Highlights
-
•
The study comprehensively evaluate predictive value of myocardial injury markers.
-
•
cTnI-ultra on admission might be the best predictor of in-hospital mortality.
-
•
Elevated cTnI-ultra was an independent biomarker of the death.
Keywords: COVID-19, In-hospital mortality, Myocardial injury biomarkers
Abstract
Objective
The aim of this study was to systematically and comprehensively evaluate the diagnostic and prognostic value of myocardial injury biomarkers in COVID-19 patients.
Methods
This is a retrospective cohort study of confirmed COVID-19 patients that were admitted to the Renmin Hospital of Wuhan University from January 30, 2020 to February 15, 2020.
Results
Receiver operating characteristic (ROC) curve analysis demonstrated that cTnI-ultra had the highest area under the curve (AUC) at 0.855, with a sensitivity of 67.3% and a specificity of 88.7% for the prediction of in-hospital mortality. Patients with higher troponin I-ultra (cTnI-ultra), creatinine kinase-myocardial band (CK-MB), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were associated with higher mortality, compared to those who lower levels. The multivariable cox regression indicated that age (hazard ratio (HR) 3.450, 95% confidence interval (CI) 1.627–7.314, P = 0.001), coronary heart disease (HR 1.855, 95% CI 1.006–3.421; P = 0.048), elevated cTnI-ultra (HR 3.083, 95% CI 1.616–5.883, P = 0.001), elevated CK-MB (HR 2.907, 95% CI 1.233–6.854; P = 0.015), and elevated NT-proBNP (HR 5.776, 95% CI 2.272–14.682; P < 0.001) were associated with in-hospital mortality.
Conclusions
cTnI-ultra might be the best predictor of in-hospital mortality among myocardial injury biomarkers. Elevated cTnI-ultra, CK-MB, and NT-proBNP were independent biomarkers of the mortality in COVID-19 patients.
1. Introduction
Since the first case of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was reported in Wuhan, Hubei Province in December 2019, COVID-19 has dramatically spread across the world [1], [2], [3]. As of 11th May 2020, 4,095,678 cases have been diagnosed with COVID-19, and 279,309 patients died of this disease over the world. Most of mild and common patients exhibit a good prognosis and present with milder clinical symptoms, while severe and critical patients might rapidly develop acute respiratory distress syndrome, acute respiratory failure, multiple organ failure, and other fatal complications, which cause a high mortality rate [4], [5]. At present, COVID-19 lacks specific and effective drugs and vaccines. Therefore, it has great clinical significance to investigate risk factors of prognosis for COVID-19 patients.
Recent studies revealed that the elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) and elevated cardiac troponin I-ultra (cTnI-ultra), which are markers of myocardial injury, were significantly associated with severe disease and higher mortality [6], [7], [8], [9], [10], [11]. Huang et al. reported that most patients with elevated cTnI-ultra required admission to the intensive care unit (ICU) [6]. In a similar single-center study, of the 138 patients, 36 (26.1%) were transferred to the ICU, and compared with patients who did not receive ICU care, their levels of creatinine kinase-myocardial band (CK-MB) and cTnI-ultra were significantly higher in patients who required ICU care [7]. Additionally, a study demonstrated that elevated troponin was associated with higher mortality [8], and also Ruan et al. found that cardiac troponin and myoglobin (MYO) levels in the non-suvivor group were significantly higher than the suvivor group [11]. However, these studies above only evaluated the relationship between several myocardial injury biomarkers and mortality, and the diagnostic value of these biomarkers were not evaluated. The aim of this study was to systematically and comprehensively investigate the potential diagnostic and prognostic value of myocardial injury biomarkers in patients with COVID-19.
2. Materials and methods
2.1. Study design and participants
A retrospective study was conducted in Renmin Hospital of Wuhan University (Wuhan, China), which served as designated hospital for COVID-19 patients diagnosis and treatment. The COVID-19 was confirmed by RNA detection of the SARS-CoV-2 using the diagnostic criteria defined in the World Health Organization (WHO) interim guidance [12]. We excluded patients if they were younger 18 years of age, pregnant women, died on admission, with missing data or were transferred to other designated hospitals. Therefore, a total of 264 patients hospitalized from January 30 to February 15, 2020 were included in the final analysis and the final follow-up date was March 21, 2020. All enrolled inpatients had a definite outcome (dead or discharged). The current study was conducted in accordance with the Declaration of Helsinki Principles, and was approved by the Ethics Committee of Renmin Hospital of Wuhan University. (WDRY2019-K056). The requirement for written informed patient consent was waived because of the anonymous nature of the data.
