Abstract
Recently, several promising treatments have emerged for neuromuscular disorders, highlighting the need for robust biomarkers for monitoring therapeutic efficacy and maintenance of the therapeutic effect. Several studies have proposed circulating and tissue biomarkers, but none of them has been validated to monitor acute and long-term drug response. We previously described how the myostatin (MSTN) level is naturally downregulated in several neuromuscular diseases, including Duchenne muscular dystrophy (DMD). Here, we show that the dystrophin-deficient Golden Retriever muscular dystrophy (GRMD) dog model also presents an intrinsic loss of Mstn production in muscle. The abnormally low levels of Mstn observed in the GRMD dog puppies at 2 months were partially rescued at both mRNA and protein level after adeno-associated virus (AAV)-microdystrophin treatment in a dose-dependent manner. These results show that circulating Mstn is a robust and reliable quantitative biomarker, capable of measuring a therapeutic response to pharmaco-gene therapy in real time in the neuromuscular system, as well as a quantitative means for non-invasive follow-up of a therapeutic effect. Moreover, a 2-year follow-up also suggests that Mstn could be a longitudinal monitoring tool to follow maintenance or decrease of the therapeutic effect.
Keywords: myostatin, biomarker, neuromuscular disorders, clinical trials, Duchenne muscular dystrophy (DMD), GDF8, therapy
Graphical Abstract
Duchenne muscular dystrophy is a severe muscle disease, and recently, several treatments have emerged highlighting the need for a blood-based biomarker. Here, Dumonceaux and colleagues demonstrated that myostatin, an inhibitor of muscle growth secreted by skeletal muscles, is a reliable and quantitative biomarker for short- and long-term monitoring of therapy effects.
Introduction
Neuromuscular disorders are a group of heterogeneous diseases leading to muscle wasting and weakness.1 Several promising treatment strategies using pharmaco-gene therapies have emerged for some of them, including Duchenne muscular dystrophy (DMD).2, 3, 4 DMD patients present mutations in the dystrophin gene, leading to an absence of the dystrophin protein. The consequences are a progressive muscle degeneration and weakness. Recently, 2 oligo-antisense drugs, eteplirsen and golodirsen, were approved by the US Food and Drug Administration (FDA), and several clinical trials are ongoing, including 3 systemic adeno-associated virus (AAV) trials administering microdystrophin for DMD (“ClinicalTrials.gov: NCT03368742, NCT03362502, and NCT03375164”).
Primary endpoints for clinical trials using pharmaco-gene therapy commonly use clinical scales, such as the North Star Ambulatory Assessment; timed tests, such as the 6-min walk test; or respiratory measures, such as Forced Vital Capacity.5, 6, 7, 8 These measures suffer from important methodological limitations, such as dependence on motivation and floor and ceiling effects for individual items, and importantly, also show age-related or nonlinear behavior over the disease time course, such as evidenced for loss of ambulation with drastic deterioration of 6 minute walk test (MWT) performances.9,10 These shortcomings are only partially compensated by quantifiable secondary or exploratory endpoints, such as muscle magnetic resonance imaging (MRI),11 which does not provide a quantitative summary readout for the whole neuromuscular system.
To overcome this limitation, molecular biomarkers have also been proposed (for review, see Wilson et al.12), but the quantification of some of these biomarkers, such as dystrophin, needs invasive investigations (muscle biopsy) and are limited to the small sampled area, and their quantitative relation to a clinically observable treatment response remains to be established.
Ideally, easily accessible circulating biomarkers, which in a quantitative and robust manner reflect the state of muscle well-being, would be preferable for noninvasive monitoring of treatment effects and should be applicable across the entire range of clinical severity of affected patients. To that effect, promising results have been obtained for serum biomarkers, such as myomesin-3 or serum or urine titin, or for a host of microRNAs (miRNAs), in particular, the muscle-specific miRNAs (myomiRs) in DMD (for review, see Aartsma-Rus and Spitali13). All of the above biomarkers suffer from quite variable ranges across individuals with different epigenetic background and disease-states, and directly or indirectly reflect specific parts of the pathogenetic process such as degradation of the elastic sarcomere scaffold in DMD (myomesin-3, titin) or inflammatory aspects of the disease (miRNAs). None of these molecules has been validated as biomarkers for monitoring drug response.
