Abstract
Objective:
Increased muscle activity during rapid eye movement (REM) sleep (i.e. REM sleep without atonia) is common in people with Parkinson’s disease (PD). This study tested the hypotheses that people with PD and REM sleep without atonia (RSWA) would present with more severe and symmetric rigidity compared to individuals with PD without RSWA and age-matched controls.
Methods:
Sixty-one individuals participated in this study (41 PD, 20 controls). An overnight sleep study was used to classify participants with PD as having either elevated (PD-RSWA+) or normal muscle activity (PD-RSWA-) during REM sleep. Quantitative measures of rigidity were obtained using a robotic manipulandum that passively pronated and supinated the forearm.
Results:
Quantitative measures of forearm rigidity were significantly higher in the PD-RSWA+ group compared to the control group. Rigidity was significantly more asymmetric between limbs in the PD-RSWA− group compared with controls, while there was no significant difference in symmetry between the control and PD-RSWA+ groups.
Conclusion:
In people with mild to moderate PD, RSWA is associated with an increased and more symmetric presentation of upper limb rigidity.
Significance:
Dysfunction of brainstem systems that control muscle tone during REM sleep may contribute to increased rigidity during wakefulness in people with PD.
Keywords: Parkinson’s disease, rigidity, REM sleep without atonia, REM sleep behavior disorder
1. Introduction
Rigidity is one of the cardinal motor signs of Parkinson’s disease (PD) and is characterized by a marked increase in muscle activity in response to imposed muscle stretch. Increasing rigidity correlates with greater disease severity, yet, there is considerable heterogeneity in the expression of rigidity both within and across individuals with PD, even in individuals who are considered to have an akinetic-rigid predominant phenotype of PD (Vu et al., 2012). Heterogeneity in rigidity is particularly evident in the early stages of disease. The mechanisms contributing to the expression of rigidity are poorly understood, but alterations in the excitability of both cortical and subcortical pathways mediating long-latency stretch reflexes (Rothwell et al., 1983; Tatton and Lee, 1975) and stretch induced co-activation of agonist-antagonist muscles (Xia, 2011; Xia et al., 2009, 2006; Xia and Rymer, 2004) are considered to contribute to increased resistance during imposed movements.
Muscle activity is also abnormally elevated during rapid eye movement (REM) sleep in a substantial percentage (approximately 40%) of people with PD (Chahine et al., 2014). Increased phasic or tonic muscle activity during REM sleep is termed REM sleep without atonia (RSWA) and, in conjunction with the phenomenon of dream enactment, characterizes the parasomnia of idiopathic REM sleep behavior disorder (iRBD). Idiopathic RBD affects approximately 1% of the general population, and its prevalence is near 5% in the elderly (Boeve et al., 2013; Haba-Rubio et al., 2018). It is now well recognized that the presence of iRBD is highly predictive of progression to a neurodegenerative disease with alpha-synuclein pathology. It has been estimated that more than 75% of people with iRBD will develop PD, multiple system atrophy, or dementia with Lewy bodies over the course of 12 years (Postuma et al., 2019; Schenck et al., 2013). In a cohort of patients with de novo PD, 51% exhibited movement events during REM sleep (Mollenhauer et al., 2013). It is estimated that over 40% of individuals with PD have RSWA and more than 20% have a diagnosis of iRBD (Chahine et al., 2014; Zhang et al., 2017). Individuals with PD and iRBD are more likely to present with a non-tremor dominant subtype of PD (Folle et al., 2019; Kumru et al., 2007; Postuma et al., 2008; Romenets et al., 2012), and are more likely to develop postural instability, gait problems including freezing of gait, and orthostatic symptoms compared to individuals with PD alone (Postuma et al., 2008; Romenets et al., 2012). Higher levels of phasic muscle activity during REM sleep is associated with increased severity and more symmetric expression of disease (Bliwise et al., 2010). These findings suggest that among people with PD, RSWA is associated with increased pathology of both nigrostriatal and non-dopaminergic pathways.
The increased prevalence of the akinetic-rigid phenotype in people with PD and idiopathic RBD suggests that mechanisms mediating abnormally increased phasic and tonic muscle activity during REM sleep may also contribute to alterations in muscle activity regulation during wakefulness, and thus the expression of rigidity. Increased brainstem pathology in conjunction with a higher rate of disease progression may also lead to a more symmetric presentation of rigidity in people with elevated REM sleep muscle tone. Degeneration in brainstem regions with bilateral descending projections that impact muscle tone, such as the locus coeruleus, caudal raphe, and medullary magnocellular region of the reticular formation (Braak et al., 2006), would be expected to contribute to a more symmetrical presentation of rigidity. PD symptoms also tend to become more symmetric with disease progression, reflecting increasingly bilateral degeneration of nigrostriatal dopaminergic pathways, so more rapid disease progression may lead to earlier presentation of symmetric rigidity (Marinus and van Hilten, 2015). To date, no study has used quantitative measures to compare rigidity between people with PD with and without RSWA. The purpose of this study was to examine the level and symmetry of forearm rigidity during pronation-supination using quantitative metrics in a cohort of individuals with mild to moderate PD. We hypothesized that people with PD and RSWA would present with more severe and symmetric rigidity compared with individuals with PD without RSWA and age-matched controls.
2. Methods
2.1. Participants
Forty-one people with PD (26 males, 15 females, age = 64 ± 7.5) and 20 healthy controls (8 males, 12 females, age = 60.2 ± 7.4) were included in this study (demographic summary in Table 1). Clinical diagnosis of PD was determined by movement disorders neurologists according to the Movement Disorder Society Clinical Diagnostic Criteria for PD (Postuma et al., 2015). Individuals in this study were part of a long-term prospective study, and those who were subsequently diagnosed with another form of parkinsonism were removed from this dataset. Exclusion criteria included: dementia diagnosis and/or a Montreal Cognitive Assessment score below 22 to screen for capacity to consent (Karlawish et al., 2013), history of a musculoskeletal disorder that significantly affects upper limb movement, other significant neurological disorders, implanted deep brain stimulator or other neurosurgery to treat PD, and insufficiently treated sleep apnea. All participants with PD performed movement tasks after a 24-hour withdrawal period from extended release antiparkinson medications and a 12-hour withdrawal period from immediate release antiparkinson medications. All study procedures were approved by the University of Minnesota Institutional Review Board and all participants provided written informed consent according to the Declaration of Helsinki.
Table 1.
