Abstract
Objective
Coronavirus disease 2019 (COVID-19) is a novel infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several reports highlighted the risk of infection and disease in pregnant women and neonates. To assess the risk of clinical complications in pregnant women and neonates infected with SARS-CoV-2 carrying out a systematic review and meta-analysis of observational studies.
Data sources
Search of the scientific evidence was performed using the engines PubMed and Scopus, including articles published from December 2019 to 15 April 2020.
Study eligibility criteria
Only observational studies focused on the assessment of clinical outcomes associated with pregnancy in COVID-19 women were selected.
Study appraisal and synthesis methods
The first screening was based on the assessment of titles and abstracts, followed by the evaluation of full-texts. Qualitative variables were summarized with frequencies, whereas quantitative variables with central and variability indicators depending on their parametric distribution. Forest plots were used to describe point estimates and in-between studies variability. Study quality assessment was performed.
Results
Thirteen studies were selected. All of them were carried out in China. The mean (SD) age and gestational age of pregnant women were 30.3 (1.5) years and 35.9 (2.9) weeks, respectively. The mean (SD) duration from the first symptoms to the hospital admission and to labour were 5.5 (2.0) and 9.5 (8.7) days, respectively. Patients mainly complained of fever and cough (pooled (95 % CI) proportions were 76.0 % (57.0 %–90.0 %) and 38.0 (28.0 %–47.0 %), respectively). Several antibiotics, antivirals, and corticosteroids were prescribed in different combinations. The pooled prevalence of maternal complications and of caesarean section were 45.0 % (95 % CI: 24.0 %–67.0 %) and 88.0 % (95 %CI: 82.0 %–94.0 %). A proportion of pregnant women less than 20 % were admitted to ICU. The pooled proportion of preterm infants was 23.0 % (95 %CI: 11.0 %–39.0 %). The most frequent neonatal complications were pneumonia and respiratory distress syndrome. The pooled percentage of infected neonates was 6.0 % (95 %CI: 2.0 %–12.0 %).
Conclusions
The present study suggests a high rate of maternal and neonatal complications in infected individuals. However, the current scientific evidence highlights a low risk of neonatal infection. Multicentre, cohort studies are needed to better elucidate the role of SARS-CoV-2 during pregnancy.
Keywords: Pregnancy, Neonate, COVID-19, SARS-CoV-2, Vertical transmission
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a novel virus which can cause severe pulmonary and extra-pulmonary disease and death. SARS-CoV-2-related disease (COVID-19) can affect more frequently elderly persons and individuals with cardiologic, respiratory, renal, and metabolic comorbidities [1]. Pregnancy can compromise the immune system and potentially SARS-CoV-2 infection can increase the risk of pneumonia in pregnant women in comparison to non-pregnant women [2]. No clear scientific evidence has been provided on the vertical transmission of SARS-CoV-2 (infection in utero from an infected mother to her infant before birth) [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. The aim of the present systematic review and meta-analysis is to assess the clinical features and outcomes of pregnant women and neonates following SARS-CoV-2 infection, as well as the risk of vertical transmission, if any.
Methods
Search strategy
Two electronic search engines (i.e., PubMed and Scopus) were chosen to detect the manuscripts on the topic “COVID-19 and pregnancy”.
The selection of the scientific evidence was based on the identification of mother-, fetus-, and newborn-outcomes. The search included articles published from December 2019 to 15 April 2020. The search was restricted to manuscripts written in English language and published in peer-reviewed journals.
Several keywords, combined in different strings depending on the electronic database, were chosen: “pregnancy”, “mother”, “child”, “mother-to-child transmission”, “COVID-19”, and “SARS-CoV-2”.
To improve the diagnostic accuracy of the search a manual assessment of the list of references was performed.
Reports published in the grey literature or in the social and conventional media were excluded following the risk of unreliable and poor scientific information on the adopted methodology.
Study selection
Manuscripts describing clinical outcomes associated with a pregnancy (both for the mother and the foetus/neonate) and a concomitant COVID-19 were considered suitable for the present analysis.
All the studies with an observational design were selected: case-report, case-series, cross-sectional, case-control, and cohort (both prospective and retrospective).
The following exclusion criteria were adopted: 1) editorials, reviews, correspondences; 2) studies performed in vitro; 3) studies which recruited animals.
Titles and abstracts (if any) were screened to evaluate the suitability of the manuscript based on the above-mentioned inclusion and exclusion criteria.
The first assessment, carried out by two investigators (LS and AP), was supervised by a third investigator (GS). The assessment of the selected full texts, as well as the extraction of the outcome and independent variables, was performed by the same investigators.
Data extraction
An ad hoc electronic form (excel file) was prepared to collect qualitative and quantitative variables. Two investigators (LS and AP) independently retrieved data from the results sections of the selected articles. Discrepancies (<5%) were discussed and addressed by a third investigator (GS). The inter-rater agreement was ∼100 %.
Anonymized information was retrieved from the full-texts articles. In the majority of the cases data were aggregated. On this basis, a local ethical approval was not needed.
The following study-, pregnant, and neonate-related variables were collected: 1) first author; 2) publication year; 3) study period; 4) country/ies where the study was performed; 5) epidemiological study design; 6) clinical setting/s; 7) gender of the neonate; 8) age of the mother and of the neonate; 9) mode of delivery; 10) Complications of the mother and of the neonate/s; 11) therapy prescribed to the pregnant and to the neonate/s; 12) Apgar score; 13) SARS-CoV-2 infection of the neonate/s; 14) clinical, laboratory, radiological variables associated with the infection of the mother and of the neonate; 15) clinical outcomes of the mother and of the newborn.
Study quality assessment
The steps of the review process, from study selection to data collection, were performed by investigators whose agreement was ∼100 %. Both the systematic review and meta-analysis were conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Moher D, et al. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009).
The Scottish Intercollegiate Guidelines Network was adopted to assess the quality of the studies with the observational design [13]. Case-report and case-series were evaluated following the ad hoc quality scale “Methodological quality and synthesis of case-series and case-reports” [14].
Statistical analysis
Qualitative variables were summarized with absolute and relative (percentage) frequencies, whereas quantitative variables were described with means (standard deviations, SD, or ranges).
Forest plots were used to describe point (95 % confidence intervals, CI) estimates and in-between studies’ variability. The inconsistency indicator (I2) was chosen to show the relationship between true variability and overall variation, with low, medium, and high heterogeneity in case of the following values: <25 %, ≥25 %-<50 %, and ≥50 %, respectively.