Here, we investigated if circulating myostatin (Mstn) could be a quantitative biomarker for treatment efficacy in DMD. Mstn is a secreted protein, produced and released by the skeletal muscle, which acts as an inhibitor of muscle growth.14 We have previously demonstrated that circulating Mstn levels are dramatically reduced in patients affected by a muscle wasting or atrophying disease, both in the human and in preclinical models of myotubular myopathy.15 In particular, we observed that whereas intramuscular (i.m.) levels of Mstn are dramatically low in the Mtm1 knockout (KO) mouse model for X-linked myotubular myopathy, AAV-Mtm1 gene therapy leads to an increase of Mstn and to the decrease of its natural inhibitor, follistatin. This suggested that the Mstn pathway expression could be used to monitor muscle health and disease, therefore opening the possibility for essential effectors of this pathway (Mstn, activin receptors, follistatin, etc.) to reflect quantitatively the effects of therapy in neuromuscular patients.
In this study, we have investigated this hypothesis in the Golden Retriever muscular dystrophy (GRMD) canine model for DMD and DMD-treated patients. Indeed, we have recently demonstrated that locoregional or systemic administration of an AAV vector coding a canine microdystrophin (cMD1) reduces the physiological decline in muscle strength/function of treated limbs in the treated animals in a dose-dependent manner.3 Here, we show that in dogs, Mstn levels were sufficient to distinguish control, untreated, low-dose-, and high-dose-treated GRMD dogs, confirming the results obtained in clinical evaluation and in the muscle dystrophin quantitation. These data show that circulating Mstn is a reliable and quantitative biomarker for short- and long-term monitoring of therapy effects in DMD and may be in other neuromuscular patients.
Results
Circulating Levels of Mstn in Control and GRMD Dogs
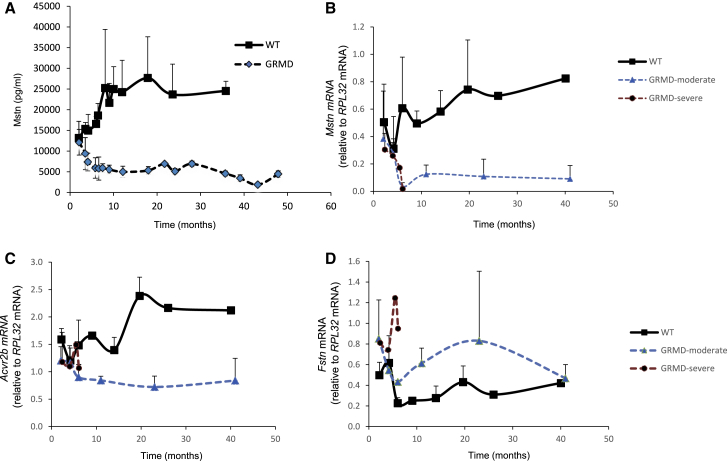
Mstn circulating serum concentrations were determined in control dogs or dogs affected by dystrophin deficiency (GRMD) at different ages, starting at 2 months (Figure 1A). In control animals, Mstn levels increased until the age of 10 months, when a plateau was reached. In the GRMD dogs, Mstn levels rapidly decreased, and already at 6 months, the minimum value was reached. Whereas the data in the wild-type (WT) animals showed some variability, the levels observed in GRMD dogs are highly homogeneous, and the two cohorts never overlap after the age of 2 months (Figure 1A). At the age of 2 months, Mstn levels are similar in the WT and GRMD animals (13,173 pg/mL ± 4,008 versus 12,138 pg/mL ± 3,103, respectively, p > 0.05), but at 4 months, the measure of circulating Mstn is sufficient to distinguish WT and GRMD animals (14,960 pg/mL ± 3,905 versus 7,350 pg/mL ± 2,140, respectively, p = 0.03), and at 6 months, the difference is even larger (16,582 pg/mL ± 1,794 versus 5,924 pg/mL ± 2,463, respectively, p = 0.003) (Figure 1A). Some of the GRMD dogs were clinically stratified as moderate (n = 10) or severe (n = 4) disease progressors, according to the ambulatory status at the age of 6 months (ambulant versus nonambulant, respectively).16 The levels of circulating Mstn were not significantly different between moderate and severe progressors at the ages of 2, 4, and 6 months (Figure S1).
