Abstract
Intracranial subdural abscess is a rare condition. Although brain abscess is often reported in relation to dental infection, reports of intracranial subdural abscess are few. Actinomyces spp. forms part of the normal flora of the oral, gastrointestinal, and genital tract, and is rarely the cause of intracranial infection; moreover, the pathogen Actinomyces meyeri is very rare. We report an exceptional case of intracranial subdural abscess caused by A. meyeri and related to dental treatment.
A 57-year-old woman initially presented with a 5-day history of headache. Because left arm numbness and weakness became apparent, she was admitted to our department. She had a history of hypertension and dental problems requiring tooth extractions. Diffusion-weighted imaging (DWI) showed a 1-cm right convexity hyperintense mass above the postcentral gyrus. A post-gadolinium T1-weighted image showed a thin hypointense area with peripheral rim enhancement in the right subdural space that appeared to partially thicken in the same location as the DWI-positive mass. She underwent emergent navigation-guided drainage and 4 mL of pus was obtained. Postoperatively, left arm numbness and weakness disappeared. Cultures showed growth of A. meyeri and Fusobacterium nucleatum. She was started on intravenous penicillin G and metronidazole. After a 4-week course of the intravenous antibiotics, her headache gradually improved and the abscess in the subdural space subsided.
To our best knowledge this is the first case report of intracranial subdural abscess caused by A. meyeri associated with dental treatment.
Keywords: Actinomyces meyeri, intracranial subdural abscess, dental treatment
Introduction
Intracranial subdural abscess is a rare condition, accounting for 15%-20% of localized intracranial infections.1,2) Dental infection is recognized as a rare source of intracranial infection and the etiology may be hematogenous, or may develop through extension of oral-cervicofacial disease.1) Although brain abscess related to dental infection is often reported, there are few reports of intracranial subdural abscess.
Actinomycosis is a chronic infection caused by organisms of the genus Actinomyces.3) Actinomyces spp. form part of the normal flora of the oral, gastrointestinal, and genital tracts, and is rarely a cause of intracranial infection,3,4) moreover, the pathogen Actinomyces meyeri itself is very rare, accounting for 1% of all Actinomyces spp. involved in human pathogenesis.4) A. meyeri often causes pulmonary infection and shows a tendency for hematogenous dissemination.5) However, only a few cases of brain abscess related to A. meyeri have been reported.4–6)
Here we report an exceptional case of intracranial subdural abscess caused by A. meyeri and related to dental treatment.
Case Report
A 57-year-old woman initially presented with a 5-day history of headache. After left arm numbness and weakness became apparent, she was admitted to our department. Her medical history consisted of hypertension and dental problems requiring tooth extractions, and outpatient oral antibiotic courses 2 months prior to presentation. She had smoked a pack of cigarettes daily for 37 years, consumed approximately two bottles of beer daily, and had no known drug allergies. Her temperature was 36.8 °C, blood pressure 140/86 mmHg, pulse rate 81 beats/min, and respiration rate 17 breaths/min. Neurological examination revealed mild left arm paralysis and decreased sensation. Dental examinations were normal. Tympanum and external otic meatus status were also normal.
