Abstract
Purpose of review:
Traditional methods to assess antiretroviral adherence, such as self-report, pill counts, and pharmacy refill data, may be inaccurate in determining actual pill-taking to both antiretroviral therapy (ART) or pre-exposure prophylaxis (PrEP). HIV viral loads serve as surrogates of adherence on ART, but loss of virologic control may occur well after decreases in adherence and viral loads are not relevant to PrEP
Recent findings:
Pharmacologic measures of adherence, electronic adherence monitors, and ingestible electronic pills all serve as more objective metrics of adherence, surpassing self-report in predicting outcomes. Pharmacologic metrics can identify either recent adherence or cumulative adherence. Recent dosing measures include antiretroviral levels in plasma or urine, as well as emtricitabine-triphosphate in dried blood spots (DBS) for those on tenofovir-emtricitabine-based therapy, A urine tenofovir test has recently been developed into a point-of-care test for bedside adherence monitoring. Cumulative adherence metrics assess adherence over weeks to months and include measurement of tenofovir-diphosphate in peripheral blood mononuclear cells or DBS, as well as ART levels in hair. Electronic adherence monitors and ingestible electronic pills can track pill bottle openings or medication ingestion, respectively.
Summary:
New and objective approaches in adherence monitoring can be used to detect nonadherence prior to loss of prevention efficacy or virologic control with PrEP or ART, respectively.
Keywords: Adherence metrics, PrEP, ART, Pharmacologic metrics, Electronic adherence monitors, Ingestible sensors
Introduction:
Advances in antiretroviral therapy (ART) have led to increasingly well-tolerated and potent ART regimens, resulting both in less toxicity and greater tolerance of non-adherence prior to loss of virologic control. Although a virally-suppressed patient was once assumed to be an adherent patient, as low as 50% adherence may be sufficient for some individuals on the most potent antiretroviral regimens to achieve virologic suppression, particularly with prior sustained viral suppression [1–4]. However, a decrease in adherence can precede loss of virologic control by weeks to months [5, 6]. Moreover, adherence can be a challenge over time with nearly 20% stopping ART altogether after five years in sub-Saharan Africa [7]. Increasing evidence has also demonstrated the importance of optimal (currently daily) adherence to ART, even upon achieving virologic suppression, given the relationship between suboptimal adherence and systemic inflammation [8–10]. Additional tools to identify individuals at risk of loss of virologic control and non-persistence as early as possible are also needed to maximize the impact of treatment as prevention [11, 12]. Furthermore, objective adherence data can enhance interpretation of an unsuppressed viral load, permitting more rapid treatment switching or intensification and triaging of costly resistance testing [13, 14].
Pre-exposure prophylaxis (PrEP) is a highly effective HIV prevention strategy, but requires adequate adherence[15]. The only currently approved agents for PrEP are oral and include daily or intermittent[16] tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) or daily tenofovir alafenamide (TAF)/FTC.[17] For PrEP, given the strong relationship between adherence and prevention efficacy, objective adherence measures have established themselves as key predictors and outcomes in clinical trials and demonstration projects,[18–24] and increasingly, to drive adherence interventions [25].
Traditional adherence measures, such as self-reported adherence, have played key roles in ART implementation to date [26–28]. Although self-reported adherence remains the most widely used method to assess adherence, particularly in real-world clinical settings, it is subject to multiple limitations including social desirability and recall biases (Table 1) [28–32]. Researchers and clinicians have attempted to combine self-reported adherence with pill counts and pharmacy refill data to improve accuracy, although this approach adds additional time, staff, and cost, without necessarily achieving higher accuracy[28, 33–36]. Directly observed therapy, if implemented well, is the gold-standard of adherence monitoring, but is rarely used outside the setting of multidrug resistant tuberculosis due to cost, staffing, and inconvenience to patients or study participants [14]. However, use of artificial intelligence via mobile health technologies and smartphone cameras could mitigate these downsides, although additional data on acceptability and feasibility is needed [37, 38]. Recent advances in adherence metrics seek to address the limited accuracy of traditional measures of adherence. Pharmacologic measures of adherence, electronic adherence monitors, and ingestible electronic pills seek to objectively assess both cumulative adherence as well as adherence patterns. Techniques to make objective adherence data available as rapidly as possible to clinicians (at the point of care) are being developed, to obtain both accurate and actionable information to immediately direct adherence counseling and interventions.
Table 1:
Comparison of Adherence Metrics
| Measure | Advantages | Limitations | Implementation Concerns |
|---|---|---|---|
| HIV viral load |
|
|
|
| Self-report |
|
|
|
| Pill Counts |
|
|
|
| Pharmacy Refills |
|
|
|
| Electronic Adherence Monitors |
|
|
|
| Ingestible electronic pills |
|
|
|
| Pharmacologic measures (recent adherence: plasma, FTC-TP in DBS, urine) |
|
|
|
| Pharmacologic measures (cumulative adherence: TFV-DP in PBMCs and DBS, antiretrovirals (ARVs) in hair) |
|
|
|
Pharmacologic Metrics of Adherence, Including Cumulative Measures and Point-of-Care Assays
Pharmacologic metrics of adherence involve measuring drug concentrations in a biomatrix such as plasma [39], urine [40], peripheral blood mononuclear cells (PBMCs) [41], hair [42–89], and dried blood spots (DBS) [90], most frequently via liquid chromatography tandem mass spectrometry (LC-MS/MS)-based methods (Figure 1). Pharmacologic measures of adherence have been critical to the interpretation of PrEP clinical trials and demonstration projects. For instance, the efficacy of PrEP in iPrEx, the first clinical trial to demonstrate this finding among men who have sex with men (MSM) and transgender women, rose from 44% to 92% among those with detectable drug levels in plasma and PBMCs [91]. In the VOICE and FEM-PrEP trials among women in sub-Saharan Africa, women in both trials reported >95% adherence to study drug, but random plasma tenofovir (TFV) levels among women on active drug were detectable in fewer than 30% of participants [20, 18].
Figure 1: Time Frames Examined by Pharmacologic Measures of Adherence.
