Abstract
The coronavirus disease COVID-19 is a public health emergency caused by a novel coronavirus named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). SARS-CoV-2 infection uses the angiotensin-converting enzyme 2 (ACE2) receptor, and typically spreads through the respiratory tract. Invading viruses can elicit an exaggerated host immune response, frequently leading to a cytokine storm that may be fueling some COVID-19 death. This response contributes to multi-organ dysfunction. Accumulating data points to an increased cardiovascular disease morbidity, and mortality in COVID-19 patients. This brief review explores potential available evidence regarding the association between COVID-19, and cardiovascular complications.
Keywords: COVID-19, SARS-CoV-2, Myocardial injury, ACE2, Severe acute respiratory syndrome, Troponin
1. Introduction
Epidemic Corona Virus Disease 2019 (COVID-19) is gradually spreading by human-to-human transmission. The pathogen has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], [2]. In COVID-19, particular attention has been given to the role of angiotensin-(Ang) converting enzyme 2 (ACE2), and the binding site for SARS-CoV-2 cellular entry [3]. Besides severe lung involvement, invasion of the virus into circulation elicits an exaggerated host immune response, frequently leading to a cytokine storm that is associated with in-hospital death [4]. At present, there are very few evidences supporting cardiac involvement during SARS-Cov-2 infection, and they arose mainly from observational studies. Accumulating data points to the implication of the cardiovascular (CV) system on multiple levels linking COVID-19 with increased morbidity, and mortality from cardiovascular disease (CVD). In this review article, we explore SARS-CoV-2 associated infection mechanisms with a special focus on CVD and provide an overview of this topic.
2. Structural properties
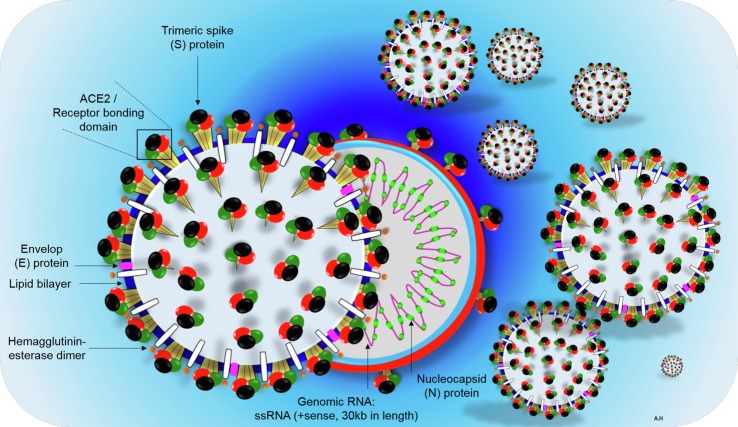
Corona viruses are miniature in size (60–140 nm in diameter) and contain a single positive-stranded ribonucleic acid (RNA), typically ranging from 26 to 32kbs in length. The SARS-CoV-2 is a novel β-coronavirus category that takes a round or elliptic pleomorphic form (Fig. 1 ). Metagenomics analysis from next-generation sequencing convincingly demonstrate this virus consists of six major open-reading frames (ORFs) that are common to coronaviruses, and a number of other accessory genes [5]. Further analysis indicates some of the expressed genes share less than 80% nucleotide sequence identity to earlier SARS-CoV [6]. The SARS-CoV-2 RNA genome contains 29,891 nucleotides, encoding for 9860 amino acids [6]. Although its probable origins are not completely understood, genomic analyses suggest that it probably evolved from a strain found in bats [7], [8]. Similar to most other coronaviruses, the outer membrane spike glycoprotein of SARS-Cov-2, is the prime interacting protein with host cell target receptors (such as ACE2, CD26, Ezrin, cyclophilins) which are important for cell adhesion, and virulence [9]. Under an electron microscope, the SARS-CoV-2 surface morphology possesses multiple polyproteins, nucleoproteins, and membrane proteins, such as spike glycoproteins S [10]. The latter involves homotrimers protruding far from the viral surface, giving it a halo like appearance or corona as illustrated in Fig. 1.
Fig. 1.
Schematic structure of virion of COVID-2019, and its major structural proteins. ssRNA; single-stranded ribonucleic acid, ACE2; angiotensin-converting enzyme 2, RBD; receptor binding domain.
