Abstract
Basilar artery perforator aneurysms (BAPAs) are a rare cause of subarachnoid hemorrhage (SAH), and the natural history is still unknown. Herein, we report a case of ruptured BAPA that appeared during the observation period and then spontaneously disappeared; we have also conducted a review of the literature and performed an analysis based on the type of management. This case of BAPA had a unique course, and our observations may help establish a treatment strategy. A 60-year-old man presented with acute diffuse SAH, World Federation of Neurosurgical Societies (WFNS) Grade II and Fisher Grade 3. Initial three-dimensional digital subtraction angiography (DSA) did not show the source of the hemorrhage. DSA performed on day 39 showed a BAPA with a diameter of 3 mm at the posterior surface of the upper third of the basilar artery. Conservative treatment was chosen. DSA performed on day 64 showed complete resolution of the aneurysm. BAPAs are likely pseudoaneurysms, and not saccular aneurysms, caused due to dissection of basilar perforator arteries. BAPAs are often not recognized on initial imaging, and hence, it is necessary to repeat the DSA examination. Considering the relatively high rate of spontaneous resolution, we chose conservative management. When BAPAs enlarge or do not disappear after conservative treatment, additional therapy such as multiple stents should be considered.
Keywords: basilar artery perforator aneurysm, subarachnoid hemorrhage
Introduction
Basilar artery perforator aneurysms (BAPAs), first described by Ghogawala et al.1) in 1996, are a rare cause of subarachnoid hemorrhage (SAH). BAPA is an aneurysm with the neck located entirely on a perforating artery without directly involving the basilar trunk.2) Although the number of cases being reported in recent times is increasing, the natural history is still unknown. We report a specific clinical case in which the source of bleeding was not identified at the onset of SAH; a BAPA appeared during the observation period and then spontaneously disappeared. This case of BAPA had a unique clinical course, and our observations may help establish a treatment strategy. We have also conducted a review of the literature and performed an analysis based on the type of management.
Case Report
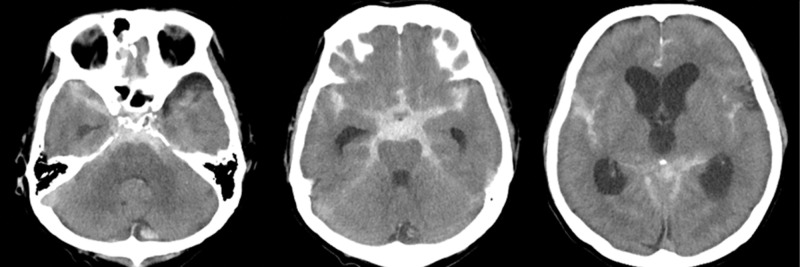
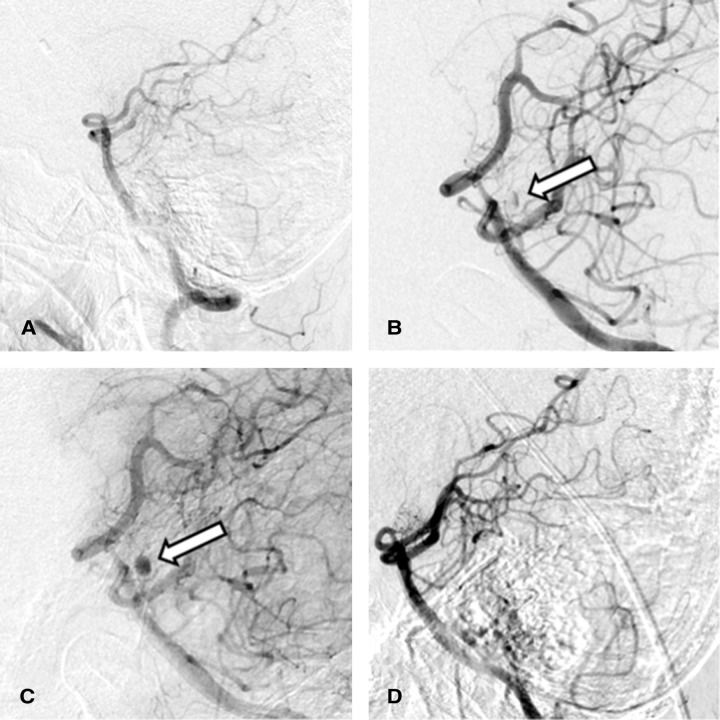
A 60-year-old man with a history of hypertension and type 2 diabetes mellitus was admitted to the hospital for sudden headache in the occipital area. He was drowsy on admission, but there were no other neurologic deficits (World Federation of Neurosurgical Societies [WFNS] Grade II). A head computed tomography (CT) scan showed diffuse SAH mainly in the posterior fossa with hydrocephalus (Fisher Grade 3) (Fig. 1). Computed tomographic angiography (CTA) and three-dimensional digital subtraction angiography (DSA) did not show the source of bleeding (Fig. 2A). We only performed ventricular drainage at first, and decided to re-examine the bleeding source later. A second CTA performed on day 9 also did not show the source of bleeding. Cerebral infarction due to vasospasm occurred in the bilateral frontal lobes on day 9, resulting in disturbance in consciousness and quadriparesis. A ventriculoperitoneal (VP) shunt was performed for hydrocephalus on day 31. DSA performed on day 39 showed an