Fixed and random-effects models were computed keeping into consideration the expected between-study heterogeneity. Inconsistency among the studies was evaluated with the chi-squared test for heterogeneity.
Bias assessment plots and Egger weighted regression test methods were used to assess the publication bias.
Statistically significance was considered when a two-tailed p-value was <0.05.
All the analyses were carried out with the statistical softwares StatsDirect version 3.1.12 (StatsDirect Ltd.) and STATA version 15 (StatsCorp, College Station, Texas, USA).
Results
Study selection
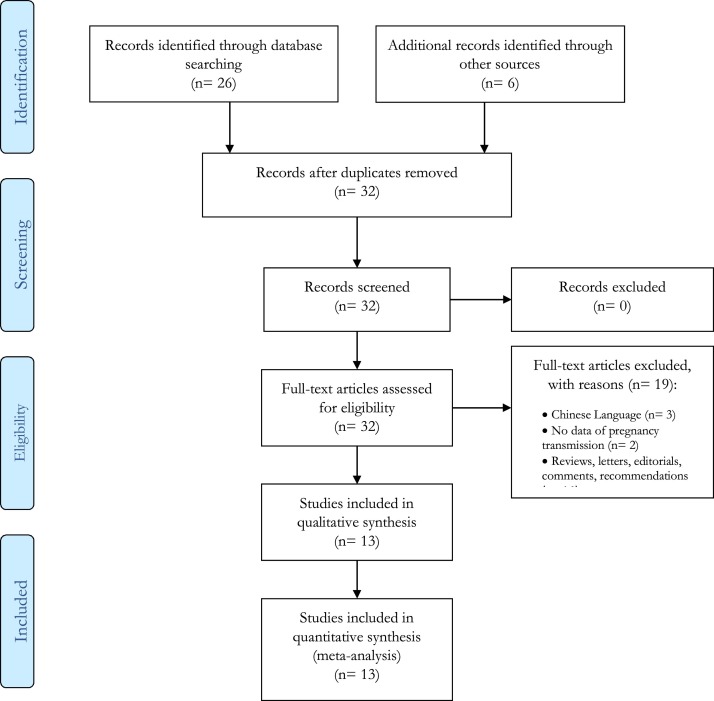
The records found in the search databases were 32 (Fig. 1 ), with 32 full texts carefully evaluated for their inclusion. Only 13 (40.6 %) were considered suitable for the qualitative and quantitative analyses. They other papers were excluded for the following reasons: articles written in Chinese language (n = 3), missing information on pregnancy and transmission of SARS-CoV-2 (n = 2), reviews, letters, editorials, commentaries, recommendations (n = 14).
Fig. 1.
PRISMA 2009 Flow Diagram.
Characteristics of the selected studies
All the studies were performed in the years 2019 [15] and 2020 [[2], [3], [4],[6], [7], [8], [9], [10],[15], [16], [17], [18]], (from December 2019 [15] to March 2020 [18]) and the manuscripts were published in the year 2020 [2,9,10,[15], [16], [17], [18]], (Table 1 ). The types of the studies were case-reports (5, 38.5 %) [2,4,5,9,18], case-series (2, 15.4 %) [3,8], and observational studies (6, 46.2 %) [6,7,10,[15], [16], [17]]. The design of the observational studies was described as retrospective and as cohort study in five (85.7 %) [6,7,10,15,16] and in two (28.6 %) [7,17] manuscripts, respectively.
Table 1.
Description of the characteristics of the selected studies.
| First author | Title | Publication data | Type of study | Centre | Study period |
|---|---|---|---|---|---|
| Yang Li [2] | Lack of Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. | Mar 05, 2020 | Case report | The First Affliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China | Feb 6-Feb 24, 2020 |
| Huijun Chen [3] | Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records | Feb 12, 2020 | Case series | Zhongnan Hospital of Wuhan University, Whuan, China | Jan 20-Jan 31, 2020 |
| Cuifan Fan [4] | Perinatal Transmission of COVID-19 Associated SARS-CoV-2: Should We Worry? | Mar 17, 2020 | Case report | Department of Obstetrics, Renmin Hospital of Wuhan University, Hubei, Wuhan, China | Jan 25-Feb 19, 2020 |
| Yan Chen [5] | Infants Born to Mothers With a New Coronavirus (COVID-19) | Mar 16, 2020 | Case report | Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China | – |
| Huaping Zhu [6] | Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia | Feb 10, 2020 | Retrospective observational study | 5 hospitals in Hubei | Jan 20-Feb 5, 2020 |
| Lei Zhang [7] | [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province]. | Mar 7, 2020 | Retrospective cohort study | Department of Obstetrics, Renmin Hospital of Wuhan University, Wuhan, China-Department of Obstetrics, The Central Hospital of Qianjiang City, Qianjiang, China. | – |
| Weiyong Liu [8] | Coronavirus Disease 2019 (COVID-19) During Pregnancy: A Case Series | Feb 25, 2020 | Case series | Obstetric ward of Tongji Hospital affiliated to Huazhong University of science and technology, Wuhan, China. | Feb 2-Feb 5, 2020, |
| Xiaotong Wang [9] | A Case of 2019 novel coronavirus in a pregnant woman with preterm delivery | Feb 28, 2020 | Case report | Suzhou Municipal Hospital, China | Feb 2-Feb 18, 2020, |
| Dehan Liu [10] | Pregnancy and Perinatal Outcomes of Women with Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis | Mar 7, 2020 | Retrospective observational study | Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China | Jan 20-Feb 10, 2020 |
| Yangli Liu [15] | Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy | Feb 27, 2020 | Retrospective observational study | Zhejiang, Cities of Hubei, Fujian, Shanxi, Beijing, Guangdong, Jiangxi, Heilongjiang and Anhui | Dec 8, 2019, and Feb 25, 2020 |
| Nan Yu [16] | Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study | Mar 24, 2020 | Retrospective observational study | Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China | Jan 1-Feb 8, 2020 |
| Lingkong Zeng [17] | Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China | Mar 26, 2020 | Cohort study | Wuhan Children's Hospital, Wuhan, China | Jan-Feb 2020 |
| Lan Dong [18] | Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn | Mar 26, 2020 | Case report | Renmin Hospital,Wuhan, China | Jan 28-Mar 19, 2020 |
The studies were conducted in China [[2], [3], [4], [5], [6], [7], [8], [9], [10],[15], [16], [17], [18]], mainly in Hubei province [[3], [4], [5], [6], [7], [8],10,[15], [16], [17], [18]]. Monocentre studies were eleven (84.6 %) [[2], [3], [4], [5],7,[3], [4], [5], [6], [7], [8], [9], [10],16,16,17,18], whereas only two (15.4 %) [6,15] were multicentre studies.