3. Mechanisms of COVID-19 infection
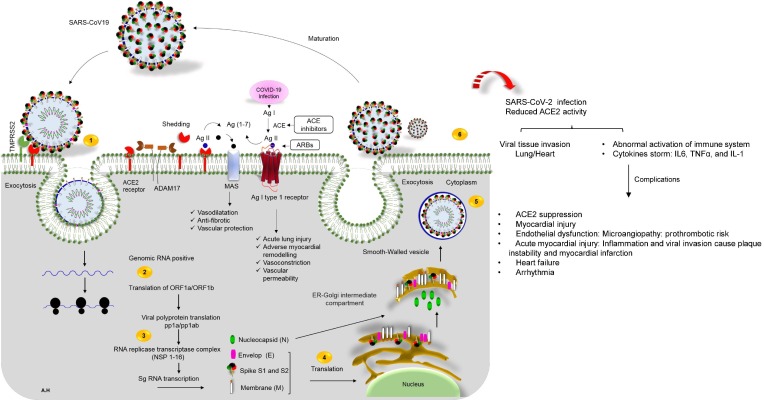
COVID-19 patients presented with mild flu-like symptoms, and a few patients rapidly develop acute respiratory distress syndrome, respiratory failure, multiple organ failure, and even deaths (Table 1 ). In the initial step of the infection, studies support a potential interaction between SARS-CoV-2, and ACE2 receptor as a portal of infection (Fig. 2 ) [9], [11]. ACE2 is a type 1 transmembrane protein predominantly expressed by epithelial cells of the lung [12]. When compared to other viruses that cause SARS, there are some differences in the precise amino acids belong to spike glycoprotein used to bind SARS-CoV-2 to that ACE2 receptor. Distinctly, a larger sequence difference (~55% identity) between SARS-CoV-2, and SARS-CoV was found in the S1 domain of the spike glycoprotein S (aa01–aa550) [9]. This domain is recognized for host cell target contact [9]. The receptor-binding domain (RBD) of SARS-CoV-2, and SARS-CoV interacts with ACE2 receptor [9]. Interface comparison between the RBD in SARS-CoV-2, and SARS-CoV indicates that the most prominent alteration is the substitution of Val404 in the SARS-CoV-RBD with Lys417 in the SARS-CoV-2-RBD [11]. The high infectivity of the SARS-CoV-2 virus is in part related to new mutations in the RBD, and acquisition of a furin cleavage site. The latter mutation is inserted at the boundary of the S1/S2 subunits of the spike S-protein [13]. Moreover, the furin binding site can enhance the virus ability to internalize into cells [12]. Initially, membrane bound ACE2 proteins are cleaved by A Disintegrin And Metalloproteases 17 (ADAM17) that is upregulated by endocytosed SARS-CoV-2 spike S proteins (Fig. 2). Moreover, ACE2 contains an enzymatic domain located on the cell surface where it converts Ang II (1–9) to Ang 1–7 [14]. At this phase negative regulation of the renin-Ang system, with down regulation of ACE2 by SARS-CoV-2 may magnify the cytokine storm, resulting in an overwhelming inflammatory response (Fig. 2) [12]. In order to be active, the ACE2 complex is assembled as a stable dimer of heterodimers through interactions of the collectrin-like domain of ACE2 [11]. The resultant homodimer is able to bind two SARS-CoV-2 S protein trimers simultaneously. In this interaction, the cleavage of S protein into subunits, S1 and S2 facilitates target cell internalization following activation of the spike protein by transmembrane protease serine 2 (TMPRSS2) [12]. The S1 subunit of S protein contains the RBD which allows coronaviruses to directly bind to the peptidase domain (PD) of ACE2 [11]. S2 likely plays a role in membrane fusion where S1 domain potentially interacts with the human CD26 that is involved in the T-cell immune response [15]. It is speculated that increased expression of ACE2 could potentially facilitate SARS-CoV-2 infection [16]. The SARS-CoV-2 binding site shows that it has improved binding stability, and potentially enhanced ACE2 receptor binding affinity [11]. Lastly, structure-based rational affinity interaction to either ACE2 or the S protein of SARS-CoV-2 provides invaluable insights into the molecular basis for coronavirus recognition, and infection. These mechanisms may uncover a potential target of novel neutralizing antibodies to suppress infection by new viruses.
