Highlights
-
•
Treatment initiation of T2-T3 rectal cancers with Contact (CXB) provides a fast clinical complete response.
-
•
In T2N0< 3 cm tumors, CXB first with chemoradiotherapy can achieve local control in more than 85%.
-
•
The Phase III OPERA trial should bring robust data in favor of CXB as initial treatment of T2N0< 3 cm.
Keywords: Organ preservation, Rectal cancer, Neoadjuvant treatment, Contact X-ray brachytherapy, Watch and Wait
Abstract
Introduction
A neoadjuvant treatment aimed at rectal preservation should achieve a clinical complete response. This study comparing neoadjuvant treatment initiated with Contact X-ray (CXB) or External Beam radiotherapy (EBRT) is evaluating the influence of the time/dose parameter on clinical response during the first six months.
Materials and methods
This retrospective consecutive series included T2-3 rectal adenocarcinoma staged using digital examination (DRE), endoscopy, magnetic radiation imaging and/or endorectal ultrasound. All patients were treated with organ preservation intent. Treatment protocol combined CXB (80–110 Gy/3–4 fractions) and EBRT ± concurrent capecitabine. In tumor exceeding 3.5 cm treatment was often initiated using EBRT. Clinical response was assessed (DRE, proctoscopy ± imaging) at very close interval between 2 weeks and 6 months after treatment initiation.
Results
Between 2002 and 2017, 61 patients (T2: 31; T3: 30) M0 (median age: 76 years) were treated. Treatment was initiated in 40 patients (T2: 28, T3: 12) with contact X-ray and in 21 (T2: 4, T3: 17) with EBRT. Using contact X-ray or EBRT first treatment, clinical complete (or near complete) response at week 14(±1) was respectively 88% [95CI:74–96] and 33% [95CI:15–57]. In multivariate analysis the treatment chronology was the most significant factor influencing cCR (OR: 7.53). At 6 months, with contact X-ray first all patients were in clinical complete response and five with EBRT remained in partial response. With 61 months median follow-up time, the local recurrence rate was 10% [95% CI: 6–16] at 5 years. T3 and fungating tumors were at higher risk of local recurrence. Organ preservation with good function was achieved in 95% of cases.
Conclusion
This non randomized study tends to show that in early T2-3 tumors, a strategy using upfront contact therapy, which is reducing the overall treatment time, is an option allowing a more favorable outcome than EBRT first.
1. Introduction
Proctectomy, often combined with external beam chemoradiotherapy (EBCRT), is the standard treatment for T2-3 rectal cancer. Rectal preservation is a field of interest in clinical research to improve patient quality of life [1]. To achieve rectal conservation, a neoadjuvant treatment must achieve clinical complete response (cCR). Two strategies are used for this purpose: neoadjuvant EBCRT [2], [3], [4], [5] or contact X- ray Brachytherapy (CXB) combined with EBCRT [6], [7], [8], [9]. The assessment of cCR remains a dilemma when using digital rectal examination (DRE), proctoscopy and imaging. cCR is defined as the disappearance of the rectal tumor opposed to partial response (PR) in which a persisting tumor is observed. A “grey zone” exists between cCR and PR called near clinical complete response (ncCR) [10]. The optimal timing to assess this clinical response remains controversial [11]. The goal of this study is to analyze the influence of the dose/time factor on the clinical response during the first six months after treatment initiation in a cohort of patients treated using CXB or EBCRT delivered first.
2. Material and methods
This retrospective study evaluated the clinical outcomes of patients presenting rectal cancer treated with organ preservation intent using neoadjuvant treatment initiated with either CXB or EBCRT. Selection criteria for this strategy were: tumors, located in the distal or middle rectum, biopsy-proven adenocarcinoma, T2-T3 not exceeding 5 cm in largest dimension and/or half rectal circumference, N0-N1, M0, any age, operable or inoperable patient. Work-up included colonoscopy, rectal imaging using endo-rectal ultrasound (ERUS) and/or magnetic resonance imaging (MRI). General work-up used thoraco-abdomino-pelvic CT scan and biology tests. Tumors < 3 cm largest diameter were treated with CXB first. For larger tumors, treatment was often initiated with EBCRT.