2.2. Data collection
Two investigators (PD and BQ) independently extracted data including demographic data, comorbidities, laboratory data and outcome data from all of the included studies using a standardized data collection form for analysis and, all data of the patients were checked by two investigators (PD and LY) independently to verify data accuracy.
The hematological parameters (including red blood cell count, platelet count, hemoglobin, white blood cell, lymphocyte count, neutrophil count, and monocyte count) were determined using XN-9000 Hematology Analyzer (Sysmex, Kobe, Japan). The ADVIA 2400 Clinical Chemistry System (Siemens, Erlangen, Germany) was used to measure the albumin, alanine aminotransferase, aspartate aminotransferase, creatine kinase, creatinine (Cr), lactate dehydrogenase, total bilirubin, and blood urea nitrogen. The levels of cTnI-ultra, CK-MB, MYO, and NT-proBNP was measured on a ADVIA Centaur XP platform (Siemens, Erlangen, Germany). Coagulation parameters including plasma D-dimer and prothrombin time were measured by CA7000 Coagulation Analyzer (Sysmex, Kobe, Japan).
2.3. Statistical analysis
Categorical data were presented as n(%) and analysed using χ2 tests. Normally distributed distributed continuous variables are presented as means ± standard deviations (SD), whereas non-normally distributed variables, as median and interquartile ranges (IQR), and the unpaired Student s t-test or Mann-Whitney s U test was used for two-groups comparisons of values.
Receiver operating characteristic (ROC) curve analysis was used for calculating the optimal cut-off values, sensitivity, and specificity of myocardial injury biomarkers. Survival analysis was assessed using the Kaplan-Meier method and compared using log-rank tests. Then, univariate and multivariate Cox proportional hazard regression models were used to evaluate the survival hazard using Cox proportional hazard model with a forward stepwise procedure. All P-values were two-sided, with P values less than 0.05 indicating statistical significance. All statistical analyses and graphical representations of the data were performed using GraphPad Prism 6 software (San Diego, CA, USA) and SPSS 21.0 (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Participants’ characteristics
The basic characteristics for all participants were shown in Table 1 . Of 264 eligible patients, the median age was 64.5 years (IQR, 53.3–74.0 years), ranging from 26 years to 94 years. The patients in non-survival group were much older than those in survivor group (P < 0.001). 50.8% (134/264) patients were female. The median time from admission to discharge was 38.0 days (IQR 29.0–47.8), whereas the median time to death was 5.0 days (IQR 3.0–10.8). The most prevalent clinical symptoms recorded in the COVID-19 patients on admission were fever (93.6%), cough (77.3%), sputum production (22.0%) and fatigue (21.2%). The results also showed that more patients in non-survival group had hypertension (51.9% vs. 34.4%, P = 0.02) and coronary heart diseas (34.6% vs. 6.6%, P < 0.001), and that there was no difference in the incidence of diabetes, chronic obstructive lung disease, carcinoma, chronic kidney disease, and chronic liver disease between the two groups (P > 0.05).
Table 1.
Baseline clinical and laboratory characteristics of of patients on admission.