Table 1.
Most reported signs and symptoms of COVID-19. The severity of COVID-19 symptoms can range from very mild to severe. Some people may have only a few symptoms, and some people may have no symptoms at all.
| Level of severity | COVID-19 symptoms |
|---|---|
| The most common first symptoms among those who died: | Systemic disorders: Fever, dry cough, and fatigue [58]. Sneezing, and myalgia. Respiratory disorders: Pneumonia, and breathing difficulties (dyspnoea) |
| Less common symptoms | Systemic disorders: Headache, haemoptysis, dyspnoea lymphopenia and sputum production, rash, nausea, vomiting and diarrhea. |
| Severe symptoms | Difficulty breathing or shortness of breath Persistent chest pain or pressure Myocardial necrosis [59] Edema, and necrosis can lead to contractile dysfunction, and clinical symptom [59] Loss of speech or movement |
Fig. 2.
The life cycle of SARS-CoV-2 in host cells, and cardiovascular complications of virus infection. 1) ACE2 binds to the viral S protein, which is cleaved into two subunits, S1 and S2, by an extracellular protease. While S1 binds to ACE2, S2 is further cleaved, and activated by serine protease TMPRSS2. Together these actions result in host-viral membrane fusion, and the release of the RNA genome into the host cell cytoplasm, 2) Translation of structural, and non-structural proteins as follows: ORF1a, and ORF1ab proteins are translated to yield pp1a, and pp1ab polyproteins. These are cleaved by proteases that are encoded by ORF1a to produce 16 non-structural proteins. The polyproteins (pp1a, and pp1ab) are cleaved into 16 non-structural effector proteins by 3CLpro, and PLpro, 3) allowing them to form the replication complex together with the RNA polymerase, which synthesizes a full-length negative RNA strand template, 4) This template is used to replicate the complete RNA genome, and generate the individual sub-genomic mRNA needed for the translation of the viral structural, and accessory proteins, 5) The newly synthesized structural, and accessory proteins are then trafficked from the ER through the Golgi apparatus, after which new virions assemble in bourgeoning Golgi vesicles, 6) Finally, the mature SARS-CoV-2 virions are exocytosed, and released from the host cell. Also shown, schematic representation of the RAS-pathway in which ACE/Ang-II, and ACE/Ang 1–7/Mas-axis. Surface ACE2 is further down-regulated, resulting in unopposed angiotensin II accumulation describing the regulatory role of ACE2-Ang 1–7 axis in the cardiovascular homeostasis. Local activation of the RAAS by SARS-CoV-2 spike may mediate lung injury, and responses to viral insults such as CV complications. ACE enzyme inhibitors and angiotensin receptor blockers (ARBs) possibly rise the risk of severe COVID-19 outcomes. On the right of the panel, local complications of virus infection on cardiovascular system is presented.
4. Implication of ACE2 activity in cardiovascular complications
SARS-CoV-2 infection and its possible implication to myocarditis is still not well documented despite some case report [17]. Early data indicated that 25% of hospitalized, and treated patients in Wuhan had CVD [18]. Independent studies recognized a primary role of ACE2 in COVID-19 infection [19]. Of interest, ACE2 is localized in cardiomyocytes, cardiac fibroblasts, pericytes, vascular endothelium, and vascular smooth cells [16], [20]. Whether SARS-CoV-2 can directly proliferate in the heart is unidentified. COVID-19 patients with pre-existing CVD, hypertension, and related conditions experience disproportionately worse outcomes [21]. There are a very few pathological studies conducted on the COVID-19 patients. One of the clinical features of patients infected with SARS-CoV-2 included abnormal features such as acute cardiac injury (12%) [22]. Available clinical data revealed that 15% to 30% of the COVID‐19 patients present with hypertension, and 2.5% to 15% with coronary heart disease [18], [23]. Viral involvement of cardiomyocytes, and the effect of systemic inflammation seem to be the most common mechanisms responsible for cardiac injury [20] (Fig. 2). Thus, suggesting that inflammation may be a potential mechanism for myocardial injury. Various diseases including heart failure, hypertension, and diabetes are characterized by a relative ACE2-deficient state [24]. Genetic data shows that ACE2 is an essential regulator of heart function in vivo [25]. Activation of renin-Ang system, and the downregulation of ACE2 expression play a key role in several diseases including CV pathologies (Fig. 2) [16]. Accordingly, ACE2 blockade delayed CV damage in diabetic patients [16], [26]. Experimental evidence suggested a beneficial role of ACE2 in CV function [14]. Genetic manipulation of ACE2 expression points to the possible significance of this enzyme in cardiac function, and vulnerability to heart failure [14], [19]. In the established heart failure, expression of ACE2 on the cell surface is downregulated; however, there is an increase in circulating ACE2 levels, and activity when compared to healthy individuals [14], [27]. It is assumed that shedding of the membrane‐bound ACE2 may be responsible for the increased circulating endogenous ACE2 activity in COVID-19 patients [3]. Circulating levels of soluble ACE2 are usually low to nondetectable. Soluble ACE2 would therefore not sufficiently sequester SARS-CoV-2 in the circulation to prevent viral dissemination [19]. However, the extent to which soluble ACE2 would compete for SARS-CoV-2 binding to reduce viremia infection and alleviate tissue injury is unknown.
