Abstract
In coronavirus disease-19 (COVID-19), four major factors have been correlated with worse prognosis: aging, hypertension, obesity, and exposure to androgen hormones. Angiotensin-converting enzyme-2 (ACE2) receptor, regulation of the renin-angiotensin-aldosterone system (RAAS), and transmembrane serine protease 2 (TMPRSS2) action are critical for the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) cell entry and infectivity. ACE2 expression and RAAS are abnormal in hypertension and obesity, while TMPRSS2 is overexpressed when exposed to androgens, which may justify why these factors are overrepresented in COVID-19.
Among therapeutic targets for SARS-CoV-2, we hypothesized that spironolactone, a long used and safe mineralocorticoid and androgen receptors antagonist, with effective anti-hypertensive, cardioprotective, nephroprotective, and anti-androgenic properties may offer pleiotropic actions in different sites to protect from COVID-19. Current data shows that spironolactone may concurrently mitigate abnormal ACE2 expression, correct the balances membrane-attached and free circulating ACE2 and between angiotensin II and Angiotensin-(1-7) (Ang-(1-7)), suppress androgen-mediated TMPRSS2 activity, and inhibit obesity-related RAAS dysfunctions, with consequent decrease of viral priming. Hence, spironolactone may provide protection from SARS-CoV-2, and has sufficient plausibility to be clinically tested, particularly in the early stages of COVID-19.
Keywords: COVID-19, Spironolactone, SARS-CoV-2, ACE2, TMPRS22, Pandemic
Introduction
Specific characteristics of SARS-CoV-2 may explain the relative unsuccessfulness of the virus contention policies, including long virus shedding (20 days on average) [1] and period of incubation (up to 11 days) [2], the existence of multiple asymptomatic infections [1], [2], [3], and the prolonged surviving in surfaces and aerosol.
Although preliminary, consistent data elude to the fact that three major factors are atypically correlated with worse prognosis in SARS-CoV-2: hypertension, obesity, and androgen hormones.
Indeed, hypertension alone seems to be overrepresented in patients with acute respiratory distress syndrome (ARDS) due to Covid-19 (23.2%) when compared to type 2 diabetes mellitus (T2DM) (16.2%) and established coronary heart disease (5.8%), in addition to the approximate increase of 10% risk per year of age [4], [5]. In China, where the prevalence of metabolic syndromes including obesity and T2DM are lower compared to Europe and USA, hypertension emerged as the strongest risk predictor for SARS-CoV-2 [6].
Obesity has been further identified as an important risk factor in SARS-CoV-2, which have been noticed after the pandemic spread to countries with higher prevalence of obesity. However, hypertension remained as an independent risk factor for respiratory severe manifestations in COVID-19 in obese patients, and was still prevailing in COVID-19 intensive care units (ICUs) in regions where obesity is highly prevalent.
Strong epidemiological data also supports the hypothesis that androgens are directly related to SARS-CoV-2 infectivity and pathogenesis. The larger incidence of severe COVID-19 in men compared to women is not fully justified by differences in the prevalence of metabolic disorders (obesity, T2DM, hypertension). Accordingly, pre-pubertal children show an imperative protection from SARS-CoV-2 infection, once this population is not exposed to androgens. Further, an indirect marker of dihydrotestosterone (DHT) activity and androgen receptor (AR) regulation and sensitivity, baldness has been clinically observed to be particularly present in men that develop ARDS in COVID-19.
