Abstract
Objectives
To describe the clinical manifestations and outcomes of critically ill children with coronavirus disease-19 (COVID-19) in New York City.
Study design
Retrospective observational study of children 1 month to 21 years admitted March 14 to May 2, 2020, to 9 New York City pediatric intensive care units (PICUs) with severe acute respiratory syndrome coronavirus 2 infection.
Results
Of 70 children admitted to PICUs, median age was 15 (IQR 9, 19) years; 61.4% male; 38.6% Hispanic; 32.9% black; and 74.3% with comorbidities. Fever (72.9%) and cough (71.4%) were the common presenting symptoms. Twelve patients (17%) met severe sepsis criteria; 14 (20%) required vasopressor support; 21 (30%) developed acute respiratory distress syndrome (ARDS); 9 (12.9%) met acute kidney injury criteria; 1 (1.4%) required renal-replacement therapy, and 2 (2.8%) had cardiac arrest. For treatment, 27 (38.6%) patients received hydroxychloroquine; 13 (18.6%) remdesivir; 23 (32.9%) corticosteroids; 3 (4.3%) tocilizumab; and 1 (1.4%) anakinra; no patient was given immunoglobulin or convalescent plasma. Forty-nine (70%) patients required respiratory support: 14 (20.0%) noninvasive mechanical ventilation, 20 (28.6%) invasive mechanical ventilation (IMV), 7 (10%) prone position, 2 (2.8%) inhaled nitric oxide, and 1 (1.4%) extracorporeal membrane oxygenation. Nine (45%) of the 20 patients requiring IMV were extubated by day 14 with median IMV duration of 218 (IQR 79, 310.4) hours. Presence of ARDS was significantly associated with duration of PICU and hospital stay, and lower probability of PICU and hospital discharge at hospital day 14 (P < .05 for all).
Conclusions
Critically ill children with COVID-19 predominantly are adolescents, have comorbidities, and require some form of respiratory support. The presence of ARDS is significantly associated with prolonged PICU and hospital stay.
Keywords: pediatric ARDS, pediatric respiratory failure, pediatric viral sepsis
Abbreviations: AKI, Acute kidney injury; ARDS, Acute respiratory distress syndrome; BMI, Body mass index; COVID-19, Coronavirus disease 2019; IMV, Invasive mechanical ventilation; LOS, Length of stay; MIS-C, Multisystem inflammatory syndrome in children; OI, Oxygenation index; OSI, Oxygen saturation index; PICU, Pediatric intensive care unit; PIM-2, Pediatric Index of Mortality-2; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
As the novel coronavirus disease 2019 (COVID-19) pandemic began in December 2019, children were reported to be at lower risk of developing severe symptoms or critical illness compared with adults with a large study from China demonstrating critical illness in <1% of children.1 , 2 Preliminary studies from Europe and the US have provided early data on critically ill children.3, 4, 5, 6, 7
The clinical manifestations, including pulmonary findings, and outcomes of critically ill children with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been described only in a small number of cases, and the factors associated with development of progressive critical illness and mortality are unclear. The objectives of this study are to describe the clinical manifestations of critically ill children with COVID-19 admitted to pediatric intensive care units (PICUs) across New York City during the first wave of the US pandemic and to identify factors associated with PICU and hospital length of stay (LOS).
Methods
The Albert Einstein College of Medicine institutional review board reviewed and approved this multicenter retrospective observational study. The institutional review boards at each individual center approved this study, as applicable. Only deidentified data were transmitted and analyzed. Informed consent was waived.
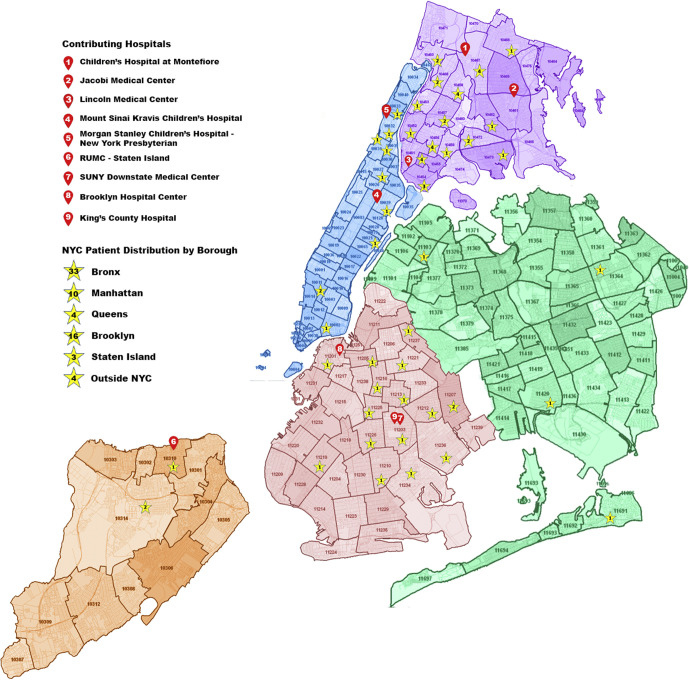
We identified critically ill pediatric patients 1 month to 21 years of age with confirmed SARS-CoV-2 infection from March 14 to May 2, 2020 (7 weeks) admitted to 9 New York City teaching hospitals (Children's Hospital at Montefiore, New York-Presbyterian Morgan Stanley Children's Hospital of New York, Kravis Children's Hospital at Mount Sinai, State University of New York Downstate Medical Center, Jacobi Medical Center, Kings County Medical Center, Lincoln Medical and Mental Health Center, The Brooklyn Hospital Center, and Richmond University Medical Center) located in 4 of 5 boroughs of New York City with a total catchment area encompassing all 5 boroughs (Figure 1; available at www.jpeds.com). These 9 of 25 PICUs (approximately 46% of the PICU beds) in New York City were included as collaborators were able to obtain institutional review board approval in time for enrollment.
Figure 1.
New York City–wide COVID-19 case distribution.