2.1. Clinical tumor assessment at baseline (week 0)
This evaluation was performed by two experienced radiation oncologists (JPG, KB) using DRE and rigid proctoscopy with a patient in the knee-chest position. Objective tumor measurement was performed using data from DRE, initial colonoscopy, ERUS and/or MRI. Depending on MRI and/or ERUS, T3 tumors were sub-classified as T3a, b, c or d. Using all these examinations the tumor was defined as a half-sphere when mostly polypoid or as a cylinder (described as fungating when presenting exophytic edges with deep ulceration). Volume was calculated in cm3 using 3D measurements and geometrical formula (half sphere: 4/3 π r3 × 0.5; cylinder: π r2 × thickness). For tumor length, MRI sagittal view was considered as the most reliable measure. For tumor thickness MRI or ERUS was used as the reference.
2.2. Treatments
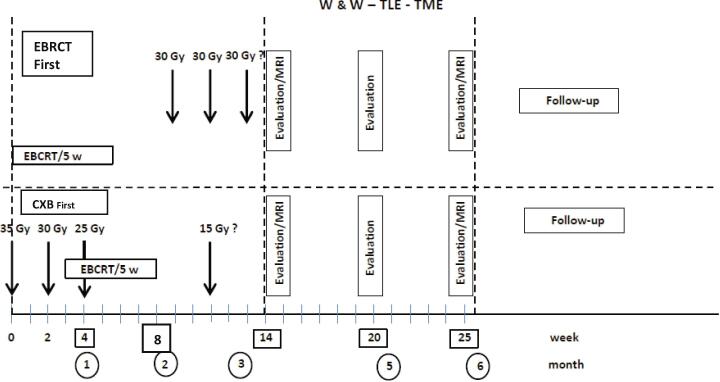
Following the Papillon experience [12], CXB was given first in 3 sessions over 4 weeks delivering a total dose of 80 to 90 Gy combined after the second or third session with EBRT. For EBRT technique, 3D conformal or IMRT was used into a restricted posterior pelvic volume with the upper limit of CTV below S2 or S1 and exclusion of most of the anal canal. Total dose over 5 weeks was 45 Gy or 50 Gy (with shrinking field after 44 Gy). Concurrent chemotherapy with capecitabine (800 mg/m2/BID) was given on radiation days and was omitted in frail patients. When tumor response was slow, a last session could be given 3 weeks after the end of EBRCT (total dose: 100–110 Gy/4 fr). EBRT (±capecitabine) was the initial treatment, mostly after 2013 when organ preservation became more popular among oncologists. CXB was initiated 3 to 5 weeks after completion of EBCRT. Depending on tumor response, CXB dose ranged between 60 Gy (after cCR) and 110 Gy delivered in 3 to 4 fractions [12]. A transanal local excision (TLE) was done in some patients on an individual decision of the attending surgeon and/or multidisciplinary team (MDT) (Fig. 1).
Fig. 1.
Timing of treatment and response assessment. EBRCT: external beam radiation-chemotherapy treatment; w: week; MRI: magnetic radiologic imaging. RXT: radiation therapy; W&W: watch and wait; TLE: transanal local excision; TME: total mesorectal excision (proctectomy); Gy: gray.
2.3. Clinical response evaluation (W2 to W25)
When treatment was initiated using CXB, clinical evaluation was performed at each CXB fraction on week 2 and 4 after first treatment. When it was initiated with EBCRT, clinical response was done at time of the first CXB fraction at week 8 (±1). In all cases this clinical response evaluation was performed by the radiation oncologist using DRE and rigid proctoscopy. On week 14 (±1) in all cases a response evaluation was performed using DRE and rigid proctoscopy often combined with MRI. MRI response was reported using the TRG score [15]. Diffusion weighting imaging (DWI) was frequently used and a diffusion restriction influenced the diagnosis toward PR. In case of cCR at week 14(±1) close surveillance was advised every 3 months (DRE, rigid proctoscopy ± MRI) for 2 years then every 6 months until year five. In case of ncCR at week 14 (±1), a new evaluation (DRE, proctoscopy ± MRI or PET-CT) was made every month until cCR was achieved. In case of PR depending on tumor response and/or patient context, a radical proctectomy or a new clinical evaluation one month later was proposed. For decision- making ncCR was considered as a cCR and no radical proctectomy was decided if ncCR was recorded. In a few frail patients, still with PR at week 25 (±2), a more prolonged surveillance was decided, with monthly evaluation.
According to RECIST criteria [13], definitions of cCR, ncCR, partial response (PR) and stable disease are given in Table 1.
Table 1.