Demographics of study participants. Age was compared with a one-way ANOVA. Disease duration and levodopa daily equivalent were compared using t-tests and Bonferroni corrected for multiple comparisons. There was a significant difference in levodopa daily equivalent between the Parkinson’s disease with RSWA (PD-RSWA+) and Parkinson’s disease without RSWA (PD-RSWA−) groups (* p = 0.009). Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) total scores and rigidity subscores were compared between groups using independent samples Kruskal-Wallis tests. These scores were significantly higher in both Parkinson’s disease groups compared to the control group (* p < 0.001).
| Control (n=20) |
PD-RSWA− (n=19) |
PD-RSWA+ (n=22) |
|
|---|---|---|---|
| Male/Female | 8/12 | 13/6 | 13/9 |
| Age (years) | 60.2 ± 7.4 | 62.6 ± 8.6 | 65.1 ± 6.4 |
| Disease duration (years) | N/A | 2.1 ± 1.5 | 2.8 ± 2.2 |
| MDS-UPDRS III Total Score (OFF medications) * | 3.5 ± 2.5 | 35.5 ± 11.9 | 40.1 ± 13.8 |
| MDS-UPDRS III Section 3.3 Rigidity Subscore * | 1.0 ± 1.2 | 6.1 ± 3.0 | 7.3 ± 3.6 |
| Levodopa daily equivalent (mg) * | N/A | 225 ± 168 | 505 ± 416 |
Note: RSWA: REM sleep without atonia.
2.2. Group assignment
All participants underwent an overnight polysomnography (PSG) study at the Sleep Center at the University of Minnesota, Fairview. Participants with PD were allowed to take antiparkinson medications for this portion of the study. The sleep studies were performed using standard video-based PSG procedures, including electromyography (EMG) recordings from the chin, forearms, and legs as well as electroencephalography recordings from 10 scalp electrodes. PSG data was analyzed by a blinded rater who is board certified in sleep medicine (A.V.). Sleep stages and percentage of REM sleep with RSWA were scored according to the American Academy of Sleep Medicine Manual for the Scoring of Sleep (Berry et al., 2018). Participants with PD were stratified into low and high RSWA groups based on the percentage of time during REM sleep when RSWA was present. The thresholds to be considered RSWA positive were derived from the distributions of the RSWA PSG scores across subjects. Participants with RSWA in at least 1 muscle group and/or clear evidence of dream enactment were assigned to the PD-RSWA+ group. There was a significant effect of group for both the tonic and phasic chin RSWA levels (F(2,58) = 15.4 (tonic), 22.6 (phasic), p < 0.001) as well as for phasic arm RSWA levels (F(2,51) = 15.1, p < 0.001) driven by significantly higher RSWA levels in the PD-RSWA+ group compared with the PD-RSWA− group and controls (p < 0.001) (Figure 1). All Control participants had normal levels of EMG activity below the determined cutoffs.
Fig. 1:
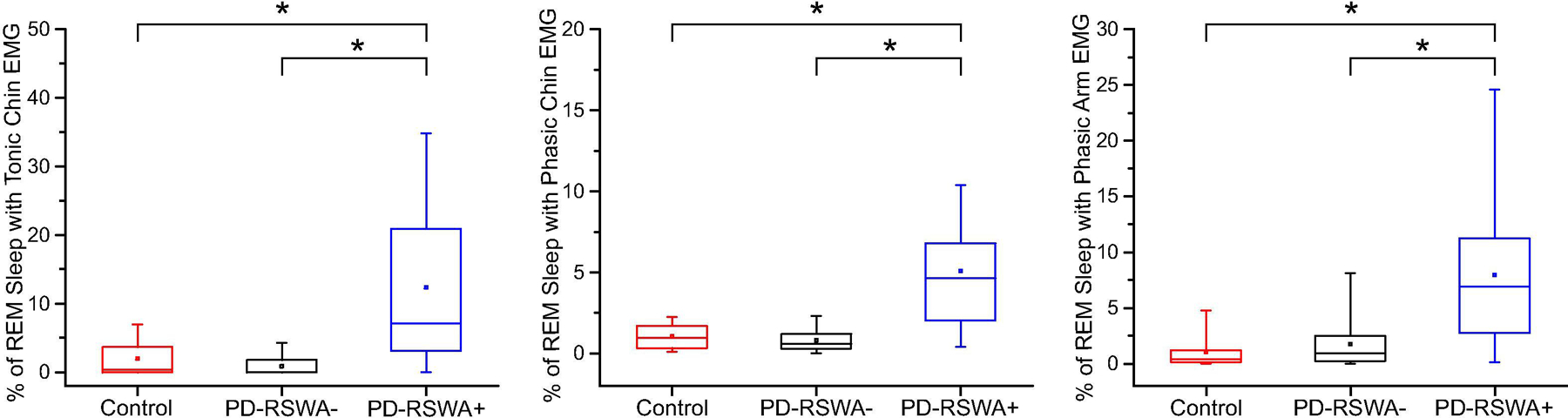
Rapid eye movement (REM) sleep without atonia (RSWA) values across groups. Participants were grouped by the percentage of REM sleep in which they exhibited RSWA for each of the measured muscles (submentalis or flexor digitorum superficialis). Participants who exhibited RSWA in at least one muscle group were categorized as Parkinson’s disease with RSWA (PD-RSWA+) while those with no RSWA were categorized as Parkinson’s disease without RSWA (PD-RSWA−). There was a significantly higher level of RSWA in the PD-RSWA+ group compared to the PD-RSWA− and control groups (One-way ANOVA, Bonferroni corrected post hoc comparisons, * p < 0.001). Boxes range from the first to the third quartile, whiskers extend to 95% confidence intervals, median is indicated by a line across the box, and mean is indicated by a square marker.
2.3. Data collection and analysis
All participants underwent a blinded assessment of motor symptom severity using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDSUPDRS) Part III evaluation. This data, as well as demographic data, were stored and managed using REDCap electronic data capture tools (Harris et al., 2009). Quantitative measures of rigidity were obtained used a custom-built robotic manipulandum (Entact Robotics Inc., Toronto, CA, Figure 2A). The device passively imposed sinusoidal movements of the forearm about the pronation-supination axis through a ± 40° range of motion at 1.5 Hz while measuring the angular displacement and resistive torque required to move the limb. Data was collected using a 1401 data acquisition interface paired with Signal software (Cambridge Electronic Design, Ltd., Cambridge, UK) at a sampling rate of 2000 Hz. Trials were collected separately on the right and left sides, with or without an activation maneuver (tapping the contralateral hand on the leg), a technique that is used clinically to elicit or enhance rigidity. In individuals with mild PD, rigidity can often only be detected during an activation maneuver. Each trial was 45 seconds long. Calculation of rigidity measures was performed using custom MATLAB software (MathWorks, Natick, MA, USA).