aneurysm with a diameter of 3 mm at the posterior surface of the upper third of the basilar artery. The blood flow into the aneurysm was very slow; therefore, it was observed in the late arterial phase. Although the aneurysm was close to the basilar artery, the artery was not directly involved (Figs. 2B and 2C). We diagnosed this aneurysm as BAPA and chose conservative management. DSA performed on day 64 showed complete resolution of the aneurysm (Fig. 2D). Although the patient was able to talk and was conscious, quadriparesis was still present (modified Rankin Scale [mRS] score = 5), and he was transferred to a rehabilitation hospital.
Fig. 1.
Non-contrast CT of the head demonstrated ventriculomegaly and diffuse subarachnoid hemorrhage predominantly in the perimesencephalic cistern (Fisher Grade 3). CT: computed tomography.
Fig. 2.
(A) Initial DSA was negative for the source of hemorrhage. (B) DSA performed on day 39 showed a 3 mm aneurysm (arrow) at the posterior surface of the upper third of the basilar artery. (C) The aneurysm (arrow) was observed in the late arterial phase of the same DSA, as shown in (B), without directly involving the basilar trunk. (D) DSA performed on day 64 showed complete resolution of the aneurysm. DSA: digital subtraction angiography.
Discussion
Although BAPAs are a rare cause of SAH, increasing number of cases are being reported due to the evolution in imaging technology. A review of 36 cases concluded that BAPAs are very small with a diameter ranging from 0.5 to 7 mm (mean 2.5 mm).3) BAPAs are thought to be of dissecting origin for two reasons: (1) the shape of the aneurysm is often described as dolichoectatic (fusiform), or with a large base and (2) variation is observed in the size of the aneurysm between closely repeated angiograms.4) The blood flow into the aneurysm is quite slow, which was also observed in our case; further, intraluminal thrombus was found in a previous report.5) A hypertension-induced dissecting aneurysm has been reported in a lenticulostriate artery, and the disrupted internal elastic lamina caused by hemodynamic stress has been confirmed histologically.6) Although we did not find any histological report of BAPA, hypertension may play a role in BAPA formation. In our case, the aneurysm was not visualized on the initial DSA, but appeared on day 39, and disappeared on day 61. The shape of the aneurysm had changed, and the aneurysm was separated from the basilar artery. The aneurysm may not have been a saccular aneurysm but a pseudoaneurysm caused due to dissection of a basilar perforator artery. The aneurysm that was observed in the late arterial phase seemed to have a high possibility of causing occlusion by thrombus, and conservative treatment would be appropriate. Basilar perforator arteries are divided into 3 groups: proximal, middle, and distal. All BAPAs are associated with the middle or distal perforators. Moreover, in contrast to proximal perforating artery aneurysms, distal perforating artery aneurysms are extremely rare.3,5) The average diameter of the middle and distal perforators is thicker than that of proximal perforators. The branch angle from the basilar artery is upward to horizontal in the middle and distal perforators, but in the proximal perforators the angle is downward.7) These may affect hemodynamic stress and define the site of BAPAs.