| Total (264) | Survival (212) | Non-survival (52) | P-value | |
|---|---|---|---|---|
| Total | 264 | 212 | 52 | |
| Male, n(%) | 130 (49.2) | 97 (45.8) | 33 (63.5) | 0.022 |
| Age, years | 64.5 (53.3–74.0) | 62.5 (52.0–70.0) | 74.5 (65.3–81.8) | <0.001 |
| Time from hospital admission to outcome, days |
35.0 (20.0–44.8) | 38.0 (29.0–47.8) | 5.0 (3.0–10.8) | <0.001 |
| Comorbidity | ||||
| Hypertension (n[%]) |
100 (37.9) | 73 (34.4) | 27 (51.9) | 0.02 |
| Diabetes, n(%) |
41 (15.5) | 33 (15.6) | 8 (15.4) | 0.964 |
| Coronary heart diseas, n(%) | 32 (12.1) | 14 (6.6) | 18 (34.6) | <0.001 |
| Chronic obstructive lung disease, n(%) |
8 (3.0) | 6 (2.8) | 2 (3.8) | 0.702 |
| Carcinoma, n(%) | 12 (4.5) | 9 (4.2) | 3 (5.8) | 0.637 |
| Chronic kidney disease n(%) | 9 (3.4) | 7 (3.3) | 2 (3.8) | 0.847 |
| Chronic liver disease n(%) | 14 (5.3) | 10 (4.7) | 4 (7.7) | 0.392 |
| Other n(%) | 80 (30.3) | 66 (31.1) | 14 (26.9) | 0.554 |
| symptom | ||||
| Fever (temperature ≥ 37.3 °C) n(%) | 247 (93.6) | 197 (92.9) | 50 (96.2) | 0.538 |
| Cough n(%) | 204 (77.3) | 160 (75.5) | 44 (84.6) | 0.159 |
| Sputum n(%) | 58 (22.0) | 46 (21.7) | 12 (23.1) | 0.830 |
| Myalgia n(%) | 32 (12.1) | 25 (11.8) | 7 (13.5) | 0.741 |
| Fatigue n(%) | 56 (21.2) | 44 (20.8) | 12 (23.1) | 0.714 |
| Diarrhoea n(%) | 10 (3.8) | 7 (3.3) | 3 (5.8) | 0.419 |
| Nausea or vomiting n(%) | 8 (3.0) | 6 (2.8) | 2 (3.8) | 0.658 |
| Red blood cell, ×1012/L | 4.0 ± 0.6 | 4.0 ± 0.6 | 4.0 ± 0.7 | 0.603 |
| Hemoglobin, g/dL | 123.4 ± 17.2 | 123.0 ± 16.2 | 124.9 ± 21.1 | 0.549 |
| White blood cell, ×109/L | 5.9 (4.0–8.1) | 5.4 (3.7–7.2) | 8.7 (5.8–13.5) | <0.001 |
| Lymphocyte, ×109/L | 0.9 (0.6–1.2) | 0.9 (0.7–1.3) | 0.6 (0.4–0.9) | <0.001 |
| Neutrophil, ×109/L | 4.4 (2.4–6.5) | 3.9 (2.3–5.5) | 7.7 (4.7–11.9) | <0.001 |
| Monocyte, ×109/L | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.5) | 0.617 |
| Platelet, ×109/L | 204.0 (155.3–269.8) | 212.0 (162.0–275.0) | 181.5 (107.3–227.5) | <0.001 |
| Albumin, g/L | 35.6 ± 4.4 | 36.3 ± 4.0 | 32.7 ± 5.0 | <0.001 |
| Alanine aminotransferase, U/L | 26.5 (18.0–47.8) | 26.5 (18.0–46.0) | 26.5 (18.3–57.5) | 0.564 |
| Aspartate aminotransferase, U/L | 31.0 (23.0–47.0) | 29.0 (23.0–41.0) | 49.0 (28.3–74.8) | <0.001 |
| Creatine kinase, U/L | 66.0 (38.3–113.8) | 61.5 (37.3–106.8) | 85.0 (57.0–140.8) | 0.005 |
| Lactate dehydrogenase, U/L | 307.0 (233.0–436.8) | 282.5 (224.3–381.0) | 544.0 (367.3–748.8) | <0.001 |
| Total bilirubin, μmol/L | 10.2 (7.9–15.1) | 9.6 (7.5–14.0) | 15.2 (10.1–24.2) | <0.001 |
| Blood urea nitrogen, mmol/L | 5.2 (4.0–8.2) | 4.9 (3.8–7.2) | 8.9 (6.6–15.8) | <0.001 |
| D-dimer, μg/L | 1.1 (0.5–4.1) | 0.9 (0.4–1.9) | 7.1 (1.7–17.1) | <0.001 |
| Prothrombin time, s | 12.2 (11.6–12.7) | 12.0 (11.5–12.6) | 12.7 (12.0–13.8) | <0.001 |
| Cardiac troponin I-ultra, ng/mL | 0.006 (0.005–0.016) | 0.006 (0.005–0.010) | 0.051 (0.012–0.346) | <0.001 |
| Creatinine kinase–myocardial band, ng/mL | 1.115 (0.770–2.058) | 1.010 (0.730–1.558) | 2.670 (1.415–1.040) | <0.001 |
| Myoglobin, μg/L | 48.9 (29.8–100.2) | 42.3 (27.9–76.0) | 122.1 (77.1–404.6) | <0.001 |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 227.7 (79.3–647.9) | 155.0 (64.4–460.3) | 943.2 (402.3–2397.5) | <0.001 |
| Creatinine, μmol/L | 60.5 (50.3–79.0) | 59.0 (50.0–72.0) | 74.5 (52.0–112.8) | 0.005 |
Data are mean ± SD, median (IQR), n (%), or n/N (%). p values were calculated by t test, Mann-Whitney U test, χ2 test, or Fisher’s exact test, as appropriate. IQR: inter-quartile range.