Fig. 2:
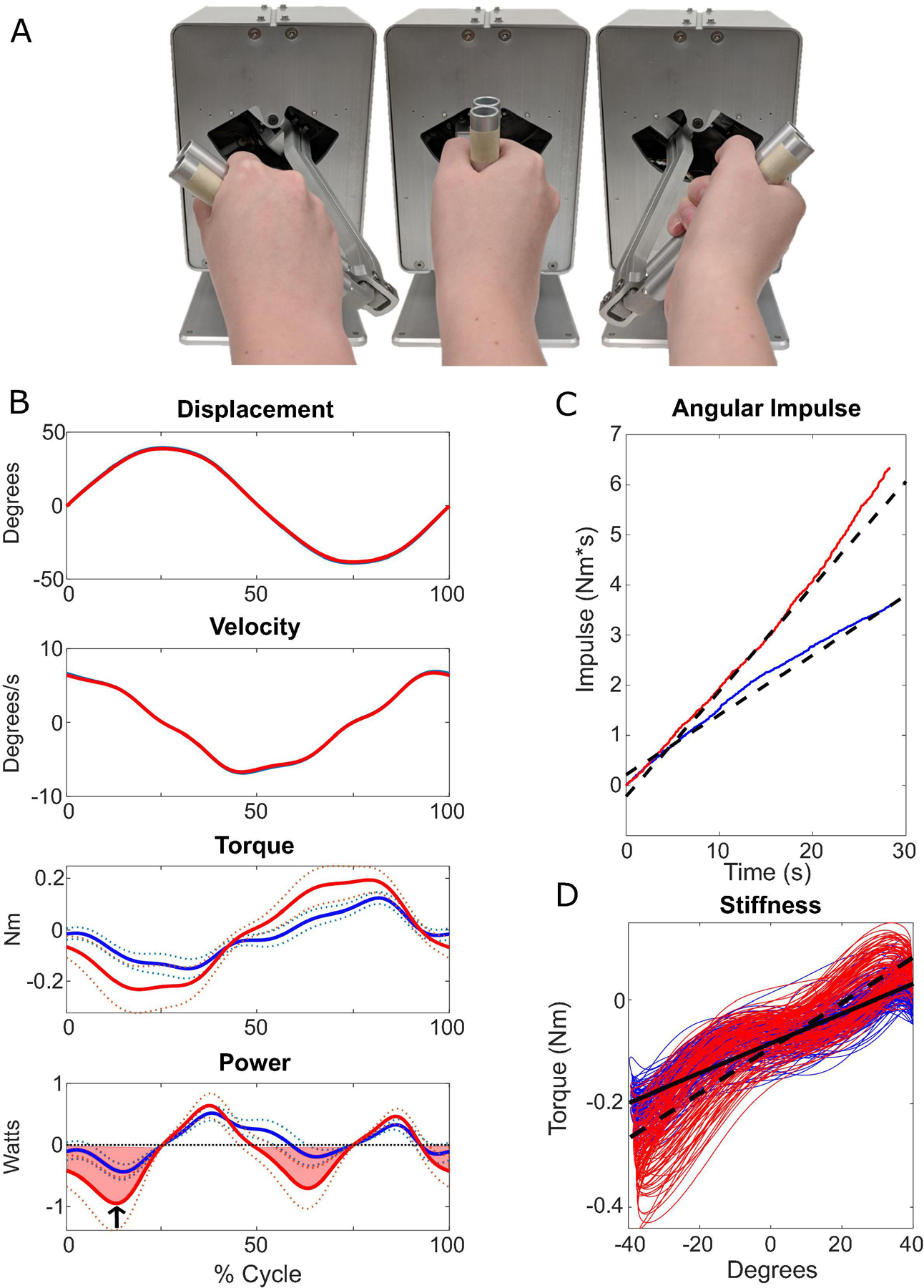
Robotic manipulandum and quantitative rigidity metrics from a single subject. A) The robotic manipulandum demonstrating approximate pronation, neutral, and supination positions (left to right, respectively). Participants were instructed to hold the handle as the motor rotated the forearm through ± 40 degrees of pronation-supination. Resistive torque was measured through a strain gage attached to the motor. B-D) Quantitative metrics are shown from two 45 second trials, with higher levels of torque measured during the trial shown in red due to the use of an activation maneuver. B) Plots showing one cycle of displacement and torque, measured from the robotic manipulandum, as well as velocity and power, which are calculated based on the displacement and torque. In the torque and power plots, the solid line shows the mean values with the standard deviation shown by the dotted lines. The shaded region of the power plot represents the negative work measure. The arrow indicates the peak negative power measure for this cycle. C) Red and blue lines show integrated torque over time, or impulse. The 1st order regression lines are indicated by the black dashed lines. The slope of the dashed line was used as a measure of angular impulse. D) The slope versus displacement plots are shown in red and blue. The slope of the 1st order regression lines (solid for blue, dotted for red) gives the measure of stiffness.
Four primary measures of rigidity were calculated from the torque, angular displacement, and time data collected by the robotic manipulandum:
- Angular impulse – a 1st order regression line was fit to the rectified torque data, and the slope of that line was used as the measure of angular impulse (Figure 2C).
- Peak negative power – the average peak negative power, calculated as the product of torque and angular velocity (derived from displacement) (Figure 2B “Power”, arrow). Final value is the average of 30 cycles.
- Negative work – the integral of negative power (Figure 2B “Power”, shaded region).
- Stiffness – the slope of the torque vs. displacement (Figure 2D).
All measures were calculated using 30 seconds of each trial, discarding the first 5 seconds to allow for stabilization of the imposed trajectory. Torque signals were filtered using a 4th order lowpass Butterworth filter with a 20 Hz cutoff frequency.
Angular impulse, stiffness, and work are rigidity metrics previously used in the literature that correlate well with clinical ratings (Fung et al., 2000; Patrick et al., 2001; Perera et al., 2019; Prochazka et al., 1997). Peak negative power is a unique measure that we implemented in order to address concerns related to the possibility of voluntary movements impacting the validity of our quantitative rigidity measures. Peak negative power represents the maximal rate of negative work done (energy absorption) when the resistive torque of the forearm opposes the direction of the angular velocity. Positive power occurs when the torque and angular velocity are the same direction and may reflect output during which the participant is actively assisting the motion of the robot arm. For this reason, negative power was used to specifically exclude periods of time when the participant may have been actively driving the manipulandum.
2.4. Statistical Analysis
Demographic features (age, disease duration, MDS-UPDRS Part III total score and rigidity subscore) were compared between groups using a one-way ANOVA (age), t-test (disease duration, levodopa daily equivalent) or an independent samples Kruskal-Wallis test (MDS-UPDRS III total score and rigidity subscore). MDS-UPDRS III upper limb rigidity subscores were compared between sides (more vs. less affected) using a Wilcoxon signed-rank test. Post hoc comparisons were Bonferroni corrected. Spearman’s rank correlation coefficients were calculated to assess the correlation between quantitative rigidity measures (without an activation maneuver) and clinical scores of rigidity (MDS-UPDRS III upper limb rigidity subscores) for all participants with PD. The distribution of the quantitative rigidity outcome measures (angular impulse, peak negative power, negative work, stiffness) across subjects (particularly in the PD-RSWA+ group) had an upward skew toward higher rigidity values and did not meet assumptions for normality (Shapiro-Wilk test) or homogeneity of variance (Levene’s test). For this reason, data were logarithmically (log10) transformed to achieve a normal distribution. A mixed model ANOVA was run to test for main effects of group (PD-RSWA+, PD-RSWA-, controls), side (more vs. less affected), activation condition (passive vs. with contralateral activation) and interactions between group × side, group × activation, and group × side × activation condition. The primary hypotheses were tested using planned comparisons of rigidity magnitude and symmetry (differences in measures between the more and less affected sides) between groups. In the PD groups, the more and less affected sides were determined by comparing the lateralized scores of the MDS-UPDRS III, which were calculated by summing the scores of all right-side symptoms and left-side symptoms separately. In the control participants, sides were randomly assigned to group 1 or 2 in order to match the distribution seen in the PD participants. Planned comparisons were conducted using Tukey’s Honest Significant Difference test. Interaction effects were further explored using post hoc pairwise comparisons, which were Bonferroni corrected. The significance level for all tests was set at α = 0.05. Statistical analysis was performed using IBM SPSS 25 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Clinical Measures
There were no significant differences in age between the three groups or disease duration between PD groups. However, the levodopa daily equivalent (LDE) was significantly different between the PD groups, with the PD-RSWA+ group having higher LDE than the PD-RSWA− group (p=0.009). The MDS-UPDRS Part III total score and rigidity subscore showed a significant effect of group (p < 0.001), with both scores significantly higher in both PD groups compared to controls (p < 0.001), but there was no significant difference in total scores or rigidity subscores between the PD-RSWA+ and PD-RSWA− groups. Upper limb rigidity subscores were compared between the more and less affected side for each group. There was no significant difference in scores between limb sides in controls. There was a significant difference in upper limb rigidity subscores between the more and less affected limbs in both the PD-RSWA− (p = 0.001) and PD-RSWA+ (p = 0.008) groups.