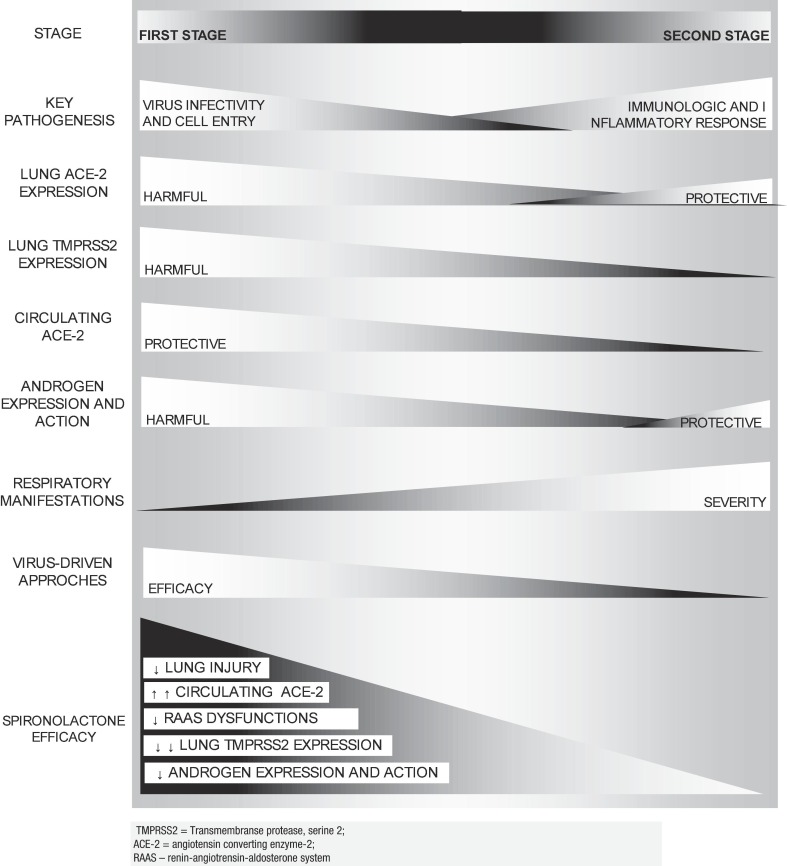
The presence of angiotensin-converting enzyme-2 (ACE2) receptor and transmembrane serine protease 2 (TMPRSS2) is mandatory for SARS-CoV-2 cell entry, while ACE2 and TMPRSS2 expression may modulate its infectivity. ACE2 and TMPRSS2 expressions abnormalities in hypertension and obesity, and exposure to androgens, respectively, may help justify why these are risk factors for COVID-19, as proposed by recent theories [7], [8], [9], [10]. ACE2 and TMPRSS2 altered expressions can be concomitantly addressed by spironolactone and other mineralocorticoid antagonists, due to their actions in the renin-angiotensin-aldosterone system (RAAS) and as androgen antagonists, respectively, providing potential protection against SARS-CoV-2. Key characteristics of COVID-19 according to the timing of the infection is presented in Fig. 1 .
Fig. 1.
COVID-19: Stages, key characteristics, ACE2 expression, and approaches.
Hypothesis
Spironolactone: May the multi-purpose drug provide protection from SARS-CoV-2?
Spironolactone, a safe anti-hypertensive and anti-androgenic drug used since 1959 that acts as potassium-sparing diuretic drug by antagonizing mineralocorticoid receptors (MRs), tend to disclose favorable patterns of the RAAS and ACE2 expression, reduces TMPRSS2 activity due to its antiandrogenic activity, and may prevent acute lung injuries due to its pleiotropic effects. We hypothesize that spironolactone my offer protection for some of the populations at highest risk for severe COVID-19, including obesity, bald males (that present enhanced TMPRSS2 expression), and hypertension.
Evaluation of the hypothesis
ACE2 expression in COVID-19: why hypertension and obesity are risk factors?
Whether the atypical increased risk related to hypertension in COVID-19 is derived from hypertension per se, due to secondary lung alterations resulted from the effects of increased blood pressure on tissues, or if the unexpected risk related to hypertension is actually secondary to the effects of chronic exposure to speicific classes of anti-hypertensives, is uncertain.