Laboratory parameters were analyzed and the results showed that the levels of myocardial injury biomarkers, including cTnI-ultra, CK-MB, MYO, NT-proBNP, and Cr were higher in the non-survivor group than those in the survivor group. In terms of blood coagulation indexes, the non-survivor group showed significantly higher D-dimer and prothrombin time compared with the survivor group. Meanwhile, white blood cell, neutrophil, aspartate aminotransferase creatine kinase, creatinine, lactate dehydrogenase, total bilirubin, and blood urea nitrogen were remarkably higher, whereas lymphocyte, platelet, and albumin was significantly lower in the non-survivor group than those in the survivor group (Table 1).
3.2. Diagnostic value of in-hospital mortality of myocardial injury biomarkers in COVID-19 patients
ROC curves were constructed to evaluate predictive value of in-hospital mortality of myocardial injury biomarkers in patients with COVID-19 on admission (Fig. 1 ). Table 2 showed that the area under the curve (AUC) of cTnI-ultra, CK-MB, MYO, NT-proBNP and Cr were 0.855 (95% confidence interval (CI): 0.798–0.912), 0.785 (95%CI: 0.710–0.859), 0.809 (95%CI: 0.744–0.873), 0.822 (95%CI: 0.761–0.884), and 0.624 (95%CI: 0.528–0.721), respectively. The optimal cutoff value were 0.020 ng/mL, 2.190 ng/mL, 82.085 μg/L, 340.00 pg/mL, and 69.50 μmol/L for cTnI-ultra, CK-MB, MYO, NT-proBNP and Cr, respectively. The highest sensitivity and specificity of cTnI-ultra, CK-MB, MYO, NT-proBNP and Cr for predicting in-hospital mortality were 67.3% and 88.7%, 83.5% and 85.8%, 75.0% and 76.9%, 84.6% and 68.4%, and 55.8% and 72.6%, respectively.
Fig. 1.
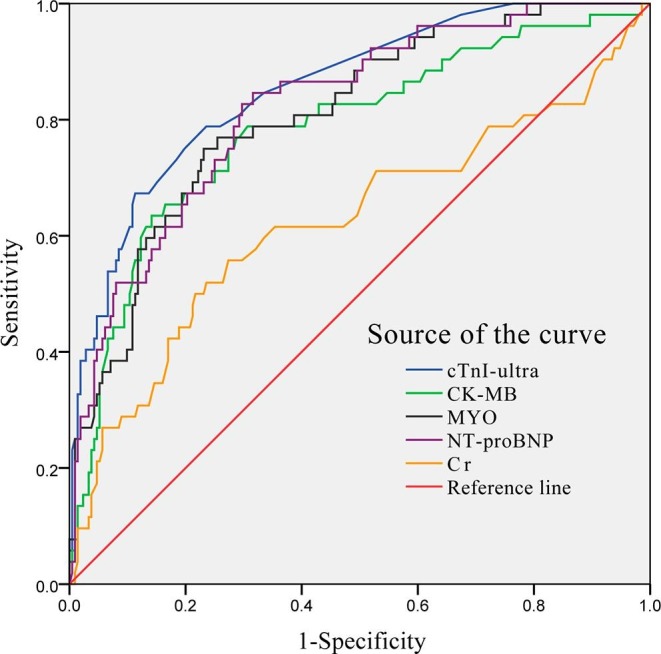
Receiver operator characteristic curve for myocardial injury biomarkers to predict in-hospital mortality.
Table 2.