Figure 1.
Myostatin Expression in GRMD and WT Dogs
(A) Mstn levels were measured in both WT and GRMD dogs at different ages. (B–D) mRNA expression of Mstn (B), Acvr2b (C), and Fstn (D) was measured in WT, GRMD-moderate, and GRMD-severe dogs at different ages. The number of samples for each point is detailed in Table S1. Error bars are SD (no SD when n < 3).
Mstn Pathway mRNA Levels in Control and GRMD Dogs
Mstn expression was next measured at the mRNA level in biceps femoris and sartorius muscle biopsies at different ages (Figures 1B and S2). In the WT animals, Mstn levels slightly increased over time, whereas in the GRMD animals, a significant decrease was observed in the first months of age to reach minimum levels, which are maintained until 40 months (Figure 1B). No difference was noted between moderate and severe GRMD disease progressors. These results confirmed the observations made in the sera. The mRNA levels of other components of the Mstn pathway were also measured. At the age of 6 months, a lower expression of the Mstn receptor Acvr2b was observed in the GRMD dogs compared to the WT animals (1.48 ± 0.46 for the WT, 0.95 ± 0.23 for the moderate progressor, and 1.06 ± 0.06 for the severe progressor; Figure 1C) associated with a higher expression of the Mstn antagonist Fstn (0.23 ± 0.05 for the WT, 0.43 ± 0.01 for the moderate progressor, and 0.95 ± 0.9 for the severe progressor; Figure 1D). In the sartorius muscle, similar expression patterns were observed (Figure S2). These results demonstrated the shutdown of the Mstn pathway in the GRMD dogs at both protein and at mRNA levels in the serum and skeletal muscle tissue. respectively. Mstn may therefore be a reliable biomarker of the anabolic homeostatic state of the muscular tissue. The GRMD dog is thus a good model to evaluate if components of the Mstn pathway may be used as biomarkers of therapeutic efficacy for systemic therapies in DMD.
Mstn Is a Biomarker to Monitor Drug Response in GRMD Dogs
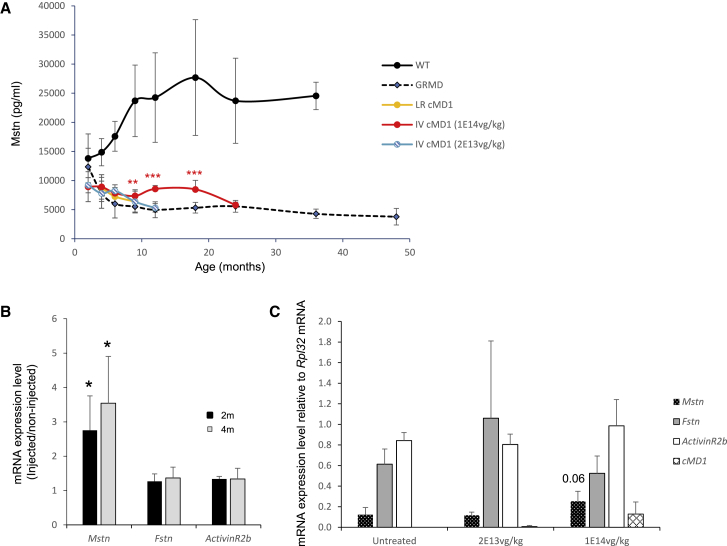
Circulating Mstn levels were assessed in GRMD dogs, locoregionally or systemically treated with a recombinant AAV2/8 (rAAV2/8) vector coding a cMD1, as described previously.3 After loco-regional (1e13 vg/kg) or low-dose intravenous (i.v.) injection (2e13 vg/kg), no modification of circulating Mstn was observed (Figure 2A). After high-dose i.v. injection (1e14 vg/kg), levels of circulating Mstn increased significantly above baseline level but remained below the levels of healthy animals. GRMD serum Mstn levels were maintained until the age of 18 months, when they started to decline (Figure 2A). One possible explanation for this decrease is the progressive clearance of the AAV-mediated transgene in a continuously regenerating muscle, leading to a lowered expression of cMD1. A larger number of samples would be necessary to answer this question definitely.