The large percentage of patients with hypertension undertaking angiotensin-converting enzyme inhibitors (ACEi’s) or angiotensin receptor blockers (ARBs) [11], [12], [13] has been hypothesized to be the main underlying mechanism that could justify the peculiar severity of COVID-19 in hypertension, due to their inherent actions in the RAAS, that could indirectly increase the availability of surface-attached ACE-2 in the lung endothelium, potentially leading to enhanced coupling of SARS-CoV2 to ACE-2 and its consequent cell entry in the early stage of COVID-19. However, despite the initial reports that have correlated ACEi and ARB with increased risk of severe manifestations in COVID-19, further findings including systematic reviews and meta-analyses failed to demonstrate these correlations [14], [15], [16].
While ARB and ACEi disclosed inconsistent findings on COVID-19 related outcomes, the fact that hypertension, regardless of its control, has been shown to be an important and independent risk factor for COVID-19 severity, remains to be explained. Unlike diabetes, which the level of control based on the HbA1c predicts COVID-19 outcomes, hypertension did not show the same sort of correlation, and this should be considered for further analyses [5].
Among the multiple mechanisms proposed to justify the severity of COVID-19 related to obesity, the disruption of the RAAS system, ACE2 expression and activity, and the balance between the hypertensive and pro-inflammatory angiotensin II and angiotensin receptor-1 (AT1) axis, and the anti-inflammatory Angiotensin-[1-7] and its Mas receptor, a G-coupled receptor, may explain the particular increased SARS-CoV-2 infectivity in this population.
Hypertension and obesity are two major representatives of the key importance of the dysfunctions-related RAAS abnormalities for the development of the SARS-CoV-2 infectiveness.
In regards with ACE2 expression, membrane-attached ACE2 should be differentiated from freely circulating ACE2 due to their likely distinct roles in RAAS and COVID-19. While membrane ACE2 is expressed broadly [17], [18], [19], [20], particularly in the lungs, kidney, heart, testicles, and gut, circulating ACE2 levels are low and its functional role in the lungs seems not to be clinically relevant, when under physiological conditions [21]. Despite the discussion on the clinical relevance under physiological conditions, the fact that epithelial type II cells of the lungs represent up to 80% of the total ACE2 expression in the body [22] is of clinical importance in pathologies that require the participation of ACE2 to occur. Indeed, absence of ACE2 expression led to full resistance to SARS-CoV infection [23], [24]. Particularly, the receptor binding domain (RBD) of the SARS-CoV2 has a stronger interaction with angiotensin converting enzyme 2 (ACE2), compared to other virus infections from the same family [25], [26], [27], [28], [29], and any increase of ACE2 expression may potentially amplify the virus capacity to entry the cells.
While expression and availability of attached ACE2 is directly correlated with Covid-19 severity during the first stage of viral replication, the free circulating form of ACE2 may couple to SARS-CoV2 and hamper its entry in the pulmonary endothelium. It has been hypothesized that recombinant human soluble ACE2 could play a protective role against the development of severe manifestations, ARDS, and death in Covid-19, which has been clinically demonstrated by the beneficial effects of recombinant ACE2 in the prevention of coronaviruses-induced lung injury [30], [31], [32], [33], [34], despite its unaffordability for regular medical use. Under physiological conditions, circulating plasma ACE2 may not be present in levels sufficient to protect membrane-attached ACE2 from coupling to SARS-COV2 [30]. However, under particular conditions including soluble ACE2 and drugs that increase its levels in the plasma, its protective actions may become relevant. Hence, a speculative ratio between attached ACE2 availability and expression, and freely circulating ACE2 could predict the lung pathogenicity of Covid-19, although methods to assess this ratio have not been developed and validated to date. Hence, as mentioned, the differences between plasma and membrane ACE2 should not be ignored.
Together with the aberrancies in ACE2 expression, another hypothesis has postulated that an unbalance between angiotensin II and angiotensin 1–7 levels and effects would also be responsible for the severity of clinical manifestations [35], [36], [37].