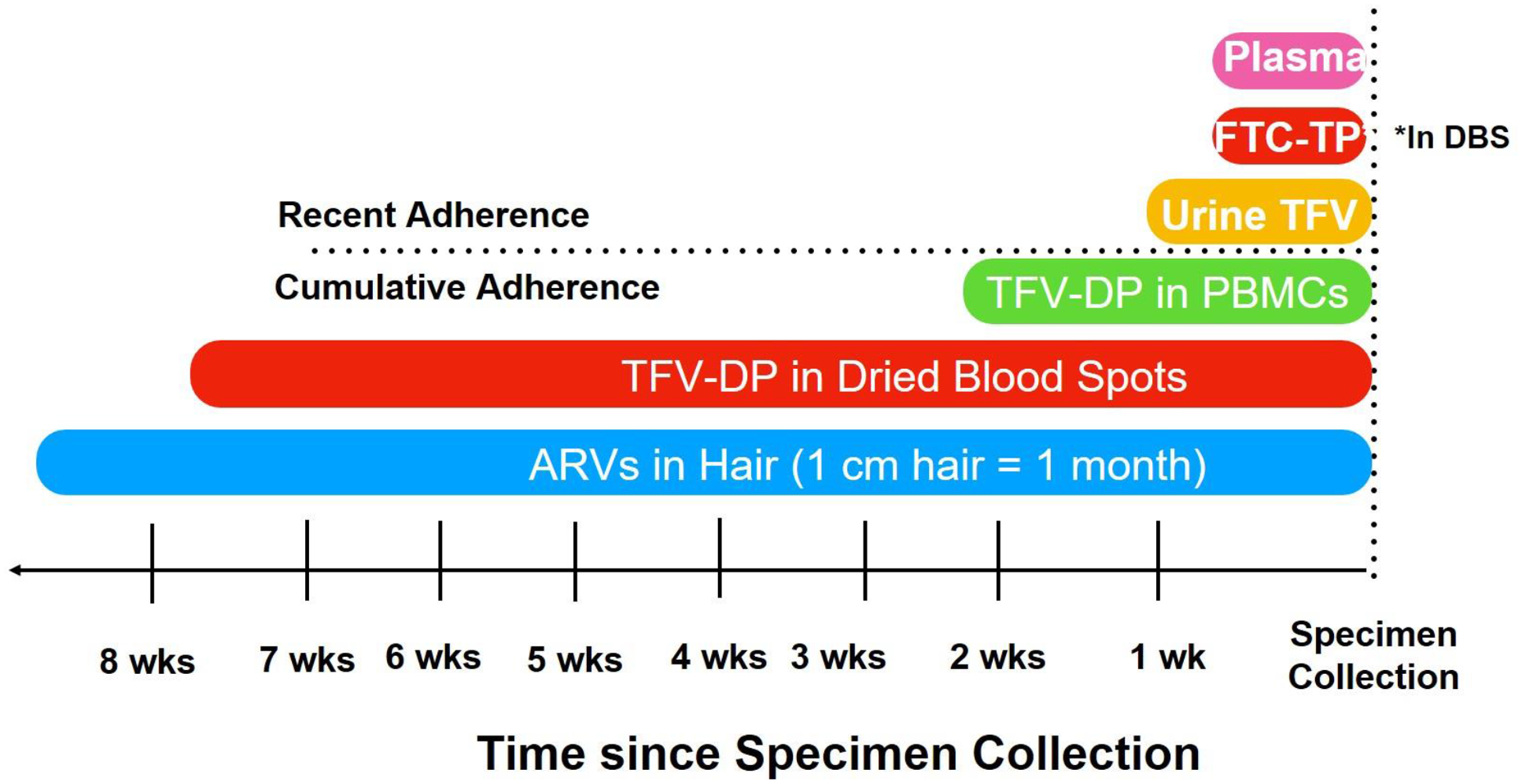
Drug-level measurement is also increasingly being used to interpret outcomes on ART, given that an elevated HIV viral load can represent either non-adherence or ART resistance (Figure 2[92, 93]). For instance, a nondetectable drug level with virologic failure is more suggestive of non-adherence than a detectable drug level with failure, which can be more suggestive of resistance. However, single plasma drug-levels, like single glucose measurements when evaluating diabetes, are limited because they reflect only a short duration of exposure [94–96], can have significant day-to-day variation [94], and are subject to “white coat” adherence (where adherence improves transiently prior to a visit) [97]. Tenofovir-diphosphate (TFV-DP) levels in PBMCs relay information on exposure over longer periods (7–14 days), although processing, isolating and counting PBMCs are costly and technically challenging. In a manner analogous to how glycosylated hemoglobin A1C provides information on average glucose levels over long periods of time, cumulative adherence measures, such as measurement of TFV-DP in DBS or drug levels in hair, examine average adherence over weeks to months [65, 90] (Figure 1). Finally, the recent development of antibody-based TFV drug-level detection or measurement in urine can allow for real-time measurement.[98–102] Immunoassays are first translated into a lateral flow immunoassay (LFA) format[101] (like a urine pregnancy test), which allows recent TFV ingestion to be captured at the point-of-care at low cost without specialized training [101].
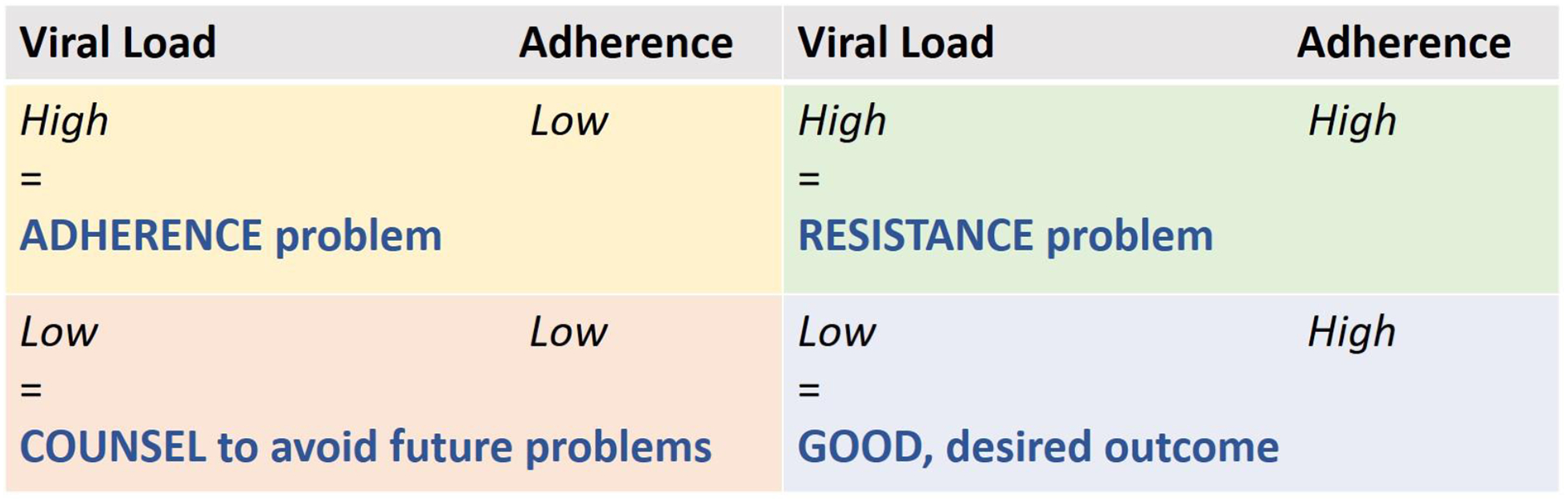
Figure 2: Clinical Interpretation of Paired HIV Viral Load and Objective Adherence Data for ART.
Tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate (FTC-TP) in Dried Blood Spots
Similar to the process within peripheral blood mononuclear cells (PBMCs), tenofovir and emtricitabine are phosphorylated to TFV-DP and FTC-TP in red blood cells (RBCs), which are abundant in dried blood spots (DBS). Each one of these phosphorylated anabolites exhibits unique pharmacokinetic parameters, which confers them distinct application as adherence measures. For example, TFV-DP–-which is quantified both with TDF and TAF-based therapy–-accumulates 25-fold from first dose to steady state with a long intracellular half-life (~17 days) in RBCs [90, 103–106]. These pharmacologic properties have been leveraged to develop adherence gradients to quantitate average TDF and TAF dosing over the preceding two months in healthy volunteers [90, 105, 106], which reflect both biology (pharmacokinetics) and behavior (adherence). Comparatively, FTC-TP has a shorter half-life in RBCs (~35 hrs.) [2, 107], and reflects recent TDF/FTC or TAF/FTC dosing (within 48–72 hrs.), similar to the look-back period duration of plasma and urine drug concentrations (Figure 1). Collectively, TFV-DP and FTC-TP in DBS provide a comprehensive measure of cumulative adherence and recent dosing, allowing for the identification of adherence patterns such as “white-coat” adherence where TFV-DP would be low but FTC-TP would be quantifiable.
The utility of TFV-DP and FTC-TP in DBS as pharmacologic measures of adherence has been well established in research and clinical settings. For example, TFV-DP in DBS has consistently shown to be a powerful predictor of efficacy to PrEP among high-risk individuals taking TDF and TAF-based PrEP [108–111], with concentrations of 700 femtomole (fmol)/3 mm punch and 900 fmol/two 7mm punches corresponding to high protection against HIV for TDF [108] and TAF [111], respectively. This strong predictive value has led to the integration of this measure to quantify adherence in PrEP studies and in clinical cases of suspected PrEP failure [112, 113]. For treatment, the clinical utility of TFV-DP was recently demonstrated in a large clinical cohort of people living with HIV (PLWH) on TDF, where increasing adjusted odds (aOR) for HIV virologic suppression (<20 copies/mL) were identified with higher concentrations of TFV-DP in DBS (highest adjusted odds ratio (aOR) 73.5 [95% Confidence interval (CI)=25.7, 210.5] for a TFV-DP ≥1850 fmol/punch compared to <350 fmol/punch). The predictive utility of this pharmacologic measure on outcomes was stronger than self-reported adherence,[114] which has been seen with most objective metrics.[75, 73] Recently, TFV-DP in DBS was also evaluated as a predictor of future viremia among participants on TDF/FTC-based ART. Among PLWH who were virologically-suppressed, the aOR of future viremia were 4.2 (95% CI=1.5,12.0) and 2.2 (95% CI=1.2, –4.0) for a concentration of 0–800 fmol/punch and 800–1649 fmol/punch compared to a TFV-DP concentration ≥1650 fmol/punch [115]. These findings highlight the potential wide clinical applications of TFV-DP and FTC-TP in DBS, not only as adherence biomarkers for PrEP or ART, but also as tools to predict clinical outcomes (i.e., viremia) among PLWH receiving tenofovir/ emtricitabine-based ART.