Characteristics of the study samples
The number of enrolled individuals ranged from 1 (23.1 %) [2,9,18] to 33 (7.7 %) [17] (Table 2 ). The total number of individuals recruited in the present analysis was 114, who were COVID-19 positive. The mean (SD) age was 30.3 (1.5) years, ranging from 22 [15] to 40 [3,10] years. The mean (SD) gestational age was 35.9 (2.9) weeks. The information on contact with a contagious patient was reported in 8 (61.5 %) [[2], [3], [4],8,9,15,16,18], studies, whereas data on infectious patients in the family was described by 5 (38.5 %) [[2], [3], [4],15,18], studies. The mean (SD) duration from the start of the symptoms and clinical signs to the hospital admission was 5.5 (2.0) days, but it was reported only by 5 (38.5 %) [2,4,8,10,18] studies. The mean duration from the occurrence of the first symptoms and clinical signs to the delivery was 9.5 (8.7) days, even if the information was provided only by six studies [[2], [3], [4],6,8,18].
Table 2.
Demographic, epidemiological, and clinical characteristics of pregnant women infected by SARS-CoV-2.
| Study | Sample size | Mean (range) age, years | Mean (range) gestational age, weeks | Epidemiological history, n (%) | Other family members affected | Mean (range) symptom onset at admission | Mean (range) symptom to delivery, days |
|---|---|---|---|---|---|---|---|
| Li Y., et al. [2] | 1 | 30 | 35 | 1 (100) | 1 (100) | 2 | 5 |
| Chen H., et al. [3] | 9 | 29.9 (26−40) | 37.1 (36−39) | 9 (100) | 4 (44.4) | 3.3 (1−7) | |
| Fan C., et al. [4] | 2 | 31.5 (29−34) | 36.5 (36−37) | 1 (50.0) | 1 (50.0) | 6.5 (3−10) | 11.5 (8−15) |
| Chen Y., et al. [5] | 4 | 29 (23−34) | 37.8 (37−39) | – | – | – | – |
| Zhu H., et al. [6] | 9 | 30.9 (25−35) | 35 (31−39) | – | – | – | 3 (1−6) |
| Zhang L., et al. [7] | 16 | 29.3 (24−39) | 38.7 (35−41) | – | – | – | – |
| Liu W., et al. [8] | 3 | 32.6 (30−34) | 38 (37−40) | 3 (100) | – | 7 (1−12) | 8 (1−15) |
| Wang X., et al. [9] | 1 | 28 | 30 | 1 | – | – | – |
| Liu D., et al. [10] | 15 | 32 (23−40) | 32 (12−38) | – | – | (2−10) | – |
| Liu Y., et al. [15] | 13 | 29.7 (22−36) | 33.4 (25−38) | 12 (92.3) | 6 (46.2) | – | – |
| Yu N., et al. [16] | 7 | 32 (29−34) | 39 (37−41) | 7 (100) | – | – | – |
| Zeng L., et al. [17] | 33 | – | – | – | – | – | – |
| Dong L., et al. [18] | 1 | 29 | 38 | 1 (100) | 0 (0.0) | 6 | 26 |
| Study | Cough, n (%) | Fever at admission, n (%) | Temperature, range | Myalgia, n (%) | Malaise, n (%) | Sore throat, n (%) | Dyspnoea, n (%) | Lymphopenia (<1.0 × 109 cells/l), n (%) |
|---|---|---|---|---|---|---|---|---|
| Li Y., et al. [2] | 1 | 1 (100) | 37.2 | – | – | – | – | – |
| Chen H., et al. [3] | 4 (44.4) | 7 (77.8) | 36.5−38.8 | 3 (33.3) | 2 (22.2) | 2 (22.2) | 1 (11.1) | 5 (55.6) |
| Fan C., et al. [4] | – | 2 (100) | 37.3−38.5 | – | – | 1 (50.0) | – | – |
| Chen Y., et al. [5] | 2 (50.0) | 3 (75.0) | – | 2 (50.0) | – | – | 2 (50.0) | 2 (50.0) |
| Zhu H., et al. [6] | 4 (44.4) | 8 (88.9) | – | – | – | 1 (11.1) | – | – |
| Zhang L., et al. [7] | 3 (18.8) | – | – | – | – | – | – | – |
| Liu W., et al. [8] | 2 (66.7) | 3 (100) | 37.0−38.0 | – | – | – | – | – |
| Wang X., et al. [9] | – | – | – | – | – | – | – | – |
| Liu D., et al. [10] | 9 (60.0) | 13 (86.5) | 37.6−39.0 | 3 (20.0) | 4 (26.7) | 1 (6.7) | 1 (6.7) | 12 (80.0) |
| Liu Y., et al. [15] | – | 10 (76.9) | 37.3−39.0 | – | 10 (76.9) | – | 3 (23.1) | – |
| Yu N., et al. [16] | 1 (14.3) | 6 (85.7) | – | – | – | – | – | – |
| Zeng L., et al. [17] | 10 (30.3) | 8 (24.2) | – | – | – | – | – | – |
| Dong L., et al. [18] | – | 1 (100) | 37.9 | – | – | – | 1 (100) | – |
The most prevalent symptoms and clinical signs were cough [2,3,[5], [6], [7], [8],10,16,17], (9, 69.2 %), fever [[2], [3], [4], [5], [6],8,10,[15], [16], [17], [18]] (11, 84.6 %), myalgia [3,5,10] (3, 23.1 %), fatigue [3,10,15] (3, 23.1 %), sore throat [3,4,6,10] (4, 30.8 %), dyspnoea [3,5,10,15] (4, 30.8 %), and lymphopenia [3,5,10] (3, 23.1 %). Body temperature in pregnant women ranged from 36.5 °C [3] to 39.0 °C [10,15].
The pooled proportion of fever and cough was 76.0 % (95 % CI: 57.0 %–90.0 %; I2: 70.9 %) and 38.0 % (95 % CI: 28.0 %–47.0 %; I2: 25.3 %), respectively (Fig. 2, Fig. 3 ).
Fig. 2.
Fever at admission in pregnant patients.
Fig. 3.
Cough in pregnant patients.