Due to the high level of uncertainty regarding the expression and activity of ACE2 in different organs during COVID-19, hypotheses regarding the correlations between ACE2 and COVID-19 severity should be generated from epidemiological data, which is consistent with the overrepresentation of obesity and hypertension. Fig. 2 depicts a hypothetical model of the first stage of (SARS-CoV-2 infectivity and replication) under different scenarios of physiological, untreated hypertension, use of different drug classes, and hyperandrogenism.
Fig. 2.
Simplified hypothetical model of the proposed scenarios in the first stage of COVID-19, during SARS-CoV-2 cell infection and replication period.
Androgen-driven theory to explain overrepresentation of males in severe COVID-19
Despite the lack of clinical data of the androgen effects on SARS-CoV-2, its strong mechanistic plausibility and corresponding epidemiological data provide theoretical basis for an androgen mediated SARS-CoV-2 infectiveness [8].
The androgen-regulated transmembrane protease serine 2 (TMPRSS2) is the important enzyme that cleaves the spike protein of the virus and the ACE2 receptor for viral cell entry [8]. Male mice are known to be extremely more vulnerable to SARS-CoV infection [38].
Recent American data showed a drastic difference of over 6 times more male fatalities in a very productive age range (40–49 years) and approximately two times more male admissions from 30 to 49 years [39].
SARS-CoV-2 entry into type II pneumocytes in the lung is driven by androgens [8], [9], [10]. This theory explains the findings that females and pre-puberty subjects are relatively protected from COVID-19 ARDS. Interestingly, infants under one year old may have increased risk of COVID-19 complications, which is in accordance with the occurrence of the mini-puberty i.e., a transient expression of androgens at this age. In their report, the authors provide a detailed hypothesis of the molecular mechanisms of COVID-19 disease mediated by androgens. Among the drugs mentioned as potential candidates to test, spironolactone, a steroidal androgen receptor competitive blocker, was singled out as a possible safe and potentially effective alternative. Fig. 2 shows a hypothetical scenario of the first stage of COVID-19 in hyperandrogenic states.
Of great interest regarding the pre-clinical data regarding how androgen blockade impacts survival, the non-steroidal androgen receptor blocker flutamide was the only drug to date that demonstrated in experimental model male mice survival from lethal dose of SARS-CoV [40]. Other drugs, such as remdesivir, failed to demonstrate male mice survival and based their conclusions on female mice analysis [41].
Consequences of the hypothesis and discussion
Spironolactone: a multi-purpose drug that may provide protection from SARS-CoV-2
Spironolactone is currently the main representative of the potassium-sparing diuretic class of drugs, may be as effective as ACEi and ARB to maintain normal blood pressure [42], [43], addresses heart function, and provides cardio- and renoprotection [44], [45], [46], [47], [48], [49]. Unlike ACEi and ARB, that specifically increase lung membrane-attached ACE2 expression, spironolactone tend to disclose favorable patterns of ACE2 expression, including a more extensive increase of circulating ACE2 when compared to membrane-attached ACE2, enhancing its potential protective role in SARS-CoV-2, once plasma ACE2 may couple to SARS-CoV-2 and avoid its entry in the cells [50], [51], [52], [53], [54], [55], and may downregulate the androgen-mediated TMPRSS2 due to its antiandrogenic activity [56], [57], [58], without the adverse events of male sexual castration. In addition, spironolactone has been demonstrated to mitigate the detrimental effects of obesity on the RAAS [58], [59], [60], [61], [62], [63], [64], [65], including hyperactivation of the RAAS due to increased angiotensinogen production by adipose tissue and imbalance towards angiotensin-2-AT-1 axis, possibly reducing obesity-related COVID-19 complications, and has direct specific anti-inflammatory effects in the lungs, possibly reducing acute lung injuries [66], [67], [68], [69], [70], [71], [72], [73].
Notably, since spironolactone mostly targets the virus entry in the cells, which is the hallmark of the first phase of Covid-19, spironolactone should be preferably administrated in the earlier stages of the infection, prior to the complications of respiratory manifestations, when SARS-CoV-2 activity becomes secondary and the exacerbated inflammatory response is the responsible for the Covid-19 induced ARDS.