Diagnostic value of in-hospital mortality of cTnI-ultra, CK-MB, MYO, NT-proBNP and Cr in COVID-19 patients.
| Biomarkers | AUC (95%CI) | Cutoff values | SE, % | SP, % | PPV (95%CI) | NPV (95%CI) |
|---|---|---|---|---|---|---|
| cTnI-ultra | 0.855 (0.798–0.912) | 0.020 | 67.3 | 88.7 | 54.4 (43.9–64.5) | 93.1 (90.1–95.3) |
| CK-MB | 0.785 (0.710–0.859) | 2.190 | 63.5 | 85.8 | 47.3 (37.8–57.0) | 92.1 (89.1–94.4) |
| MYO | 0.809 (0.744–0.873) | 82.085 | 75.0 | 76.9 | 39.4 (32.7–46.5) | 93.9 (90.5–96.1) |
| NT-proBNP | 0.822 (0.761–0.884) | 340.00 | 84.6 | 68.4 | 34.9 (29.9–40.3) | 95.7 (92.1–97.7) |
| Cr | 0.624 (0.528–0.721) | 69.50 | 55.8 | 72.6 | 29.0 (22.7–36.1) | 89.1 (85.7–91.8) |
Abbreviation: cTnI-ultra, cardiac troponin I-ultra; CK-MB, creatinine kinase–myocardial band; MYO, myoglobin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; Cr, creatinine; AUC, area under curve; SE, sensitivity; SP, specificity; PPV, Positive Predictive Value; NPV, negative predictive value; CI, confidence interval.
3.3. Association of myocardial injury biomarkers with in-hospital mortality in patients with COVID-19
To identify the independent risk factors associated with in-hospital mortality in. COVID-19 patients on admission, Kaplan-Meier univariate survival analysis and multivariate cox regression were used to determine prognostic factors. On univariate analysis, elevated cTnI-ultra, CK-MB, MYO, and NT-proBNP along with four other variables (age, female gender, hypertension, and coronary heart disease) were significantly related to shorter survival. Meanwhile, multivariate analysis demonstrated that independent predictors of in-hospital mortality in COVID-19 patients on admission were age (hazard ratio (HR) 3.450, 95% CI 1.627–7.314, P = 0.001), coronary heart disease (HR 1.855, 95% CI 1.006–3.421; P = 0.048), elevated cTnI-ultra (HR, 3.083; 95% CI, 1.616–5.883; P = 0.001), elevated CK-MB (HR, 2.907; 95% CI, 1.233–6.854; P = 0.015), and elevated NT-proBNP (HR, 5.776; 95% CI, 2.272–14.682, P < 0.001) (Table 3 ). Furthermore, Fig. 2 showed that the survival period of patients with high levels of cTnI-ultra, CK-MB and NT-proBNP were both significantly shorter compared to those with low levels (P < 0.001).
Table 3.
Univariate and multivariate cox regression analysis of mortality risk factors for patients with COVID-19.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 7.434 (3.817–14.478) | <0.001 | 3.450 (1.627–7.314) | 0.001 |
| Gender | 0.515 (0.293–0.907) | 0.021 | 0.074 | |
| Hypertension | 1.926 (1.118–3.319) | 0.018 | 0.533 | |
| Coronary heart disease | 4.546 (2.563–8.065) | <0.001 | 1.855 (1.006–3.421) | 0.048 |
| cTnI-ultra | 3.479 (1.883–6.427) | <0.001 | 3.083 (1.616–5.883) | 0.001 |
| CK-MB | 6.292 (2.802–14.126) | <0.001 | 2.907 (1.233–6.854) | 0.015 |
| MYO | 3.225 (1.818–5.719) | <0.001 | 0.069 | |
| NT-proBNP | 6.326 (2.508–15.954) | <0.001 | 5.776 (2.272–14.682) | <0.001 |
| Cr | 2.723 (1.572–4.717) | 0.120 | 0.081 | |
Abbreviation: cTnI-ultra, cardiac troponin I-ultra; CK-MB, creatinine kinase–myocardial band; MYO, myoglobin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; Cr, creatinine; HR, hazard ratio; CI, confidence interval.
Fig. 2.
