10), 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pancera M et al. (2017) Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat Chem Biol 13 (10), 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si Z et al. (2004) Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci U S A 101 (14), 5036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q et al. (2005) Identification of N-phenyl-N’-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology 339 (2), 213–25. [DOI] [PubMed] [Google Scholar]
- 39.Schon A et al. (2006) Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 45 (36), 10973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melillo B et al. (2016) Small-molecule CD4-mimics: structure-based optimization of HIV-1 entry inhibition. ACS Med Chem Lett 7 (3), 330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madani N et al. (2017) Activation and inactivation of primary human immunodeficiency virus envelope glycoprotein trimers by CD4-mimetic compounds. J Virol 91 (3), e301880–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haim H et al. (2009) Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathogens 5 (4), e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madani N et al. (2014) CD4-mimetic small molecules sensitize human immunodeficiency virus to vaccine-elicited antibodies. J Virol 88 (12), 6542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard J et al. (2015) CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112 (20), E2687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herschhorn A et al. (2016) Release of gp120 restraints leads to an entry-competent intermediate state of the HIV-1 envelope glycoproteins. MBio 7 (5), e01598–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herschhorn A et al. (2017) The beta20-beta21 of gp120 is a regulatory switch for HIV-1 Env conformational transitions. Nat Commun 8 (1), 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacheco B et al. (2017) Residues in the gp41 ectodomain regulate HIV-1 envelope glycoprotein conformational transitions induced by gp120-directed inhibitors. J Virol 91 (5), e02219–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zolla-Pazner S et al. (2016) Structure/function studies involving the V3 region of the HIV-1 envelope delineate multiple factors that affect neutralization sensitivity. J Virol 90 (2), 636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell RLR et al. (2017) Plasticity and epitope exposure of the HIV-1 envelope trimer. J Virol 91 (17), e00410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haim H et al. (2011) Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog 7 (6), e1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman TL et al. (1999) Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci U S A 96 (11), 6359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolchinsky P et al. (2001) Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J Virol 75 (7), 3435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haim H et al. (2013) Virus- and antibody-specific factors governing neutralization of human immunodeficiency virus (HIV-1). Cell Host Microbe 14 (5), 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGee K et al. (2014) The selection of low envelope glycoprotein reactivity to soluble CD4 and cold during simian-human immunodeficiency virus infection of rhesus macaques. J Virol 88 (1), 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medjahed H et al. (2013) The HIV-1 gp120 major variable regions modulate cold inactivation. J Virol 87 (7), 4103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X et al. (2006) Antibody binding is a dominant determinant of the efficiency of human immunodeficiency virus type 1 neutralization. J Virol 80 (22), 11404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labrijn AF et al. (2003) Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77 (19), 10557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upadhyay C et al. (2014) Distinct mechanisms regulate exposure of neutralizing epitopes in the V2 and V3 loops of HIV-1 envelope. J Virol 88 (21), 12853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan N et al. (1998) Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol 72 (8), 6332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding S et al. (2016) A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J Virol 90 (4), 2127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alsahafi N et al. (2019) An asymmetric opening of HIV-1 envelope mediates antibody-dependent cellular cytotoxicity. Cell Host Microbe 25 (4), 578–587 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwong PD et al. (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393 (6686), 648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan Y et al. (2013) Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110 (1), E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizzuto C et al. (1998) A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280 (5371), 1949–53. [DOI] [PubMed] [Google Scholar]
- 65.Finzi A et al. (2010) Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell 37 (5), 656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veillette M et al. (2014) Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88 (5), 2633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anand SP et al. (2019) Antibody-induced internalization of HIV-1 Env proteins limits surface expression of the closed conformation of Env. J Virol 93 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stamatatos L et al. (2009) Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15 (8), 866–70. [DOI] [PubMed] [Google Scholar]
- 69.Sather DN et al. (2009) Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83 (2), 757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen F et al. (2019) VH1–69 antiviral broadly neutralizing antibodies: genetics, structures, and relevance to rational vaccine design. Curr Opin Virol 34, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwong PD and Mascola JR (2018) HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 48 (5), 855–871. [DOI] [PubMed] [Google Scholar]