A COVID-19 case was defined as due to SARS-CoV-2 infection by a positive real-time reverse transcription polymerase chain reaction test of a specimen using nasopharyngeal swab, using one of several different testing platforms due to institutional preference, as well as limited testing kits in the early phase of the pandemic (Abbott Laboratories, Abbot Park, Illinois; Bioreference Laboratories, Elmwood, New Jersey; Cepheid Xpert Xpress, Sunnyvale, California; Hologic Panther Fusion, San Diego, California; LabCorp, Raritan, New Jersey; Luminex Aries; Northwell Health Laboratories, Lake Success, New York; New York City Department of Health and Mental Hygiene Public Health Laboratory; Roche diagnostics, Basel, Switzerland). Patients with the more recently recognized entity of multisystem inflammatory syndrome in children (MIS-C) were not represented in this study because the description was defined, and cases began to occur after the study time period.
Data from each institution's electronic medical record were obtained through a research form in Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, Tennessee). Demographic and clinical data and laboratory and radiologic results were obtained. All tests and treatments were performed at the discretion of the treating physicians.
A total of 31 of the 70 patients described in this study were included in other published reports, including 3 patients who met criteria the Centers for Disease Control use definition for MIS-C.5 , 6 , 8, 9, 10
Definitions
Acute respiratory distress syndrome (ARDS) was defined using Pediatric Acute Respiratory Distress criteria11: oxygenation index (OI) 4-8 or oxygen saturation index (OSI) 5-7.5 as mild-severity ARDS, OI 8-16 or OSI 7.5-12.3 as moderate-severity ARDS, and OI >16 or OSI >12.5 as severe ARDS. Management of ARDS was at the discretion of the treating physician and generally was based on Pediatric Acute Respiratory Distress recommendations of low tidal volume and limiting plateau pressure to ≤30 cm of water.11 , 12 Acute kidney injury (AKI) was defined using the Kidney Disease: Improving Global Outcomes classification based upon a change in serum creatinine level and creatinine clearance.13 Sepsis, severe sepsis, and septic shock were defined using the Pediatric Surviving Sepsis Guidelines.14 Virus-associated sepsis was defined as the presence of ≥2 systemic inflammatory syndrome criteria; severe sepsis as sepsis with organ dysfunction or tissue hypoperfusion; and septic shock as severe sepsis with volume-refractory hypotension.14 Obesity was considered a comorbidity and was defined as body mass index (BMI) >30 kg/m2. Asthma was considered a respiratory comorbidity. Severity of illness was described using Pediatric Index of Mortality-2 (PIM-2) scores, and standardized mortality ratio was calculated as standardized mortality ratio = observed mortality of our cohort/expected mortality using PIM-2 score.15
Outcomes
Outcomes reported included need for invasive mechanical ventilation, PICU LOS, hospital LOS, and mortality within the first 14 days and 28 days of PICU admission.
Statistical Analyses
Demographic and clinical characteristics were summarized as frequencies and percentages for categorical variables, as medians and IQRs for continuous variables, and compared between patients with and without ARDS by using χ2 test or Fisher exact test, and Wilcoxon rank-sum test for categorical and continuous variables, respectively. Patients were followed up at 14 days and 28 days from PICU admission, and Kaplan–Meier curves of PICU and hospital LOS were compared in patients with and without ARDS using log-rank tests. Cox proportional hazards regression was employed to investigate the association of presence of ARDS with PICU and hospital discharges, adjusting for need for mechanical ventilation during PICU stay and platelet count measured on admission day 1 in multivariable analyses. P < .05 was considered statistically significant. Data were analyzed by using SAS software (version 9.4; SAS Institute Inc, Cary, North Carolina).
Results
Seventy children with COVID-19 were hospitalized in the PICUs of the 9 participating hospitals in New York City from March 14 to May 2, 2020. Thirty-three (47%) patients were from the borough of the Bronx and 16 (22.8%) from the borough of Brooklyn (Figure 1).
Of the 70 patients, 21 (30%) had a diagnosis of ARDS within the first 14 days of hospitalization. Demographics and baseline characteristics of the whole cohort as well as for those with and without ARDS are shown in Table I . Forty-three (61.4%) patients were male; 27 (38.6%) were Hispanic, and 23 (32.9%) were black. Median age was 15 years (IQR 9, 19); median weight was 56.6 kg (IQR 27.7, 96.1) and BMI was 22.7 kg/m2 (IQR 19.1, 32.7). Fifty-two patients (74.3%) had at least 1 comorbidity. Severity of illness on admission was not significantly different between the ARDS and non-ARDS groups, as reflected by PIM-2 scores on PICU admission with overall risk of mortality of 0.8% (IQR 0.2%, 1.4%).
Table I.