Tumour response classification DRE: digital rectal examination; cCR: clinical complete response; PR: partial response; SD: stable disease; TRG: tumor regression grade.
| cCR | Near cCR | PR or SD | |
|---|---|---|---|
| DRE | –No palpable Tumor | -Palpable hard sessile tumor | |
| -Rectal wall supple | -Rectal wall: firm | -Rectal wall: Hard induration |
|
| –No ulceration | -Ulceration: superficial, smooth edge, regular fundus | -Ulceration: Irregular/deep |
|
| –No nodularity | -Nodularity: Small-supple/firm |
-Nodularity: hard or firm | |
| Endoscopy | –No visible tumor and normal appearance of mucosa. | -Visible malignant tumor | |
| Rigid rectoscopy | |||
| Flexible colonoscopy or sigmoidoscopy | -Scar appearance -White scar -Radiation Erythematous mucositis -Small radiation Inflammatory vessels |
-Ulceration: irregular and deep | |
| MRI | TRG1 | TRG2 | TRG 3–4-5 |
2.4. Other study endpoints
When a surgery was performed pathological examination was recorded according to the Dworak classification [14].
Local control was defined as no detectable tumor in the rectum or pelvis after 6 months.
Local recurrence was any tumor relapse in the rectum or pelvis after cCR or ncCR.
Distant metastases and survival (overall, and cancer specific) were evaluated.
Bowel function was recorded using the MSKCC score (in 4 categories) [16] or since 2014 with the LARS score [17].
Toxicity was scored using the common terminology criteria for adverse events (CTCAE) version 4.03.
2.5. Statistical analysis
Qualitative data, such as response rate, were presented as absolute frequency and relative frequency with a 95% confidence interval [95 CI]. These data were compared using Chi2 test or a Fisher exact test in case of failure to meet the Chi2 application rules.
Quantitative data were presented as mean, standard deviation, median and range. Quantitative data were compared using the Student T test or the Wilcoxon test in case of non-respect of the application conditions of the Student T test.
Clinical response was evaluated using the last observation carried forward methods (LOCF). As every patient was not evaluated every week neither at the same time, we used the LOCF method for imputation of missing data. LOCF consisted in completing the data at week n (if missing) with the data from week n-1. This imputation method is justified in this study as it does not increase the effect of the treatment.
For evaluation of the clinical response rate or the tumor volume and diameter, patients were censored at the time of Trans-anal Local Excision (TLE) if performed.
Survival distributions were estimated with the Kaplan–Meier method. Comparisons were performed using the log-rank test. Univariate and multivariate analysis was performed for prognostic factors. The significant factors found in univariate analysis were introduced in a multivariate model, from which a final model was constructed using bachward stepwise regression. P-value and curves were estimated using logistic regression model. All analyses were performed at 5% alpha risk with bilateral hypothesis, using R software version 3.5.1.
3. Results
3.1. Baseline characteristics
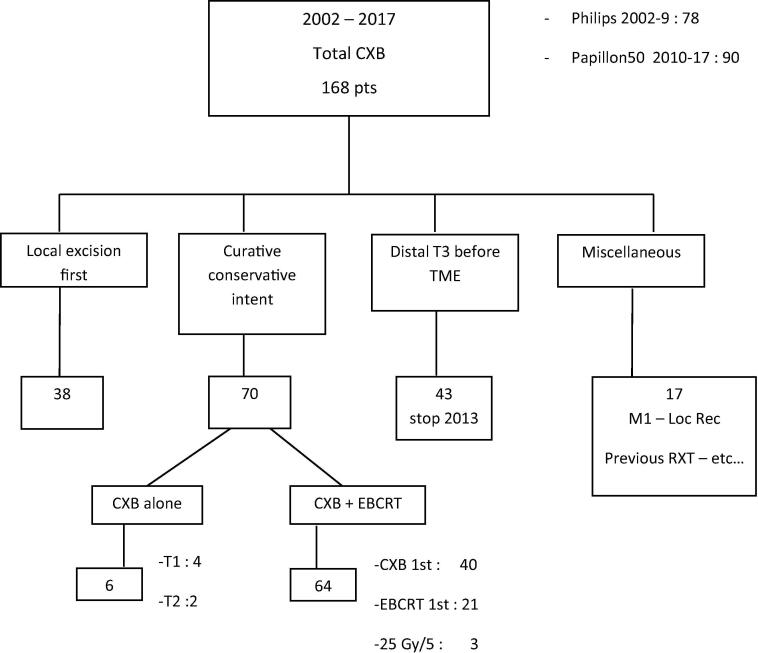
Between 2002 and 2017, 168 patients presenting rectal cancer were treated at our institution using CXB (Fig. 2). Among them 70 consecutive patients were treated with organ-preserving intent. Three patients treated with short course EBRT (25G y/5) and six with CXB alone were excluded. The study cohort comprised 61 patients with median age 78 years. Classification was done using ERUS alone in 15 patients, MRI alone in 17 and ERUS with MRI in 29. T2 and T3 were reported respectively in 31 and 30 cases. Treatment was initiated respectively with CXB and EBCRT in 40 and 21 cases. The two groups were imbalanced with in EBRCT first more T3, larger diameters, more fungating appearance. Table 2 gives patients baseline characteristics and treatments description. Median follow-up time was 61 months.