The occurrence of fulminant myocarditis, and cardiogenic shock seems low in patients with COVID-19 [28], [29]. Despite this, a predisposition to acute cardiac complications related to underlying atherosclerotic CVD may significantly increase the severity of COVID-19 in vulnerable individuals [29]. The exact mechanism of cardiac involvement in COVID-19 is still under investigation. As presented above, one potential mechanism directing myocardial involvement is mediated by ACE2. Other mechanisms of COVID-19 morbidity related to cardiac complications are discussed below.
5. Potential therapeutic approaches based on renin–angiotensin–aldosterone system inhibition
Renin–angiotensin–aldosterone system (RAAS) inhibitors are predominantly including angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II-receptor blockers (ARBs) (Fig. 2). RAAS inhibitors are often prescribed to treat patients with hypertension, or diabetes who are infected with SARS-CoV-2 [30]. Data from experimental mouse models, revealed that injection of SARS-CoV-1 induced acute lung injury, which is limited by blocking the RA pathway [27]. Intravenous recombinant human ACE2 (rhACE2; APN01, GSK2586881) was given to healthy subjects in a randomized clinical trial phase 2 (ClinicalTrials.gov number NCT01597635) safely reduced angiotensin II levels, is evaluated in humans with acute respiratory distress syndrome. In this trial, the observed restoration of ACE2 through the administration of (GSK2586881), appeared to attenuate acute lung injury [31]. However, this study was not adequately powered to determine changes in acute physiology or clinical outcomes [32]. Furthermore, in COVID-19 as with SARS‐CoV, higher ACE2 expression might lead to a higher risk of SARS‐CoV‐2 infection [3], [33]. Therefore, targeted disruption of ACE2 in mice caused the development of abnormal heart function [25]. Yet, increasing the expression of ACE2 may prevent, and reverse heart failure. Consequently, a therapeutic approach that will amplify the ACE2-Ang (1–7) axis could provide further protection against the progression of CVD [34]. Basing on these data, some authors have proposed an association between the use of RAAS inhibitors and COVID-19. Nevertheless, this association has been rejected due to the lack of evidence on the efficacy of this approach in reducing adverse outcomes in Covid-19 patients [35], [36]. Even if RAAS inhibitors modify ACE2 levels or activity (or both) in target tissue, there are not enough clinical reports to indicate whether this would in turn facilitate greater entry of SARS-CoV-2 proteins. Further mechanistic studies are required to better define the unique interplay between SARS-CoV-2 and the RAAS network [37].