Figure 2.
Myostatin Expression in AAV-cDM1-Treated GRMD Dogs
(A) Mstn levels were measured in blood samples isolated from either WT or GRMD dogs injected via loco-regional route (LR) or systemic i.v. (IV) routes with a high (1e14 vg/kg) or low (2e13 vg/kg) dose of the AAV vector coding a sequence-optimized cMD1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Student’s t test, compared to untreated GRMD dogs. (B) 3- to 4-month-old GRMD dogs (n = 4) were loco-regionally treated with 1e13 vg/kg, and Mstn, Fstn, and Acvr2b were analyzed at mRNA levels in muscle biopsies, 2 and 4 months after treatment. For each mRNA, results are expressed as the ratio, injected leg/noninjected leg. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Student’s t test. (C) Mstn (black), Fstn (gray), AcvR2b (ActivinR2b; white), and cMD1 (diamond) mRNA levels were analyzed in untreated or low- or high-dose i.v.-treated GRMD animals at the age of 10 months. The transcript amounts were normalized using Rpl32 dog ribosomal RNA. Treated dogs were AAV injected at the age of 2–2.5 months. The number of samples for each point is detailed in Table S1. Error bars are SEM. A one-way ANOVA, followed by Newman-Keuls test was performed.
The levels of different components of the Mstn pathway were also evaluated on muscle biopsies at mRNA levels. The expression levels of Mstn, Fstn, and Acvr2b were compared in 4 loco-regionally injected and in untreated leg muscles. Because we had access to muscle biopsies harvested, 2 and 4 months after injection (T1 and T2 respectively) but not before injection, we calculated a ratio-injected limb/noninjected limb for each gene in each dog’s extensor carpi radialis muscle (Figure 2B). This ratio was 2.7 ± 1 at T1 (p = 0.03) and 3.5 ± 1.3 at T2 (p = 0.05) for Mstn. This shows that Mstn is significantly expressed at higher levels in the treated leg, thus demonstrating that dystrophin rescue induces Mstn re-expression at the mRNA level. Indeed, the mean percentage of cMD1-positive fibers was 50% in the treated forelimb and 2% in the noninjected one.17 This observation is also true for Fstn and Acvr2b, which are also increased subsequent to microdystrophin therapy but to a lesser extent (T1: 1.26 ± 0.21 and 1.33 ± 0.07; T2: 1.36 ± 0.31 and 1.33 ± 0.3, respectively).
The Mstn network was also analyzed after systemic injection in 10-month-old dogs, treated with either 2e13 vg/kg or 1e14 vg/kg and compared to age-matched untreated dogs (Figure 2C). No difference in Mstn mRNA was observed in the untreated and 2e13 vg/kg-treated animals (0.12 ± 0.068 and 0.11 ± 0.03, respectively), whereas in high-dose-treated animals, Mstn levels were increased two-fold (0.25 ± 0.1; p = 0.06). Expression levels of either Fstn or Acvr2b did not show a modification of expression in the treated animals. The Mstn pathway was thus reactivated in the high-dose-treated animals only, where cMD1 is also expressed (Figures 2C).