Discussion
The current COVID-19 pandemic urges for rapid responses to change its course and mortality rate. The learnings of the mechanisms of SARS-CoV-2 cell entry and infectivity, which meet corresponding epidemiological data and identification of risk factors, allowed more precise predictions regarding efficacy of drugs proposed to protect from COVID-19.
Among current options, researchers should preferably focus on existing drugs that have been long used, i.e., to repurpose old drugs for COVID-19, due to five major reasons that are inherent to these molecules: 1. The short- and long-term safety profile of these drugs are known, whereas can only be obtained after longer studies with new molecules; 2. Risks are known, which allow directed monitoring and allows, in opposition to newly released drugs, for which multi-organ and multi-risk monitorization is recommended due to the little known effects, particularly because very few have been tested in large populations; 3. Contraindications are known, avoiding the use on those that may present harms, in contrast to the lack of awareness of the contraindications of new drugs, among which several contraindications can only be detected in large-scale studies; 4. Clinicians are familiarized with the clinical management of old drugs, including posology, monitoring for risks, contraindications, and potential complications, which is desirable since the number of infected subjects does not allow COVID-19 to be managed within specialized centers; on the contrary, general practitioners, family medicine physicians, and all medical doctors should be skilled to manage these patients, which is unfeasible in case novel molecules unfamiliar to medical community is largely used; 5. The cost of new, patented drugs is unaffordable by public health systems, and unlikely has favorable cost-effectiveness, since the level of evidence of benefits is too weak compared to costs due to the insufficient time of studies to provide supporting data for their use massively.
The above-mentioned advantages of old drugs are imperative for the massive clinical use of these drugs against COVID-19. In this regard, oppositely to other alternatives, that may cause undesired harms that led to questionings regarding their use, spironolactone offers solid safety, extensive effectiveness, and has strong plausibility that meets according in vitro, in vivo, and clinical evidence that spironolactone provides active protection for those organs centrally affected in COVID-19: lungs, heart, and kidneys.
In addition, as demonstrated in the present article, unlike the majority of the current proposed drugs, that only offer potential protection in the first stage of COVID-19, during SARS-CoV-2 viral replication and spread, spironolactone has demonstrated actions that may provide protection during all three stages of COVID-19, since it may reduce SARS-CoV-2 infectivity, inhibit cytokine and inflammatory storm as an overresponse to the virus, and alleviate lung, heart, and kidney injuries, for the first, second, and third stages of COVID-19, respectively.
To us, there is sufficient data to support the employment of spironolactone for large-scale studies, and an empirical alternative for compassionate use due to the lack of relevant risks [74], [75].
Perspectives and conclusion
Abnormal ACE2 expression, angiotensin II and angiotensin 1–7 imbalance, and androgen activity seem to be key regulators of SARS-CoV-2 infectivity, in accordance with epidemiological observations. Since spironolactone exhibits concurrent actions in the modulation of ACE2 expression that would direct- and indirectly avoid SARS-CoV-2 cell entry, in the increase of angiotensin 1–7 levels, that can attenuate the harms of the overexpression of angiotensin II-AT-1 axis, and as an anti-androgenic agent, that would decrease viral priming through TMPRSS2 activity, spironolactone seems to be an ideal candidate drug for the prophylactic and early treatment of SARS-CoV-2.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We acknowledge all researchers who have provided insightful and helpful information aiming to overcome the COVID-19 pandemic, as well as all front-line health providers who are directly dealing with COVID-19 infected patients, exposing themselves at risk for this potentially severe infection.
Data availability statement and data deposition
There is no additional data associated with the present manuscript.