Demographics and baseline characteristics of critically ill pediatric patients with COVID-19
| Characteristics | Total (n = 70) | No ARDS (n = 49) | ARDS (n = 21) | P value∗ |
|---|---|---|---|---|
| Age, y, median (IQR) | 15.0 (9.0, 19.0) | 15.0 (11.0, 18.0) | 14.0 (9.0, 19.0) | .7237 |
| Sex | ||||
| Female, n (%) | 27 (38.6) | 19 (38.8) | 8 (38.1) | .9573 |
| Male, n (%) | 43 (61.4) | 30 (61.2) | 13 (61.9) | |
| Race | ||||
| Black, n (%) | 23 (32.9) | 19 (38.8) | 4 (19.0) | .1699 |
| White, n (%) | 7 (10.0) | 6 (12.2) | 1 (4.8) | |
| Latino, n (%) | 27 (38.6) | 15 (30.6) | 12 (57.1) | |
| Other, n (%) | 13 (18.6) | 9 (18.4) | 4 (19.0) | |
| Weight, kg, median (IQR) | 56.6 (27.7, 96.1) | 63.4 (27.7, 96.1) | 45.2 (28.0, 83.8) | .8526 |
| BMI, kg/m2, median (IQR) [n = 66] | 22.7 (19.1, 32.7) | 23.5 (19.2, 32.7) | 21.4 (19.1, 30.6) | .9561 |
| PIM-2 score: % risk of mortality, median (IQR) [n = 69] | 0.8 (0.2, 1.4) | 0.8 (0.2, 1.8) | 0.8 (0.4, 1.3) | .6417 |
| Comorbidities | ||||
| Obesity (BMI>30), n (%) [n = 66] | 20 (30.3) | 13 (28.9) | 7 (33.3) | .7144 |
| BMI >35, n (%) [n = 66] | 14 (21.2) | 9 (20.0) | 5 (23.8) | .7534 |
| BMI >40, n (%) [n = 66] | 12 (18.2) | 8 (17.8) | 4 (19.0) | 1.0000 |
| BMI >50, n (%) [n = 66] | 4 (6.1) | 2 (4.4) | 2 (9.5) | .5865 |
| Respiratory, n (%) | 17 (24.3) | 10 (20.4) | 7 (33.3) | .2478 |
| Heart disease, n (%) | 4 (5.7) | 3 (6.1) | 1 (4.8) | 1.0000 |
| Hematologic/malignancy/immunosuppression, n (%) | 12 (17.1) | 8 (16.3) | 4 (19.0) | .7432 |
| Diabetes/prediabetes, n (%) | 9 (12.9) | 7 (14.3) | 2 (9.5) | .7140 |
| Neurologic, n (%) | 10 (14.3) | 5 (10.2) | 5 (23.8) | .1536 |
| 2 or more comorbidities, n (%) | 18 (25.7) | 12 (24.5) | 6 (28.6) | .7203 |
| 3 or more comorbidities, n (%) | 2 (2.9) | 0 (0.0) | 2 (9.5) | .0870 |
| Presenting symptoms/history | ||||
| Cough, n (%) | 50 (71.4) | 34 (69.4) | 16 (76.2) | .5637 |
| Fever, n (%) | 51 (72.9) | 34 (69.4) | 17 (81.0) | .3187 |
| Shortness of breath, n (%) | 45 (64.3) | 26 (53.1) | 19 (90.5) | .0028 |
| Headache, n (%) | 15 (21.4) | 9 (18.4) | 6 (28.6) | .3564 |
| Rhinorrhea, n (%) | 9 (12.9) | 4 (8.2) | 5 (23.8) | .1155 |
| Nausea/vomiting, n (%) | 24 (34.3) | 20 (40.8) | 4 (19.0) | .0787 |
| Diarrhea, n (%) | 18 (25.7) | 12 (24.5) | 6 (28.6) | .7203 |
| Myalgias, n (%) | 13 (18.6) | 10 (20.4) | 3 (14.3) | .7410 |
| Known sick contacts, n (%) [n = 11 missing] | 30 (50.8) | 19 (47.5) | 11 (57.9) | .4555 |
| Travel history in past month, n (%) | 2 (2.9) | 1 (2.0) | 1 (4.8) | .5130 |
| Duration of symptoms before admission, d, median (IQR) [n = 1 missing] | 5.0 (2.0, 7.0) | 4.0 (2.0, 8.5) | 5.0 (3.0, 7.0) | .7626 |
| Vital signs on admission | ||||
| Temp, °C, median (IQR) | 37.5 (36.9, 38.3) | 37.4 (36.9, 38.3) | 37.5 (37.0, 37.9) | .7923 |
| Heart Rate, bpm, median (IQR) | 107.0 (99.0, 129.0) | 107.0 (101.0, 131.0) | 107.0 (99.0, 123.0) | .6628 |
| SBP, mm Hg, median (IQR) | 114.0 (101.0, 126.0) | 116.0 (102.0, 126.0) | 105.0 (95.0, 124.0) | .1421 |
| DBP, mm Hg, median (IQR) | 68.5 (58.0, 77.0) | 69.0 (58.0, 79.0) | 68.0 (58.0, 72.0) | .6909 |
| RR, bpm, median (IQR) | 26.0 (22.0, 38.0) | 25.0 (22.0, 36.0) | 32.0 (26.0, 42.0) | .0724 |
| SpO2 percentage, median (IQR) | 98.0 (96.0, 99.0) | 98.0 (96.0, 99.0) | 97.0 (94.0, 99.0) | .2375 |
| Admission diagnosis | ||||
| Respiratory failure, n (%) | 47 (67.1) | 26 (53.1) | 21 (100.0) | .0090 |
| Shock, n (%) | 5 (7.1) | 5 (10.2) | 0 (0.0) | |
| Sepsis, n (%) | 5 (7.1) | 5 (10.2) | 0 (0.0) | |
| DKA, n (%) | 7 (10.0) | 7 (14.3) | 0 (0.0) | |
| Seizures/neuro, n (%) | 5 (7.1) | 5 (10.2) | 0 (0.0) | |
| Other, n (%) | 1 (1.4) | 1 (2.0) | 0 (0.0) |
bpm, breaths per minute; DBP, diastolic blood pressure; DKA, diabetic ketoacidosis; RR, respiratory rate; SBP, systolic blood pressure; SpO2, arterial oxygen saturation.
χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
Fever (72.9%) and cough (71.4%) were the most common presenting symptoms. Ninety percent of patients who developed ARDS came to medical attention with dyspnea, compared with 53.1% who did not develop ARDS (P = .003). Median duration of symptoms before hospitalization was 5 days (IQR 2.0, 7.0), and a known sick contact was reported in 50.8% of patients.
Table II summarizes the laboratory and radiographic testing results. The median total white blood cell count for the entire cohort of patients was 8.8 (IQR 6.3, 13.0) k/μL; median absolute lymphocyte count was 1.1 (IQR 0.6,2.0) k/μL. Patients with ARDS had significantly lower platelet counts compared with those without ARDS (169 [IQR 110, 242] k/μL vs 216 [IQR 159, 309] k/μL, P = .04). Serum levels of C-reactive protein, procalcitonin, lactate, pro-B type natriuretic peptide, and interleukin-6 were elevated among patients with ARDS, but levels were statistically significantly different than patients without ARDS only for interleukin-6 (78.7 [IQR 34, 201.5] vs 16.4 [IQR 12.1, 66] pg/mL, P = .03). Eight patients had positive blood cultures; 3 patients had positive respiratory tract cultures, and 5 patients had positive urine cultures; concomitant respiratory infections were reported in 3 patients (4.3%) with rhinovirus in 2 and Bordetella pertussis in 1.