Interventions prescribed to pregnant women during the hospital stay were: corticosteroids (5, 38.5 %) [2,4,9,16,18], antibiotics (8, 61.5 %) [[2], [3], [4],[8], [9], [10],16,18], antivirals (9, 69.2 %) [[2], [3], [4],6,[8], [9], [10],16,18], and oxygen therapy (5, 38.5 %) [3,8,10,16,18] (Table 3 ). In particular, oseltamivir was prescribed in two (15.4 %) [4,16] studies, lopinavir/ritonavir in two (15.4 %) [2,9] studies, inhaled atomized peg-interferon and ganciclovir in two (15.4 %) [8,16] studies, and arbidol in three (23.1 %) [8,9,16] studies. Methylprednisolone was administered in two (15.4 %) [2,4] studies, whereas dexamethasone in one (7.7 %) [9] study. The following antibiotics were given: cefoperazone sulbactam sodium in one (15.4 %) [2,9] studies, azithromycin and ceftazidime in one (7.7 %) [4] study, cephalosporins combined with fluoroquinolones and macrolides in one (7.7 %) [16] study.
Table 3.
Therapy prescribed to pregnant women infected by SARS-CoV-2.
| Study | Sample size | Antiviral, n (%) |
Corticosteroids, n (%) |
Antibiotics, n (%) |
Oxygen therapy, n (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-partum | Post-partum | Pre-partum | Post-partum | Pre-partum | Post-partum | Pre-partum | Post-partum | ||
| Li Y., et al. [2] | 1 | 11 | 11 | – | 12 | – | 13 | – | – |
| Chen H., et al. [3] | 9 | 6 (66.7) | – | 9 (100) | 9 (100) | ||||
| Fan C., et al. [4] | 2 | 2 (100)4 | – | 2 (100)5 | – | 2 (100)6 | – | – | |
| Chen Y., et al. [5] | 4 | – | – | – | – | – | – | – | – |
| Zhu H., et al. [6] | 9 | – | 6 (55.6)7 | – | – | – | – | – | – |
| Zhang L., et al. [7] | 16 | – | – | – | – | – | – | – | – |
| Liu W., et al. [8] | 3 | – | 3 (100)8 | – | – | – | 2 (66.7) | – | 3 (100) |
| Wang X., et al. [9] | 1 | 19 | – | 110 | – | 13 | – | – | – |
| Liu D., et al. [10] | 15 | – | 11 (73.3) | – | – | 15 (100) | – | 14 (93.3) | – |
| Liu Y., et al. [15] | 13 | – | – | – | – | – | – | – | – |
| Yu N., et al. [16] | 7 | 7 (100)11 | – | – | 5 (71.4)5 | 7 (100)12 | – | 7 (100) | – |
| Zeng L., et al. [17] | 33 | – | – | – | – | – | – | – | – |
| Dong L., et al. [18] | 1 | 1 (100) | – | 1 (100) | – | 1 (100) | – | 1 (100) | – |
1. Oral lopinavir 200 mg and ritonavir 50 mg, each 2×/d.
2. Methylprednisolone (40 mg 1×/d).
3. Cefoperazone sodium/sulbactam sodium (intravenous drip, 2 g/ 8 h).
4. Oseltamivir (75 mg, PO daily).
5.Methylprednisolone (20 mg 4×/d).
6. Azithromycin/Ceftazidime.
7. Oral oseltamivir.
8.1. Atomized inhalation of interferon (40 μg, bid) and ganciclovir (0.25 g, IV); 8.2. Oral Arbidol hydrochloride; 8.3 Arbidol hydrochloride 3 g, qid orally.
9. Arbidol (0.2 g administered orally every 8 h), Lopinavir and Ritonavir Tablets (400/100 mg administered orally every 8 h).
10. Dexamethasone;
11. Oseltamivir (75 mg every 12 h, orally), ganciclovir (0·25 g every 12 h, intravenously), and interferon (40 μg daily, atomisation inhalation) and arbidol tablets (200 mg three times daily, orally;
12. Cephalosporins, quinolones, and macrolides.
Clinical and laboratory characteristics in pregnant women were variable: pneumonia was clinically diagnosed in all recruited women in all selected studies [[2], [3], [4], [5], [6],[8], [9], [10],[16], [17], [18]], with the only exception of one [7] study where only 6.3 % showed a pneumonia and one [15] study where the information on pneumonia was not provided (Table 4 ). CT-based diagnosis of pneumonia was performed in 88.9 % [3]–100 % [2,4,[3], [4], [5], [6],[8], [9], [10],[16], [17], [18]], women per single study.
Table 4.
Clinical and laboratory characteristics of pregnant women infected by SARS-CoV-2.