Similar to other pharmacologic measures, TFV-DP is influenced by demographic, clinical (including hemoglobin concentrations) and behavioral characteristics. Previous studies identified that TFV-DP in DBS was overall higher in PLWH compared to healthy volunteers, and that it was 36% higher in women than men [105, 115, 114]. Similarly, TFV-DP in DBS in PLWH was found to be 14% and 22% higher in Whites and Hispanic PLWH compared to Blacks, respectively, likely due to lower hemoglobin levels among Blacks. TFV-DP is also influenced by body mass index, showing a strong inverse correlation, and by ART class, demonstrating higher concentrations with concomitant use of a pharmacologic booster (i.e. ritonavir or cobicistat) [116]. Despite its powerful associations with clinical outcomes, understanding the influence of these patient-specific characteristics on the variability of TFV-DP in DBS will allow for a more accurate characterization of this biomarker as a measure of TFV. Studies are underway to implement TFV-DP and FTC-TP testing clinically to improve outcomes on PrEP and ART, including the application of a bench-top near-real time technique for assaying TFV-DP in DBS [117].
Hair drug-level measurement
Hair drug-level measurement is a technique, similar to TFV-DP in DBS, that can measure cumulative adherence to ART or PrEP over weeks to months[42–89] (Figure 1), with one centimeter of hair equivalent to one month of drug ingestion.[77, 80] Hair concentrations provides long-term exposure information on multiple antiretrovirals (ARVs) [60, 43, 44, 64] and do not require the medication to be processed intracellularly, like tenofovir. Hair concentrations of ARVs reliably predict virologic success in large prospective cohorts [46–54, 82], and clinical trials [55, 83] among PLWH, providing pharmacodynamic relevance for the longitudinal exposure data provided by hair samples. Hair levels of ARVs are stronger predictors of treatment outcomes than self-reported adherence [47–49, 54, 51, 82], or single plasma ARV concentrations [46, 47]. Furthermore, ARV hair levels have been shown to reflect adherence intervention effects when compared pre- and post-implementation [61, 62].
A linear relationship is observed between TFV dose and concentrations of TFV in hair among HIV-noninfected volunteers under directly observed therapy conditions.[65] Moreover, a strong correlation between hair levels of TFV and DBS concentrations of TFV-DP has been demonstrated, paving the way for the use of hair measures in the setting of PrEP.[66–79, 89, 80] Hair levels of TFV are also associated with PrEP-related toxicities in open-label studies, specifically declines in renal function.[68, 71] Hair concentrations of TFV are similar among men and women under conditions of directly observed therapy.[79] Therefore, the same range of hair levels can be used to quantitate adherence to PrEP/ART in both men and women[83] [79]. Finally, segmental analysis of hair samples allows for the assessment of adherence at various time points over preceding months (depending on the length of the participant’s hair), which can be useful in the context of PrEP failure [77, 80]. For example, segmental hair analysis can be used to measure adherence patters month-by-month, permitting examination of adherence patterns prior to and around the estimated time of seroconversion on PrEP[77, 80].
Hair collection is noninvasive and does not require specific skills like phlebotomy, sterile equipment, or specialized storage conditions. Hair sample collection requires only a pair of scissors and storage at room temperature. Hair collection has shown high rates of acceptability and feasibility (>95%) for hair ARV monitoring in African and Asian settings [47, 50, 53, 66, 57, 83], and among U.S. adolescents [72], and women [48, 49, 54]. Acceptability of hair collection has been lower among MSM in the U.S.[71] and among children in Africa [58]. Moreover, self-collection of hair samples (which may enhance feasibility of collection)[118] provides equivalent ARV concentration data to hair samples collected by field staff [70].
Point-of-care (POC) Urine Tenofovir Measurement
Although drug levels in DBS and hair have an advantage over traditional pharmacologic methods given their ability to measure cumulative adherence, they are limited in their scalability within real-world clinical settings. Traditional methods to measure drug levels for pharmacologic monitoring, regardless of biomatrix, require expensive spectrometry-based equipment, usually liquid chromatography/tandem mass spectrometry (LC-MS/MS) machines. Moreover, LC-MS/MS involves specialized personnel and can be labor-intensive [99]. Therefore, an easy-to-perform point-of-care test that could measure adherence objectively would be of interest to the field.
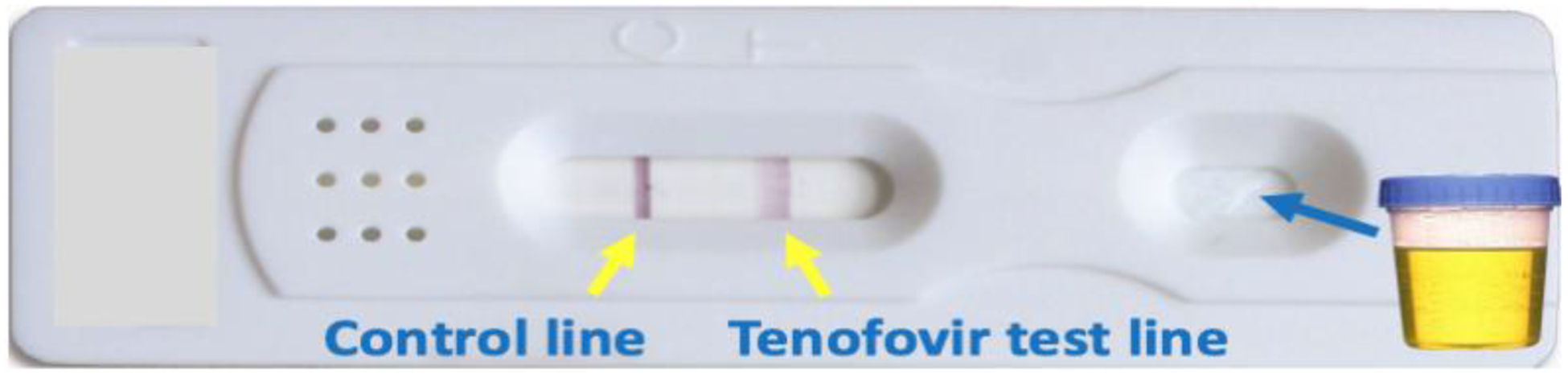
A urine-based point-of-care (POC) test, which qualitatively assesses recent adherence to TDF-based regimens over the past 4–7 days, analogous to the duration of exposure provided by FTC-TP measurement in DBS, [101] (Figure 1) has been recently developed.[98–102] Rather than using spectrometry-based methods, this assay leverages a very selective antibody raised against TFV, which is the metabolite of both TDF and TAF excreted in the urine. The antibody-based assay has now been packaged into a lateral flow immunoassay, analogous to a urine pregnancy test or tuberculosis urine galactomannan test (Figure 3) [99]. The POC strip test provides information on recent adherence to TDF-based regimens in a few minutes, requires no special training, and is projected to be low-cost for use in resource-limited settings. The POC test has excellent performance characteristics in terms of sensitivity and specificity (both >99%) in comparison to both LC-MS/MS[101] and laboratory-based enzyme-linked immunoassays (ELISA)[102], minimizing the risk for any misclassification of adherence.
Figure 3: Prototype for first lateral flow assay for tenofovir detection in urine.