Table II.
Results of laboratory imaging studies on admission to PICU
| Variables | Total (n = 70) | No ARDS (n = 49) | ARDS (n = 21) | P value∗ |
|---|---|---|---|---|
| WBC, k/μL, median (IQR) [n = 69] | 8.8 (6.3, 13.0) | 8.8 (6.3, 11.7) | 8.9 (6.0, 14.3) | .9105 |
| Absolute lymphocyte count, cells/μL, median (IQR) [n = 69] | 1117.4 (610.9, 1998.4) | 1001.6 (604.5, 2258.7) | 1177.0 (766.3, 1914.9) | .3936 |
| Hemoglobin, g/dL, median (IQR) [n = 69] | 12.2 (10.8, 14.6) | 12.9 (11.1, 14.7) | 11.9 (9.6, 13.2) | .1815 |
| Platelets, k/μL, median (IQR) [n = 69] | 198.0 (147.0, 264.0) | 216.0 (159.0, 309.0) | 169.0 (110.0, 242.0) | .0430 |
| AST, U/L, median (IQR) [n = 60] | 36.0 (28.0, 60.0) | 33.0 (27.0, 57.0) | 40.0 (32.0, 61.0) | .3691 |
| ALT, U/L, median (IQR) [n = 60] | 26.0 (14.5, 49.5) | 30.0 (15.0, 56.0) | 20.0 (12.0, 45.0) | .1870 |
| Total bilirubin, mg/dL, median (IQR) [n = 60] | 0.4 (0.2, 0.9) | 0.4 (0.2, 1.0) | 0.3 (0.1, 0.9) | .1987 |
| BUN, mg/dL, median (IQR) | 11.0 (8.0, 14.0) | 10.0 (8.0, 13.0) | 11.0 (7.0, 15.0) | .6528 |
| Creatinine, mg/dL, median (IQR) | 0.7 (0.5, 0.9) | 0.7 (0.5, 0.9) | 0.6 (0.4, 0.9) | .7728 |
| C-reactive protein, mg/dL, median (IQR) [n = 48] | 6.8 (2.6, 14.9) | 6.0 (2.5, 26.0) | 8.1 (3.5, 11.8) | .8716 |
| Procalcitonin, ng/mL, median (IQR) [n = 34] | 0.2 (0.1, 0.9) | 0.2 (0.1, 0.6) | 0.4 (0.2, 1.1) | .1778 |
| Pro-BNP, pg/mL, median (IQR) [n = 14] | 940.0 (92.0, 4289.0) | 440.8 (60.0, 4289.0) | 1734.0 (1112.0, 15 000.0) | .1195 |
| Troponin, ng/mL, median (IQR) [n = 32] | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.1) | .6109 |
| CPK, U/L, median (IQR) [n = 25] | 120.0 (74.0, 302.0) | 128.5 (74.0, 316.0) | 99.0 (60.0, 302.0) | 1.0000 |
| Lactate, mmol/L, median (IQR) [n = 43] | 1.5 (1.2, 2.5) | 1.6 (1.2, 2.6) | 1.5 (1.3, 2.3) | .5972 |
| D-dimer, μg/mL FEU, median (IQR) [n = 35] | 2.3 (0.8, 161.0) | 3.1 (0.8, 161.0) | 2.0 (0.7, 20.0) | .8663 |
| LDH, U/L, median (IQR) [n = 39] | 370.0 (289.0, 524.0) | 318.0 (276.0, 461.0) | 421.0 (344.0, 569.0) | .0413 |
| IL-6, pg/mL, median (IQR) (n = 23) | 42 (13.7, 112) | 16.4 (12.1, 66) | 78.7 (34, 201.5) | .0316 |
| +Blood culture, n (%) | 8 (11.8) | 4 (8.5) | 4 (19.0) | .2403 |
| +Respiratory culture, n (%) | 3 (5.2) | 0 (0.0) | 3 (14.3) | .0431 |
| +Urine culture, n (%) | 5 (8.2) | 1 (2.5) | 4 (19.0) | .0437 |
| Chest radiograph | ||||
| Clear, n (%) | 12 (17.1) | 12 (24.5) | 0 (0.0) | .0133 |
| B/L infiltrates, n (%) | 35 (50.0) | 20 (40.8) | 15 (71.4) | .0189 |
| Pleural effusions, n (%) | 4 (5.7) | 1 (2.0) | 3 (14.3) | .0776 |
| Pneumothorax, n (%) | 1 (1.4) | 0 (0.0) | 1 (4.8) | .3000 |
| Other, n (%) | 16 (22.9) | 10 (20.4) | 6 (28.6) | .5383 |
| LV dysfunction by echocardiogram, n (%) | 6 (40.0) | 3 (33.3) | 3 (50.0) | .6224 |
| LV EF percentage, median (IQR) [n = 3] | 44.1 (25.0, 48.7) | 48.7 (48.7, 48.7) | 34.6 (25.0, 44.1) | .5403 |
| RV dysfunction by echocardiogram, n (%) [n = 3] | 3 (20.0) | 2 (22.2) | 1 (16.7) | 1.0000 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; B/L, Bilatera; BUN, blood urea nitrogen; CPK, creatine phosphokinase; EF, ejection fraction; FEU, Fibrinogen equivalent unit; IL, interleukin; LV, left ventricular; pro-BNP, pro-B type natriuretic peptide; LDH, lactate dehydrogenase; RV, right ventricular; WBC, white blood cell count.
χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
On chest radiography, 71% of patients with ARDS had bilateral infiltrates compared with 41% of those without ARDS (P = .02). Fifteen patients had documented formal echocardiograms performed, of who 6 had left ventricular dysfunction, and 3 had right ventricular dysfunction.
Medical Therapy and Outcomes
As presented in Table III , 57 patients met criteria for sepsis, of whom 12 patients met criteria for severe sepsis. Ten of these 12 patients meeting severe sepsis criteria also had ARDS compared with 2 in the non-ARDS group (P < .0001). Fourteen patients (20%) required vasopressor support: 11 in the ARDS group vs 3 in the non-ARDS group (P < .0001), with norepinephrine being the most frequently used vasopressor medication. Nine patients (12.9%) had AKI, and 1 patient required renal-replacement therapy.