| Study | Sample size | CT evidence of pneumonia, n (%) | Pneumonia, n (%) | Pregnancy complication*, n (%) | C- section, n (%) | ICU admission, n (%) | Cure rate at discharge, n (%) | Death, n (%) | * |
|---|---|---|---|---|---|---|---|---|---|
| Li Y., et al. [2] | 1 | 1 (100) | 1 (100) | 1 (100.0) | 1 (100.0) | 0 (0.0) | 1 (100) | 0 (0.0) | non-reassuring fetal testing |
| Chen H., et al. [3] | 9 | 8 (88.9) | 9 (100) | 7 (77.8) | 9 (100) | – | – | 0 (0.0) | Influenza 1; Gestational hypertension1; pre-eclampsia 1; non-reassuring fetal testing 2; PROM 2. |
| Fan C., et al. [4] | 2 | 2 (100) | 2 (100) | 0 (0.0) | 2 (100) | 0 (0.0) | 1 (50.0) | 0 (0.0) | – |
| Chen Y., et al. [5] | 4 | 4 (100) | 5 (100) | 2 (50.0) | 3 (75.0) | 1 (25.0) | – | 0 (0.0) | Cholecystitis 1; placenta previa 1. |
| Zhu H., et al. [6] | 9 | 9 (100) | 4 (100) | 7 (77.8) | 7 (77.8) | – | – | – | Non-reassuring fetal testing 6; PROM 3; Abnormal amniotic fluid 2; umbilical cord abnormalities 2; placenta previa 1 |
| Zhang L., et al. [7] | 16 | – | 1 (6.3) | – | 16 (100) | – | – | – | Gestational diabetes (3), PROM (3), preterm delivery (3), uterine rupture (2), B-Lynch/compression suture procedure (2), severe preeclampsia (1), non-reassuring fetal testing (1), fetal asphyxia (1), meconium staining (1) |
| Liu W., et al. [8] | 3 | 3 (100) | 3 (100) | – | 2 (66.7) | 0 (0.0) | 3 (100) | 0 (0.0) | – |
| Wang X., et al. [9] | 1 | 1 (100) | 1 (100) | – | 1 (100) | 1 (100) | 1 (100) | 0 | – |
| Liu D., et al. [10] | 15 | 15 (100.0) | 16 (100.0) | 3 (20.0) | 10/11 (90.9) | – | – | 0 (0.0) | Thalassemia and gestational diabetes 1; Mitral valve and tricuspid valve replacement 1; placenta previa 1. |
| Liu Y., et al. [15] | 13 | – | – | 5 (38.5) | 10/10 (100) | 1/13 (7.7) | – | 0 (0.0) | Non-reassuring fetal testing 3; PROM 1; Stillbirth 1; |
| Yu N., et al. [16] | 7 | 7 (100) | 7 (100) | 3 (42.9) | 7 (100) | 0 (0.0) | 7 (100) | 0 (0.0) | Uterine scarring 3 |
| Zeng L., et al. [17] | 33 | 33 (100) | 33 (100) | 3 (9.1) | 26 (78.8) | – | – | 0 (0.0) | PROM 3 |
| Dong L., et al. [18] | 1 | 1 (100) | 1 | – | 1 (100) | 0 (0.0) | – | 0 (0.0) | – |
The most frequent complications during pregnancy were: non-reassuring fetal testing (5, 38.5 %) [2,3,6,7,15], premature rupture of membranes (5, 38.5 %) [3,6,7,15,17], placenta praevia (3, 23.1 %) [5,6,10], preeclampsia (2, 15.4 %) [3,7], uterine rupture (2, 15.4 %) [7,16], gestational diabetes (2, 15.4 %) [7,10], hypertension (1, 7.7 %) [3], cholecystitis (1, 7.7 %) [5], abnormal amniotic fluid (1, 7.7 %) [6], umbilical cord abnormalities (1, 7.7 %) [6], fetal asphyxia (1, 7.7 %) [7], meconium staining (1, 7.7 %) [7], and stillbirth (1, 7.7 %) [15].
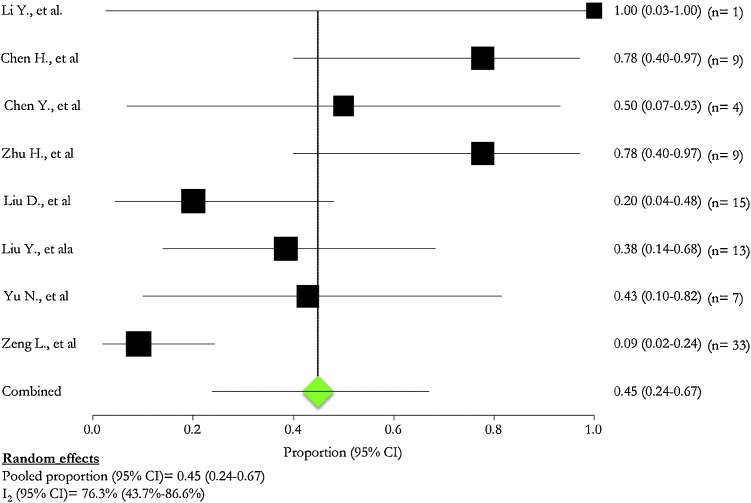
The pooled prevalence of complications in pregnant women was 45.0 % (95 % CI: 24.0 %–67.0 %; I2: 76.3 %) (Fig. 4 ).
Fig. 4.
Complications in pregnant patients.
Caesarean section was described in all studies [[2], [3], [4], [5], [6], [7], [8], [9], [10],[13], [14], [15], [16]], and was carried out from 66.7 % [8] to 100 % [[2], [3], [4],7,13,14,16], (53.9 % of all studies) women. The pooled proportion of caesarean section was 88.0 % (95 % CI: 82.0 %–94.0 %; I2: 9.2 %) (Fig. 5 ).
Fig. 5.
Caesarean section.
The information on admission to intensive care units was provided by eight (61.5 %) [2,4,5,8,9,13,16,18] studies: from 0% [2,4,8,16,18] to 100 % [9] women were admitted for severe disease per single study. The pooled percentage of women admitted to ICU was 13.0 % (95 % CI: 4.0 %–25.0 %; I2: 0.0 %) (Fig. 6 ).
Fig. 6.
ICU admission.
Cure rate (i.e., clinical and/or virologic recovery) was described in five (38.5 %) studies. No women (0.0 %) died [[2], [3], [4], [5], [6], [7], [8], [9], [10],[15], [16], [17], [18]].
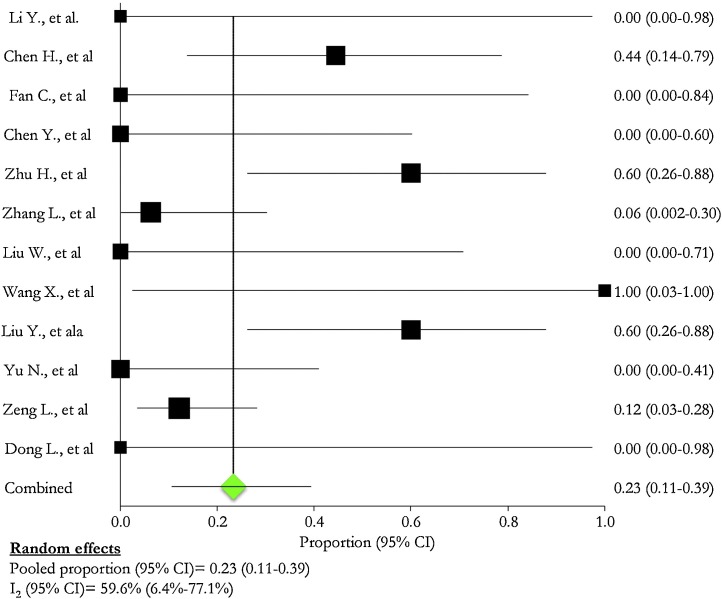
Preterm neonates were described in all [[2], [3], [4], [5], [6], [7], [8], [9],[15], [16], [17], [18]], studies than one [10]: their proportion ranged from 0.0 % [2,4,5,8,16,18] to 100.0 % [9] (Table 5 ). The pooled proportion of preterm infants was 23.0 % (95 % CI: 11.0 %–39.0 %; I2: 59.6 %) (Fig. 7 ).