BAPAs are often not recognized on the initial imaging due to the elevation of intracranial pressure caused by bleeding. In a single-center study, it was observed that only 22% of ruptured aneurysms were visualized on the initial imaging.8) In 45% of cases, the hemorrhage was located in the perimesencephalic or prepontine region.4) In cases of SAH around the brainstem with no aneurysm on the initial imaging, it is necessary to repeat the DSA, keeping the possibility of BAPAs in mind. In SAH due to ruptured BAPAs, rebleeding or vasospasm has been reported, and the clinical course is clearly different from that of benign perimesencephalic SAH; distinguishing between the two is important especially in mild SAH.
We performed an analysis of previous reports and our own case based on the type of treatment provided; 23 patients who received conservative management (Table 1)2-4,8-13) and 18 who underwent endovascular treatment (Table 2)3,8,10,11,13-18) were analyzed. Three patients were excluded because they were described in two different articles.8,10) Although there have been cases wherein direct surgery was performed, many perioperative complications have been reported.1,5,19-21) As BAPAs are often located on the anterior surface of the brainstem, performing direct surgery is very difficult. Furthermore, the fact that BAPAs are perforator pseudoaneurysms may be one of the reasons for numerous complications in direct surgery.
Table 1. Characteristics of previously reported basilar artery perforator aneurysms managed with conservative treatment.
| Study | Age(years)/Sex | WFNS/H&H Grade | Fisher Grade | Bleed pattern | Detection on initial angiogram | Time until aneurysm detection | Size(mm) | Origin of the perforator on the basilar artery | Location of aneurysm from the origin of the perforator artery | Treatment | Pontine stroke | Complications | FU(months) | Time until aneurysm disappearance | GOS/mRS Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Park et al. (2009)9) | 54/F | WFNS I | 2 | pm | Yes | 1 | Distal 1/3 | Proximal | Conservative | No | Third nerve palsy, hemiparesis, vasospasm | 16 | 16 months | GOS 5 | ||
| Park et al. (2009)9) | 67/M | WFNS I | 2 | pm | Yes | 1 | Distal 1/3 | Proximal | Conservative | No | None | 15 | 16 months | GOS 5 | ||
| Park et al. (2009)9) | 53/M | H&H 1 | 2 | pm | Yes | 1 | Distal 1/3 | Proximal | Conservative | No | None | 1 | 1 month | GOS 5 | ||
| Ding et al. (2013)10) | 55/NA | H&H 2 | 3 | Diffuse | No | 1 week | 1.8 | Distal 1/3 | Proximal | Conservative | No | None | 19 | 6 months | GOS 5 | |
| Chavent et al. (2014)4) | 55/M | WFNS I | 3 | Diffuse | No | 8 days | 1.7 | Distal 1/3 | Proximal | Conservative | No | None | 6 | 3 months | mRS 0 | |
| Chavent et al. (2014)4) | 39/F | WFNS I | 2 | pm | No | 8 days | 1.5 | Distal 1/3 | Proximal | Conservative | No | None | 12 | 3 months | mRS 0 | |
| Chavent et al. (2014)4) | 56/M | WFNS I | 3 | Diffuse | No | 8 days | 1 | Distal 1/3 | Proximal | Conservative | No | None | 12 | 3 months | mRS 0 | |