6. COVID-19 pathogenesis in relation to the cardiovascular system
SARS-CoV-2 seems to damage the heart's muscle tissue and can cause myocarditis [38]. Accordingly, after SARS-CoV-2 infection various cases of severe myocarditis with reduced systolic function have been reported [17]. Although the mechanisms of myocardial involvement in COVID-19 are still under investigation, they probably include direct viral infection, hypoxia-induced apoptosis, and cytokine storm-related cell damage in the body [4]. Excessive inflammation (IL6, TNFα, and IL-1) can further modulate the function of several cardiomyocyte ion channels, specifically K + and Ca++ channels, leading to inflammatory cardiac channelopathies [39]. Excessive intracellular calcium promotes cardiomyocyte apoptosis [40]. Moreover, myocardial injury might represent a main driver of enhanced arrhythmic risk in these patients [41]. With the constantly evolving recognition of the interplay between COVID-19 and the CV system, available clinical data have shown that between 8 and 28% of patients with COVID-19 infections will manifest acute cardiac injury. This condition is assessed by increase in cardiac biomarkers release such as elevated troponin I (TnT), N-terminal pro–brain natriuretic peptide (NT-proBNP) or B-type natriuretic peptide (BNP) (Table 2 ) [22], [42], [43]. Evidence of myocardial injury was recommended on the basis that TnT elevation in patients with COVID-19 is significantly associated with fatal outcomes [44]. Numerous mechanisms may elucidate this phenomenon mostly viral myocarditis, cytokine-driven myocardial injury, and microangiopathy (Table 2). Yet, none of these mechanisms have been recognised to be the key driver of troponin rise and/or myocardial injury. Moreover, given the presence of abundant distribution of ACE2 in cardiomyocytes [20], some have suggested that myocarditis might elucidate rise of TnT in some cases, mainly as acute left ventricular failure in some cases [44], [45]. Notably, a rise of cardiac TnT should not be considered evidence for an acute MI [46]. This should be based on clinical assessment, and ECG, where cardiac troponin elevations of cardiac TnT can inform the diagnosis of a number of cardiac conditions related to COVID-19 [45].
Table 2.
Biomarkers for the detection and diagnosis of myocardial injury in patients with COVID-19.
| Potential mechanism of myocardial injury in COVID-19 | Biomarkers of diagnosis |
|---|---|
| Acute coronary syndrome; Myocardial infarction condition | Trajectory of TnT concentration, and ECG changes (defined as ST segment elevation/ST-T0); coronary angiography Haemodynamic stress, quantified by BNP, and NT-proBNP concentrations Raised serum CK-MB levels are correlated with injury size, but are predictors of poor prognosis in COVID-19 patient [52] |
| Heart failure [43] | Elevated d-dimer, TnT, LDH, and IL plasma levels |
| Cytokine storm: myocardial dysfunction | Inflammatory, and cardiac biomarker testing (often need to exclude coexisting cardiac diagnoses) TnT, NT-pro BNP tests (for the analysis of potential myocardial injury, myocarditis, and cardiac dysfunction). |
| Myocarditis | Triple elevation in cardiac TnT (over 0.12 ng/mL) plus abnormalities on echocardiography, and/or ECG [60]. Cardiac MRI for tissue characterisation (Lake Louise criteria); endomyocardial biopsy in selected cases [61] |
| Stress cardiomyopathy | Cardiac imaging patterns; diagnosis of exclusion (typically after excluding coronary artery disease) POCUS: assessment of the left ventricle in a case of cardiomyopathy [62]. |
TnT, troponin; ECG, electrocardiogram; BNP, B-type natriuretic peptide; NT-proBNP, N-terminal B type natriuretic peptide; CK-MB, creatine Kinase-MB; COVID-19, corona virus disease 2019; LDH, lactate dehydrogenase; IL, interleukin; MRI, magnetic resonance imaging; Point-of-care ultrasound, POCUS.