Fig. 2.
Consort diagram of 168 patients treated using CXB. Among the 64 patients treated with conservative intent with CXB + EBCRT, a short course regimen (25 Gy/5) was used in 3 patients. These 3 patients are not included in the present study. CXB: contact X-Ray brachytherapy; pts: patients; TME: total mesorectal excision; Loc Rec: local recurrence; EBRCT: external beam radiation-chemotherapy treatment; RXT: radiation therapy.
Table 2.
Baseline characteristics of 61 patients (Curative-conservative intent) and treatment PS: performance status; T: tumor; N: node; NK: not known.
| CXB 1st(40) | EBRT 1st(21) | Total (61) | P-value | ||
|---|---|---|---|---|---|
| Gender | Male | 30 | 11 | 41 | 0.136 |
| Female | 10 | 10 | 20 | ||
| Age | Median [range] | 76 [ 39-93 ] | 82 [ 30-98 ] | 78[30-98] | 0.386 |
| Operable | Yes | 16 | 6 | 22 | 0.295 |
| High risk | 21 | 12 | 33 | ||
| Inoperable | 3 | 3 | 6 | ||
| PS | 0-1 | 36 | 16 | 52 | 0.114 |
| 2-3 | 4 | 5 | 9 | ||
| Histology | Well differentiated | 19 | 10 | 29 | 0.903 |
| Moderately | 12 | 5 | 17 | ||
| Poorly | 2 | 0 | 2 | ||
| Unspecified | 6 | 4 | 10 | ||
| cT | T2 | 27 | 4 | 31 | < 0.001 |
| T3 : a | 1 | 3 | 4 | ||
| b | 9 | 3 | 12 | ||
| c | 0 | 4 | 4 | ||
| d | 0 | 1 | 1 | ||
| x | 3 | 6 | 9 | ||
| T3 Total | 13 | 18 | 30 | ||
| cN | N0 | 29 | 15 | 44 | 0.788 |
| N1 | 9 | 6 | 15 | ||
| N2 | 2 | 0 | 2 | ||
| Site | Anterior | 14 | 8 | 22 | 0.877 |
| Posterior | 12 | 5 | 17 | ||
| lateral | 14 | 8 | 22 | ||
| Distance Anal verge (cm) | < 6 | 26 | 13 | 39 | 0.808 |
| ≥ 6 | 14 | 8 | 22 | ||
| T diameter (cm) | mean (SD) | 3.1(0.8) | 3.6 (0.9) | 3.2(0.9) | 0.051 |
| median [range] | 3 [1.8-4.6] | 3.4 [2-5] | 3.2 [1.8-5] | ||
| Tumour volume (cm3) | mean [95 CI] | 10.3 [7.4] | 14.6 [11] | 11.8 (9.3) | 0.0128 |
| median (range) | 8.5 (1.5-29) | 10 (2.1-38) | 10.3 [1.5-38] | ||
| Circumference % | mean [95 CI] | 29.9 [10] | 44.1 [12] | < 0.001 | |
| median (range) | 28 [15-60] | 45 [25-75] | |||
| Clinical aspect | Polypoid | 31 | 8 | 39 | 0.002 |
| fungating | 9 | 13 | 22 | ||
| Shape | ½ sphere | 28 | 11 | 39 | < 0.001 |
| cylinder | 12 | 10 | 22 | ||
| CXB | Machine | ||||
| Philips RT | 23 | 2 | 25 | <0.001 | |
| Papillon 50 | 17 | 19 | 36 | ||
| Dose | |||||