- 72.Bonsignori M et al. (2017) Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275 (1), 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCoy LE and Burton DR (2017) Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev 275 (1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward AB and Wilson IA (2017) The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev 275 (1), 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu M et al. (2019) Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568 (7752), 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blattner C et al. (2014) Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40 (5), 669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sattentau QJ and Moore JP (1991) Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med 174 (2), 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakrabarti BK et al. (2011) Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J Virol 85 (16), 8217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madani N et al. (2004) Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J Virol 78 (7), 3742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding S et al. (2019) A new family of small-molecule CD4-mimetic compounds contacts highly conserved aspartic acid 368 of HIV-1 gp120 and mediates antibody-dependent cellular cytotoxicity. J Virol 93 (24), e01325–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madani N et al. (2008) Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 16 (11), 1689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madani N et al. (2018) A CD4-mimetic compound enhances vaccine efficacy against stringent immunodeficiency virus challenge. Nat Commun 9 (1), 2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fellinger CH et al. (2019) eCD4-Ig limits HIV-1 escape more effectively than CD4-Ig or a broadly neutralizing antibody. J Virol 93 (14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardner MR et al. (2015) AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519 (7541), 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis-Gardner ME et al. (2017) eCD4-Ig promotes ADCC activity of sera from HIV-1-infected patients. PLoS Pathog 13 (12), e1006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kilby JM et al. (1998) Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med 4 (11), 1302–7. [DOI] [PubMed] [Google Scholar]
- 87.Matthews T et al. (2004) Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov 3 (3), 215–25. [DOI] [PubMed] [Google Scholar]
- 88.Wyss S et al. (2005) Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J Virol 79 (19), 12231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abrahamyan LG et al. (2005) The cytoplasmic tail slows the folding of human immunodeficiency virus type 1 Env from a late prebundle configuration into the six-helix bundle. J Virol 79 (1), 106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barouch DH et al. (2013) Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503 (7475), 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horwitz JA et al. (2013) HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110 (41), 16538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klein F et al. (2012) HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492 (7427), 118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shingai M et al. (2013) Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503 (7475), 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Margolis DM et al. (2017) HIV antibodies for treatment of HIV infection. Immunol Rev 275 (1), 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caskey M et al. (2015) Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522 (7557), 487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lynch RM et al. (2015) Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7 (319), 319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scheid JF et al. (2016) HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535 (7613), 556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bar KJ et al. (2016) Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 375 (21), 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bar-On Y et al. (2018) Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 24 (11), 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendoza P et al. (2018) Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561 (7724), 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao HX et al. (2013) Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496 (7446), 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doria-Rose NA et al. (2014) Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509 (7498), 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonsignori M et al. (2016) Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165 (2), 449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Q et al. (2019) A single substitution in gp41 modulates the neutralization profile of SHIV during in vivo adaptation. Cell Rep 27 (9), 2593–2607. [DOI] [PubMed] [Google Scholar]
- 105.Dingens AS et al. (2017) Comprehensive mapping of HIV-1 escape from a broadly neutralizing antibody. Cell Host Microbe 21 (6), 777–787 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wren LH et al. (2013) Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 138 (2), 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baum LL et al. (1996) HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157 (5), 2168–73. [PubMed] [Google Scholar]