Correlations between all four quantitative measures and the MDS-UPDRS III upper limb rigidity subscores were significant (p < 0.001) with Spearman’s rho values as follows: angular impulse, ρ = 0.489; peak negative power, ρ = 0.532; negative work, ρ = 0.441; and stiffness, ρ = 0.518.
3.2. Quantitative Rigidity Measures
For all four quantitative rigidity measures (angular impulse, peak negative power, negative work, and stiffness) the mixed model ANOVA revealed significant main effects of group (F(2,58) > 4.0, p < 0.023), side (F(1,58) > 8.2, p < 0.006), and activation condition (F(1,58) > 33.5, p < 0.001). A significant group × side interaction (F(2,58) > 3.7, p < 0.030) was shown for measures of peak negative power, negative work, and stiffness, while a group × activation condition interaction was significant (F(2,58) > 3.8,p < 0.029) for measures of angular impulse, peak negative power, and stiffness. Comprehensive results of this analysis are shown in Table 2, and an example plot of peak negative power values is shown in Figure 3.
Table 2.
Mixed ANOVA Results. F-statistics and p-values are shown for main effects and interactions analyzed for each quantitative rigidity metric. Significant effects (p < 0.05) are indicated with an asterisk. Results of post hoc testing are described in the right column. Participants who exhibited rapid eye movement sleep without atonia (RSWA) in at least one muscle group were categorized as Parkinson’s disease with RSWA (PD-RSWA+) while those with no RSWA were categorized as Parkinson’s disease without RSWA (PD-RSWA−).
| Main effect of Group | Main effect of Side | Main effect of Activation | Interaction of Group × Side | Interaction of Group × Activation | Post hoc comparisons | |
|---|---|---|---|---|---|---|
| Angular impulse | F=4.32, p=0.018* |
F=9.86, p=0.003* |
F=33.11, p<0.001* |
F=2.76, p=0.072 |
F=4.78, p=0.012* |
Group: PD-RSWA+ > Controls Side: More affected > less affected Activation: Activation > no activation Group × Activation: Activation > no activation in PD |
| Peak negative power | F=4.31, p=0.018* |
F=14.59, p<0.001* |
F=47.08, p<0.001* |
F=4.61, p=0.014* |
F=3.75, p=0.029* |
Group: PD-RSWA+ > Controls Side: More affected > less affected Activation: Activation > no activation Group × Side: More affected > less affected in PD Group × Activation: Activation > no activation in PD |
| Negative work | F=4.03, p=0.023* |
F=8.24, p=0.006* |
F=33.48, p<0.001* |
F=3.74, p=0.03* |
F=2.59, p=0.084 |
Group: PD-RSWA+ > Controls Side: More affected > less affected Activation: Activation > no activation Group × Side: More affected > less affected in PD |
| Stiffness | F=4.68, p=0.013* |
F=10.59, p=0.002* |
F=33.2, p<0.001* |
F=6.88, p=0.002* |
F=5.69, p=0.006* |
Group: PD-RSWA+ > Controls Side: More affected > less affected Activation: Activation > no activation Group × Side: More affected > less affected in PD-RSWA− Group × Activation: Activation > no activation in PD |
Note: RSWA: REM sleep without atonia.
Fig. 3:
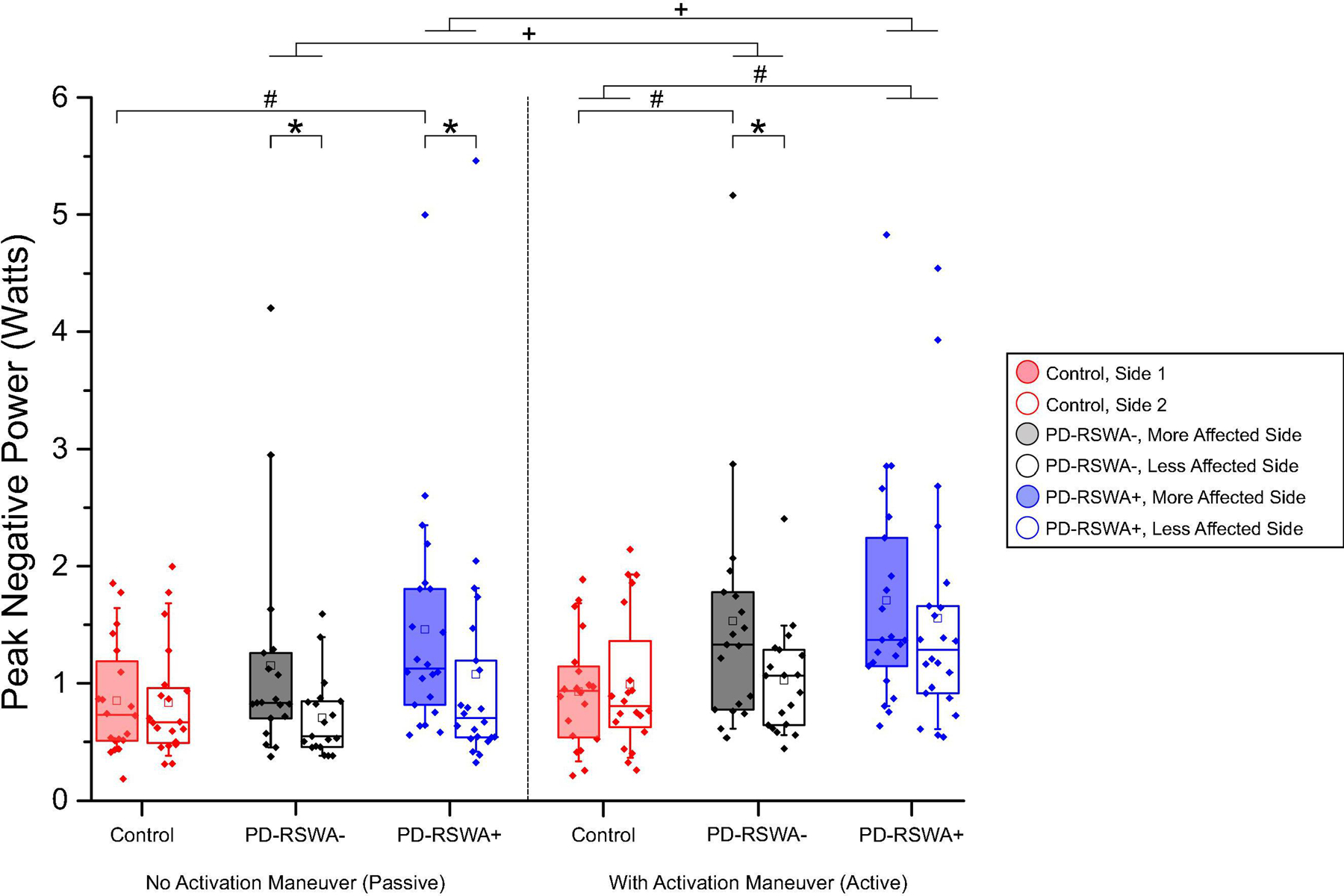
Peak negative power scores for all conditions. Scores for the more (shaded) and less affected limbs are shown for each group, with scores measured without an activation maneuver on the left and scores measured with an activation maneuver on the right. Left and right limbs in the control group were randomly assigned to Side 1 or Side 2 in order to match the distribution of left and right limbs in the more and less affected categories for participants with Parkinson’s disease. Data presented in the box plots are original, untransformed data. Statistical significance was determined using a mixed ANOVA of log transformed data, with Bonferroni corrected post hoc pairwise comparisons. Significant between group differences (# p < 0.027), within group differences between the more and less affected sides (* p < 0.008), and within group differences between the activation maneuver conditions (+ p < 0.001) are indicated with significance bars. Participants who exhibited rapid eye movement sleep without atonia (RSWA) in at least one muscle group were categorized as Parkinson’s disease with RSWA (PD-RSWA+) while those with no RSWA were categorized as Parkinson’s disease without RSWA (PD-RSWA−). Boxes range from the first to the third quartile, whiskers extend to 90% confidence intervals, median is indicated by a line across the box, and mean is indicated by a square marker. Individual data points are indicated with diamonds.
3.2.1. Difference between groups in rigidity magnitude