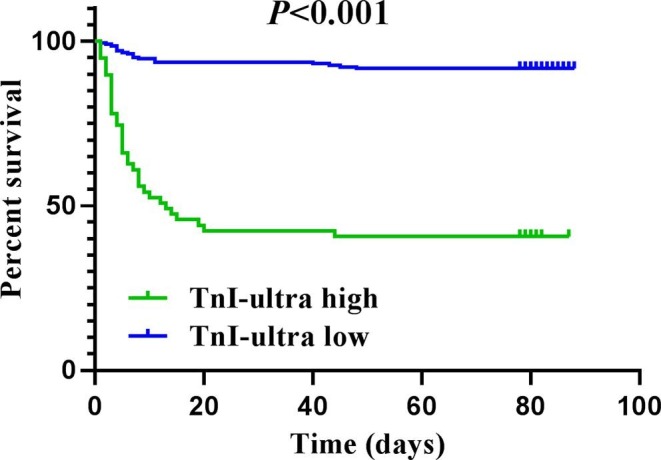
Kaplan-Meier curves of survival analysis for COVID-19 patients by cTnI-ultra levels. cTnI-ultra high and low are defined as the levels of cTnI-ultra greater than 0.02 ng/mL and less than 0.02 ng/mL, respectively. P values for survival analysis are derived by the log-rank test.
4. Discussion
This study, to our best knowledge, was the first to investigate the potential diagnostic and prognostic value of myocardial injury biomarkers in patients with COVID-19. The present study showed that the clinical characteristics of these patients are similar to those reported in previous studies. The most dominant symptoms and comorbidities recorded in the COVID-19 patients on admission were fever (93.6%) and hypertension (37.9%), respectively. The results also showed that the overall in-hospital mortality was 19.70% among COVID-19 patients.
In current study, we found that the levels of myocardial injury biomarkers including cTnI-ultra, CK-MB, MYO, NT-proBNP, and Cr in the non-survivor group were significantly higher than the survivor group. We also established the ROC curve for myocardial injury biomarkers to evaluate their diagnostic roles, and the results showed that cTnI-ultra had the highest AUC at 0.855, with a sensitivity of 67.3% and a specificity of 88.7% for predicting in-hospital mortality of COVID-19, which was seems better than other myocardial injury biomarkers. Notably, 14.15% (30/212) patients had cTnI-ultra levels greater than the 99th-percentile upper reference limit in survivor group, while 28.85% (15/52) patients in non-survivor group. We also observed that elevated cTnI-ultra, CK-MB, and NT-proBNP both could be considered independent biomarkers of the mortality in COVID-19 patients. Contrary to the our study, Shi et al. reported that only elevated cTnI-ultra was an independent risk of the death in COVID-19 patients [9]. The reasons for the difference between the studies might be associated with different underlying diseases, sample size, the type of patients, and biomakers examination time.
The results above indicated that the levels of myocardial injury biomarkers were significantly higher in the non-survivor group compared with the survivor group. There might be several reasons for this finding. According to previous research reports, angiotensin converting enzyme 2 (ACE2), which is highly expressed in lung and heart, is a functional receptors for severe acute respiratory syndrome coronavirus (SARS-CoV), and is likely to play a critical role in infection of the myocardium [13], [14]. SARS-CoV-2 may have the same mechanism of infection as SARS-COV because the viruses are highly homologous in genome [15], [16], [17]. At the same time, recent studies have demonstrated that ACE2 has a high binding affinity with spike protein of SARS-CoV-2 [18], [19], [20]. Therefore, mycardial injury caused by COVID-19 might proceed directly via ACE2. Several studies have demonstrated that the levels of peripheral pro-inflammatory cytokines or other inflammatory markers, such as C-reactive protein, procalcitonin, leukocytes, interleukin (IL)-2, IL-7, and IL-10 were elevated in patients suffering from cardiac injury [6], [8]. The overproduction and activation of these inflammatory cytokines can induce apoptosis or necrosis of cardiomyocyte. Huang et al. highlighted that the imbalance of T helper 1 (Th1) and T helper 2 (Th2) responses triggered a cytokine storm in COVID-19 patients, which may cause myocardial injury [6]. In addition, previous studies found that patients with cardiovascular diseases might be more susceptible to heart injury when infected by SARS-CoV-2 [9], [21]. The present study showed that approximately 34.6% and 51.9% of the patients in non-survivor group had a history of coronary heart disease and hypertension, respectively, which were significantly higher than that in the survivor group. Similarly, Shi et al. reported the history of coronary heart disease and hypertension in patients with myocardial injury as 30% and 60% of patients, respectively [9]. These results were similar to those of our present study. Gold et al. discovered that elderly patients with underlying diseases, especially with hypertension, coronary heart disease, and diabetes, are more susceptible to SARS-CoV-2 infection and often have greater severity of illness [21]. The exact pathophysiological mechanisms of myocardial injury caused by COVID-19 presently are are still elusive at present and Further experiments need to be done to investigate the detail mechanisms.