Table III.
Therapies and clinical outcomes
| Characteristics | Total (n = 70) | No ARDS (n = 49) | ARDS (n = 21) | P value∗ |
|---|---|---|---|---|
| Medical therapy | ||||
| Hydroxychloroquine, n (%) | 27 (38.6) | 14 (28.6) | 13 (61.9) | .0087 |
| Azithromycin, n (%) | 23 (32.9) | 16 (32.7) | 7 (33.3) | .9557 |
| Remdesivir, n (%) | 13 (18.6) | 6 (12.2) | 7 (33.3) | .0494 |
| Corticosteroid, n (%) | 23 (32.9) | 13 (26.5) | 10 (47.6) | .0852 |
| IL-6 inhibitor, n (%) | 3 (4.3) | 3 (6.1) | 0 (0.0) | .5488 |
| IL-1 inhibitor, n (%) | 1 (1.4) | 1 (2.0) | 0 (0.0) | 1.0000 |
| Antibiotics <48 h, n (%) | 20 (28.6) | 17 (34.7) | 3 (14.3) | .0833 |
| Antibiotics >48 h, n (%) | 28 (40.0) | 13 (26.5) | 15 (71.4) | .0004 |
| Vasopressor use | ||||
| Any inotrope use, n (%) | 14 (20.0) | 3 (6.1) | 11 (52.4) | <.0001 |
| Epinephrine, n (%) | 3 (4.3) | 1 (2.0) | 2 (9.5) | .2123 |
| Norepinephrine, n (%) | 8 (11.4) | 2 (4.1) | 6 (28.6) | .0074 |
| Dopamine, n (%) | 5 (7.1) | 1 (2.0) | 4 (19.0) | .0259 |
| Dobutamine, n (%) | 1 (1.4) | 1 (2.0) | 0 (0.0) | 1.0000 |
| Milrinone, n (%) | 3 (4.3) | 2 (4.1) | 1 (4.8) | 1.0000 |
| Respiratory support | ||||
| NC oxygen, n (%) | 30 (42.9) | 18 (36.7) | 12 (57.1) | .1138 |
| Duration of NC oxygen, h, median (IQR) | 27.5 (9.0, 36.0) | 28.0 (10.0, 48.0) | 15.5 (6.5, 25.5) | .3125 |
| HFNC oxygen, n (%) | 21 (30.0) | 14 (28.6) | 7 (33.3) | .6903 |
| Duration of HFNC oxygen, h, median (IQR) | 96.0 (59.0, 108.0) | 96.0 (87.0, 108.0) | 8.0 (8.0, 8.0) | .1349 |
| Non-IMV, CPAP, or BiPAP, n (%) | 14 (20.0) | 4 (8.2) | 10 (47.6) | .0004 |
| Total duration of non-IMV/CPAP/BiPAP, h, median (IQR) | 79.5 (41.5, 121.5) | 156.0 (156.0, 156.0) | 72.0 (11.0, 87.0) | .3711 |
| IMV, n (%) | 20 (28.6) | 2 (4.1) | 18 (85.7) | <.0001 |
| Extubated, n (%) | 9 (45.0) | 1 (50.0) | 8 (44.4) | 1.0000 |
| Duration of IMV, h, median (IQR) [among 9 extubated patients] | 163.0 (79.0, 308.7) | 79.0 (79.0, 79.0) | 191.4 (107.5, 309.5) | .5613 |
| Prone positioning while awake, nonintubated, n (%) | 4 (5.7) | 3 (6.1) | 1 (4.8) | 1.0000 |
| Prone positioning while intubated, n (%) | 3 (15.0) | 0 (0.0) | 3 (16.7) | 1.0000 |
| Neuromuscular blockade, n (%) | 15 (75.0) | 0 (0.0) | 15 (83.3) | .0526 |
| Mechanical support | ||||
| ECMO, n (%) | 1 (1.4) | 1 (2) | 0 (0.0) | .1053 |
| RRT, n (%) | 1 (1.4) | 0 (0.0) | 1 (4.8) | .3000 |
| Outcomes | ||||
| Sepsis syndrome, n (%) | 57 (81.4) | 38 (77.6) | 19 (90.5) | .3173 |
| Severe sepsis syndrome, n (%) [n = 12] | 12 (21.1) | 2 (5.3) | 10 (52.6) | <.0001 |
| AKI, n (%) | 9 (12.9) | 5 (10.2) | 4 (19.0) | .4366 |
| Cardiac arrest, n (%) | 2 (2.9) | 0 (0) | 2 (2.9) | |
| Status on hospital day 14, n (%) | <.0001 | |||
| Discharged | 47 (69.1) | 42 (89.4) | 5 (23.8) | |
| Hospitalized (floor) | 5 (7.4) | 4 (8.5) | 1 (4.8) | |
| Hospitalized (PICU) | 14 (20.6) | 1 (2.1) | 13 (61.9) | |
| Mortality | 2 (2.9) | 0 (0.0) | 2 (9.5) | |
| Status on hospital day 28, n (%)† [n = 70] | ||||
| Discharged | 55 (78.5) | 46 (93.8) | 9 (42.9) | |
| Hospitalized (floor) | 4 (5.7) | 2 (4.1) | 2 (9.5) | |
| Hospitalized (PICU) | 9 (12.8) | 1 (2) | 8 (38.1) | |
| Mortality | 2 (2.8) | 0 | 2 (9.5) | |
BiPAP, bi-level positive airway pressure; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation; NC, nasal cannula; RRT, renal-replacement therapy.
χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
Seven patients did not reach 28 days from PICU admission as they were discharged before this benchmark.
Hydroxychloroquine, remdesivir, and antibiotics >48 hours were used more often in the ARDS group (P < .05 for all). Corticosteroids were used in 23 patients and 4 patients were treated with immunomodulatory therapies (3 with tocilizumab and 1 with anakinra); none received intravenous immunoglobulin or convalescent plasma.