Comparisons between groups showed that rigidity scores were significantly higher in the PD-RSWA+ group than in controls (p < 0.015) for all rigidity measures. When the more and less affected sides were analyzed separately, peak negative power, negative work, and forearm stiffness were significantly increased in the PD-RSWA+ group compared with controls for all sides and conditions (p < 0.033), with the exception of the less affected arm without an activation maneuver. The impulse slope measure was also significantly increased in the PD-RSWA+ group compared with controls in both the more and less affected arms during the activation maneuver condition (p < 0.013). In contrast, significant differences in forearm rigidity between the PD-RSWA− and control group were only observed in the more affected limb during the activation maneuver condition (p < 0.027). Forearm stiffness was significantly higher in the PD-RSWA+ compared with the PD-RSWA− group in the less affected arm during both activation conditions (p < 0.045).
3.2.2. Rigidity symmetry between limbs
The main effect of side indicated that the more affected limb was significantly more rigid than the less affected limb for all four quantitative measures. Analyses of the group × side interaction revealed that there was a significant difference in rigidity scores between the more and less affected sides in the PD-RSWA− group for both the passive and activation maneuver conditions (p < 0.028). In the PD-RSWA+ group, there was a significant difference between sides only for the passive (no activation maneuver) condition (p < 0.022). There was no significant difference between sides in the control group for any condition. Planned between-group comparisons of rigidity score symmetry for each quantitative measure (more affected side score – less affected side score) were also performed. Rigidity scores measured without an activation maneuver showed no significant differences in symmetry between the PD-RSWA− and PD-RSWA+ groups or between the control and PD-RSWA+ groups. There was a significant difference in symmetry between the control and PD-RSWA− groups for measures of stiffness and negative work (p < 0.046), with the PD-RSWA− group showing greater asymmetry. With the activation maneuver, rigidity score symmetry was significantly different between the control and PD-RSWA− groups for all quantitative measures (p < 0.033), with the PD-RSWA− group showing greater asymmetry than controls. There was also a significant difference in symmetry between the PD-RSWA− and PD-RSWA+ groups (p < 0.039) for measures of angular impulse and stiffness, with the PD-RSWA− group showing greater asymmetry. There were no significant differences between the control and PD-RSWA+ group symmetry scores with the activation maneuver. Symmetry scores for stiffness are shown in Figure 4.
Fig. 4:
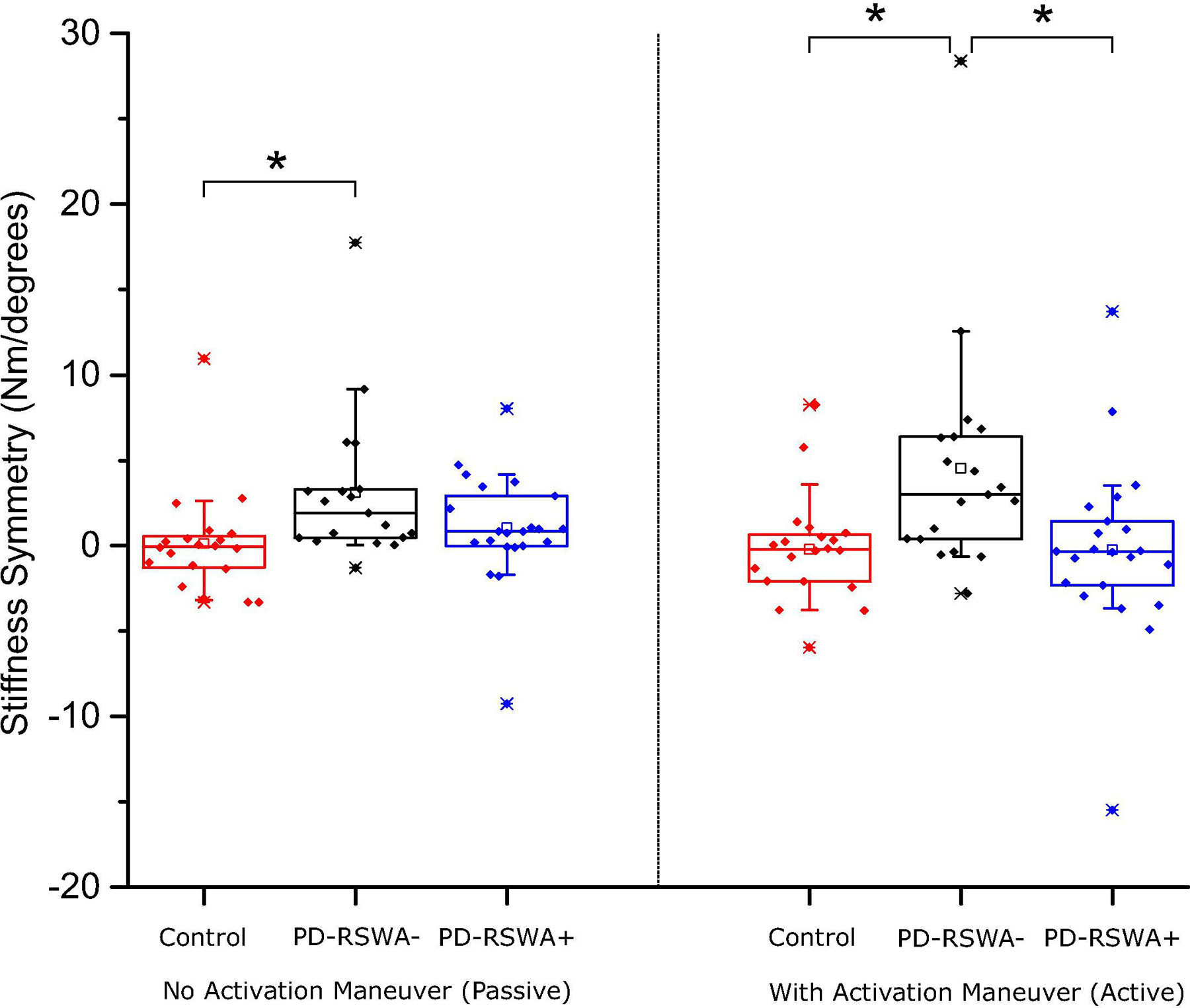
Symmetry scores for the stiffness measure. Symmetry scores were determined by subtracting the quantitative rigidity measure of the less affected limb from the more affected limb, thus values closest to zero indicate the greatest symmetry. Symmetry scores were significantly higher in the Parkinson’s disease without RSWA (PD-RSWA−) group compared to the control group (Tukey’s Honestly Significant Difference test, * p = 0.031) in the no activation maneuver condition. In the condition with an activation maneuver, the PD-RSWA− group had significantly higher symmetry scores than both the Parkinson’s disease with RSWA (PD-RSWA+) and control groups (Tukey’s Honestly Significant Difference test, * p < 0.018). Boxes range from the first to the third quartile, whiskers extend to 90% confidence intervals, median is indicated by a line across the box, and mean is indicated by a square marker. Individual data points are indicated with diamonds.
RSWA: REM sleep without atonia.
3.2.3. Enhancement of rigidity with an activation maneuver
The main effect of the activation condition showed that rigidity scores for all four outcome measures were significantly higher with an activation maneuver (p < 0.001). Post hoc analyses of the group × activation condition interaction found significantly higher rigidity scores for both PD groups on both sides with activation when compared to the no activation condition (p < 0.015). There was no significant difference between activation conditions for the control group.
4. Discussion