Table 5.
Clinical outcomes of neonates born from women infected by SARS-CoV-2.
| Study | New-borns, n | Pre-term, n (%) | Mean (range) birthweight, g | Apgar score 1 min, range | Apgar score 5 min, range | Complication*, n (%) | SARS-CoV2 RNA | Death, n (%) | * |
|---|---|---|---|---|---|---|---|---|---|
| Li Y., et al. [2] | 1 | 0 | – | – | – | 0 (0.0) | 0 | 0 | – |
| Chen H., et al. [3] | 9 | 4 (44.4) | 3011 (1880−3820) | 8−9 | 9−10 | 2 (22.2) | 0 (0.0) | 0 (0.0) | Low birthweight 2 |
| Fan C., et al. [4] | 2 | 0 (0.0) | 3145 (2890−3400) | 9 | 10 | 2 (100) | 0 (0.0) | 0 (0.0) | Fever and abdominal distension and lymphopenia 1; pneumonia and lymphopenia 1 |
| Chen Y., et al. [5] | 4 | 0 (0.0) | 3000 (3050−3800) | 7−8 | 8−9 | 2 (50.0) | 0/3 (0.0) | 0 (0.0) | Skin rash 2; Oedema 1; Transient tachypnoea of the newborn 1. |
| Zhu H., et al. [6] | 10 | 6 (60.0) | 2423 (1520−3800) | 7−10 | 8−10 | 9 (90.0) | 0 (0.0) | 1 (10.0) | shortness of breath 6; fever 2; Rapid heart rate 1; Gastrointestinal symptoms 4; Infections 4; neonatal respiratory distress syndrome (NRDS) 2; pneumothorax 1 |
| Zhang L., et al. [7] | 16 | 1 (6.3) | 3139 (2300−3750) | – | – | 3 (18.8) | 0/10 (0.0) | 0 (0.0) | Pneumoniae 3 |
| Liu W., et al. [8] | 3 | 0 (0.0) | 3390 (3250−3670) | 8 | 9 | 2 (66.7) | 0 (0.0) | 0 (0.0) | Meconium Stained Amniotic Fluid (MSAF) 1; Slight decreased responsiveness and muscle tension 1. |
| Wang X., et al. [9] | 1 | 1 | 1830 | 9 | 10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Liu D., et al. [10] | 11 | – | – | 8 | 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Liu Y., et al. [15] | 10 | 6 (60.0) | – | – | – | – | – | 1 (10.0) | – |
| Yu N., et al. [16] | 7 | 0 (0.0) | 3264 (3000−3500) | 8−9 | 9−10 | 1 (14.3) | 1/3 (33.3) | 0 (0.0) | SARS-CoV2 positivity with mild pulmonary infection. |
| Zeng L., et al. [17] | 33 | 4 (12.1) | – | – | – | No individual data | 3 (9.1) | 0 (0.0) | Fever 2, SARS-Cov2 pneumoniae 3; Shortness of breath 4; Respiratory distress syndrome 4; Cyanosis 3; Feeding intolerance 3 |
| Dong L., et al. [18] | 1 | 0 | 3120 | 9 | 10 | 1 | 0 (0.0) | 0 (0.0) | Abnormal cytokine test results 2 h after birth |
Fig. 7.
Pre-term born.
The mean (SD) weight at birth was 2,924.7 (490.8) g in 9 (69.2 %) [[3], [4], [5], [6], [7], [8], [9]] [16,18], studies. Apgar score at one and ten minutes after birth ranged from 7 [5,6] to 10 [6] and from 8 [5,6] to 10 [3,4,6,9,16,18], respectively.
Complications of the neonates were: fever was described in 3 (23.1 %) [4,6,17] studies, pneumonia in 3 (23.1 %) [4,7,17] studies, respiratory distress syndrome in 2 (7.7 %) [6,17] studies, infection in 2 (7.7 %) [6,16] studies, low birth weight in 1 (7.7 %) [3] study, lymphopenia in 1 (7.7 %) [4] study, abdominal distension in 1 (7.7 %) [4] study, skin rash in 1 (7.7 %) [5] study, oedema in 1 (7.7 %) [5] study, transient tachypnoea in 1 (7.7 %) [5] study, shortness of breath in 1 (7.7 %) [17] study, rapid heart rate in 1 (7.7 %) [6] study, gastro-intestinal symptoms in 1 (7.7 %) [6] study, feeding intolerance in 1 (7.7 %) [15] study, pneumothorax in 1 (7.7 %) [6] study, meconium stained amniotic fluid in 1 (7.7 %) [8] study, slight decreased responsiveness and muscle tension in 1 (7.7 %) [8] study, cyanosis in 1 (7.7 %) [15] study, and abnormal cytokine test results in 1 (7.7 %) [4] study.
The pooled proportion of neonates born with complications was 39.0 % (95 % CI: 18.0 %–63.0 %; I2: 71.8 %) (Fig. 8 ).
Fig. 8.
Complications in newborn babies.
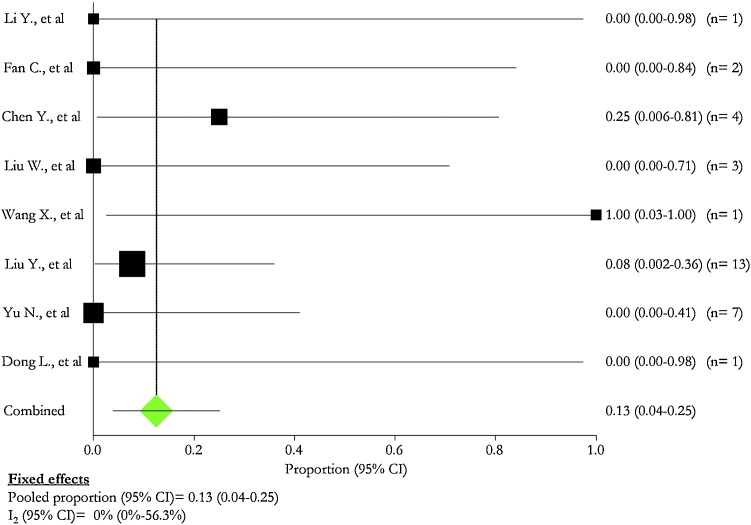
SARS-CoV-2 infection was found in four neonates and was described in two (15.4 %) studies [16,17], whereas 2 died in two (15.4 %) studies [6,15]. One patient developed refractory shock, multiple organ failure, and disseminated intravascular coagulation eight days after birth; no information was provided for the other one. The pooled percentage of infected neonates was 6.0 % (95 % CI: 2.0 %–12.0 %; I2: 0.0 %) (Fig. 9 ).