Prone positioning was used in 7 (10%) of patients, of whom 4 had ARDS. A greater proportion of patients with ARDS received noninvasive mechanical ventilation than patients without ARDS (47.6% vs 8.2%, P = .0004). Twenty patients (28.6%) required invasive mechanical ventilation (IMV; 85.6% in the ARDS group vs 4.1% in non-ARDS group, P < .0001), 4 of whom were intubated before PICU admission. Of the 16 who required IMV in a PICU, respiratory support before intubation included oxygen by nasal cannula in 4, high-flow nasal cannula oxygen in 4, and bi-level positive airway pressure in 7 patients.
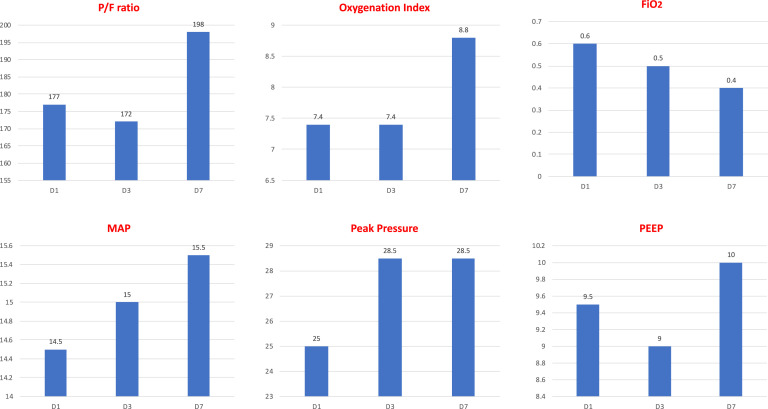
Of the 21 patients with ARDS, 4 had mild, 12 had moderate, and 5 had severe ARDS. Of these, 18 required IMV: 3 with mild ARDS, 10 with moderate ARDS, and 5 with severe ARDS (Figure 2 ). The 3 patients with mild ARDS who required IMV had significant comorbidities, which included malignancy in 2 patients and severe obesity (BMI 51 kg/m2) in 1 patient. The median duration of mechanical ventilation in the ARDS group was 218.9 (139.8, 310.4) hours with 9 (50%) of these 18 patients still hospitalized and requiring invasive mechanical ventilation on day 14, and 7 (38.9%) on day 28 of hospitalization (Table III). Inhaled nitric oxide was used in 2 patients (2.8%).
Figure 2.
Oxygenation and ventilatory characteristics of intubated patients with COVID-19. FiO2, fraction of inspired oxygen; MAP, mean arterial pressure; PEEP, positive end-expiratory pressure; P/F, arterial pO2 divided by the FIO2; pO2, partial pressure of oxygen.
One patient (1.4%) was cannulated to venoarterial extracorporeal membrane oxygenation support for acute decompensated heart failure in the setting of the previously diagnosed condition of dilated cardiomyopathy. Two patients (2.9%) required cardiopulmonary resuscitation, 1 of whom survived. Extracorporeal cardiopulmonary resuscitation was not used in any center for this cohort of patients.
By hospital day 14, 49 patients (70%) were discharged home; 14 (20%) remain hospitalized in PICU, and 5 (7.1%) remain hospitalized out of the PICU. Two patients (2.9%) died, one after withdrawal of life-sustaining therapies associated with end-stage osteosarcoma with extensive pulmonary metastases. The second death occurred in a patient with hemoglobinopathy who suffered from a hypoxic bradycardic arrest without return of spontaneous circulation after cardiopulmonary resuscitation.
By hospital day 28, 55 patients (78.6%) were discharged home; 9 (12.9%) remained hospitalized in PICU, and 4 (5.7%) remained hospitalized out of the PICU. Mortality remained at 2 deaths (2.9%) on hospital day 28. The patient on extracorporeal membrane oxygenation remained cannulated on day 28 awaiting cardiac transplant/ventricular assist device placement. The standardized mortality rate at hospital day 14 and 28 day for our cohort is 3.4, which represents 3.4 times excess mortality than that predicted by the PIM-2 score.
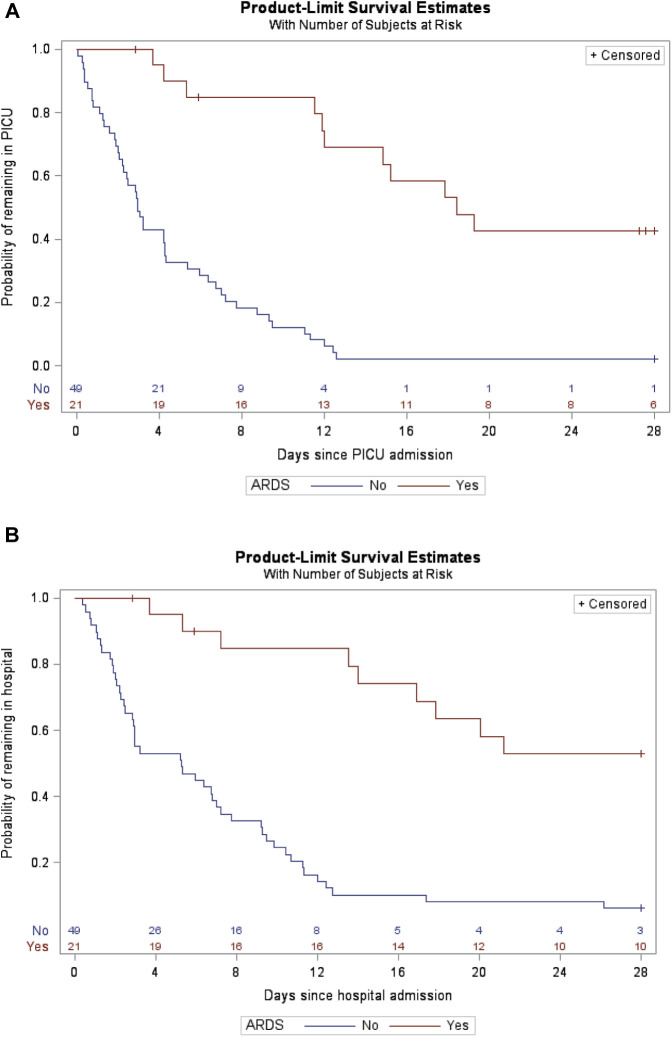
The presence of ARDS was associated with significantly longer PICU and hospital duration of stay (both P < .0001, Figure 3, A and B [available at www.jpeds.com]). In a multivariable analysis of time to discharge, ARDS (but not race or comorbidity) was independently associated with lower probability of PICU and hospital discharge (P = .001 for both) and black/Latino race was associated with greater probability of hospital discharge by day 28 (P = .04) (Tables IV and V ).