| 50-79 | 4 | 7 | 11 | ||
| 80-110 | 32 | 10 | 42 | 0.00115 | |
| >110 | 4 | 4 | 8 | ||
| EBRT | Dose | ||||
| 44-50 | 36 | 20 | 56 | 0.826 | |
| <44 | 4 | 1 | 5 | ||
| Conc. Chemo (EBRT) | Capecitabine | 34 | 18 | 52 | 1 |
| Local excision | 11 | 1 | 12 |
3.2. Clinical tumor response during the first six months
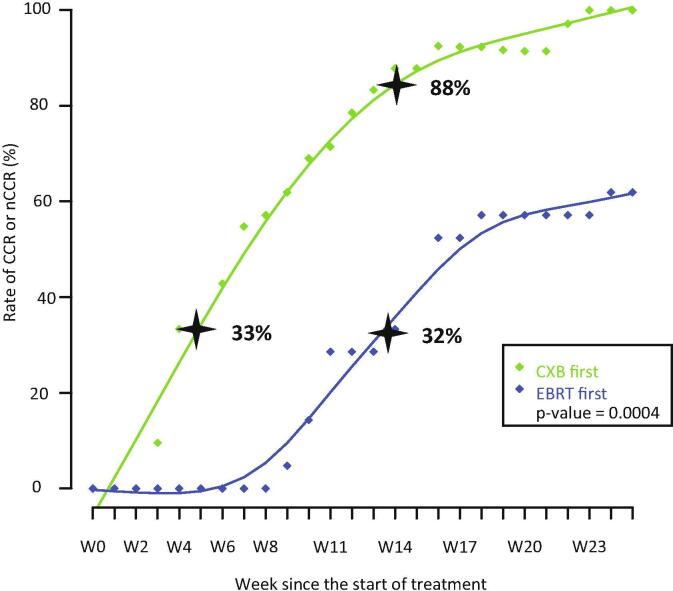
When CXB was the first treatment, at week 4 (±1) at time of third CXB session, cCR or ncCR was observed in 33% of cases. When EBCRT was the first treatment, at week 8 (±1) at time of first CXB session, cCR or ncCR was observed in 14% of cases. Out of 23 T2-T3a ≤ 3 cm treated using CXB first, 19 were in cCR (or ncCR) at week 4. Out of 7 T2-T3a ≤ 3 cm with EBCRT first, two were in ncCR and 5 in PR at week 8(±1). At week 14(±1), three weeks after end of all radiotherapy treatments, cCR or ncCR was seen, respectively, in CXB and EBCRT group in 88% [95 CI : 74–96] and 32% [95 CI: 15–57] of cases (chi 2: p < 0.001) (Fig. 3) (suppl File Fig. 1).
Fig. 3.
Rate of clinical response and time. W: week; CXB: contact X-Ray brachytherapy; EBCRT: external beam chemoradiotherapy. CCR: clinical complete response nCCR: near clinical complete response CXB: contact X-ray brachytherapy EBRT: External beam radiation therapy.
TLE was performed on 12 patients (CXB: 11, EBCRT: 1) between weeks 12 and 25 after treatment initiation. Eight patients were in cCR, 3 in ncCR and 1 in PR. Pathology showed ypT0 in 6, yp T1 in 4 and ypT2 in 2. All TLE were R0. One patient had a local recurrence at 7 years.
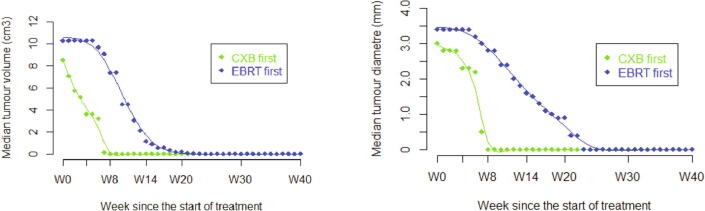
At week 20 to 25 (±2), a cCR or ncCR was seen in all patients treated with CXB first. Five patients with EBCRT first were still in PR, four of these achieved cCR between month 6 and 8 and did not develop local recurrence. The other patient received palliative treatment and died without requiring a diverting stoma. When evaluating tumor volume or diameter during this 6-months period, the same difference in rapidity of response was observed in patients receiving CXB first. At week 14 (±1) mean volume or diameter for CXB-first patients was close to zero and was still measurable 4.94 cm3 (SD 8.1) and 1.6 cm (SD 1.3)) for EBCRT-first patients (Fig. 4).
Fig. 4.