Fig. 9.
SARS-CoV 2 positivity in newborn babies.
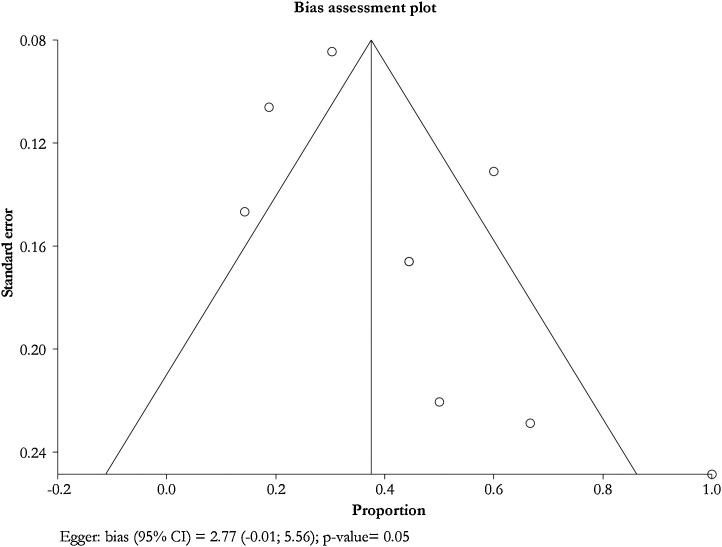
Publication bias and quality assessment are described in the Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17 and Table 6, Table 7 . The methodological quality was poor.
Fig. 10.
Cough in pregnant patients.
Fig. 11.
Fever at admission in pregnant patients.
Fig. 12.
Complications in pregnant patients.
Fig. 13.
Caesarean section.
Fig. 14.
ICU admission.
Fig. 15.
Pre-term born.
Fig. 16.
Complications in newborn babies.
Fig. 17.
SARS-CoV 2 positivity in newborn babies.
Table 6.
Tool for evaluating the methodological quality of case-reports and case-series.
| Domains | Leading explanatory questions | Li Y., et al. (2) | Chen H., et al. (3) | Fan C., et al. (4) | Chen Y., et al. (5) | Zhu H., et al. (6) | Liu W., et al. (8) | Wang X., et al. (9) | Liu Y., et al. (15) | Yu N., et al. (16) | Dong L., et al. (18) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | 1. Does the patient(s) represent(s) the whole experience of the investigator (centre) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? | No | No | No | No | – | Yes | No | Yes | Yes | No |
| Ascertainment | 2. Was the exposure adequately ascertained? | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | No |
| 3. Was the outcome adequately ascertained? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| Causality | 4. Were other alternative causes that may explain the observation ruled out? | – | – | – | – | – | – | – | – | – | |
| 5. Was there a challenge/rechallenge phenomenon? | – | – | – | – | – | – | – | – | – | ||
| 6. Was there a dose–response effect? | – | – | – | – | – | – | – | – | – | ||
| 7. Was follow-up long enough for outcomes to occur? | Yes | – | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | |
| Reporting | 8. Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
Questions 4, 5 and 6 are mostly relevant to cases of adverse drug events.
Table 7.
Checklist for observational cohort studies (1), according to the Scottish Intercollegiate Guidelines Network.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Score | Grade of evidence (2) |
|---|---|---|---|---|---|---|---|
| Zhang L., et al. [7] | Yes | Yes | No | No | No | 2 | – |
| Liu D., et al. [10] | Yes | Yes | No | No | No | 2 | – |
| Zeng L., et al. [17] | Yes | Yes | No | No | No | 2 | – |
1 One score for each checkpoint:
Q1 Are both groups selected from the same and well-defined cohort?
Q2 Is the proportion of dropout in each group known, and if so, is it <15 % in each?
Q3 Any comparison between full participants and those lost to follow-up?
Q4 Main potential confounders identified and considered?
Q5 Any confidence interval?
2.Grading was refined with a ‘+’ sign to suggest a low risk of bias for a score of 4 or 5, a ‘–’sign to suggest a high risk of bias for a score of 1 or 2, and no sign to suggest a moderate risk of bias for a score of 3.
Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developer’s handbook. Edinburgh, UK: SIGN, 2014.
Discussion
The present systematic review and meta-analysis showed a high risk of severe maternal and neonatal complications in case of SARS-CoV-2 infection. COVID-19 is an infectious disease whose incidence is highest in elderly individuals and in patients with cardiovascular, respiratory, renal, and metabolic comorbidities [[19], [20], [21], [22], [23]]. Immunocompromised individuals could be at risk of pulmonary and/or extra-pulmonary conditions during respiratory infections [24,25]. Pregnant women can show a qualitative and quantitative impairment of their immune system, which can increase the probability of respiratory infections and, then, severe infectious diseases [26,27]. The first cases of severe COVID-19 were described at the end of December 2019 and its incidence has been seemed heterogeneous [28].
Cases of SARS-CoV-2 infection and disease of pregnant women have been described in reports and series [[2], [3], [4], [5],8,9,18]. Concerns have been raised on the risk of severe disease, vertical transmission, and foetal and neonatal complications.
The present systematic review and meta-analysis is aimed at describing the current scientific evidence on COVID-19 in pregnant females and on the neonatal risk of infection and disease. To the best of our knowledge, this is the first study focused on the assessment of the clinical characteristics of COVID-19 in pregnancy.
All the scientific studies were performed in China [[2], [3], [4], [5], [6], [7], [8], [9], [10],[15], [16], [17], [18]], mainly in the most affected province of Hubei [[3], [4], [5], [6], [7], [8],10,[15], [16], [17], [18]]. The number of the described cases was not high both for pregnant women and neonates. However, a first focus on the clinical impact of COVID-19 during pregnancy can help clinicians and policymakers to better manage future cases of disease. Fever and cough seem the most prevalent symptoms and clinical signs of the disease in the population group of pregnant women. Other respiratory symptoms were reported, with few or absent extra-pulmonary manifestations. Cases were probably selected and do not adequately describe the real impact of the disease in the population group: pneumonia was diagnosed in about all selected studies.