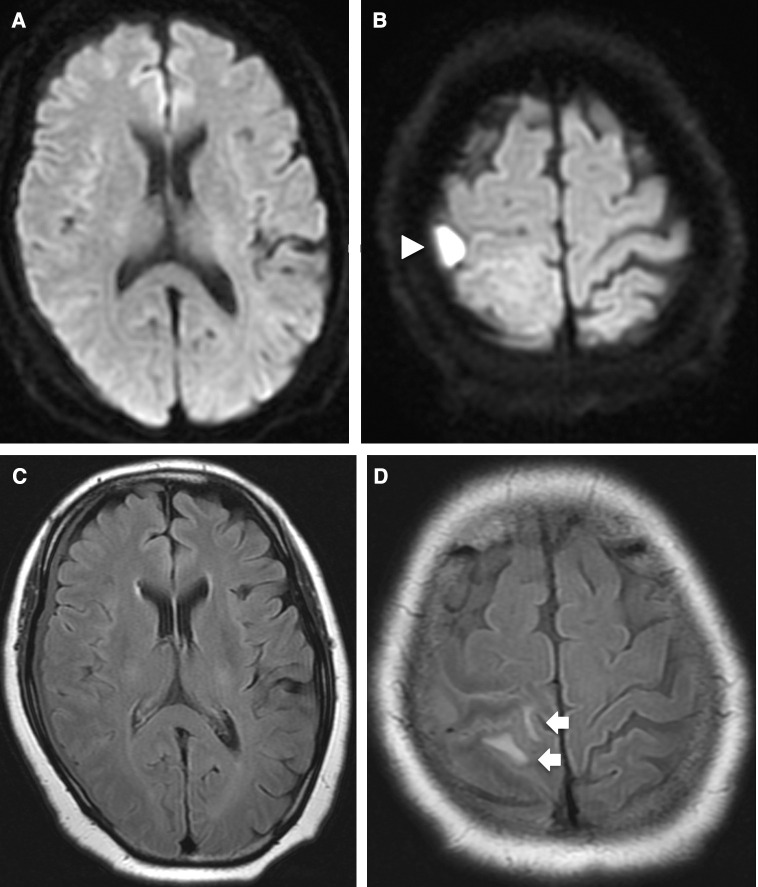
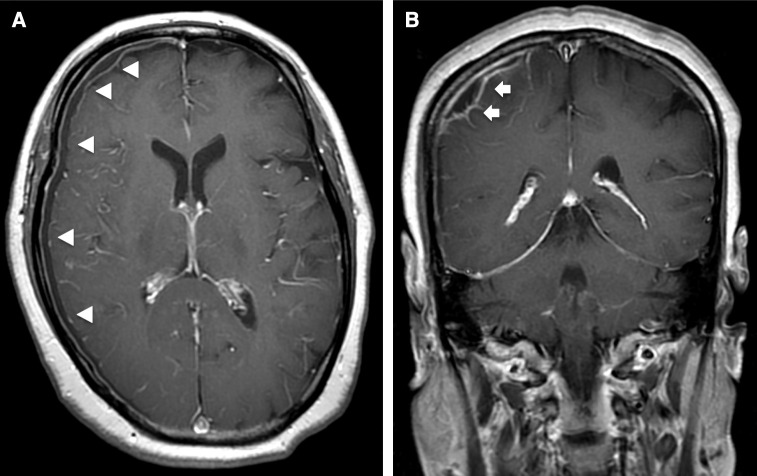
Blood tests on admission showed the following: leukocytes, 5700/mm3; hemoglobin, 12.2 g/dL; platelets, 431000/mm3; C-reactive protein, 14 mg/dL; creatinine, 0.8 mg/dL. Blood cultures were negative and chest X-ray was normal. Because computed tomography (CT) of the brain showed localized edema in the right parietal lobe, magnetic resonance imaging (MRI) was performed. Diffusion-weighted imaging (DWI) showed a 1-cm right convexity hyperintense mass above the postcentral gyrus (Figs. 1A and B). Both fluid-attenuated inversion recovery (FLAIR) and CT showed localized edema around the DWI-positive mass (Figs. 1C and D). A post-gadolinium T1-weighted image (T1WI) showed a thin hypointense area with peripheral rim enhancement in the right subdural space that, on coronal view, thickened partially in the same location as the DWI-positive mass (Figs. 2A and B). There were no radiological findings of sinusitis. Enhanced CT of the chest and abdomen showed no lesions. Transthoracic echocardiography did not show any abnormalities. Intracranial subdural abscess was suspected as the preoperative diagnosis.
Fig. 1. (A and B) DWI shows a 1-cm right convexity hyperintense mass (white arrowhead) above the postcentral gyrus. (C and D) FLAIR shows localized edema (white arrows) in the parietal lobe around the DWI-positive mass. DWI: diffusion-weighted imaging, FLAIR: fluid-attenuated inversion recovery.
Fig. 2. (A and B) Post-gadolinium T1WI shows thin hypointense area with peripheral rim enhancement in the right subdural space (white arrowheads); on coronal view, the hypointense area is partially thick in the same location as the DWI-positive mass (white arrows). TIWI: T1-weighted image, DWI: diffusion-weighted imaging.