There were two main findings from this experiment. First, measures of forearm rigidity were significantly increased in the PD-RSWA+ group compared to control subjects. In contrast, significant differences in forearm rigidity between the PD-RSWA−and control group were only observed in the more affected limb during the activation maneuver condition. Second, asymmetry in the expression of forearm rigidity (calculated as the difference in quantitative scores between the more and less affected sides) was higher in the PD group without RSWA (PD-RSWA−) relative to controls and, for some variables, more asymmetric than the PD-RSWA+ group, particularly with the use of an activation maneuver. There were no significant differences in asymmetry between controls and the PD-RSWA+ group. These findings demonstrate that the loss of atonia during REM sleep is associated with increased bilateral expression of forearm rigidity in people with PD.
Two primary, albeit not mutually exclusive, explanations might account for the increased rigidity in the cohort with early PD and RSWA. First, increased expression and symmetry of rigidity may reflect increased disease severity and more extensive degeneration of nigrostriatal dopaminergic neurons bilaterally. Rigidity increases in severity as PD progresses, and in individuals with akinetic-rigid predominant PD, rigidity progresses faster than in the tremor predominant subtype (Vu et al., 2012). In addition, motor signs, including rigidity, typically become more symmetrical with disease progression (Marinus and van Hilten, 2015). This explanation aligns with previous work suggesting that the co-expression of idiopathic RBD and PD is associated with increased severity and faster disease progression (Chahine et al., 2014; Folle et al., 2019). In the present study, there was no significant statistical difference in age or disease duration between the PD groups. However, the disease duration was longer (on average by 0.7 years) and disease severity was worse in the RSWA+ group (on average by 5 points on the MDS-UPDRS Part III). Given the inherent inaccuracies of estimating disease duration based on time since diagnosis or time from first symptoms, we cannot rule out the possibility that the PD-RSWA+ group has had a longer disease duration thus providing more time to manifest increases in bilateral rigidity.
A second possibility is that increased expression of symmetric rigidity in the PD-RSWA+ participants may be mediated by the early degeneration of brainstem structures that contribute to the regulation of muscle tone. The circuits responsible for the control of REM sleep muscle tone involve multiple interconnected brainstem nuclei (Boeve et al., 2007). Portions of this circuit overlap with nuclei that contribute to spinal motor neuron excitability, postural control, and locomotion during wakefulness, such as the pedunculopontine nucleus, pontomedullary reticular formation, locus coeruleus and caudal raphe nucleus. Degeneration in nuclei that control REM sleep muscle tone (e.g. sublaterodorsal nucleus) likely precedes the loss of neurons that contribute to alterations in muscle tone regulation during wakefulness such as the locus coeruleus, caudal raphe, magnocellular and gigantocellular reticular formation (Braak Stage 2) and the pedunculopontine nucleus (Braak stage 3) (Boeve et al., 2007; Braak et al., 2006, 2003). This may explain why overt signs of rigidity are not expressed until 3–5 years prior to a diagnosis of PD in people with idiopathic RBD (Fereshtehnejad et al., 2019).
It has been hypothesized that parkinsonian rigidity may be driven, in part, by dysfunctional reticulospinal tract output originating in the nucleus reticularis giganto cellularis (NRGc) (Delwaide, 2001). Caudal regions of the pedunculopontine nucleus send cholinergic projections to both the NRGc and ventromedial medullary reticular formation (Martinez-Gonzalez et al., 2011). Reticulospinal neurons in these regions project bilaterally to the spinal cord and can influence muscle tone via direct and indirect connections to alpha and gamma motoneurons in the spinal cord (Carpenter, 1991; MacKinnon, 2018). Accordingly, the symmetrical expression of rigidity may reflect disordered control of motor neuron excitability due to pedunculopontine nucleus and/or reticular formation pathology. In keeping with this idea, Bliwise et al. showed that elevated phasic muscle activity during REM sleep is associated with a more symmetric presentation of disease in a cohort with comparable disease duration (Bliwise et al., 2010).