Although women were heterogeneously treated (from antibiotics, to antivirals, to corticosteroids, in different combinations), 45.0 % of infected women shows pregnancy-related complications, with non-reassuring fetal testing, premature rupture of membranes, and placenta praevia being the most frequently reported. Caesarean section was performed in more than 50 % of the cases, but a clear explanation of the rationale or reasons behind that surgical intervention were not provided. A relatively high pooled proportion of pregnant women (13.0 %) were admitted to the ICU. No cases of deaths were notified. The absence of deaths can be explained by the young age of the infected patients; it has been proved that the mortality rate in COVID-19 patients is high in elderly individuals and in those patients with at least one comorbidity. We hypothesize that pregnancy-related immunological changes do not significantly affect the response against SARS-CoV-2.
The proportion of infected neonates was low (6%), highlighting a low probability of vertical transmission. However, two neonates died. Furthermore, a pooled neonatal complication rate of 39.0 % (95 % CI: 18.0 %–63.0 %) was found. The types of clinical conditions diagnosed in newborns were variable: fever, pneumonia, and respiratory distress syndrome were the most incident, underscoring a respiratory tropism of the virus in neonates and their mothers.
The percentage of preterms varied from 0% to 100 % in the selected studies (pooled percentage was 23.0 %), whereas the weight at birth was less than 3 Kg in the majority of the selected studies. The health of the neonate was good during the labour and outside the mother’s womb, with the 1- and 10-minute Apgar score ranging from seven to ten.
Timing of the infection was not clarified by the studies. This point needs to be evaluated in future incidence studies in order to understand if there is a more frequent vulnerability period. It is unclear whether the infection is acquired in uterine, during vaginal delivery of after birth. A recent article on ten not pregnant women with severe COVID-19 pneumonia stated that SARS-CoV-2 virus is not found in vaginal fluids. Thus, in theory, vaginal delivery could be allowed [29]. However, the pooled proportion of caesarean section was 88.0 %. A systematic review and meta-analysis of Di Mascio et al. [30] reported that COVID-19 is associated with a high rate of miscarriage, preterm birth, pre-eclampsia, caesarean, and perinatal death. A systematic review on 51 women described a high rate of preterm caesarean delivery [31]. Furthermore, a study on 116 Chinese pregnant women showed that no increased risk of spontaneous abortion and preterm birth was found [32].
Several study limitations can be raised: the findings are based on studies whose methodological quality is poor (e.g., case-reports and case-series). All the studies were carried out in China and the inference can be affected by genetic and environmental factors which could have influenced the natural history of the disease. The sample size of the pooled cohort and of the single study is associated with a poor statistical power: potentially significant differences could not have been assessed. The retrieved scientific reports do not keep into consideration important clinical variables (e.g., maternal comorbidities) which could play a relevant role in the prognosis of the disease.
Samples seem recruited by convenience. The women described show clinically diagnosed pneumonia in all articles, with the only exception of two studies. Then, this Authors’-driven selection could partially describe the role of SARS-CoV-2 during pregnancy. The majority of the studies are cross-sectional and retrospective; consequently, they do not appropriately describe the follow-up of infected women and neonates.
However, the present scientific evidence can provide useful clinical elements which should be carefully monitored during the assessment of pregnant women. The first experiences carried out in Asia could help better tailor clinical (e.g., diagnostic protocols, prophylactic and therapeutic regimens) and public health (e.g., home and hospital confinement) interventions.
Observational prospective, cohort, multicentre studies should be promptly implemented to collect standardized epidemiological and clinical data to better address the challenge of COVID-19 in pregnant women [19].
In conclusion, COVID-19 can cause significant maternal and neonatal morbidity. Public health interventions should be carefully implemented and tailored on those important susceptible groups to reduce the incidence of infection and, then, the risk of major complications. A regular and intensive follow-up is required to detect early the occurrence of clinical conditions.
The estimated second epidemic wave should be faced keeping into account the available scientific information to reduce the burden of disease in vulnerable population groups, including pregnant women and neonates.
Declaration of Competing Interest
The Authors state that they do not have any conflict of interest
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(March (1)):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(June (6)) doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(March (10226)):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., et al. Perinatal Transmission of COVID-19 Associated SARS-CoV-2: Should We Worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., et al. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8(March 16):104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(February (1)):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J., et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55(March (0)):E009. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 8.Liu W., Wang Q., Zhang Q., Chen L., Chen J., Zhang B., et al. 2020. Coronavirus Disease 2019 (COVID-19) During Pregnancy: A Case Series. Preprints, 2020020373. [Google Scholar]
- 9.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020;(February) doi: 10.1093/cid/ciaa200. pii: ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;(March):1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 11.Chen S., Liao E., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020;(March) doi: 10.1002/jmv.25789. [Epub ahead of print] PubMed PMID: 32222119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D., Yang H., Cao Y., Cheng W., Duan T., Fan C., et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;(March) doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scottish Intercollegiate Guidelines Network . SIGN; Edinburgh, UK: 2014. SIGN 50: a guideline developer’s handbook. [Google Scholar]
- 14.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020;(March 4) doi: 10.1016/j.jinf.2020.02.028. pii: S0163-4453(20)30109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;(March 24) doi: 10.1016/S1473-3099(20)30176-6. pii: S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;(March) doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;(March) doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 - studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams M.L., Katz D.L., Grandpre J. Population-based estimates of chronic conditions affecting risk for complications from coronavirus disease, United States. Emerg Infect Dis. 2020;26(8) doi: 10.3201/eid2608.200679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 24.Jose R.J., Dickey B.F., Brown J.S. Infectious respiratory disease in non-HIV immunocompromised patients. Br J Hosp Med (Lond) 2014;75(12):685–690. doi: 10.12968/hmed.2014.75.12.685. [DOI] [PubMed] [Google Scholar]
- 25.Corti M., Palmero D., Eiguchi K. Respiratory infections in immunocompromised patients. Curr Opin Pulm Med. 2009;15(3):209–217. doi: 10.1097/MCP.0b013e328329bd2c. [DOI] [PubMed] [Google Scholar]
- 26.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathad J.S., Gupta A. Pulmonary infections in pregnancy. Semin Respir Crit Care Med. 2017;38(2):174–184. doi: 10.1055/s-0037-1602375. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization WHO timeline - COVID-19. https://www.who.int/news-room/detail/08-04-2020-who-timeline---covid-19
- 29.Qiu L., Liu X., Xiao M., Xie J., Cao W., Liu Z., et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;(April 2) doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., et al. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. COVID19 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.013. pii: S0002-9378(20)30438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., et al. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]