Figure 3.
A, Kaplan–Meier plot: time to PICU discharge for patients with and without ARDS (N = 70, Log-rank test P < .0001). Censored for mortality. Patients still in PICU at study end are censored at last day of follow-up (up to hospital day 28). B, Kaplan–Meier plot: time to hospital discharge for patients with and without ARDS (N = 70, log-rank test P < .0001). Censored for mortality. Patients still in hospital at study end are censored at last day of follow-up (up to hospital day 28).
Table IV.
Multivariable Cox proportional hazards model of outcome: time to PICU discharge (N = 70)
| Variables | AHR (95% CI) | P value |
|---|---|---|
| ARDS (reference = no) | 0.08 (0.03-0.21) | <.0001 |
| Black/Latino (reference = white) | 1.78 (0.71-4.48) | .2210 |
| Other race (reference = white) | 0.91 (0.33-2.51) | .8539 |
| Any comorbidity (reference = no) | 1.29 (0.68-2.45) | .4377 |
AHR, adjusted hazard ratio.
Table V.
Multivariable Cox proportional hazards model of outcome: time to hospital discharge (N = 70)
| Variables | AHR (95% CI) | P value |
|---|---|---|
| ARDS (reference = no) | 0.10 (0.04-0.27) | <.0001 |
| Black/Latino (reference = white) | 2.76 (0.93-8.24) | .0685 |
| Other race (reference = white) | 1.00 (0.29-3.42) | .9951 |
| Any comorbidity (reference = no) | 0.98 (0.51-1.91) | .9620 |
Discussion
In this study, we describe clinical manifestations of critically ill children with COVID-19 disease admitted to PICUs in New York City, the epicenter of the COVID-19 pandemic in the US. This study adds to the growing literature on critical illness and outcomes in children with COVID-19, especially that associated with ARDS.
The exact PICU admission rate in children with COVID-19 remains unknown, with previous reports ranging from 1.3% to 28.2%.1 , 5 , 7 , 13 A systematic review of early studies from China and Singapore identified only 1 critically ill child among 1065 children (0.1%) with confirmed COVID-19.3 In Spain, only 4 of 41 (16%) confirmed cases were admitted to PICU.4 In a single-center cohort of 46 hospitalized children positive for SARS-CoV-2 in New York City, 13 (28%) were critically ill, requiring admission to the PICU; 77% of PICU patients developed ARDS; and 61.5% were discharged home.5 Another study reported with less detail than our study the early experience of critically ill children in a 2-week cross-sectional study of children with COVID-19 admitted to 46 North American PICUs between March 14 and April 3, 2020.6 Götzinger et al7 reported an 8% PICU admission rate in their multinational study from Europe. Although some of these studies show a greater rate of critical illness than previously reported, detailed clinical characteristics and multicenter longitudinal outcomes were not reported.
Our study focused on multiple PICUs in the catchment area that includes all of New York City, the epicenter of the COVID-19 pandemic in the US. A previous report was only from 1 of 25 PICUs in New York City.6 Although our cohort represents approximately 46% of PICU beds, the proportion of critically ill children represented by our cohort is substantially greater because multiple hospitals' PICU beds were repurposed to accommodate critically ill adults during the pandemic surge. Similarly, a report from China that did not include the epicenter of Wuhan2 underestimated the presence of critical illness in children compared with studies from Wuhan, the Chinese epicenter.1 Studies that do not emanate from an epicenter may not represent the experience of a city consumed by pandemic infection. In addition, we report comprehensive details of ventilatory support and oxygenation markers, with concurrent standard scoring of patient criteria for severe sepsis,14 ARDS,11 , 12 and AKI.13 Compared with previous reports, we provide comprehensive details of patients' multiorgan involvement, longer enrollment duration (7 weeks vs 2 weeks), and longer outcome follow-up (14 and 28 days vs 8 days).
The median age in our cohort was 15 years, different from early reports that suggested infants and preschool-aged children may be at greater risk of critical illness with COVID-19 infection,1 but similar to a more recent cross-sectional point-prevalence report from North America.6 Similar to previous findings,5 approximately 50% of our critically ill patients reported no known sick contact, indicating a high rate of community spread, which may be explained partially by the high population density of New York City.16
There was no difference in the median PIM-2 scores in patients with and without ARDS with a median risk of expected mortality of 0.8%. The overall cohort mortality of 2.8% (all in patients with ARDS) is greater than that expected by the PIM-2 scores. In addition, patients with ARDS had significantly greater prevalence of severe sepsis and required greater levels of respiratory and hemodynamic support.
One of the known risk factors for the development of critical illness in adults is the presence of comorbidities.17, 18, 19, 20 A high percentage (74%) of our cohort of critically ill children had at least 1 comorbidity, consistent with data from both pediatric and adult studies.5 , 6 , 16, 17, 18, 19 Despite the presence of significant comorbidities, however, these comorbidities were not associated with ARDS in our cohort.