| Forbrig et al. (2016)11) | 71/F | WFNS V | 4 | Diffuse | Yes | 7 | Middle 1/3 | Proximal | Conservative(failed end) | Yes | Mild hemiparesis, hydrocephalus, VP shunt | 11 | 7 days | mRS 1 | ||
| Forbrig et al. (2016)11) | 65/M | WFNS I | 4 | Diffuse | No | 8 days | 1 | Middle 1/3 | Proximal | Conservative | Yes | Mild hemiparesis, hydrocephalus, VP shunt | 15 | Unknown | mRS 1 | |
| Forbrig et al. (2016)11) | 82/M | WFNS V | 4 | Diffuse | Yes | 2 | Distal 1/3 | Proximal | Conservative(failed end) | Yes | Rebleeding 20 days after SAH, severe hemiparesis, dysarthria | 6 | Unknown | mRS 5 | ||
| Forbrig et al. (2016)11) | 60/F | WFNS I | 3 | Diffuse | Yes | 2.5 | Middle 1/3 | Proximal | conservative(failed End) | Yes | Vasospasm | 78 | 2 months | mRS 0 | ||
| Forbrig et al. (2016)11) | 53/M | WFNS I | 3 | Diffuse | No | 47 days | 1 | Distal 1/3 | Proximal | Conservative | No | None | 6 | 3 months | mRS 0 | |
| Aboukais et al. (2016)2) | 67/M | WFNS I | 2 | pm | No | 6 days | 3 | Middle 1/3 | Proximal | Conservative | No | None | 1.5 | 6 weeks | mRS 0 | |
| Daruwalla et al. (2016)12) | 76/M | H&H 4 | 4 | pm | Yes | 2.5 | Middle 1/3 | Proximal | Conservative | No | Died on day 16 because of poor general condition, acute hydrocephalus, EVD | NA | 4 days | mRS 6 | ||
| Finitsis et al. (2017)3) | 59/M | WFNS I | 3 | Diffuse | No | 9 days | 0.5 | Distal 1/3 | Proximal | Conservative | No | None | 2 | 6 weeks | mRS 0 | |
| Finitsis et al. (2017)3) | 62/F | WFNS II | 4 | Diffuse | No | 4 days | 1 | Middle 1/3 | Proximal | Conservative/end (FD) | No | Rebleeding day 10 post-SAH, ptosis, hemiparesis, hypoacusis | 3 | 3 months | mRS 0 | |
| Finitsis et al. (2017)3) | 78/M | WFNS IV | 4 | Diffuse | Yes | 16 days | 3 | Distal 1/3 | Proximal | Conservative | Yes | Quadriparesis | 14 | unknown | mRS 5 | |
| Finitsis et al. (2017)3) | 53/F | WFNS II | 3 | NA | No | 7 days | 1.2 | Middle 1/3 | Proximal | Conservative | No | None | 2 | 6 weeks | mRS 0 | |
| Buell et al. (2018)8) | NA/NA | H&H 2 | NA | NA | No | 7 days | 1 | Distal 1/3 | NA | Conservative | No | None | 2 | NA | mRS 1 | |
| Buell et al. (2018)8) | NA/NA | H&H 3 | 4 | Diffuse | No | 5 days | 2 | Distal 1/3 | Proximal | Conservative | No | Acute hydrocephalus, EVD, VP shunt | 42 | 1 week | mRS 1 | |
| Buell et al. (2018)8) | NA/NA | H&H 3 | NA | NA | No | 5 days | 1.7 | Middle 1/3 | NA | Conservative | No | None | 62 | NA | mRS 0 | |
| Chau et al. (2018)13) | 69/M | WFNS IV | 4 | Diffuse | No | 2 months | 2.5 | Distal 1/3 | Proximal | Conservative | No | Acute hydrocephalus, EVD | 12 | 12 months | mRS 0 | |
| Current study | 60/M | WFNS II | 3 | Diffuse | No | 39 days | 3 | Distal 1/3 | Proximal | Conservative | No | Acute hydrocephalus, EVD, VP shunt, quadriparesis from vasospasm | 19 | 9 weeks | mRS 5 |
End: endovascular, EVD: extraventricular drainage, F: female, FD, flow diverter, FU: follow-up, GOS: Glasgow Outcome Scale, H&H: Hunt and Hess, M: male, mRS: modified Rankin Scale, NA: not available, pm: perimesencephalic, SAH: subarachnoid hemorrhage, VP: ventriculoperitoneal, WFNS: World Federation of Neurosurgical Societies.