- 108.Lambotte O et al. (2009) Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23 (8), 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haynes BF et al. (2012) Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366 (14), 1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomaras GD et al. (2013) Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110 (22), 9019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Bredow B et al. (2016) Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol 90 (13), 6127–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bruel T et al. (2016) Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7, 10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anand SP et al. (2019) Two families of Env antibodies efficiently engage Fc-gamma receptors and eliminate HIV-1-infected cells. J Virol 93 (3), e01823–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams KL et al. (2019) Identification of HIV gp41-specific antibodies that mediate killing of infected cells. PLoS Pathog 15 (2), e1007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Richard J et al. (2018) Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses. MBio 9 (2), e00358–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aiken C et al. (1994) Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76 (5), 853–64. [DOI] [PubMed] [Google Scholar]
- 117.Willey RL et al. (1992) Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol 66 (12), 7193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kimura T et al. (1994) Intracellular membrane traffic of human immunodeficiency virus type 1 envelope glycoproteins: vpu liberates Golgi-targeted gp160 from CD4-dependent retention in the endoplasmic reticulum. J Biochem 115 (5), 1010–20. [DOI] [PubMed] [Google Scholar]
- 119.Sanders RW et al. (2013) A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9 (9), e1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castillo-Menendez LR et al. (2018) Comparison of uncleaved and mature human immunodeficiency virus membrane envelope glycoprotein trimers. J Virol 92 (12), e00277–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castillo-Menendez LR et al. (2019) Conformational differences between functional human immunodeficiency virus envelope glycoprotein trimers and stabilized soluble trimers. J Virol 93 (3), e01709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pauthner M et al. (2017) Elicitation of robust Tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46 (6), 1073–1088 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torrents de la Pena A et al. (2018) Immunogenicity in rabbits of HIV-1 SOSIP trimers from clades A, B, and C, given individually, sequentially, or in combination. J Virol 92 (8), e01957–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanders RW et al. (2015) HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349 (6244), aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klasse PJ et al. (2016) Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from clades A, B and C. PLoS Pathog 12 (9), e1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Taeye SW et al. (2015) Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163 (7), 1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu JK et al. (2015) Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J Virol 89 (20), 10383–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feng Y et al. (2016) Thermostability of well-ordered HIV spikes correlates with the elicitation of autologous Tier 2 neutralizing antibodies. PLoS Pathog 12 (8), e1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pancera M et al. (2017) How HIV-1 entry mechanism and broadly neutralizing antibodies guide structure-based vaccine design. Current Opinion in Hiv and Aids 12 (3), 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dubrovskaya V et al. (2019) Vaccination with glycan-modified HIV NFL envelope trimer-liposomes elicits broadly neutralizing antibodies to multiple sites of vulnerability. Immunity 51 (5), 915–929 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu K et al. (2018) Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat Med 24 (6), 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen HT et al. (2019) Effects of the SOS (A501C/T605C) and DS (I201C/A433C) disulfide bonds on HIV-1 membrane envelope glycoprotein conformation and function. J Virol 93 (12), e00304–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alsahafi N et al. (2018) SOSIP changes affect human immunodeficiency virus Type 1 envelope glycoprotein conformation and CD4 engagement. J Virol 92 (19), e01080–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alsahafi N et al. (2015) Effects of the I559P gp41 change on the conformation and function of the human immunodeficiency virus (HIV-1) membrane envelope glycoprotein trimer. PLoS One 10 (4), e0122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JH et al. (2016) Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351 (6277), 1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Julien JP et al. (2013) Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342 (6165), 1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lyumkis D et al. (2013) Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342 (6165), 1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pancera M et al. (2014) Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514 (7523), 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Torrents de la Pena A et al. (2019) Similarities and differences between native HIV-1 envelope glycoprotein trimers and stabilized soluble trimer mimetics. PLoS Pathog 15 (7), e1007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan J et al. (2020) Cryo-EM structure of full-length HIV-1 Env bound with the Fab of antibody PG16. J Mol Biol. (online ahead of print DOI: 10.1016/j.jmb.2019.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kabat D et al. (1994) Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol 68 (4), 2570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moore JP et al. (1992) Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol 66 (1), 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sok D et al. (2017) Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 548 (7665), 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stanfield RL et al. (2018) The unusual genetics and biochemistry of bovine immunoglobulins. Adv Immunol 137, 135–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prévost J et al. (2018) Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology 515, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]