ARDS is reported to occur in 2%-3% of critically ill children21 and in 17% of critically ill children with viral infections.22 We found a 30% ARDS prevalence in our COVID-19 cohort, which is substantially greater. When patients with COVID-19 develop ARDS, their intubation rates (86% in our study) are greater than that for other viral infection. One possible reason for a greater intubation rate is the early recommendation to limit use of noninvasive ventilation and to consider early intubation of patients with COVID-19 in an attempt to limit aerosolization of the virus.23 , 24
In patients who required mechanical ventilation, a lung-protective strategy of using a low tidal volume and limiting the plateau pressure as recommended by Pediatric Acute Lung Injury Consensus Conference (PALICC)11 seem to be effective in COVID-19 ARDS. The moderate median positive end-expiratory pressure of 9.5 cm of water in our cohort is similar to that reported by Grasselli et al17 in their subcohort of patients <20 years of age and is not unusual in critically ill children.25 This is in comparison with the median positive end-expiratory pressure of 14 cm water in adults aged 20-80 years with COVID-19 in the same study,17 which may reflect relatively preserved compliance of pediatric lungs in the early phase of COVID-19–associated ARDS. Preserved compliance is probably also reflected in the low rate of prone positioning used in our study (only 16.7% for intubated patients with ARDS), again similar to Grasselli et al, who reported a 27% use of prone positioning, with no use in patients younger than 20 years of age.17 This also may reflect a greater adherence to the PROSEVA study26 recommendations of prone positioning as well as an absence of conclusive data supporting prone positioning in pediatric patients with ARDS.
More than 30% of the patients in our cohort were from a single borough of New York City, the Bronx. This patient distribution is consistent with a report showing a disproportionate involvement of Bronx County in New York City COVID-19 hospitalizations and deaths, despite the Bronx having the greatest number of hospital beds per 100 000 population and the greatest number of tests performed per 100 000 population of all 5 boroughs of New York City.27 This may reflect the unique sociodemographic factors of Bronx County,16 particularly the high density of population/transmission and high rate of poverty, which has been associated with prolonged hospital stay and PICU admission.28
Reassuringly, our observed discharge rate (70% by 14 days and 78.6% by 28 days) is greater than that reported in adults.17, 18, 19, 20 Those with ARDS, however, had longer PICU and hospital LOS compared with those without ARDS.
Our cohort had mortality rates similar to other pediatric studies5 , 6 but lower than that reported in adults.17, 18, 19, 20 However, our 3.5-fold excess standardized mortality of COVID-19 patients is noteworthy. Whether excess mortality ratio is truly related to COVID-19 or is rather an artifact due to low patient numbers or a reflection of sociodemographic factors of New York City is not clear and may be clarified by future large multicenter studies. This early mortality rate of 2.9% in our cohort, however, must be considered in the context of 18.6% of our patients still remaining hospitalized at day 28 and 38.9% of children with ARDS still receiving invasive ventilatory support at day 28.
This study has the limitations of being a retrospective study of a relatively small sample size. Our results may not be applicable to other parts of the country and world, considering the unique demographics of New York City. In addition, this study had not been designed to identify patients with MIS-C, as the first reports to the New York State Department of Health were outside the time frame of our study period.8 Applying the case definition retroactively following data collection identified some patients who met inflammatory criteria for MIS-C, likely reflecting an overlap in pathophysiology of COVID-19 lung disease, MIS-C, and sepsis. Our cohort primarily represents COVID-19 lower respiratory tract disease, an uncommon feature of MIS-C. Further studies are needed to better understand underlying pathophysiologies and potential spectrum vs distinctive clinical conditions. Further, larger national and international studies are necessary to determine the true incidence and risk factors for critical illness from SARS-CoV-2 infection and the development of ARDS in the pediatric population.
Acknowledgments
We thank the multidisciplinary teams for their expert care of these patients, particularly the divisions of Pediatric Infectious Disease, Pediatric Rheumatology, Pediatric Hematology, Pediatric Hospital Medicine, and Pediatric Cardiology at the participating institutions.
Footnotes
The spouse of M.G. discloses financial relationships (stocks) with Sorrento Therapeutics INC COMM, Asterias Biotherapeutics INC (purchased by Biotime), Synergy Pharmaceuticals Del Com, and Trulance (plecanatide). The other authors declare no conflicts of interest.
Appendix
References
- 1.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145:e20200702. [Google Scholar]
- 2.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:P689–P696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 4.Tagarro A., Epalza C., Santos M., Sanz-Santaeufemia F.J., Otheo E., Moraleda C. Screening and Severity of Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr. 2020;223:14–19.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1948. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Götzinger F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New York State Department of Health Health advisory: pediatric multi-system inflammatory syndrome temporally associated with COVID-19 interim case definition in New York State. https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf
- 9.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah P., Johnson C.L., Halabi K.C., Ahn D., Sen A.I., Fischer A. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2430. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric Acute Lung Injury Consensus Conference Group Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Kellum J.A., Lameire N., KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss S.L., Peters M.J., Alhazzani W., Agus M.S.D., Flori H.R., Inwald D.P. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 15.Slater A., Shann F., Pearson G. For the PIM Study Group PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 16.US Census QuickFacts: Bronx County (Bronx Borough), New York. https://www.census.gov/quickfacts/fact/table/bronxcountybronxboroughnewyork/IPE1 20218#IPE120218
- 17.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khemani R.G., Smith L., Lopez-Fernandez Y.M., Kwok J., Morzov R., Klein M.J. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study [published correction appears in Lancet Respir Med 2018 Nov 13;] [published correction appears in Lancet Respir Med 2019;7:e12] Lancet Respir Med. 2019;7:115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh J.W.J.C., Wong J.J., Sultana R., Wong P.P.C., Mok Y.H., Lee J.H. Risk factors for mortality in children with pneumonia admitted to the pediatric intensive care unit. Pediatr Pulmonol. 2017;52:1076–1084. doi: 10.1002/ppul.23702. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization.https://apps.who.int/iris/handle/10665/331446. License: CC BY-NC-SA 3.0 IGO Accessed June 2, 2020. [Google Scholar]
- 24.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khemani R.G., Parvathaneni K., Yehya N., Bhalla A.K., Thomas N.J., Newth C.J.L. Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med. 2018;198:77–89. doi: 10.1164/rccm.201707-1404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 27.Wadhera R.K., Wadhera P., Gaba P., Figueroa F.J., Maddox K.E.J., Yeh R.W. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrist E., Riley C.L., Brokamp C., Taylor S., Beck A.F. Neighborhood poverty and pediatric intensive care use. Pediatrics. 2019;144:e20190748. doi: 10.1542/peds.2019-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]