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published online ahead of print, 2020 Mar 11] [published correction appears in Lancet. 2020 Mar 12;:]. Lancet. 2020;S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed]
- 2.Lauer SA, Grantz KH, Bi Q, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application [published online ahead of print, 2020 Mar 10]. Ann Intern Med. 2020;10.7326/M20-0504. [DOI] [PMC free article] [PubMed]
- 3.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China [published online ahead of print, 2020 Mar 13]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 4.Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease [published online ahead of print, 2020 Feb 28]. Chin Med J (Engl). 2020;10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed]
- 5.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020 March 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 6.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:28. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? Am J Physiol Endocrinol Metab. 2020;318(5):E587–E588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wambier CG, Goren A. SARS-COV-2 infection is likely to be androgen mediated [published online ahead of print, 2020 Apr 10]. J Am Acad Dermatol. 2020;S0190-9622(20)30608-3.
- 9.Goren A, McCoy J, Wambier CG, et al. What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy [published online ahead of print, 2020 Apr 1]. Dermatol Ther. 2020;e13365. [DOI] [PMC free article] [PubMed]
- 10.Goren A, Vano-Galvan S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain – a potential clue to the role of androgens in COVID-19 severity [published online ahead of print, 2020 Apr 16]. J Cosmet Dermatol. 2020;10.1111/jocd.13443. [DOI] [PubMed]
- 11.Sommerstein R, Gräni C. Preventing a COVID-19 pandemic: ACE inhibitors as a potential risk factor for fatal COVID-19. BMJ 2020;368:m810-m810(https://www.bmj.com/content/368/bmj.m810/rr-2. opens in new tab). [DOI] [PubMed]
- 12.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens 2020 March 11 (Epub ahead of print). [DOI] [PubMed]
- 13.Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020 March 18 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 14.Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 with Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients with Hypertension:A Meta-Analysis [published online ahead of print, 2020 May 27]. Hypertension. 2020;10.1161/HYPERTENSIONAHA.120.15572. [DOI] [PubMed]
- 15.Zhang X., Yu J., Pan L.Y., Jiang H.Y. ACEI/ARB use and risk of infection or severity or mortality of COVID- 19: A systematic review and meta-analysis [published online ahead of print, 2020 May 15] Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey K, King VJ, Gurley S, et al. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults [published online ahead of print, 2020 May 15]. Ann Intern Med. 2020;10.7326/M20-1515. [DOI] [PMC free article] [PubMed]
- 17.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas G.C., O’Bryan M.K., Hedger M.P., Lee D.K.L., Yarski M.A., Smith A.I. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult leydig cells of the testis. Endocrinology. 2004 Oct;145(10):4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 19.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mary Donoghue, Frank Hsieh, Elizabeth Baronas, Kevin Godbout, Michael Gosselin, Nancy Stagliano. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 21.Serfozo P., Wysocki J., Gulua G. Ang II (angiotensin II) conversion to angiotensin-(1–7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75:173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 Jan 26;2020.01.26.919985.