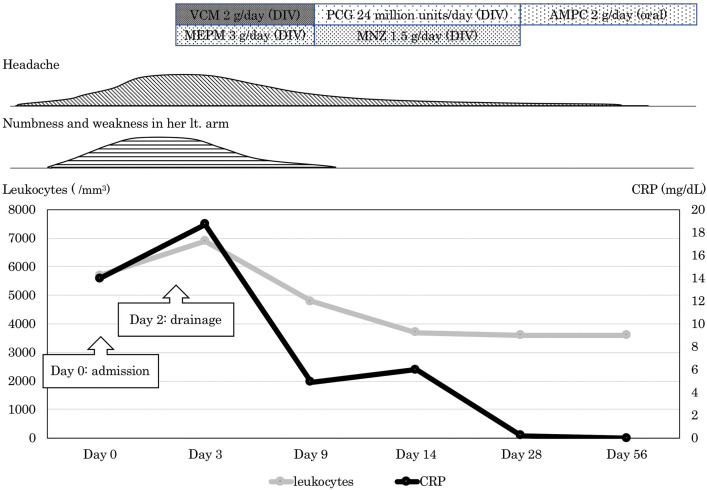
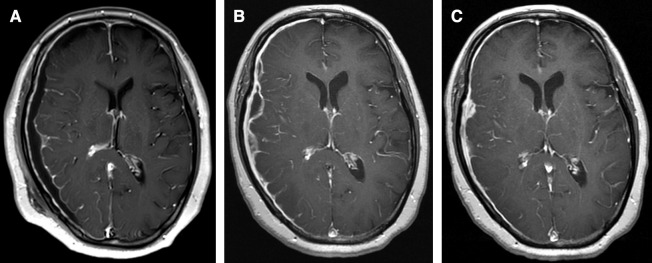
Because her headache and left arm paralysis worsened on the day after admission, the patient underwent emergent navigation-guided drainage. A burr hole was drilled and the dura mater incised. 4 mL of pus was obtained from the subdural space and we confirmed that the surface of the brain was intact. The definitive diagnosis was intracranial subdural abscess, although more pus that had spread into the subdural space could not be acquired because of septum. Figure 3 shows the patient’s clinical course. Postoperatively, left arm numbness and weakness subsides. The patient was started on intravenous vancomycin, 1 g every 12 h and meropenem, 1 g every 8 h. MRI on the day following surgery showed disappearance of the DWI-positive mass, but the abscess in the subdural space was slightly larger (Fig. 4A). Cultures taken 1 week after the operation showed growth of A. meyeri and Fusobacterium nucleatum. Once cultures results were available, vancomycin and meropenem were discontinued and the patient was started on intravenous penicillin G, 4 million units every 4 h and metronidazole, 500 mg every 8 h. After a 4-week course of the intravenous antibiotics, her headache gradually improved and the abscess in the subdural space decreased in size (Figs. 4B and C). In addition, C-reactive protein became negative. She was discharged home and continued taking oral amoxicillin, 500 mg every 6 h, and no recurrence occurred at 6 months.
Fig. 3. Clinical course of the patient. VCM: vancomycin, DIV: drip infusion in vein, MEPM: meropenem, PCG: penicillin G, MNZ: metronidazole, AMPC: amoxicillin, Lt: left, CRP: C-reactive protein.
Fig. 4. (A) Post-gadolinium T1WI on the day following surgery shows that the abscess in the subdural space is slightly enlarged. (B and C) Post-gadolinium T1WI after administration of intravenous antibiotic (B: 1 month later; C: 2 months later) shows gradual decrease in the size of the abscess. TIWI: T1-weighted image.