Elevated TnT is likely to be multifactorial, and less likely to be attributable to atherothrombotic coronary occlusion [46]. Indeed, 16% of patients with underlying CVD but with normal TnT levels had a relatively favorable outcome [42]. Available data on the role of cardiac biomarkers, supported the role of assessing TnT and natriuretic peptides (NT-proBNP and BNP) for cardiac risk stratification and prognostication of patients with severe COVID-19 [47], Based on the evidence of myocardial injury and the presence of CV complications, this might improve CV care among COVID-19 patients, as well as decrease the risk of infection, but there could be some limitations [48]. Nevertheless, additional novel biomarkers are needed for CV disease diagnosis, risk stratification, and management in COVID-19
7. Inflammation and CV complications in COVID-19
Rise in inflammation in the myocardium can result in CV complications, such as myocarditis, heart failure, cardiac arrhythmias, and acute coronary syndrome (ACS) (Fig. 2) [17]. However, elevated high sensitivity TnT with other inflammatory vascular biomarkers (d-dimer, ferritin, IL-6, and lactate dehydrogenase) may rise the possibility that these changes are specific effects of the virus, reflects a cytokine storm, or is merely an isolated myocardial injury (Table 2). As an example of this statement, one case reported a man presenting with chest pain, and ST-segment elevation without coronary obstruction as well as elevated cardiac biomarkers (TnT serum levels > 10 ng/mL, NT-proBNP > 21,000 pg/mL) [40]. Another study found IL-6 over 4.3 pg/mL, and d-dimer (a fibrin degradation product) over 0.28 µg/L, and had a possible predictive value for the severity of SARS‐COV‐2 infection [49]. The finding of increased d-dimer levels in patients with severe COVID-19 has prompted questions regarding the existence of disseminated intravascular coagulation which may predispose patients to thrombosis risk [4]. These abnormalities may then lead to ACS [50]. Patients with COVID-19 are also at an increased risk of venous thromboembolic event (Fig. 2). The most consistent hemostatic abnormalities with COVID-19 include mild thrombocytopenia, and increased d-dimer levels [51]. Thus, demonstrating a promise to hold prognostic value of d-dimers in COVID-19 patients [52]. However, the reason behind elevated d-dimer levels whether is thrombosis/hypercoagulability or proinflammatory response remains unclear until now.
More recently, inflammatory response, hypoxic abnormalities, and electrolytic disturbance are found to be one of the physiologic sequelae of COVID-19 leading to arrhythmic risk. Notably, heart palpitation was reported as one of the most common initial symptoms in 137 patients presenting with COVID-19 (7.3%) [41]. In addition, the elevation of serum Ang exhibits a direct correlation to the viral load, and lung injury [53]. At this point, plasma levels of angiotensin II may offer a novel method of predicting disease severity [52]. Indeed, activation of ADAM-17 by Ang II, and SARS-CoV-2 binding leads to a loss of membrane-bound ACE2, and its release into the circulation (Fig. 2). However, intracellular ACE levels are not depleted [54]. These conditions limit the effects of Ang II diverse in tissues, culminating in CV, renal, and lung diseases. Higher plasma ACE2 levels were associated with a history of atrial fibrillation, and coronary artery bypass graft, and elevated heart rate specially in man [14]. Moreover, reduced ACE2 levels in plasma was associated with higher left ventricular ejection fraction, and systolic blood pressure [55].
In summary, there is a mounting evidence implicating an excessive cytokine storm with the pathophysiology of severe COVID-19, where systemic inflammation potentiates/complicates the coexisting CVD in these patients [56]. Such complications included hypertension, heart failure, myocardial injury, and atherosclerosis [57]. In these patients, the pathogenic mechanism that produces CV complications seem to be remarkably complex.
8. Conclusion
Significance of the SARS-CoV-2 infection in the CV system is reflected through incidences of acute myocardial injury, arrhythmias, ACS, sepsis, septic shock, viral myocarditis, and heart failure. Myocardial injury underscore significant association with fatal outcomes, whereas the prognosis of patients with underlying CVD but without myocardial injury seems quite favorable. We speculate that while COVID-19 disease begins with respiratory condition, it hastily involves the CV system through an imbalance of the renin-Ang system mediated by ACE2 depletion. This mechanism possibly complicates the clinical course through the inflammatory response, endothelial dysfunction, and microvascular damage where inflammation may be associated with myocardial injury. However, additional study of these mechanisms is clearly needed and may influence the search for ways to prevent myocardial complications. More evidence from laboratories, and clinical research is needed to learn more about the impact of COVID-19 on the CV system.