Mstn Levels in DMD Patients Treated i.m. with Morpholino-Induced Exon 51 Skipping
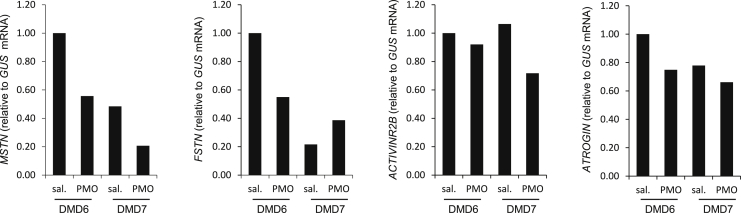
Mstn levels were next measured in the muscle biopsies of DMD patients, intramuscularly treated with the morpholino (phosphorodiamidate morpholino oligomer [PMO]) antisense oligonucleotide AVI-4658 (eteplirsen) that induces skipping of exon 51 in dystrophin mRNA.18 Seven patients were previously treated during the trial (“ClinicalTrials.gov: NCT00159250”), but we only had access to the biopsies of 2 patients who received 0.9 mg intramuscular eteplirsen in one extensor digitorum brevis (EDB) muscle. MSTN, FSTN, ACVR2B, and ATROGIN expressions were analyzed in the PMO and saline-treated muscles, 3 (patient 6) or 4 (patient 7) weeks after treatment, and correlated with dystrophin levels. The PMO-treated muscles did not show a difference in the mRNA levels of these genes compared to the saline-treated ones (Figure 3). Of note, only a limited amount of dystrophin was expressed (63%–65% of dystrophin-positive fibers with a relative intensity of 22%–25% of that in healthy muscle),18 in contrast to the >50% microdystrophin expression in GRMD muscles where Mstn was partially rehabilitated,3 suggesting that certain threshold levels of correction have to be achieved in order to restore intramuscular Mstn.
Figure 3.
Mstn Pathway in DMD Patients Intramuscularly Treated with AVI-4658 PMO (PMO)
Patients 6 and 7 have been described previously.18 mRNA levels of MSTN, FSTN, ACVR2B, and ATROGIN have been analyzed by quantitative real-time PCR in both AVI-4658 PMO and saline-injected (sal.) EDBs.
Discussion
In our study, we evaluated MSTN as a biomarker for treatment efficacy in DMD. Indeed, we had previously observed low levels of serum MSTN in these patients.15 Furthermore, our results indicated that the regulation of the Mstn network was, at least partly, driven by muscle atrophy/inactivity, because patients affected by the most atrophying conditions showed the strongest downregulation of the Mstn pathway. In the present study, we demonstrate that Mstn was significantly increased following treatment with a disease-modifying drug, which further validates Mstn as a biomarker.
Because we could not have access to serum from systemically treated DMD patients in current clinical trials, we used samples generated in preclinical microdystrophin trials conducted in 12 GRMD dogs.3 Very low levels of MSTN were measured in the serum of untreated GRMD dogs, suggesting that the Mstn pathway may be intrinsically downregulated in these dogs to counterbalance the wasting process mediated by the absence of dystrophin, as we observed in humans.15 The GRMD dog is thus a good model to evaluate if components of the Mstn pathway may be used as biomarkers of therapeutic efficacy for DMD. Our results indicate that indeed, MSTN levels may be a reliable biomarker to monitor drug response in neuromuscular patients, including DMD and MTM1 (this work and Mariot et al.15). This is based on several observations. (1) We have previously observed in the MTM1-KO mouse model that Mstn levels are massively decreased compared to controls but increased after MTM1 rescue.15 (2) Mstn is a blood-based biomarker sensitive to change, as an increase of circulating Mstn occurred only in GRMD dogs systemically treated with a high dose of the cMD1-coding AAV vector. After loco-regional dystrophin rescue in the GRMD dogs, there was an increase of Mstn mRNA in the muscle biopsies but no change in circulating Mstn, likely due to the limited systemic effect of only one injected leg. Interestingly, intramuscular injection of the PMO AVI-4658 (eteplirsen) in DMD patients did not lead to an increase of MSTN. This is likely due to the fact that only one small foot muscle was injected in this proof-of-principle study and that this muscle only expressed a limited amount of dystrophin (63%–65% of