- 23.Imai Y., Kuba K., Ohto-Nakanishi T., Penninger J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J. 2010;74(3):405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 24.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV [published online ahead of print, 2020 Feb 17]. Biochem Biophys Res Commun. 2020;S0006-291X(20)30339-9. [DOI] [PMC free article] [PubMed]
- 26.Hoffmann M., Kleine-Wever H., Kruger N., Muller M., Drotsten C., Pholhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry in target cells. Cell. 2020;181:1–10. [Google Scholar]
- 27.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell MC, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Med 2005; 11:875–879. [DOI] [PMC free article] [PubMed]
- 28.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8–15. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega JT, Serrano ML, Pujol FH, Rangel HR. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J. 2020;19:410–417. Published 2020 Mar 18. [DOI] [PMC free article] [PubMed]
- 30.Zou Z., Yan Y., Shu Y. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5 doi: 10.1038/ncomms4594. 3594 3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu H., Xie Z., Li T. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6 doi: 10.1038/srep19840. 19840-19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan A., Benthin C., Zeno B. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21 doi: 10.1186/s13054-017-1823-x. 234-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batlle D., Wysocki Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki J., Ye M., Rodriguez E., Gonzalez-Pacheco F.R., Barrios C., Evora K. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin ii-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020March 4 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 37.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1). doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed]
- 40.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 41.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online ahead of print, 2020 Feb 27]. J Med Virol. 2020;10.1002/jmv.25728. [DOI] [PMC free article] [PubMed]
- 42.Georgianos P.I., Vaios V., Eleftheriadis T., Zebekakis P., Liakopoulos V. Mineralocorticoid antagonists in ESRD: an overview of clinical trial evidence. Curr Vasc Pharmacol. 2017;15(6):599–606. doi: 10.2174/1570161115666170201113817. [DOI] [PubMed] [Google Scholar]
- 43.Hermidorff M.M., Faria Gde O., Amâncio Gde C., de Assis L.V., Isoldi M.C. Non-genomic effects of spironolactone and eplerenone in cardiomyocytes of neonatal Wistar rats: do they evoke cardioprotective pathways? Biochem Cell Biol. 2015;93(1):83–93. doi: 10.1139/bcb-2014-0110. [DOI] [PubMed] [Google Scholar]
- 44.Nakano S., Kobayashi N., Yoshida K., Ohno T., Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005;28(11):925–936. doi: 10.1291/hypres.28.925. [DOI] [PubMed] [Google Scholar]
- 45.Dieterich H.A., Wendt C., Saborowski F. Cardioprotection by aldosterone receptor antagonism in heart failure. Part I. The role of aldosterone in heart failure. Fiziol Cheloveka. 2005;31(6):97–105. [PubMed] [Google Scholar]
- 46.Taira M., Toba H., Murakami M. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. Eur J Pharmacol. 2008;589(1–3):264–271. doi: 10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 47.Schjoedt K.J. The renin-angiotensin-aldosterone system and its blockade in diabetic nephropathy: main focus on the role of aldosterone. Dan Med Bull. 2011;58(4):B4265. [PubMed] [Google Scholar]
- 48.Kong E.L., Zhang J.M., An N., Tao Y., Yu W.F., Wu F.X. Spironolactone rescues renal dysfunction in obstructive jaundice rats by upregulating ACE2 expression. J Cell Commun Signal. 2019;13(1):17–26. doi: 10.1007/s12079-018-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda Y., Zhu A., Yoneda T., Usukura M., Takata H., Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2007;20(10):1119–1124. doi: 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Zhu A., Yoneda T., Demura M. Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J Hypertens. 2009;27(4):800–805. doi: 10.1097/HJH.0b013e328325d861. [DOI] [PubMed] [Google Scholar]
- 51.Keidar S., Gamliel-Lazarovich A., Kaplan M. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97(9):946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 52.Te Riet L., van Esch J.H., Roks A.J., van den Meiracker A.H., Danser A.H. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116(6):960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 53.Hamming I., Cooper M.E., Haagmans B.L. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 55.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sert M., Tetiker T., Kirim S. Comparison of the efficiency of anti-androgenic regimens consisting of spironolactone, Diane 35, and cyproterone acetate in hirsutism. Acta Med Okayama. 2003;57(2):73–76. doi: 10.18926/AMO/32820. [DOI] [PubMed] [Google Scholar]