Funding
This research received no funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J., Huang Z., Lin L., Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet. Respir. Med. 2020;8(6) doi: 10.1016/S2213-2600(20)30216-2. e47–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England journal of medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England). 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8):1–19. doi: 10.1371/journal.ppat.1007236. e1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P.P., Blet A., Smyth D., Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020;142(1,7):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 13.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176(104742):1–5. doi: 10.1016/j.antiviral.2020.104742. 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keidar S., Kaplan M., Gamliel-Lazarovich A. ACE2 of the heart: From angiotensin I to angiotensin (1–7) Cardiovasc. Res. 2007;73(3):463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Vankadari N., Wilce J.A. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerging Microbes Infect. 2020;9(1):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiology. 2020;5(7):745–747. doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 17.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. American Journal of Physiology-Heart and Circulatory Physiology. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojkova D., Wagner J.U.G., Shumliakivska M., Aslan G.S., Saleem U., Hansen A., et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. BioRxiv. 2020 doi: 10.1101/2020.06.01.127605. 2020.06.01.127605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P.P., Blet A., Smyth D., Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020 Jul;7142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 22.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical Characteristics of Coronavirus Disease 2019 in China.N Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan T., Xiao R., Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 2020;34(5):6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 26.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 Expression within Different Organs of the. NOD Mouse. International journal of molecular sciences. 2017;18(563):1–13. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal M. Cardiovascular disease and COVID-19. Diabetes & metabolic syndrome. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020:1. doi: 10.1093/eurheartj/ehaa248. ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn K.L., Fralick M., Zipursky J.S., Stall N.M. Renin-angiotensin-aldosterone system inhibitors and COVID-19. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2020;192(20):E553–E554. doi: 10.1503/cmaj.200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Critical care (London, England). 2017;21(234):1–9. doi: 10.1186/s13054-017-1823-x. 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divani A.A., Andalib S., Di Napoli M., Lattanzi S., Hussain M.S., Biller J., et al. Coronavirus Disease 2019 and Stroke: Clinical Manifestations and Pathophysiological Insights. Journal of Stroke and Cerebrovascular Diseases. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. 104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., et al. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319(4):1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemnes A.R., Rathinasabapathy A., Austin E.A., Brittain E.L., Carrier E.J., Chen X., et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. The European respiratory journal. 2018;51(6) doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet (London, England). 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. The New England journal of medicine. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat. Rev. Immunol. 2019;19(1):63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 40.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Jul;5(7):1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J. Cardiac Fail. 2020;26(6):470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Januzz JL. Troponin and BNP Use in COVID-19. American College of Cardiology Mar 18, 2020 Accessed July 1, 202. https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid190.
- 46.Chapman A.R., Bularga A., Mills N.L. High-Sensitivity Cardiac Troponin Can Be an Ally in the Fight Against COVID-19. Circulation. 2020;141(22):1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan K., Chand Negi P., Ganju N., Asotra S. Cardiac biomarker-based risk stratification algorithm in patients with severe COVID-19. Diabetes & metabolic syndrome. 2020;14(5):929–931. doi: 10.1016/j.dsx.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X.-Y., Zhang F., Zhang C., Zheng L.-R., Yang J. The Biomarkers for Acute Myocardial Infarction and Heart Failure. Biomed Res. Int. 2020;2020(2018035):1–13. doi: 10.1155/2020/2018035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. The American journal of emergency medicine. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aboughdir M., Kirwin T., Abdul Khader A., Wang B. Prognostic Value of Cardiovascular Biomarkers in COVID- 19. A Review. Viruses. 2020;12(5) doi: 10.3390/v12050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedersen K.B., Chodavarapu H., Porretta C., Robinson L.K., Lazartigues E. Dynamics of ADAM17-Mediated Shedding of ACE2 Applied to Pancreatic Islets of Male db/db Mice. Endocrinology. 2015;156(12):4411–4425. doi: 10.1210/en.2015-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sama I.E., Ravera A., Santema B.T., van Goor H., ter Maaten J.M., Cleland J.G.F., et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur. Heart J. 2020;41(19):1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. 2020 doi: 10.1101/2020.03.24.20042655. [DOI] [Google Scholar]
- 57.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. The American journal of emergency medicine. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109(102433) doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 61.Cheng R., Leedy D. COVID-19 and acute myocardial injury: the heart of the matter or an innocent bystander? Heart. 2020:;106(15) doi: 10.1136/heartjnl-2020-317025. heartjnl-2020-317025. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L., Wang B., Zhou J., Kirkpatrick J., Xie M., Johri A.M. Bedside Focused Cardiac Ultrasound in COVID-19 from the Wuhan Epicenter: The Role of Cardiac Point-of-Care Ultrasound, Limited Transthoracic Echocardiography, and Critical Care Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2020;33(6):676–682. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]