Qualitative data from two completed PrEP demonstration projects, which tested drug-level feedback using plasma measures, emphasized that dosing cut-offs should seek to maximize accuracy for dosing within the last 24 hours, and that feedback would be most effective if available immediately [119]. Participants indicated that a cut-off should be selected to minimize the risk of telling a participant taking daily PrEP that they were non-adherent, potentially damaging the therapeutic relationship [25].Therefore, a cut-off of 1,500 ng/ml of tenofovir in urine for the TDF-based assay was chosen, because this cut-off accurately classified 98% of patients who took a dose within the last 24 hours as adherent [99]. The immunoassay has now been developed, validated against LC-MS/MS concentrations with high sensitivity and specificity, and packaged into a POC test using this cut-off [101]. In a secondary data analysis of a large PrEP demonstration project, the iPrEx open-label extension (OLE) study, low versus high urine TFV levels via the immunoassay were associated with 14-fold higher odds of future HIV seroconversion (95% CI=1.3,1197) [120]. POC adherence monitoring could be performed on urine specimens already collected for sexually transmitted infection (STI) screening for gonorrhea and chlamydia as part of routine PrEP care [121], requiring few changes in procedures and minimal time to implement in clinical settings. In the context of HIV treatment, the POC test could also be used to rapidly detect non-adherence versus possible drug resistance to HIV treatment, enhancing the interpretation of viral load testing and allowing immediate transition to second-line therapy while resistance testing is pending (Figure 2). The POC strip test is currently being evaluated as a tool to perform drug-level feedback to support PrEP adherence among young Kenyan woman (NCT03935464) and enhanced adherence counseling among young US MSM. Adherence patterns in these trials will be investigated with both recent and cumulative adherence measures (urine and hair levels, respectively) to examine possible “white coat” adherence effects. A version of the assay for use with TAF is currently being developed.
Electronic Adherence Monitors
Electronic adherence monitoring involves “smart” pill containers that record a date-and-time stamp with each opening of the container as a proxy for pill ingestion. This technology has played an important role in understanding adherence behavior in both HIV treatment and prevention.[122–124] Data can be stored on standard monitors (e.g., medication event monitoring systems (MEMS) caps) for downloading to a computer. Real-time monitors (e.g., Wisepill,[125] AdhereTech, CleverCap) have also become available in recent years and transmit data via cellular networks. Strengths of electronic adherence monitoring include its objectivity and day-to-day records, which are a powerful means to analyze adherence patterns.[73] A significant weakness, however, is the inability to measure drug ingestion; individuals may open the monitor without taking medication or take out multiple doses for later dosing, thus resulting in misclassification bias.
Electronic adherence monitors have traditionally been used to understand execution and persistence of adherence and factors that influence adherence. Other studies have used these devices to assess the estimated accuracy of alternate adherence measures, typically self-report.[73, 126] While electronic adherence monitors continue to serve these functions, recent studies have leveraged their ability to discern day-to-day adherence patterns to understand how adherence aligns with other behaviors (e.g., sexual activity)[127] and how adherence patterns evolve longitudinally. Other studies have explored the way electronic monitoring itself influences adherence and the experience of being monitored. Still others have described the use of real-time monitors in novel populations and their ability to trigger tailored/stepped adherence interventions.[128] This section of the review highlights key studies in each of these domains.
Patterns of adherence and associated behaviors
Several recent studies have used electronic adherence monitors to explore patterns of adherence and their relationship with associated behaviors such as sexual events. ADAPT 067 was a multi-national, randomized, open-label study of daily versus nondaily oral PrEP that utilized Wisepill to monitor adherence [129]. Weekly interviews guided by the electronic adherence data formed the basis for assessing coverage of sex events with pre- and post-exposure dosing and adherence. The Partners Demonstration Project was a prospective, open-label, implementation science-driven study of ART and oral PrEP for HIV prevention among high-risk heterosexual HIV serodiscordant couples in Kenya and Uganda; it used MEMS caps to assess PrEP adherence. This study was the first to explore the concept of prevention-effective adherence by demonstrating alignment of adherence with HIV risk [130, 131]. Additionally, the electronic adherence data was used to identify four distinct patterns of adherence related to HIV risk and other factors (i.e., high steady, moderate steady, late declining, early declining) [127]. Finally, electronic adherence data was combined with SMS-reported sexual activity and a population pharmacokinetic model to estimate the percent of reported sexual events likely covered by therapeutic tenofovir levels [132].
Impact of electronic monitoring on adherence and the experience of being monitored
Building on the well-known Hawthorne effect (i.e., altered behavior because of known observation), a quasi-experimental analysis showed that real-time monitoring likely has a significant intervention effect compared to standard monitoring [133]. Additionally, an ethics study explored the experience of electronic adherence monitoring; participants reported feeling pressured by the monitor to take their ART, yet also perceived the monitors as conducive to their fundamental goal of achieving high adherence [134].
Utilization of electronic adherence monitors in novel populations
Initial studies involving real-time electronic adherence monitors largely took place in sub-Saharan Africa and Asia; however, two recent studies used Wisepill in the US South among both women living with HIV and depression [135], and with youth living with HIV [126]. Both studies noted overall feasibility but with technical and acceptability challenges. Another study explored electronic adherence monitoring among injection drug users in Kazakstan [136]. Participants supported the use of these devices, although some were concerned about having their adherence tracked. Additionally, Wisepill was used in a study of patients coinfected with drug-resistant tuberculosis and HIV in South Africa [137]. Wisepill was highly acceptable, although adherence was higher for the tuberculosis medication (bedaquiline) than the ART.
Real-time adherence monitoring and triggered interventions
Recent studies have leveraged the ability of electronic adherence monitors to trigger real-time adherence interventions. A study in Chicago developed a triaged real-time intervention based on alerts of missed ART doses (via Wisepill) among young African-American men [138]. Support escalated from text messages to social supporter engagement based on the duration of the adherence interruption. A New York study explored patients’ experiences using CleverCap linked to an HIV self-management mobile phone app comprised of testimonial videos, adherence and physical activity reminders, a fitness tracker, health surveys, and wellness tasks [139]. Finally, a study in Missouri used MEMS caps to assess the efficacy of ecologic momentary assessment (i.e., repeated real-time measurement of behaviors in participants’ natural environments aimed at minimizing recall bias and maximizing ecological validity) to define patterns of alcohol use, mood, and medication adherence [140].
Ingestible Electronic Pills
Ingestible sensors combined with medications can be used to study and directly measure medication adherence and ingestion patterns. There are three major components of ingestible sensors systems. First, the ingestible sensor is coupled with an inert silver/magnesium battery and integrated into standard gelatin capsules. These capsules can be coencapsulated with medication to create a “digital pill” using a standard pharmacy pill filling machine (first component). Investigations have demonstrated bioequivalence among various ARVs when encapsulated with an ingestible sensor [141–143]. When this digitized version of drug is ingested, gastric acid catalyzes a chemical reaction between silver and magnesium to generate an electrical charge adequate to power the ingestible sensor for approximately 30 minutes. These sensors can then produce an electrochemical signal or radiofrequency signal, broadcasting ingestion of the sensor and medication.
This signal is acquired by a wearable cutaneous patch or off-body reader (e.g., a lanyard), the second component of the digital pill system. This reader device stores ingestion data gathered by each digital pill ingestion, and acts as a relay, transmitting information via low energy Bluetooth protocols to a smartphone, which then transmits the data to storage in the cloud. In countries where smartphone usage may not be ubiquitous, a third generation (3G) radio can allow for direct transmission of ingestion data to the cloud. Adherence data can also be acquired through querying the reader using a low energy Bluetooth reader.
The third component of the digital pill system is a cloud-based interface that stores and interprets adherence data transmitted from the reader. A programmable interface can be used to automate messaging surrounding nonadherence and adherence and provide other adherence support programs either asynchronously or in synchrony with medication ingestion patterns.
Feasibility and acceptability
Several investigations have demonstrated the feasibility and acceptability of using ingestible sensors to measure adherence to single medication regimens in individuals with diabetes, schizophrenia, hypertension, and tuberculosis [144–148]. These investigations deployed ingestible sensors coencapsulated with specific study drugs demonstrating the ability of various patient populations to operate the system, and general acceptability in these populations. It is also plausible to coencapsulate multiple different medications (for example an ARV plus antidepressant in a person living with HIV who has depression) and measure adherence to various regimens. Additionally, ingestible sensors can also be used to measure patterns of ingestion for medications that may be prescribed on an as needed basis. For example, individuals who experience pain can use a digitized version of their opioid analgesics, thereby reporting patterns of opioid ingestion [149]. For PrEP, a digital pill can be used to understand the contextual basis surrounding alternative PrEP regimens like on-demand PrEP. Currently, there are several ongoing clinical trials utilizing ingestible sensor systems to measure adherence to ART as well as PrEP (NCT02797262, NCT04065347, NCT03978793, NCT03842436, NCT03512418).