Tumor Volume and Diameter evolution with time. W: week; CXB: contact X-Ray brachytherapy; EBCRT: external beam chemoradiotherapy. Dots on both curves EBRT first are “virtual assessment” between W0-8.
When ncCR was reported, in both groups, between week 8 and 25, it involved ulceration in 70% of cases, firm rectal wall using DRE in 20% and residual nodularity in 10%.
MRI was not included in this evaluation study but had little influence on the decision. MRI data will be analyzed in another paper.
3.3. Local control and local recurrence
At one year after treatment initiation, local control was observed in 59 out of 61 patients. One patient achieved only PR. On account of the advanced age of the population, 3 patients died during the first year with no detectable rectal tumor. One patient (T3b) after cCR presented a local recurrence 10 months after initiation of treatment and was treated with Hartman surgery. Local recurrence was diagnosed in 7 patients. At 5 years the cumulative incidence of local recurrence was 10% [95 CI: 1–18] and organ preservation was seen in 95% of patients. Two local recurrences were observed at year six and seven. The median occurrence time of the 7 local recurrences was 22 months. All these local recurrences were found on DRE and endoscopy (Suppl File Fig. 2 Table 3).
3.4. Survival and metastases
Overall and cancer-specific survival at 5 years were respectively 54% [95 CI: 41–71] and 90% [95 CI: 81–100]. Cumulative incidence of distant metastases at 5 years was 20% [95 CI: 8–31].
3.5. Prognostic factors
When treated with CXB first all 23 patients with polypoid tumors ≤ 3 cm in diameter were in cCR (or ncCR) at week 11 ± 1 (3 weeks after end of EBCRT) and none had local recurrence. One developed isolated distant metastasis.
Taking cCR + ncCR at week 14 as the end point in univariate analysis, in addition to treatment chronology, the following parameters were significant (for 60 evaluable patients): T stage : T2 87% (27/31), T3 48% (14/29) p-value < 0.001; clinical aspect: polypoid 85% (33/39), fungating 38% (8/21) p-value < 0.001 and circumferential extension (p-value < 0.001). In multivariate analysis, chronology was the most significant parameter (OR:7.531 [95% CI 1.72–32.81] before circumferential extension (OR:1.029[95%CI 1.092–1.172] (suppl File Table 1, Table 2).
Taking the cumulative rate of local recurrence at 5 years as the end point in univariate analysis two parameters were significant: T stage: T2 0% [95%CI 0-0]-T3 21% [95%CI 2–26] p-value < 0.05 and clinical aspect: polypoid 0% [95%CI 0-0]-fungating 26% [95%CI: 3–44] p-value < 0.05. Chronology had no influence: CXB first 6% [95% CI 0–13]-EBRT first: 19% [95% CI 0–36] p-value = 0.68.
3.6. Toxicity
Main complication was rectal bleeding in 28 patients. Plasma argon coagulation was needed in 4 cases. Telangiectasia was described in 30% of patients usually 1 or 3 years after end of treatment and was responsible for the rectal bleeding which stopped after 3 years. No patient required diverting stoma for toxicity. Bowel function was estimated as good or excellent in 85% of patients and none required diverting stoma for poor function.
4. Discussion
The main original finding of this study is to show the difference in speed of clinical tumor response when CXB is used as first treatment (with a high dose over a short time in a small well-targeted tumor volume) versus EBCRT (lower dose, longer time and larger volume). At week 14 (±1), 88% vs 32% of patients respectively, were evaluated as cCR or ncCR. When treating T2 ≤ 3 cm in diameter using EBCRT first, both cCR and ncCR were infrequently observed (28%) at week 8 (±1). On the opposite, with CXB first at week 4 the rate of cCR and ncCR was already 33%. As well stressed by Habr Gama [18], there is a need for a prolonged follow-up period even after 6 months since complete tumor regression can take a long time and proctectomy should not be performed too urgently. It is logical that tumor response is faster in T2 tumor and tumor with small size (than in T3 or large volume). More interesting is the prognostic relevance of a polypoid aspect for rapid response and good local control, as already mentioned by Papillon in the 1970s [12]. When discussing treatment options for a T2 T3a ≤ 3 cm with a polypoid aspect it appears possible to propose a conservative treatment using CXB first in frail but also in fit patients. A rapid cCR observed at week 4 is a strong argument in favor of this initial option. Conversely, the use of EBCRT first is associated with a longer period of uncertainty for oncologists and of anxiety for patient during the first 6 months. The present outcome data may appear better than those published by institutions using a W-W strategy without intracavitary boost reporting a rate of cCR close to 25% with a rate of local relapse at 2 years approximating 20% [3], [4], [5]. In Habr Gama’s experience the rates of cCR for T2 and T3 are respectively, 72% and 63% and the rates of local relapse 8% and 40% [18]. This difference is probably explained by the different strategies used: in the Nice experience early tumors are treated with high radiation dose with a planned organ preservation whereas in other institutions, more locally advanced tumors are treated with lower radiation dose leading to opportunistic preservation [24]. The Montreal strategy using high dose endoluminal iridium brachytherapy is quite similar to the Nice approach and report similar results for cCR and organ preservation [22].