Early development of synucleinopathy in the locus coeruleus and caudal regions of the raphe nucleus (Braak et al., 2006) may also contribute to changes in spinal motoneuron excitability during wakefulness. The locus coeruleus and raphe nucleus send extensive noradrenergic and serotonergic projections to motor neurons in the spinal cord. These inputs play a critical role in the modulation of motor neuron firing by amplifying and prolonging synaptic input and facilitating self-sustained firing through the induction of persistent inward currents (Heckman et al., 2009, 2005). Persistent inward currents allow the motor neuron to continue firing in the absence of synaptic input. These mechanisms are considered to play an important role in postural control by reducing the need for sustained synaptic input. Accordingly, reduced descending input from this system could result in disordered control of motor neuron firing in response to sensory afferent or descending input.
The mechanisms contributing to the expression of rigidity are poorly understood but alterations in the excitability of both cortical and subcortical pathways mediating long-latency stretch reflexes (Rothwell et al., 1983; Tatton and Lee, 1975) and stretch induced co-activation of agonist-antagonist (Xia, 2011; Xia et al., 2009, 2006; Xia and Rymer, 2004) have been proposed. Early studies of rigidity pathophysiology identified that the long-latency component of the stretch reflex evoked by rapidly imposed displacements of the upper limb was abnormally enhanced in PD (Berardelli et al., 1983; Mortimer and Webster, 1979; Rothwell et al., 1983; Tatton and Lee, 1975). In the hand and wrist muscles, the long-latency stretch reflex occurs after the spinally mediated short-latency stretch reflex and is thought to be mediated, in part, by a polysynaptic transcortical pathway via the primary motor cortex (Angel and Lemon, 1975; Cheney and Fetz, 1984; MacKinnon et al., 2000; Matthews, 1991). In more proximal muscles, subcortical structures are considered to play more of a role in the generation of long latency responses to imposed stretch (F. Lenz et al., 1983; F. A. Lenz et al., 1983). Thus, changes in sensorimotor processing at the cortical or subcortical level may contribute to the expression of rigidity in the forearm during passively imposed movements. In addition to abnormally enhanced long-latency reflexes, people with PD may also show an abnormal shortening reaction, defined as an increase in activity in muscles shortened by an imposed movement (Berardelli et al., 1983; Xia and Rymer, 2004). The shortening reaction leads to co-contraction and increased joint stiffness during passive movement. The mechanisms mediating the shortening reaction in PD are poorly understood but changes in the pathways controlling short-latency autogenic inhibition, suggesting abnormalities in the excitability of Ia and Ib spinal interneurons, may contribute (Delwaide et al., 1991). These populations of interneurons are modulated by inputs from the reticulospinal tract, again suggesting that dysfunction of reticular nuclei may contribute to the expression of rigidity (Delwaide, 2001; Delwaide et al., 2000). Taken together, it is likely that all of the above mechanisms contribute and interact to increase muscle activity during imposed movements (Xia et al., 2009), but expression of rigidity may be dependent upon location and extent of cortical or subcortical degeneration for a given individual. Our data shows that rigidity is increased bilaterally in people with PD and RSWA, which could suggest increased involvement of subcortical pathways.
Contralateral movements used as an activation maneuver resulted in a significant increase in rigidity in both PD groups but not in control subjects. This finding is consistent with previous studies (Fung et al., 2000; Hong et al., 2007; Powell et al., 2011) and further supports and validates the use of the contralateral activation maneuver as a method to differentiate between healthy adults and people with parkinsonism. The relative increase in rigidity evoked by activation was substantially higher in the PD-RSWA− (55%) compared with the PD-RSWA+ group (15%), and enhanced the symmetric presentation of symptoms in the PD-RSWA+ group. The increased symmetry may be due to a ceiling effect, such that the rigidity in the PD-RSWA+ group’s more affected limb can only increase a small amount with activation, whereas the PD-RSWA−shows a large increase in rigidity in both limbs. Currently, the mechanisms mediating the increase in rigidity when contralateral movements are performed are poorly understood. Assuming that rigidity is mediated in part by a transcortical long-latency stretch reflex, it is possible that the activation maneuver enhances this reflex through changes in cortical activity. Isometric muscle contractions as well as rhythmic movements of the upper limb have been shown to increase cortical excitability of the ipsilateral motor cortex (Carson et al., 2004; Hortobágyi et al., 2003), which suggests a possible mechanism for the activation maneuver. Changes in spinal motoneuron excitability via crossed sensory afferent feedback (e.g. group lb for the activation side) may increase the sensitivity to imposed stretch or impact of the shortening reaction on measures of rigidity (Powell et al., 2011). Alternatively, the activation maneuver may facilitate bilateral descending projects from regions of the reticular formation that regular muscle tone and stretch reflex gain.
5. Conclusions
Our findings confirm that people with PD and RSWA have unique dysfunction in the regulation of muscle tone during both sleep and wakefulness. They also suggest that RSWA in the setting of PD predicts distinctive brain stem neuropathology. These discoveries are fundamentally important because they help investigators understand the physiology of motor activity and the pathophysiology of Parkinsonian disorders. To our knowledge, this is the first study to use quantitative measures to compare the level and bilateral expression of rigidity between people with PD with and without RSWA. While previous studies have found increased symptom severity in individuals with PD and idiopathic RBD, our study shows that even in mild to moderate PD, the presence of RSWA (even without a diagnosis of idiopathic RBD) is associated with increased rigidity magnitude and symmetry, especially upon use of an activation maneuver. It remains unclear whether the increased expression of rigidity reflects increased disease severity or increased involvement of subcortical pathways.
Highlights.
Muscle activity during REM sleep was associated with increased rigidity in Parkinson’s disease.
Upper limb rigidity in individuals with elevated REM sleep muscle activity was more symmetric.
These results suggest synucleinopathy in the brainstem may contribute to parkinsonian rigidity.
Acknowledgements
We would like to thank our research volunteers for their participation in this study, Joshua De Kam for his role in coordinating this research, Devin O’Connell and Minwoo Kim for assistance in data collection and administration, Drs. Nance, Johnson, Kuyper, and Parashos for referring volunteers to our study, and Dr. Lynn Eberly for statistical consultation. This work was supported by the National Institutes of Health grants R01 NS070264, R01 NS088670, P50 NS09857, T32 GM008471, 8UL1TR000114-02 and UL1TR000114, National Science Foundation fellowship DGE-1734815, the Wallin Neuroscience Discovery Fund, and MnDRIVE Research Fellowships in Neuromodulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None of the authors have potential conflicts of interest to be disclosed.