We acknowledged that this study has the following limitations. First, our study was a retrospective, single-center study with a relatively small number of enrolled patients. Second, the levels of myocardial injury biomarkers were measured only once on admission, so we may have a better understanding of the relationship between the myocardial injury biomarkers and in-hospital mortality if we detect myocardial injury biomarkers levels at the different time points. Third, This study only included cases of Chinese patients diagnosed with COVID-19, and the generalizability of the results to other ethnicities might be limited. Forth, Patients in isolation wards or ICU lack some clinical data, such as echocardiography data, electrocardiography data, and measurement of cytokine levels, which limits a comprehensive analysis of cardiac injury.
In conclusion, the current study provides convincing evidence that elevated TnI-ultra, CK-MB, and NT-proBNP on admission were independent risk factors, and the diagnostic value of cTnI-ultra was might superior to other myocardial injury biomarkers, which could help clinicians to adopt optimal treatment strategies at an early stage.
Funding
This research was supported by National Natural Science Foundation of China (No. 81802090), by Emergency Special Research Project for COVID-19 (No. 2020XGFYZR17), by New Coronary Pneumonia Prevention and Control Technology Guidance Emergency Scientific Research Project of Shiyan (No. 20Y03), and by Initial Project for Post-Graduates of Hubei University of Medicine (No. 2017QDJZR03).
Ethical approval
The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. (WDRY2019-K056). The requirement for written informed patient consent was waived because of the anonymous nature of the data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2020.07.018.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F., Tan Wenjie. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji M., Yuan L., Shen W., Lv J., Li Y., Li M., Lu X., Hu L., Dong W. Characteristics of disease progress in patients with coronavirus disease 2019 in Wuhan, China. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X.Y., Xu X.X., Yin H.S., Hu Q.M., Xiong T., Tang Y.Y., Yang A.Y., Yu B.P., Huang Z.P. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect. Dis. 2020;20(1):311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Dawei, Hu Bo, Hu Chang, Zhu Fangfang, Liu Xing, Zhang Jing, Wang Binbin, Xiang Hui, Cheng Zhenshun, Xiong Yong, Zhao Yan, Li Yirong, Wang Xinghuan, Peng Zhiyong. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.T. Guo, Y. Fan, M. Chen, X. Wu, L. Zhang, T. He, H. Wang, J. Wan, X. Wang, Z. Lu, Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19), JAMA Cardiol. (2020). [DOI] [PMC free article] [PubMed]
- 9.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7(2):91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance 2020; 28.
- 13.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavesi A. New insights into the evolutionary features of viral overlapping genes by discriminant analysis. Virology. 2020;546:51–66. doi: 10.1016/j.virol.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahmi M., Kubota Y., Ito M. Nonstructural proteins NS7b and NS8 are likely to be phylogenetically associated with evolution of 2019-nCoV. Infect. Genet. Evol. 2020;81 doi: 10.1016/j.meegid.2020.104272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang Qihui, Zhang Yanfang, Wu Lili, Niu Sheng, Song Chunli, Zhang Zengyuan, Lu Guangwen, Qiao Chengpeng, Hu Yu, Yuen Kwok-Yung, Wang Qisheng, Zhou Huan, Yan Jinghua, Qi Jianxun. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold J.A.W., Wong K.K., Szablewski C.M., Patel P.R., Rossow J., da Silva J., Natarajan P., Morris S.B., Fanfair R.N., Rogers-Brown J., Bruce B.B., Browning S.D., Hernandez-Romieu A.C., Furukawa N.W., Kang M., Evans M.E., Oosmanally N., Tobin-D'Angelo M., Drenzek C., Murphy D.J., Hollberg J., Blum J.M., Jansen R., Wright D.W., Sewell W.M., 3rd, Owens J.D., Lefkove B., Brown F.W., Burton D.C., Uyeki T.M., Bialek S.R., Jackson B.R. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(18):545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