There are several limitations associated with digital pills. First, researchers and clinicians who select digital pills as a modality to measure adherence will need to ensure that individuals also adhere to the technology. Individuals need to be trained on the operation of the relay device which collects ingestion data and transmits it to the phone. Second, individuals must ensure that the reader is paired to their smartphone via Bluetooth. As the use of Bluetooth technology becomes increasingly ubiquitous with connected speakers, smart watches and other devices, this barrier will be lowered. Second, the infrastructure requirements to successfully deploy a digital pill can be difficult to assemble. Currently, most digital pill systems are classified as a medical device and require specialty pharmacies to assemble drug prior to delivery to the patient. As digitized medications continue to evolve, integration of the ingestible sensor may be incorporated into other junctures of the supply chain, including clinical settings. Finally, the cost associated with digitized medications has yet to be estimated. While one digital medicine product, a combination aripiprazole pill with ingestible sensor (Abilify MyCite), is FDA approved and marketed, it is unclear whether there are active models for insurance to reimburse the use of this technology within clinical care.
Real-time adherence monitoring using digital pills
Like with the electronic adherence monitors described above, digital pills can measure ART or PrEP adherence in real time, but with the added advantage of documenting ingestion. These real time measures of adherence lend a contextual basis to daily medication taking behaviors in patients. Combined with other measures of activity-tracking, such as sleep or location, this contextualized adherence data can be used as a tool by patients or clinicians to support adherence habits. Additionally, digital pills also provide the unique opportunity to detect the onset of nonadherence. For individuals on ART, these suboptimal episodes of nonadherence may represent an opportunity to intervene and support these individuals prior to the development of ingrained behaviors of nonadherence and the development of viremia and/or virologic failure. Continued measurement with a digital pill can demonstrate improved adherence after intervention.
Conclusions
New approaches to the measurement of antiretroviral adherence, including pharmacologic measures, electronic adherence monitors, and ingestible electronic pills, provide more objective and accurate data on adherence than traditional measures, such as self-report, pill counts, and pharmacy refill data. The recently-developed urine-based assay to measure ART and PrEP taking at the point of care, real-time electronic adherence monitors, and ingestible electronic pills with sensors can all measure adherence behavior in real-time and trigger immediate adherence intervention. These technologies can thereby be harnessed to improve the effectiveness of PrEP and ART regimens worldwide.
Acknowledgements:
M.A.S. was supported by NIAID/NIH T32AI060530 (P.I. Havlir) and NIMH/NIH K23MH122286. J.E.H. is supported by NIMH K24MH114732. P.R.C. is supported by NIDA/NIH K23DA044874. J.C.M. is supported by NIAID R01AI145453. P.L.A is supported by NIAID R01AI122298.. M.G. is supported by NIAID R01AI143340, 2R01AI098472, and R03AI152773.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest: M.A.S., P.R.C., J.C.M., and M.G. have no relevant conflicts of interest to report. P.L.A. has received grant funding from Gilead. J.E.H. has accepted consulting funds from Merck Pharmaceuticals.
References:
- 1.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng JH et al. Tenofovir Diphosphate in Dried Blood Spots Is Strongly Associated With Viral Suppression in Individuals With Human Immunodeficiency Virus Infections. Clin Infect Dis. 2019;68(8):1335–42. doi: 10.1093/cid/ciy708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother. 2018;62(1). doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musinguzi N, Mocello RA, Boum Y 2nd, Hunt PW, Martin JN, Haberer JE et al. Duration of Viral Suppression and Risk of Rebound Viremia with First-Line Antiretroviral Therapy in Rural Uganda. AIDS Behav. 2017;21(6):1735–40. doi: 10.1007/s10461-016-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4(9):e7196. doi: 10.1371/journal.pone.0007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow M, MaWhinney S, Coyle RP, Coleman SS, Gardner EM, Zheng JH et al. Predictive Value of Tenofovir Diphosphate in Dried Blood Spots for Future Viremia in Persons Living With HIV. J Infect Dis. 2019;220(4):635–42. doi: 10.1093/infdis/jiz144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stohr W, Fidler S, McClure M, Weber J, Cooper D, Ramjee G et al. Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS One. 2013;8(10):e78287. doi: 10.1371/journal.pone.0078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas AD, Zaniewski E, Anderegg N, Ford N, Fox MP, Vinikoor M et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2). doi: 10.1002/jia2.25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ Jr., Gardner EM, Macatangay BJ et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis. 2016;63(12):1661–7. doi: 10.1093/cid/ciw650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Mancilla JR, Morrow M, Boum Y, Byakwaga H, Haberer JE, Martin JN et al. Brief Report: Higher ART Adherence Is Associated With Lower Systemic Inflammation in Treatment-Naive Ugandans Who Achieve Virologic Suppression. J Acquir Immune Defic Syndr. 2018;77(5):507–13. doi: 10.1097/QAI.0000000000001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Mancilla JR, Phillips AN, Neaton JD, Neuhaus J, Sharma S, Baker JV et al. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc. 2019;22(6):e25297. doi: 10.1002/jia2.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder SP, Liu AY. CROI 2019: advances in HIV prevention and plans to end the epidemic. Top Antivir Med. 2019;27(1):8–25. [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–9. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans LE, Steegen K, terHeine R, Schuurman R, Tempelman H, Moraba R et al. PI Drug-Level Testing as a Screening Tool for Drug Resistance in 2nd-Line ART Failure. Conference on Retroviruses and Opportunistic Infections Seattle. 2019. [#461]. [Google Scholar]
- 14.Winchester NE, Maldarelli F, Mejia Y, Dee N, Dewar R, Laidlaw E et al. 8-Day Inpatient Directly Observed Therapy for ART Failure: A Tool For Preventing Unnecessary ART Changes and Optimizing Adherence Support. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glidden DV, Amico KR, Liu AY, Hosek SG, Anderson PL, Buchbinder SP et al. Symptoms, Side Effects and Adherence in the iPrEx Open-Label Extension. Clin Infect Dis. 2016;62(9):1172–7. doi: 10.1093/cid/ciw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 17.Hare CB, Coll J, Ruane P, Molina JM, Mayer KH, Jessen H et al. The Phase 3 Discover STudy: Daily F/TAF or F/TDF for HIV Pre-Exposure Prophylaxis. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2019; Seattle Abstract 104LB. [Google Scholar]
- 18.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J Acquir Immune Defic Syndr. 2015;68(5):578–84. doi: 10.1097/QAI.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 20.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Straten A, Brown ER, Marrazzo JM, Chirenje MZ, Liu K, Gomez K et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19(1):20642. doi: 10.7448/IAS.19.1.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker Z, Javanbakht M, Mierzwa S, Pavel C, Lally M, Zimet G et al. Predictors of Over-Reporting HIV Pre-exposure Prophylaxis (PrEP) Adherence Among Young Men Who Have Sex With Men (YMSM) in Self-Reported Versus Biomarker Data. AIDS Behav. 2017. doi: 10.1007/s10461-017-1958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agot K, Taylor D, Corneli AL, Wang M, Ambia J, Kashuba AD et al. Accuracy of Self-Report and Pill-Count Measures of Adherence in the FEM-PrEP Clinical Trial: Implications for Future HIV-Prevention Trials. AIDS Behav. 2015;19(5):743–51. doi: 10.1007/s10461-014-0859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumenthal J, Haubrich R. Pre-exposure prophylaxis for HIV infection: how antiretroviral pharmacology helps to monitor and improve adherence. Expert Opin Pharmacother. 2013;14(13):1777–85. doi: 10.1517/14656566.2013.812072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–11. doi: 10.1097/QAI.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo-Mancilla JR, Haberer JE. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep. 2018;15(1):49–59. doi: 10.1007/s11904-018-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stirratt MJ, Curtis JR, Danila MI, Hansen R, Miller MJ, Gakumo CA. Advancing the Science and Practice of Medication Adherence. J Gen Intern Med. 2018;33(2):216–22. doi: 10.1007/s11606-017-4198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–82. doi: 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: an examination of key methodological issues. AIDS Behav. 