References
- Angel A, Lemon RN. Sensorimotor cortical representation in the rat and the role of the cortex in the production of sensory myoclonic jerks. J Physiol 1975;248:465–88. 10.1113/jphysiol.1975.sp010984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1983;46:45–53. 10.1136/jnnp.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.5. American Academy of Sleep Medicine; 2018. [Google Scholar]
- Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Ann Neurol 2010;68:353–9. 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Molano JR, Ferman TJ, Lin S-C, Bieniek K, Tippmann-Peikert M, et al. Validation of the Mayo Sleep Questionnaire to Screen for REM Sleep Behavior Disorder in a Community-Based Sample. J Clin Sleep Med 2013;09:475–80. 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130:2770–88. 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 2006;21:2042–51. 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211. 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Core text of neuroanatomy. 4th Edition Baltimore, MD, Williams & Wilkins, 1991. [Google Scholar]
- Carson RG, Riek S, Mackey DC, Meichenbaum DP, Willms K, Forner M, et al. Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 2004;560:929–40. 10.1113/jphysiol.2004.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Kauta SR, Daley JT, Cantor CR, Dahodwala N. Surface EMG activity during REM sleep in Parkinson’s disease correlates with disease severity. Parkinsonism Relat Disord 2014;20:766–71. 10.1016/j.parkreldis.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 1984;349:249–72. 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ. Parkinsonian rigidity. Funct. Neurol 2001;16:147–56. [PubMed] [Google Scholar]
- Delwaide PJ, Pepin J-L, De Pasqua V, de Noordhout AM. Projections from basal ganglia to tegmentum: a subcortical route for explaining the pathophysiology of Parkinson’s disease signs? J Neurol 2000;247:II75–81. 10.1007/PL00007765. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Pepin JL, De Noordhout AM. Short-latency autogenic inhibition in patients with parkinsonian rigidity. Ann Neurol 1991;30:83–9. 10.1002/ana.410300115. [DOI] [PubMed] [Google Scholar]
- Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 2019;142:2051–67. 10.1093/brain/awz111. [DOI] [PubMed] [Google Scholar]
- Folle AD, Paul KC, Bronstein JM, Keener AM, Ritz B. Clinical progression in Parkinson’s disease with features of REM sleep behavior disorder: A population-based longitudinal study. Parkinsonism Relat Disord 2019:1–7. 10.1016/j.parkreldis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung VSC, Burne JA, Morris JGL. Objective quantification of resting and activated parkinsonian rigidity: A comparison of angular impulse and work scores. Mov Disord 2000;15:48–55. . [DOI] [PubMed] [Google Scholar]
- Haba-Rubio J, Frauscher B, Marques-Vidal P, Toriel J, Tobback N, Andries D, et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 2018;41:1–8. 10.1093/sleep/zsx197. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle and Nerve 2005;31:135–56. 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: The importance of neuromodulatory inputs. Clin Neurophysiol 2009;120:2040–54. 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Perlmutter JS, Earhart GM. Enhancement of rigidity in Parkinson’s disease with activation. Mov Disord 2007;22:1164–8. 10.1002/mds.21524. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in Segmental and Motor Cortical Output With Contralateral Muscle Contractions and Altered Sensory Inputs in Humans. J Neurophysiol 2003;90:2451–9. 10.1152/jn.01001.2002. [DOI] [PubMed] [Google Scholar]
- Karlawish J, Cary M, Moelter ST, Siderowf A, Sullo E, Xie S, et al. Cognitive impairment and PD patients’ capacity to consent to research. Neurology 2013;81:801–7. 10.1212/WNL.0b013e3182a05ba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Santamaria J, Tolosa E, Iranzo A. Relation between subtype of Parkinson’s disease and REM sleep behavior disorder. Sleep Med 2007;8:779–83. 10.1016/j.sleep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lenz F, Tatton W, Tasker R. Electromyographic response to displacement of different forelimb joints in the squirrel monkey. J Neurosci 1983;3:783–94. 10.1523/JNEUROSCI.03-04-00783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Tatton WG, Tasker RR. The effect of cortical lesions on the electromyographic response to joint displacement in the squirrel monkey forelimb. J Neurosci 1983;3:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon CD. Sensorimotor anatomy of gait, balance, and falls. Handb Clin Neurol 2018;159:3–26. 10.1016/B978-0-444-63916-5.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon CD, Verrier MC, Tatton WG. Motor cortical potentials precede long-latency EMG activity evoked by imposed displacements of the human wrist. Exp Brain Res 2000;131:477–90. 10.1007/s002219900317. [DOI] [PubMed] [Google Scholar]
- Marinus J, van Hilten JJ. The significance of motor (A)symmetry in Parkinson’s disease. Mov Disord 2015;30:379–85. 10.1002/mds.26107. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat 2011;5:22 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. The human stretch reflex and the motor cortex. Trends Neurosci 1991;14:87–91. 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Sixel-Döring F, Wicke T, Ebentheuer J, Schaumburg M, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013;81:1226–34. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Webster DD. Evidence for a quantitative association between EMG stretch responses and Parkinsonian rigidity. Brain Res 1979;162:169–73. 10.1016/0006-8993(79)90768-6. [DOI] [PubMed] [Google Scholar]
- Patrick SK, Denington AA, Gauthier MJA, Gillard DM, Prochazka A. Quantification of the UPDRS rigidity scale. IEEE Trans Neural Syst Rehabil Eng 2001;9:31–41. 10.1109/7333.918274. [DOI] [PubMed] [Google Scholar]
- Perera T, Lee W-L, Jones M, Tan JL, Proud EL, Begg A, et al. A palm-worn device to quantify rigidity in Parkinson’s disease. J Neurosci Methods 2019;317:113–20. 10.1016/j.jneumeth.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–601. 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry 2008;79:1117–21. 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142:744–59. 10.1093/brain/awz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D, Hanson N, Joseph Threlkeld A, Fang X, Xia R. Enhancement of parkinsonian rigidity with contralateral hand activation. Clin Neurophysiol 2011;122:1595–601. 10.1016/j.clinph.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, et al. Measurement of rigidity in Parkinson’s disease. Mov Disord 1997;12:24–32. 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- Romenets SR, Gagnon J-F, Latreille V, Panniset M, Chouinard S, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease. Mov Disord 2012;27:996–1003. 10.1002/mds.25086. [DOI] [PubMed] [Google Scholar]
- Rothwell J, Obeso J, Traub M, Marsden C. The behaviour of the long-latency stretch reflex in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1983;46:35–44. 10.1136/jnnp.46.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744–8. 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Lee RG. Evidence for abnormal long-loop reflexes in rigid Parkinsonian patients. Brain Res 1975;100:671–6. 10.1016/0006-8993(75)90167-5. [DOI] [PubMed] [Google Scholar]
- Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol 2012;74:267–83. 10.1111/j.1365-2125.2012.04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R. Physiological and Biomechanical Analyses of Rigidity in Parkinson’s Disease In: Qayyum Rana A, editor. Etiol. Pathophysiol. Park. Dis., IntechOpen; 2011, p. 13 [Google Scholar]
- Xia R, Markopoulou K, Puumala SE, Rymer WZ. A comparison of the effects of imposed extension and flexion movements on Parkinsonian rigidity. Clin Neurophysiol 2006;117:2302–7. 10.1016/j.clinph.2006.06.176. [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. The role of shortening reaction in mediating rigidity in Parkinson’s disease. Exp Brain Res 2004;156:524–8. 10.1007/s00221-004-1919-9. [DOI] [PubMed] [Google Scholar]
- Xia R, Sun J, Threlkeld AJ. Analysis of interactive effect of stretch reflex and shortening reaction on rigidity in Parkinson’s disease. Clin Neurophysiol 2009;120:1400–7. 10.1016/j.clinph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu C-Y, Liu J. Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease. BMC Neurol 2017;17:23 10.1186/s12883-017-0795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