dystrophin-positive fibers with a relative intensity of 22%–25% of that in healthy muscle).18 (3) MSTN levels are also clinically meaningful and reflect global muscle health. MSTN is mainly produced by the skeletal muscle,19 and low levels of MSTN reflect not only muscle loss but also poor muscle health, as previously observed in a number of atrophying neuromuscular disorders.15 (4) MSTN may be a disease agnostic and treatment-agnostic biomarker, as demonstrated here for dystrophin deficiency. (5) There is a progressive decrease of Mstn levels in the long-term follow-up of AAV-treated GRMD dogs, suggesting that MSTN may be of high relevance and particular value for the longitudinal follow-up for treatment efficacy in patients, because contrary to more specific endpoints measured in clinical trials, it will reflect the functional status of the whole neuromuscular system. Indeed, it is known that dystrophin rescue using AAV-mediated microdystrophin can last only a certain time, due to ongoing transgene loss,20 and a decrease in MSTN levels may indicate that a patient needs to be retreated. However, unlike cohorts classically seen in clinical trials, our dog cohort numbers are too small to draw a definitive picture, but conclusions can be made when considering the convergent picture between clinical and preclinical models.
It is also worth noting that anti-MSTN molecules are currently used in clinical trials in DMD patients (“ClinicalTrials.gov: NCT03789734”). Since circulating MSTN levels can increase after dystrophin rescue, our results also support the concept that patients treated with disease-modifying therapies, capable of substantially ameliorating muscle health, could be better targets for an add-on anti-MSTN therapy than therapy-naive patients, where not only the target for an anti-MSTN therapy itself is low but also, the corresponding ACT2B tissue receptor is downregulated.
In conclusion, the data presented in this study suggest that MSTN is a quantifiable biomarker for monitoring pharmaco-gene therapy in neuromuscular disease, but further studies and clinical trials are needed to demonstrate definitely both the sensitivity of the marker and the capacity of pharmaco-gene therapies to restore the functional capacity of the neuromuscular system. It may be worth measuring MSTN levels in the blood of participants to different current clinical trials aiming at rescuing dystrophin levels using different approaches (such as PMOs, peptide-conjugated PMOs, stereopure antisense oligonucleotides, and AAV-microdystrophin). Indeed, MSTN might complement and eventually replace the need for invasive muscle biopsies usually performed to assess therapy effects in neuromuscular disease.
Materials and Methods
Ethic Statement and Patient Reports
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The collection of sera and biopsies was approved by ethics committees in each institution. Written, informed consents were provided for each participant. The DMD patients have been described previously.18
Animals
Dog samples were obtained from the Boisbonne Center for Gene Therapy (ONIRIS; Atlantic Gene Therapies, Nantes, France) and the Institut Mondor de Recherche Biomédicale (IMRB) Team 10 (Ecole Nationale Veterinaire de Maison Alfort, France). Samples coming from the Boisbonne Center have been already described and used previously.3 The protocol was performed without any immunosuppression regimen.
ELISA Analysis
Dog serum samples were obtained from blood collection after clotting for 30 min at room temperature, followed by a centrifugation for 30 min at 1,000 × g. Samples were aliquoted and stored at −80°C until analysis. The ELISA kit was GDF8 (#DGDF80; R&D Systems Europe, Abingdon, UK), used according to the manufacturer’s instructions. The optical density was measured using a microplate reader (Infinite 200 Pro; Tecan Group, Männedorf, Switzerland).