This study has many limitations. It is a monocentric retrospective analysis of only 61 patients. The main limitation is clearly a significant difference between the two groups regarding baseline tumors characteristics with more locally advanced tumors in the EBCRT group. Part of the difference in tumor shrinkage can be attributed to this imbalance. The ongoing OPERA trial (NCT 02505750) is designed to provide some answers on this question [24]. Although performed throughout this study by the same two radiation oncologists, clinical description and response assessment remain inherently subjective and tumor size measurements whatever the tools used remain uncertain. Tumor size measurements is difficult to assess and it is possible that the largest tumor diameter could be the most relevant measurement. MRI performed at week 14 and every 3 months subsequently is a standard recommendation but is outside the scope of this article. MRI may assist the decision-making but many experts recognize that a robust way for assessing cCR or local relapse is DRE and endoscopy [19], [20], [21].
Organ preservation for rectal cancer is still controversial. The definition of cCR may vary from one center to another [22]. There is a clear need for a common language to describe the clinical tumor responses with either DRE or endoscopy. The optimal time interval for assessing this response [11] and appropriate management of ncCR are not standardized [23]. Therefore it is possible to stress some potential benefits to be achieved by initiating treatment in early polypoid tumors using CXB first. As there is no need for long planning simulation, CXB treatment can be commenced within a day or a week following the decision. Overall treatment time can be reduced as EBCRT can be initiated after the second CXB session. The dose to the visible tumor (Gross Tumor Volume) can be higher. Taking advantage of a visible tumor, CXB targeting is improved which is not always the case when CXB is given after EBCRT. CXB first may also trigger a better immunological reaction [23].
In summary cCR or ncCR are observed more rapidly when the neoadjuvant treatment is initiated with CXB. When selecting early polypoid T2 (T3a) N0 tumor ≤ 3 cm in diameter, using upfront CXB is an option allowing a more favorable outcome. This strategy can be adopted as the first treatment followed by EBCRT. This combination rapidly provides a high rate of cCR and enables long-term local control with little toxicity. This approach can be proposed as a planned conservative option in well-informed patients. In all cases, prolonged and strict multidisciplinary surveillance is mandatory.
Disclosure
Jean-Pierre Gerard is Medical Advisor of Ariane Medical Systems, UK; All other authors have no conflict of interest.
Funding/Support
In 2009 a grant of 564 868 € was given by the French ANR (Agence Nationale de la Recherche) (ANR-08-TECS-015) to the Centre Antoine- Lacassagne to support the development of the Papillon 50 system.