2007;11(2):161–73. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. Journal of Acquired Immunodeficiency Syndromes (JAIDS). 2006;43 Suppl 1:S79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–52. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- 33.Okatch H, Beiter K, Eby J, Chapman J, Marukutira T, Tshume O et al. Brief Report: Apparent Antiretroviral Overadherence by Pill Count is Associated With HIV Treatment Failure in Adolescents. J Acquir Immune Defic Syndr. 2016;72(5):542–5. doi: 10.1097/QAI.0000000000000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5(5):e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molina JM, Charreau I, Chidiac C, Pialoux G, Cua E, Delaugerre C et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18(3):308–17. doi: 10.1016/S1473-3099(17)30725-9. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor JL, Gardner EM, Esser S, Mannheimer SB, Lifson AR, Telzak EE et al. A simple self-reported adherence tool as a predictor of viral rebound in people with viral suppression on antiretroviral therapy. HIV Med. 2016;17(2):124–32. doi: 10.1111/hiv.12284. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JT, LeGrand S, Hightow-Weidman LB, McKellar MS, Kashuba AD, Cottrell M et al. Smartphone-Based Contingency Management Intervention to Improve Pre-Exposure Prophylaxis Adherence: Pilot Trial. JMIR Mhealth Uhealth. 2018;6(9):e10456. doi: 10.2196/10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–6. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32(1):32–43. doi: 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenig HC, Mounzer K, Daughtridge GW, Sloan CE, Lalley-Chareczko L, Moorthy GS et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017;18(6):412–8. doi: 10.1111/hiv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137(8):696–7. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(21):3401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Yang Q, Yoon K, Lei Y, Shi R, Gee W et al. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(6):1923–33. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis. 2012;206(9):1453–61. doi: 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56(4):333–9. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasitsuebsai W, Kerr SJ, Truong KH, Ananworanich J, Do VC, Nguyen LV et al. Using Lopinavir Concentrations in Hair Samples to Assess Treatment Outcomes on Second-Line Regimens Among Asian Children. AIDS Res Hum Retroviruses. 2015;31(10):1009–14. doi: 10.1089/AID.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23(4):471–8. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–75. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohan D, Natureeba P, Koss CA, Plenty A, Luwedde F, Mwesigwa J et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29(2):183–91. doi: 10.1097/QAD.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS. 2015;29(7):825–30. doi: 10.1097/QAD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chawana TD, Gandhi M, Nathoo K, Ngara B, Louie A, Horng H et al. Defining a Cutoff for Atazanavir in Hair Samples Associated With Virological Failure Among Adolescents Failing Second-Line Antiretroviral Treatment. J Acquir Immune Defic Syndr. 2017;76(1):55–9. doi: 10.1097/QAI.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pintye J, Bacchetti P, Teeraananchai S, Kerr S, Prasitsuebsai W, Singtoroj T et al. Brief Report: Lopinavir Hair Concentrations Are the Strongest Predictor of Viremia in HIV-Infected Asian Children and Adolescents on Second-Line Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2017;76(4):367–71. doi: 10.1097/QAI.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baxi SM, Greenblatt RM, Bacchetti P, Jin C, French AL, Keller MJ et al. Nevirapine Concentration in Hair Samples Is a Strong Predictor of Virologic Suppression in a Prospective Cohort of HIV-Infected Patients. PLoS One. 2015;10(6):e0129100. doi: 10.1371/journal.pone.0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandhi M, Ofokotun I, Bacchetti P, Jin C, Ribaudo HJ, Haas DW et al. Antiretroviral concentrations in hair strongly predict virologic response in a large HIV treatment-naive clinical trial. Clin Infect Dis. 2019;5:1044–7. doi: 10.1093/cid/ciy764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD et al. Hair and Plasma Data Show That Lopinavir, Ritonavir, and Efavirenz All Transfer From Mother to Infant In Utero, But Only Efavirenz Transfers via Breastfeeding. J Acquir Immune Defic Syndr. 2013;63(5):578–84. doi: 10.1097/QAI.0b013e31829c48ad00126334-201308150-00006 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66(3):311–5. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olds PK, Kiwanuka JP, Nansera D, Huang Y, Bacchetti P, Jin C et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care. 2015;27(3):327–32. doi: 10.1080/09540121.2014.983452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. Journal of Clinical Pharmacology. 2014;54(2):121–32. doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gandhi M, Yang Q, Bacchetti P, Huang Y. Short communication: A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses. 2014;30(1):25–8. doi: 10.1089/AID.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gwadz M, Cleland CM, Applegate E, Belkin M, Gandhi M, Salomon N et al. Behavioral Intervention Improves Treatment Outcomes Among HIV-Infected Individuals Who Have Delayed, Declined, or Discontinued Antiretroviral Therapy: A Randomized Controlled Trial of a Novel Intervention. AIDS Behav. 2015;19(10):1801–17. doi: 10.1007/s10461-015-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hickey MD, Salmen CR, Omollo D, Mattah B, Fiorella KJ, Geng EH et al. Implementation and Operational Research: Pulling the Network Together: Quasiexperimental Trial of a Patient-Defined Support Network Intervention for Promoting Engagement in HIV Care and Medication Adherence on Mfangano Island, Kenya. J Acquir Immune Defic Syndr. 2015;69(4):e127–34. doi: 10.1097/QAI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gandhi M, Gandhi RT, Stefanescu A, Bosch RJ, Cyktor JC, Horng H et al. Cumulative Antiretroviral Exposure Measured in Hair Is Not Associated With Measures of HIV Persistence or Inflammation Among Individuals on Suppressive ART. J Infect Dis. 2018;218:234–8. doi: 10.1093/infdis/jiy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phung N, Kuncze K, Okochi H, Louie A, Benet LZ, Ofokotun I et al. Development and Validation of an Assay to Analyze Atazanavir in Human Hair Via Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Rapid Commun Mass Spectrom. 2018;March 15;32(5):431–441. doi: 10.1002/rcm.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68(1):13–20. doi: 10.1097/QAI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis. 2015;212(9):1402–6. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e8. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017;33(8):778–83. doi: 10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saberi P, Neilands TB, Ming K, Johnson MO, Kuncze K, Koss CA et al. Strong Correlation Between Concentrations of Antiretrovirals in Home-Collected and Study-Collected Hair Samples: Implications for Adherence Monitoring. J Acquir Immune Defic Syndr. 2017;76(4):e101–e3. doi: 10.1097/QAI.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS. 2017;31(16):2245–51. doi: 10.1097/QAD.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis. 2018;66(2):213–9. doi: 10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abaasa A, Hendrix C, Gandhi M, Anderson P, Kamali A, Kibengo F et al. Utility of Different Adherence Measures for PrEP: Patterns and Incremental Value. AIDS Behav. 2018;22(4):1165–73. doi: 10.1007/s10461-017-1951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K et al. Adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr. 2017;March 1:77(3):295–298. doi: 10.1097/QAI.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baxi SM, Vittinghoff E, Bacchetti P, Huang Y, Chillag K, Wiegand R et al. Comparing pharmacologic measures of tenofovir exposure in a U.S. pre-exposure prophylaxis randomized trial. PLoS One. 2018;13(1):e0190118. doi: 10.1371/journal.pone.0190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Markowitz M, Grossman H, Anderson PL, Grant R, Gandhi M, Horng H et al. Newly Acquired Infection With Multidrug-Resistant HIV-1 in a Patient Adherent to Preexposure Prophylaxis. J Acquir Immune Defic Syndr. 2017;76(4):e104–e6. doi: 10.1097/QAI.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thaden JT, Gandhi M, Okochi H, Hurt CB, McKellar MS. Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination. AIDS. 2018;32(9):F1–F4. doi: 10.1097/QAD.0000000000001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colby DJ, Kroon E, Sacdalan C, Gandhi M, Grant RM, Phanuphak P et al. Acquisition of Multidrug-Resistant Human Immunodeficiency Virus Type 1 Infection in a Patient Taking Preexposure Prophylaxis. Clin Infect Dis. 2018;31:962–4. doi: 10.1093/cid/ciy321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koss CA, Liu AY, Castillo-Mancilla J, Bacchetti P, McHugh C, Kuncze K et al. Similar tenofovir hair concentrations in men and women after directly observed dosing of tenofovir disoproxil fumarate/emtricitabine: implications for preexposure prophylaxis adherence monitoring. AIDS. 2018;32(15):2189–94. doi: 10.1097/QAD.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen SE, Sachdev D, Lee SA, Scheer S, Bacon O, Chen MJ et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV. 2018:doi: 10.1016/S2352-3018(18)30288-1. [Epub ahead of print]. doi:10.1016/S2352–3018(18)30288–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gandhi M, Devi S, Bacchetti P, Chandy S, Heylen E, Phung N et al. Measuring adherence to antiretroviral therapy via hair concentrations in India. J Acquir Immune Defic Syndr. 2019:doi: 10.1097/QAI.0000000000001993. [Epub ahead of print]. doi:10.1097/QAI.0000000000001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabb ZJ, Mmbaga BT, Gandhi M, Louie A, Kuncze K, Okochi H et al. Antiretroviral drug concentrations in hair are associated with virologic outcomes among young people living with HIV in Tanzania. AIDS. 2018;32(9):1115–23. doi: 10.1097/QAD.0000000000001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murnane PM, Bacchetti P, Currier JS, Brummel S, Okochi H, Phung N et al. Tenofovir concentrations in hair strongly predict virologic suppression in breastfeeding women. AIDS. 2019;33(10):1657–62. doi: 10.1097/QAD.0000000000002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jotwani V, Scherzer R, Glidden DV, Mehrotra M, Defechereux P, Liu A et al. Pre-exposure Prophylaxis With Tenofovir Disoproxil Fumarate/Emtricitabine and Kidney Tubular Dysfunction in HIV-Uninfected Individuals. J Acquir Immune Defic Syndr. 2018;78(2):169–74. doi: 10.1097/QAI.0000000000001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saberi P, Ming K, Legnitto D, Neilands TB, Gandhi M, Johnson MO. Novel methods to estimate antiretroviral adherence: protocol for a longitudinal study. Patient Prefer Adherence. 2018;12:1033–42. doi: 10.2147/PPA.S166380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hojilla JC, Satre DD, Glidden DV, McMahan VM, Gandhi M, Defechereux P et al. Brief Report: Cocaine Use and Pre-exposure Prophylaxis: Adherence, Care Engagement, and Kidney Function. J Acquir Immune Defic Syndr. 2019;81(1):78–82. doi: 10.1097/QAI.0000000000001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saberi P, Ming K, Legnitto D, Neilands TB, Gandhi M, Johnson MO. Feasibility and acceptability of novel methods to estimate antiretroviral adherence: A longitudinal study. PLoS One. 2019;14(1):e0210791. doi: 10.1371/journal.pone.0210791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ascher SB, Scherzer R, Estrella MM, Shigenaga J, Spaulding KA, Glidden DV et al. HIV pre-exposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine and changes in kidney function and tubular health. AIDS. 2019. doi: 10.1097/QAD.0000000000002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velloza J, Bacchetti P, Hendrix CW, Murnane P, Hughes JP, Li M et al. Short- and Long-Term Pharmacologic Measures of HIV Pre-exposure Prophylaxis Use Among High-Risk Men Who Have Sex With Men in HPTN 067/ADAPT. J Acquir Immune Defic Syndr. 2019;82(2):149–58. doi: 10.1097/QAI.0000000000002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. doi: 10.1089/AID.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yager JL, Coyle RP, Coleman SS, Ellison L, Zheng JH, Bushman L et al. Moderately High Tenofovir Diphosphate in Dried Blood Spots Indicates Drug Resistance in Viremic Persons Living with HIV. J Int Assoc Provid AIDS Care. 2019;18:2325958219888457. doi: 10.1177/2325958219888457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hermans LE, Steegen K, ter Heine R, Schuurman R, Tempelman H, Moraba R et al. PI Drug-Level Testing as a Screening Tool for Drug Resistance in 2nd-Line ART Failure. Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washingon, March 4–7 2019 (abstract 461). [Google Scholar]
- 94.Nettles RE, Kieffer TL, Parsons T, Johnson J, Cofrancesco J Jr., Gallant JE et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42(8):1189–96. [DOI] [PubMed] [Google Scholar]
- 95.Clevenbergh P, Garaffo R, Durant J, Dellamonica P. PharmAdapt: a randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS. 2002;16:2311–5. [DOI] [PubMed] [Google Scholar]
- 96.Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7(2):59–69. [DOI] [PubMed] [Google Scholar]
- 97.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV clinical trials. 2008;9(4):238–46. [DOI] [PubMed] [Google Scholar]
- 98.Gandhi M, Bacchetti P, Rodrigues WC, Spinelli M, Koss CA, Drain PK et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinical Medicine (Published by The Lancet); https://doiorg/101016/jeclinm201808004. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gandhi M, Bacchetti P, Spinelli MA, Okochi H, Baeten JM, Siriprakaisil O et al. Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test. J Acquir Immune Defic Syndr. 2019;81(1):72–7. doi: 10.1097/QAI.0000000000001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spinelli MA, Glidden DV, Rodrigues WC, Wang G, Vincent M, Okochi H et al. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a PrEP demonstration project. AIDS. 2019(5):867–72. doi: 10.1097/QAD.0000000000002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gandhi M, Wang G, King R, Rodrigues WC, Vincent M, Glidden DV et al. Development and validation of the first point-of-care assay to objectively monitor adherence to HIV treatment and prevention in real-time in routine settings. AIDS. 2020;34(2):255–60. doi: 10.1097/QAD.0000000000002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spinelli MA, Rodrigues WC, Wang G, Vincent M, Glidden DV, Okochi H, Stalter R, Defechereux P, Deutsch M, Grant RM, Ngure K, Mugo NR, Baeten JM, Gandhi M. High accuracy of a real-time urine antibody-based tenofovir point-of-care test compared to laboratory-based ELISA in diverse populations. JAIDS 2020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal. 2016;122:16–20. doi: 10.1016/j.jpba.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo-Mancilla J, Coyle RP, Zheng JH, Ellison L, Roon L, Fey J et al. Tenofovir diphosphate arising from TAF is quantifiable in dried blood spots. Poster Presented at CROI 2017. Seattle, WA Abstract 405. [Google Scholar]
- 105.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrobial agents and chemotherapy. 2018;62(1):e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yager J, Castillo-Mancilla J, Ibrahim M, Brooks K, McHugh C, MaWhinney S et al. Tenofovir diphosphate in dried blood spots following escalating TAF/FTC dosing Accepted for Themed Discusison presentation CROI 2019 Seattle, WA: 2019. [Google Scholar]
- 107.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH et al. Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob Agents Chemother. 2016;60(11):6692–7. doi: 10.1128/AAC.01017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B et al. An HIV Preexposure Prophylaxis Demonstration Project and Safety Study for Young MSM. J Acquir Immune Defic Syndr. 2017;74(1):21–9. doi: 10.1097/QAI.0000000000001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hare C, Coll J, Ruane P, Molina J, Mayer K, Jessen H, editors. The phase 3 DISCOVER Study: Daily F/TAF or F/TDF for HIV pre-exposure prophylaxis annual Conference on Retroviruses and Opportunistic Infections (CROI). Seattle; 2019. [Google Scholar]
- 112.Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. New England Journal of Medicine. 2017;376(5):501–2. [DOI] [PubMed] [Google Scholar]