RNA Extraction and Real-Time PCR
Cryopreserved tissues were transferred in tubes containing 1.4 mm ceramic beads (FastPrep; MP Biomedicals, UK) plus 1 mL of TRIzol (Thermo Fisher Scientific, Paisley, UK) and shaken once at 10,000 revolutions per minute (rpm) for 40 s. Total RNA was extracted using TRIzol, according to the manufacturer’s protocol (Thermo Fisher Scientific, Paisley, UK). The quantity of RNA was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The reverse transcription was described previously and performed on 1 μg of total RNA in a final volume of 10 μL using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Quantitative PCRs (qPCRs) were performed on a LightCycler 480 Real-Time PCR System (Roche, Basel, Switzerland) in a final volume of 9 μL with 0.4 μL of reverse transcriptase (RT) product, 0.18 μL each of forward and reverse primers (20 pmol/mL), and 4.5 μL of SYBR Green Mastermix (Roche, Basel, Switzerland). After qPCR, the PCR products were run on a 2% agarose gel and were cloned using the Topo TA cloning kit (Thermo Fisher Scientific, Paisley, UK) and sequenced. qPCR was designed according to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) standards. Primers used in this study are described in Table S2. The transcript amounts were normalized using Rpl32 dog ribosomal RNA or human GUS RNA.
Statistical Analysis
A one-way ANOVA, followed by Newman-Keuls was used, unless otherwise stated, in the figure legends, with p values of <0.05 considered significant.
Author Contributions
J.D., T.V., and V.M. conceptualized the study. J.D. and T.V. provided funding. V.M. and J.D. performed and analyzed the experiments. F.M., J.M., and S.T. were involved in the DMD clinical trials. C.L.G., I.B., M.M., and S.B. provided dog biopsies and sera. J.D., V.M., and T.V. wrote, discussed, and edited the manuscript, which was revised and approved by all authors. J.D. supervised the project.
Conflicts of Interest
F.M. reports personal fees from Pfizer, personal fees from Roche, personal fees from Biogen, personal fees from Avexis, personal fees from Sarepta, and grants from Sarepta, outside the submitted work. C.L.G. has worked as a consultant for Genethon. C.L.G. and T.V. have filed a patent application for systemic treatment of dystrophic pathologies (WO2015197869A1). T.V. is a SAB member of Constant Pharmaceuticals and a cofounder of Dinaqor AG. He has acted or is acting as a consultant for Antisense Therapeutics, Biophytis, Capricor, DebioPharm, Dynacure, Italfarmaco, Santhera, Sarepta, Servier, and Solid Biosciences. J.D. has acted as a consultant for Solid Biosciences. V.M., T.V., and J.D. are named inventors in the patent PCT/GB2018/05061 (method relating to Mstn pathway inhibition) filled by UCL. The other authors declare no competing interests.
Acknowledgments
We deeply thank all of the patients involved in this study. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR, or Department of Health. The MRC Centre for Neuromuscular Diseases Biobank is acknowledged for providing biopsy samples. V.M., J.M., S.T., F.M., T.V., and J.D. are supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The reference samples on untreated GRMD dogs were obtained, thanks to the support of the Association Française contre les Myopathies (grant #18778). BRC funding for the biobank is also acknowledged. The Muscular Dystrophy UK support to the GOSH and UCLH Neuromuscular Centres is also gratefully acknowledged.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.06.016.