Discussion
Intracranial subdural abscess is a rare condition.1,2) Because it can be difficult to diagnose owing to its nonspecific presentation, it often has a poor outcome. As improving the outcome is critically dependent on rapid diagnosis and treatment, it is important to comprehend the patient’s medical history and recognize the clinical features.1,7,8) The most common symptoms are fever, headache, and vomiting.7,8) With progression, focal neurological symptoms and seizures may result.1,7,8) The common sources of intracranial subdural abscess are neurosurgical procedures, sinusitis, otogenic sources and skull trauma.1) Dental infection is a rare source of intracranial infection and the etiology may be hematogenous, or it may develop through extension of oral-cervicofacial disease.1) Although brain abscess related to dental infection is often reported, there are few reports of intracranial subdural abscess in this context. Derin et al.9) reported a case of intracranial subdural abscess arising from tooth infection. Because the patient had left pan-sinusitis and the cultures grew Streptococcus viridans confirming the dental origin, they suspected that tooth infection spread intracranially via the frontal sinus and formed an intracranial subdural abscess. In our case, we diagnosed intracranial subdural abscess based on the radiological and intraoperative findings. Because tooth extractions were performed 2 months prior to presentation and A. meyeri, which forms part of the normal oral flora, was cultured, we diagnosed intracranial subdural abscess related to dental treatment. However, there were no findings of sinusitis. We presumed that the infection had spread from the mouth to the dura mater hematogenously. Although the blood culture was certainly negative, a course of oral antibiotics was performed after the tooth extractions, and the negative blood culture did not necessarily rule out a hematogenous infection.10) Additionally, dental extraction is considered to be a risk factor for brain abscess formation, and a chronic course of abscess formation by Actinomyces does not contradict this patient’s clinical course.4) In the present case, we clinically suspected that the intracranial subdural abscess had developed via hematogenous infection secondary to the tooth extractions. To our knowledge, no similar cases have been reported to date.
Actinomycosis is a chronic infection caused by organisms in the genus Actinomyces, with Actinomyces israelii being the most common etiologic agent.3) Actinomyces spp. is a constituent of the oral, gastrointestinal, and genital flora.3,4) Depending on the location affected, actinomycosis is categorized as an oro-cervicofacial, thoracic, or abdominopelvic disease.11) Less frequently, actinomycosis can affect the musculoskeletal system, pericardium, and central nervous system. Infection is often associated with formation of dental plaque and caries.11) Poor oral hygiene, dental procedures, aspiration of oral secretions, abdominal surgery, and acute intra-abdominal processes have been identified as risk factors for actinomycosis.11) In addition, immunocompetent individuals are often affected.5,12) Our patient was also immunocompetent, and, similar to previous reports, dental problems requiring tooth extractions were considered to be the cause of intracranial infection. Most actinomycotic infections are polymicrobic and include other anaerobic or microaerophilic flora such as Actinobacillus actinomycetemcomitans, Fusobacterium spp., and Peptostreptococcus spp.13) In our case, cultures showed growth of A. meyeri and F. nucleatum. These bacteria are reported to belong to the human oropharyngeal flora, and enhance the pathogenicity of Actinomyces spp. by reducing oxygen tension and by anaerobiosis-induced inhibition of phagocytes.14)
The A. meyeri organism itself is very rare.4) Only a few cases of brain abscess by A. meyeri have been reported,4–6) and Table 1 shows a summary of recent reported cases. Dental extraction can be a cause of intracranial infection by A. meyeri. because source of infection was dental extraction in three of four cases. A. meyeri often causes pulmonary infection and shows a tendency for hematogenous dissemination, and Park et al.5) reported a case of disseminated actinomycosis with brain abscess that was initially misdiagnosed as a metastatic tumor. If multiple lesions including lung and brain lesions are found, not only metastatic tumor but also infection, including disseminated actinomycosis caused by A. meyeri, should be ruled out. In addition, although it is extremely rare, clinicians should be aware that disseminated actinomycosis can also cause intracranial subdural abscess, as reported herein.