References
- 1.Heald RJ, Beets G, Carvalho C. Report from a consensus meeting: response to chemoradiotherapy in rectal cancer - predictor of cure and a crucial new choice for the patient: on behalf of the Champalimaud 2014 Faculty for 'Rectal cancer: when NOT to operate'. reports Colorectal Dis. 2014 May;16(5):334-7. [DOI] [PubMed]
- 2.Habr-Gama A., Gama-Rodrigues J., São Julião G.P., Proscurshim I., Sabbagh C., Lynn P.B. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822–828. doi: 10.1016/j.ijrobp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Martens M.H., Maas M., Heijnen L.A., Lambregts D.M., Leijtens J.W., Stassen L.P. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. 2016;108(12) doi: 10.1093/jnci/djw171. [DOI] [PubMed] [Google Scholar]
- 4.Renehan A.G., Malcomson L., Emsley R., Scott N. OnCoRe project investigators. Watch-and-wait versus surgical resection for patients with rectal cancer – Authors’ reply. Lancet Oncol. 2016;17(4):e134–e135. doi: 10.1016/S1470-2045(16)00166-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.J., Strombom P., Chow O.S., Roxburgh C.S., Lynn P., Eaton A. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gérard J.P., Myint A.S., Croce O., Lindegaard J., Jensen A., Myerson R. Renaissance of contact x-ray therapy for treating rectal cancer. Expert Rev Med Devices. 2011;8(4):483–492. doi: 10.1586/erd.11.28. [DOI] [PubMed] [Google Scholar]
- 7.Dhadda A.S., Martin A., Killeen S., Hunter I.A. Organ preservation using contact radiotherapy for early rectal cancer: outcomes of patients treated at a single centre in the UK. Clin Oncol (R Coll Radiol) 2017;29(3):198–204. doi: 10.1016/j.clon.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Sun Myint A., Smith F.M., Gollins S., Wong H., Rao C., Whitmarsh K. dose escalation using contact X-ray brachytherapy after external beam radiotherapy as nonsurgical treatment option for rectal cancer: outcomes from a single-center experience. Int J Radiat Oncol Biol Phys. 2018;100(3):565–573. doi: 10.1016/j.ijrobp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Gérard J.P., Barbet N., Gal J., Dejean C., Evesque L., Doyen J. Planned organ preservation for early T2–3 rectal adenocarcinoma: A French, multicentre study. Eur J Cancer. 2019;108:1–16. doi: 10.1016/j.ejca.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Lynn P.B., Strombom P., Garcia-Aguilar J. Organ-preserving strategies for the management of near-complete responses in rectal cancer after neoadjuvant chemoradiation. Clin Colon Rectal Surg. 2017;30(5):395–403. doi: 10.1055/s-0037-1606117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dossa F., Chesney T.R., Acuna S.A., Baxter N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017 Jul;2(7):501–513. doi: 10.1016/S2468-1253(17)30074-2. [DOI] [PubMed] [Google Scholar]
- 12.Papillon J. Present status of radiation therapy in the conservative management of rectal cancer. Radiother Oncol. 1990;17:275–283. doi: 10.1016/0167-8140(90)90001-d. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009 Jan;45(2):228-47. [DOI] [PubMed]
- 14.Dworak O., Keilholz L., Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 15.Nougaret S(1), Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology. 2013 Aug;268(2):330-44. [DOI] [PubMed]
- 16.Minsky B.D., Cohen A.M., Enker W.E., Sigurdson E. Phase I/II trial of pre-operative radiation therapy and coloanal anastomosis in distal invasive resectable rectal cancer. Int J Radiat Oncol Biol Phys. 1992;23(2):387–392. doi: 10.1016/0360-3016(92)90757-9. [DOI] [PubMed] [Google Scholar]
- 17.Emmertsen K.J., Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255(5):922–928. doi: 10.1097/SLA.0b013e31824f1c21. [DOI] [PubMed] [Google Scholar]
- 18.Habr-Gama A., São Julião G.P., Fernandez L.M., Vailati B.B., Andrade A., Araújo S.E.A. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think! Dis Colon Rectum. 2019;62(7):802–808. doi: 10.1097/DCR.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 19.Maas M., Lambregts D.M., Nelemans P.J., Heijnen L.A., Martens M.H., Leijtens J.W. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015;22(12):3873–3880. doi: 10.1245/s10434-015-4687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appelt A.L., Pløen J., Harling H., Jensen F.S., Jensen L.H., Jørgensen J.C. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 21.Gollub M.J., Blazic I., Bates D.D.B., Campbell N., Knezevic A., Gonen M. Pelvic MRI after induction chemotherapy and before long-course chemoradiation therapy for rectal cancer: what are the imaging findings? Eur Radiol. 2019;29(4):1733–1742. doi: 10.1007/s00330-018-5726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garant A, Magnan S, Devic S, Martin AG, Boutros M, Vasilevsky CA et al. Image-guided Adaptive Endorectal Brachytherapy in the Non-Operative management of patients with rectal cancer. Int J Radiat Oncol Phys 2019 Aug 30. Pii: S0360-3016. doi: 10.1016. [DOI] [PubMed]
- 23.Wu Q., Allouch A., Martins I., Brenner C., Modjtahedi N., Deutsch E. Modulating both tumor cell death and innate immunity is essential for improving radiation therapy effectiveness. Front Immunol. 2017;26(8):613. doi: 10.3389/fimmu.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerard J.P., Barbet N., Benezery K. Organ preservation for T2–T3 rectal cancer: opportunistic or planned strategy. Oncotarget. 2019;10:3431–3432. doi: 10.18632/oncotarget.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]