Supplemental Information
References
- 1.Mercuri E., Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 2.Aartsma-Rus A., Straub V., Hemmings R., Haas M., Schlosser-Weber G., Stoyanova-Beninska V., Mercuri E., Muntoni F., Sepodes B., Vroom E., Balabanov P. Development of Exon Skipping Therapies for Duchenne Muscular Dystrophy: A Critical Review and a Perspective on the Outstanding Issues. Nucleic Acid Ther. 2017;27:251–259. doi: 10.1089/nat.2017.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Guiner C., Servais L., Montus M., Larcher T., Fraysse B., Moullec S., Allais M., François V., Dutilleul M., Malerba A. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 2017;8:16105. doi: 10.1038/ncomms16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfano L.N., Charleston J.S., Connolly A.M., Cripe L., Donoghue C., Dracker R., Dworzak J., Eliopoulos H., Frank D.E., Lewis S. Long-term treatment with eteplirsen in nonambulatory patients with Duchenne muscular dystrophy. Medicine (Baltimore) 2019;98:e15858. doi: 10.1097/MD.0000000000015858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzone E., Martinelli D., Berardinelli A., Messina S., D’Amico A., Vasco G., Main M., Doglio L., Politano L., Cavallaro F. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2010;20:712–716. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 6.McDonald C.M., Henricson E.K., Han J.J., Abresch R.T., Nicorici A., Elfring G.L., Atkinson L., Reha A., Hirawat S., Miller L.L. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41:500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri E., Coratti G., Messina S., Ricotti V., Baranello G., D’Amico A., Pera M.C., Albamonte E., Sivo S., Mazzone E.S. Revised North Star Ambulatory Assessment for Young Boys with Duchenne Muscular Dystrophy. PLoS ONE. 2016;11:e0160195. doi: 10.1371/journal.pone.0160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricotti V., Selby V., Ridout D., Domingos J., Decostre V., Mayhew A., Eagle M., Butler J., Guglieri M., Van der Holst M. Respiratory and upper limb function as outcome measures in ambulant and non-ambulant subjects with Duchenne muscular dystrophy: A prospective multicentre study. Neuromuscul. Disord. 2019;29:261–268. doi: 10.1016/j.nmd.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Wu G., Sanderson B., Bittner V. The 6-minute walk test: how important is the learning effect? Am. Heart J. 2003;146:129–133. doi: 10.1016/S0002-8703(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 10.Alfano L., Lowes L., Berry K., Flanigan K., Cripe L., Mendell J. Role of motivation on performance of the 6-minute walk test in boys with Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2015;57:57–58. [Google Scholar]
- 11.Polavarapu K., Manjunath M., Preethish-Kumar V., Sekar D., Vengalil S., Thomas P., Sathyaprabha T.N., Bharath R.D., Nalini A. Muscle MRI in Duchenne muscular dystrophy: Evidence of a distinctive pattern. Neuromuscul. Disord. 2016;26:768–774. doi: 10.1016/j.nmd.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Wilson K., Faelan C., Patterson-Kane J.C., Rudmann D.G., Moore S.A., Frank D., Charleston J., Tinsley J., Young G.D., Milici A.J. Duchenne and Becker Muscular Dystrophies: A Review of Animal Models, Clinical End Points, and Biomarker Quantification. Toxicol. Pathol. 2017;45:961–976. doi: 10.1177/0192623317734823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aartsma-Rus A., Spitali P. Circulating Biomarkers for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2015;2(s2):S49–S58. doi: 10.3233/JND-150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.J. Extracellular Regulation of Myostatin: A Molecular Rheostat for Muscle Mass. Immunol. Endocr. Metab. Agents Med. Chem. 2010;10:183–194. doi: 10.2174/187152210793663748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariot V., Joubert R., Hourdé C., Féasson L., Hanna M., Muntoni F., Maisonobe T., Servais L., Bogni C., Le Panse R. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 2017;8:1859. doi: 10.1038/s41467-017-01486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barthélémy I., Pinto-Mariz F., Yada E., Desquilbet L., Savino W., Silva-Barbosa S.D., Faussat A.M., Mouly V., Voit T., Blot S., Butler-Browne G. Predictive markers of clinical outcome in the GRMD dog model of Duchenne muscular dystrophy. Dis. Model. Mech. 2014;7:1253–1261. doi: 10.1242/dmm.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Guiner C., Montus M., Servais L., Cherel Y., Francois V., Thibaud J.L., Wary C., Matot B., Larcher T., Guigand L. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol. Ther. 2014;22:1923–1935. doi: 10.1038/mt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinali M., Arechavala-Gomeza V., Feng L., Cirak S., Hunt D., Adkin C., Guglieri M., Ashton E., Abbs S., Nihoyannopoulos P. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S.J., McPherron A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Hir M., Goyenvalle A., Peccate C., Précigout G., Davies K.E., Voit T., Garcia L., Lorain S. AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol. Ther. 2013;21:1551–1558. doi: 10.1038/mt.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.