- 57.Steelman S.L., Brooks J.R., Morgan E.R., Patanelli D.J. Anti-androgenic activity of spironolactone. Steroids. 1969;14(4):449–450. doi: 10.1016/s0039-128x(69)80007-3. [DOI] [PubMed] [Google Scholar]
- 58.Broulik P.D., Stárka L. Antiandrogenic and antirenotropic effect of spironolactone. Endokrinologie. 1976;68(1):35–39. [PubMed] [Google Scholar]
- 59.Kawarazaki W., Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29(4):415–423. doi: 10.1093/ajh/hpw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid-Moussa N. Regulation and Functions of the Renin-Angiotensin System in White and Brown Adipose Tissue. Compr Physiol. 2017;7(4):1137-1150. Published 2017 Sep 12. [DOI] [PMC free article] [PubMed]
- 61.Yvan-Charvet L., Quignard-Boulangé A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79(2):162–168. doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- 62.Kalupahana N.S., Moustaid-Moussa N. The adipose tissue renin-angiotensin system and metabolic disorders: a review of molecular mechanisms. Crit Rev Biochem Mol Biol. 2012;47(4):379–390. doi: 10.3109/10409238.2012.694843. [DOI] [PubMed] [Google Scholar]
- 63.Bender S.B., DeMarco V.G., Padilla J. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65(5):1082–1088. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vecchiola A, Fuentes CA, Solar I, et al. Eplerenone Implantation Improved Adipose Dysfunction Averting RAAS Activation and Cell Division. Front Endocrinol (Lausanne). 2020;11:223. Published 2020 Apr 21. [DOI] [PMC free article] [PubMed]
- 65.Feraco A., Armani A., Mammi C., Fabbri A., Rosano G.M., Caprio M. Role of mineralocorticoid receptor and renin-angiotensin-aldosterone system in adipocyte dysfunction and obesity. J Steroid Biochem Mol Biol. 2013;137:99–106. doi: 10.1016/j.jsbmb.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Ji W.J., Ma Y.Q., Zhang X. Inflammatory monocyte/macrophage modulation by liposome-entrapped spironolactone ameliorates acute lung injury in mice. Nanomedicine (Lond) 2016;11(11):1393–1406. doi: 10.2217/nnm-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji WJ, Ma YQ, Zhou X, et al. Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments. PLoS One. 2013;8(11):e81090. Published 2013 Nov 19. doi: 10.1371/journal.pone.0081090. [DOI] [PMC free article] [PubMed]
- 68.Rafatian N., Westcott K.V., White R.A., Leenen F.H. Cardiac macrophages and apoptosis after myocardial infarction: effects of central MR blockade. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R879–R887. doi: 10.1152/ajpregu.00075.2014. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L., Hao J.B., Ren L.S., Ding J.L., Hao L.R. The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis. Lab Invest. 2014;94(8):839–850. doi: 10.1038/labinvest.2014.69. [DOI] [PubMed] [Google Scholar]
- 70.Ozacmak H.S., Ozacmak V.H., Barut F., Araslı M., Ucan B.H. Pretreatment with mineralocorticoid receptor blocker reduces intestinal injury induced by ischemia and reperfusion: involvement of inhibition of inflammatory response, oxidative stress, nuclear factor κB, and inducible nitric oxide synthase. J Surg Res. 2014;191(2):350–361. doi: 10.1016/j.jss.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 71.Kato Y., Kamiya H., Koide N. Spironolactone inhibits production of proinflammatory mediators in response to lipopolysaccharide via inactivation of nuclear factor-κB. Immunopharmacol Immunotoxicol. 2014;36(3):237–241. doi: 10.3109/08923973.2014.921690. [DOI] [PubMed] [Google Scholar]
- 72.Lieber G.B., Fernandez X., Mingo G.G. Mineralocorticoid receptor antagonists attenuate pulmonary inflammation and bleomycin-evoked fibrosis in rodent models. Eur J Pharmacol. 2013;718(1–3):290–298. doi: 10.1016/j.ejphar.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Fraccarollo D., Galuppo P., Schraut S. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension. 2008;51(4):905–914. doi: 10.1161/HYPERTENSIONAHA.107.100941. [DOI] [PubMed] [Google Scholar]
- 74.Yartaş Dumanlı G., Dilken O., Ürkmez S. Use of Spironolactone in SARS-CoV-2 ARDS Patients. Turk J Anaesthesiol Reanim. 2020;30 doi: 10.5152/TJAR.2020.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alifano M, Alifano P, Forgez P, et al. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30. S030090842030078X. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data associated with the present manuscript.