- 113.Markowitz M, Grossman H, Anderson PL, Grant R, Gandhi M, Horng H et al. Newly Acquired Infection with Multi-Drug Resistant HIV-1 in a Patient Adherent to Pre-Exposure Prophylaxis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng J-H et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clinical Infectious Diseases. 2018;68(8):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morrow M, MaWhinney S, Coyle RP, Coleman SS, Gardner EM, Zheng J-H et al. Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. The Journal of infectious diseases. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K et al. Adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. Journal of acquired immune deficiency syndromes (1999). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pu F, Pandey S, Bushman LR, Anderson PL, Ouyang Z, Cooks RG. Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem. 2020. doi: 10.1007/s00216-019-02304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma A, Stephenson R, Sallabank G, Merrill L, Sullivan S, Gandhi M. Acceptability and Feasibility of Self-Collecting Biological Specimens for HIV, Sexually Transmitted Infection, and Adherence Testing Among High-Risk Populations (Project Caboodle!): Protocol for an Exploratory Mixed-Methods Study. JMIR Res Protoc. 2019;8(5):e13647. doi: 10.2196/13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koester KA, Liu A, Eden C, Amico KR, McMahan V, Goicochea P et al. Acceptability of drug detection monitoring among participants in an open-label pre-exposure prophylaxis study. AIDS Care. 2015;27(10):1199–204. doi: 10.1080/09540121.2015.1039958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spinelli MA, Glidden DV, Rodrigues WC, Wang G, Vincent M, Okochi H et al. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a preexposure prophylaxis demonstration project. AIDS. 2019;33(5):867–72. doi: 10.1097/QAD.0000000000002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kebaabetswe PM, Stirratt MJ, McLellan-Lemal E, Henderson FL, Gray SC, Rose CE et al. Factors Associated with Adherence and Concordance Between Measurement Strategies in an HIV Daily Oral Tenofovir/Emtricitibine as Pre-exposure Prophylaxis (Prep) Clinical Trial, Botswana, 2007–2010. AIDS Behav. 2015;19(5):758–69. doi: 10.1007/s10461-014-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haberer JE, Kiwanuka J, Nansera D, Muzoora C, Hunt PW, So J et al. Realtime adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS. 2013;27(13):2166–8. doi: 10.1097/QAD.0b013e328363b53f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haberer JE, Robbins GK, Ybarra M, Monk A, Ragland K, Weiser SD et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16(2):375–82. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bachman Desilva M, Gifford AL, Keyi X, Li Z, Feng C, Brooks M et al. Feasibility and Acceptability of a Real-Time Adherence Device among HIV-Positive IDU Patients in China. AIDS Res Treat. 2013;2013:957862. doi: 10.1155/2013/957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Craker L, Tarantino N, Whiteley L, Brown L. Measuring Antiretroviral Adherence Among Young People Living with HIV: Observations from a Real-Time Monitoring Device Versus Self-report. AIDS Behav. 2019;23(8):2138–45. doi: 10.1007/s10461-019-02448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pyra M, Brown ER, Haberer JE, Heffron R, Celum C, Bukusi EA et al. Patterns of Oral PrEP Adherence and HIV Risk Among Eastern African Women in HIV Serodiscordant Partnerships. AIDS Behav. 2018;22(11):3718–25. doi: 10.1007/s10461-018-2221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mimiaga MJ, Kuhns LM, Biello KB, Olson J, Hoehnle S, Santostefano CM et al. Positive STEPS - a randomized controlled efficacy trial of an adaptive intervention for strengthening adherence to antiretroviral HIV treatment among youth: study protocol. BMC Public Health. 2018;18(1):867. doi: 10.1186/s12889-018-5815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grant RM, Mannheimer S, Hughes JP, Hirsch-Moverman Y, Loquere A, Chitwarakorn A et al. Daily and Nondaily Oral Preexposure Prophylaxis in Men and Transgender Women Who Have Sex With Men: The Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis. 2018;66(11):1712–21. doi: 10.1093/cid/cix1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haberer JE, Kidoguchi L, Heffron R, Mugo N, Bukusi E, Katabira E et al. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. J Int AIDS Soc. 2017;20(1):21842. doi: 10.7448/IAS.20.1.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pyra M, Haberer JE, Heffron R, Kidoguchi L, Brown ER, Bukusi EA et al. Brief Report: PrEP Use During Periods of HIV Risk Among East African Women in Serodiscordant Relationships. J Acquir Immune Defic Syndr. 2018;77(1):41–5. doi: 10.1097/QAI.0000000000001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mallayasamy S, Chaturvedula A, Fossler MJ, Sale M, Goti V, Bumpus NN et al. Tenofovir Plasma Concentration from Preexposure Prophylaxis at the Time of Potential HIV Exposure: a Population Pharmacokinetic Modeling and Simulation Study Involving Serodiscordant Couples in East Africa. Antimicrob Agents Chemother. 2019;63(8). doi: 10.1128/AAC.00446-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haberer JE, Musinguzi N, Tsai AC, Boum Y 2nd, Bwana BM, Muzoora C et al. Real-time electronic adherence monitoring plus follow-up improves adherence compared with standard electronic adherence monitoring. AIDS. 2017;31(1):169–71. doi: 10.1097/QAD.0000000000001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campbell JI, Eyal N, Musiimenta A, Burns B, Natukunda S, Musinguzi N et al. Ugandan Study Participants Experience Electronic Monitoring of Antiretroviral Therapy Adherence as Welcomed Pressure to Adhere. AIDS Behav. 2018;22(10):3363–72. doi: 10.1007/s10461-018-2200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stringer KL, Azuero A, Ott C, Psaros C, Jagielski CH, Safren SA et al. Feasibility and Acceptability of Real-Time Antiretroviral Adherence Monitoring among Depressed Women Living with HIV in the Deep South of the US. AIDS Behav. 2019;23(5):1306–14. doi: 10.1007/s10461-018-2322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davis A, Sarsembayeva L, Gulyaev V, Primbetova S, Terlikbayeva A, Mergenova G et al. If You Build It, Will They Use It? Preferences for Antiretroviral Therapy (ART) Adherence Monitoring Among People Who Inject Drugs (PWID) in Kazakhstan. AIDS Behav. 2019;23(12):3294–305. doi: 10.1007/s10461-019-02421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bionghi N, Daftary A, Maharaj B, Msibi Z, Amico KR, Friedland G et al. Pilot evaluation of a second-generation electronic pill box for adherence to Bedaquiline and antiretroviral therapy in drug-resistant TB/HIV co-infected patients in KwaZulu-Natal, South Africa. BMC Infect Dis. 2018;18(1):171. doi: 10.1186/s12879-018-3080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dworkin MS, Panchal P, Wiebel W, Garofalo R, Haberer JE, Jimenez A. A triaged real-time alert intervention to improve antiretroviral therapy adherence among young African American men who have sex with men living with HIV: focus group findings. BMC Public Health. 2019;19(1):394. doi: 10.1186/s12889-019-6689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beauchemin M, Gradilla M, Baik D, Cho H, Schnall R. A Multi-step Usability Evaluation of a Self-Management App to Support Medication Adherence in Persons Living with HIV. Int J Med Inform. 2019;122:37–44. doi: 10.1016/j.ijmedinf.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shacham E, Lew D, Xiao T, Lopez J, Trull T, Schootman M et al. Testing the Feasibility of Using Ecological Momentary Assessment to Collect Real-Time Behavior and Mood to Predict Technology-Measured HIV Medication Adherence. AIDS Behav. 2019;23(8):2176–84. doi: 10.1007/s10461-018-2378-9. [DOI] [PubMed] [Google Scholar]
- 141.Chai PR, Pereira LM, Jambaulikar GD, Carrico AW, O’Cleirigh C, Mayer KH et al. Short Communication: Bioequivalence of Tenofovir Component of Tenofovir/Rilpivirine/Emtricitabine in Digital Pills. AIDS Res Hum Retroviruses. 2019;35(4):361–3. doi: 10.1089/AID.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ibrahim ME, Brooks KM, Castillo-Mancilla JR, McHugh C, Morrow M, Brothers J et al. Short Communication: Bioequivalence of Tenofovir and Emtricitabine After Coencapsulation with the Proteus Ingestible Sensor. AIDS Res Hum Retroviruses. 2018;34(10):835–7. doi: 10.1089/AID.2018.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu H, Daar E, Wang Y, Siqueiros L, Campbell K, Shen J et al. Pharmacokinetics of Coencapsulated Antiretrovirals with Ingestible Sensors. AIDS Res Hum Retroviruses. 2020;36(1):65–74. doi: 10.1089/AID.2019.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J Med Internet Res. 2017;19(7):e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Browne SH, Behzadi Y, Littlewort G. Let Visuals Tell the Story: Medication Adherence in Patients with Type II Diabetes Captured by a Novel Ingestion Sensor Platform. JMIR Mhealth Uhealth. 2015;3(4):e108. doi: 10.2196/mhealth.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS One. 2013;8(1):e53373. doi: 10.1371/journal.pone.0053373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peters-Strickland T, Pestreich L, Hatch A, Rohatagi S, Baker RA, Docherty JP et al. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatr Dis Treat. 2016;12:2587–94. doi: 10.2147/NDT.S116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Browne SH, Umlauf A, Tucker AJ, Low J, Moser K, Gonzalez Garcia J et al. Wirelessly observed therapy compared to directly observed therapy to confirm and support tuberculosis treatment adherence: A randomized controlled trial. PLoS Med. 2019;16(10):e1002891. doi: 10.1371/journal.pmed.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chai PR, Carreiro S, Innes BJ, Chapman B, Schreiber KL, Edwards RR et al. Oxycodone Ingestion Patterns in Acute Fracture Pain With Digital Pills. Anesth Analg. 2017;125(6):2105–12. doi: 10.1213/ANE.0000000000002574. [DOI] [PMC free article] [PubMed] [Google Scholar]