The conventional antibiotic treatment for actinomycosis is intravenous penicillin, 18 to 24 million units daily for 2-6 weeks, followed by oral penicillin or amoxicillin for an extended period of 6-12 months.5,6) Surgical drainage is critical in the treatment of brain abscess caused by actinomycosis.5,6) The prognosis is favorable, even in patients with disseminated disease including brain abscess.5,6) In our case surgical drainage was useful for the successful treatment of abscess resulting from actinomycosis. We used intravenous metronidazole to cover anaerobic bacteria in addition to intravenous penicillin for 4 weeks. Although metronidazole is useful for anaerobic bacteria and protozoan, clinicians should consider the risk of metronidazole-induced encephalopathy, which is a rare but serious complication.15) Patients with diseases such as brain abscesses, which require long-term and high-dose administration of metronidazole, are at particularly high risk of metronidazole-induced encephalopathy.15) After transition to oral amoxicillin, the patient’s infection was well controlled. Intracranial subdural abscess resulting from A. meyeri should be treated by the same course as for actinomycotic brain abscess.
We experienced an exceptional case of intracranial subdural abscess caused by A. meyeri related to dental treatment. To our best knowledge this is the first case report of intracranial subdural abscess caused by A. meyeri associated with dental treatment.
Acknowledgment
We thank Hugh McGonigle, from Edanz Group (www.edanzediting.com/ac), for editing a draft of the manuscript.
Footnotes
Conflicts of Interest Disclosure
The authors report no competing interests concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1).French H, Schaefer N, Keijzers G, Barison D, Olson S: Intracranial subdural empyema: a 10-year case series. Ochsner J 14: 188–194, 2014 [PMC free article] [PubMed] [Google Scholar]
- 2).Mauser HW, Tulleken CA: Subdural empyema. A review of 48 patients. Clin Neurol Neurosurg 86: 255–263, 1984 [DOI] [PubMed] [Google Scholar]
- 3).Fazili T, Blair D, Riddell S, Kiska D, Nagra S: Actinomyces meyeri infection: case report and review of the literature. J Infect 65: 357–361, 2012 [DOI] [PubMed] [Google Scholar]
- 4).Clancy U, Ronayne A, Prentice MB, Jackson A: Actinomyces meyeri brain abscess following dental extraction. BMJ Case Rep 2015: April 13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Park HJ, Park KH, Kim SH, et al. : A case of disseminated infection due to Actinomyces meyeri involving lung and brain. Infect Chemother 46: 269–273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Vazquez Guillamet LJ, Malinis MF, Meyer JP: Emerging role of Actinomyces meyeri in brain abscesses: a case report and literature review. IDCases 10: 26–29, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).De Bonis P, Anile C, Pompucci A, Labonia M, Lucantoni C, Mangiola A: Cranial and spinal subdural empyema. Br J Neurosurg 23: 335–340, 2009 [DOI] [PubMed] [Google Scholar]
- 8).Bernardini GL: Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep 4: 448–456, 2004 [DOI] [PubMed] [Google Scholar]
- 9).Derin S, Sahan M, Hazer DB, Sahan L: Subdural empyema and unilateral pansinusitis due to a tooth infection. BMJ Case Rep 2015: Jun 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Jiang C, Lu H, Guo Y, et al. : Blood culture-negative but clinically diagnosed infective endocarditis complicated by intracranial mycotic aneurysm, brain abscess, and posterior tibial artery pseudoaneurysm. Case Rep Neurol Med 2018: Nov 8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Könönen E, Wade WG: Actinomyces and related organisms in human infections. Clin Microbiol Rev 28: 419–442, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Akhaddar A, Elouennass M, Baallal H, Boucetta M: Focal intracranial infections due to Actinomyces species in immunocompetent patients: diagnostic and therapeutic challenges. World Neurosurg 74: 346–350, 2010 [DOI] [PubMed] [Google Scholar]
- 13).Colmegna I, Rodriguez-Barradas M, Rauch R, Clarridge J, Young EJ: Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci 326: 152–155, 2003 [DOI] [PubMed] [Google Scholar]
- 14).Apothéloz C, Regamey C: Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis 22: 621–625, 1996 [DOI] [PubMed] [Google Scholar]
- 15).Kato H, Sosa H, Mori M, Kaneko T: Clinical characteristics of metronidazole-induced encephalopathy: a report of two cases and a review of 32 Japanese cases in the literature. Kansenshogaku Zasshi 89: 559–566, 2015. (Japanese) [DOI] [PubMed] [Google Scholar]