Table 2. Characteristics of previously reported basilar artery perforator aneurysms managed with endovascular treatment.
| Study | Age(years)/sex | WFNS/H&H Grade | Fisher Grade | Bleed pattern | Detection on initial angiogram | Time until aneurysm detection | Size(mm) | Origin of the perforator on the basilar artery | Location of the aneurysm from the origin of perforator artery | Treatment | Pontine stroke | Complications | FU(months) | Time until aneurysm disappearance | GOS/mRS Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2012)14) | 66/M | H&H 3 | 3 | pp | yes | 7 | Middle 1/3 | Distal | End (coiling) | No | None | 24 | Immediate | GOS 4 | ||
| Nyberg et al. (2013)15) | 45/M | NA | NA | pm | no | 2 months | NA | Middle 1/3 | Proximal | End (stents) | No | None | 14 | 2 months | GOS 5 | |
| Nyberg et al. (2013)15) | 65/F | NA | NA | pm | no | 9 weeks | 2 | Middle 1/3 | Proximal | End (stents) | No | None | 4 | 1 month | GOS 5 | |
| Ding et al. (2013)10) | 58/NA | H&H 2 | 3 | Diffuse | yes | 2 | Middle 1/3 | Proximal | End (failed) | Yes | Quadriparesis, facial nerve palsy, dysarthria | NA | Unknown | GOS 3 | ||
| Ding et al. (2013)10) | 62/NA | H&H 3 | 4 | Diffuse | no | 2 weeks | 1.9 | Distal 1/3 | Proximal | End (Onyx) | Yes | Hemiparesis, dysarthria, acute hydrocephalus, EVD, VP shunt | 22 | Immediate | GOS 3 | |
| Chalouhi. (2014)16) | NA/F | WFNS I | 4 | Diffuse | no | 3 days | 1.5 | Middle 1/3 | Proximal | End (FD) | No | None | 6 | 2 weeks | mRS 0 | |
| Peschillo et al. (2016)17) | NA/M | WFNS II | 4 | pm | yes | 1.5 | Distal 1/3 | Proximal | End (FD) | Yes | In-stent thrombosis reversed with abciximab, monoparesis | 6 | 13 days | mRS 2 | ||
| Peschillo et al. (2016)17) | NA/M | WFNS II | 4 | Diffuse | no | NA | 1.2 | Distal 1/3 | Proximal | End (stent + FD) | No | In-stent thrombosis reversed with tirofiban | 34 | 1 day | mRS 0 | |
| Peschillo et al. (2016)17) | NA/NA | WFNS IV | 4 | pm | no | NA | 1.5 | Distal 1/3 | NA | End (FD) | No | Vasospasm, acute hydrocephalus, ICH post-EVD meningitis | 6 | 1 day | mRS 2 | |
| Forbrig et al. (2016)11) | 72/M | WFNS II | 4 | Diffuse | no | 18 days | 2 | Distal 1/3 | Proximal | End (failed) | No | Mild cognitive impairment, gait disorder, vasospasm, VP shunt | 5 | Immediate | mRS 2 | |
| Forbrig et al. (2016)11) | 59/M | WFNS II | 3 | pp | no | 13 day | 2.5 | Distal 1/3 | Proximal | End (coiling) | Yes | Rebleeding 13 days after SAH, hemiparesis, VA shunt | 23 | Immediate | mRS 2 | |
| Finitsis et al. (2017)3) | 62/F | WFNS II | 4 | Diffuse | no | 4 days | 1 | Middle 1/3 | Proximal | Conservative/End (FD) | No | Rebleeding day 10 post-SAH, ptosis, hemiparesis, hypoacusis | 3 | 3 months | mRS 0 | |
| Satti et al. (2017)18) | 52/M | H&H III | 4 | Diffuse | no | 8 days | 1.8 | Middle 1/3 | Proximal | End (stents/stent) | Yes | Acute hydrocephalus, EVD, vasospasm, growth of aneurysm, hemiparesis, abducens nerve palsy, dissection | 7 | Immediate | mRS 0 | |
| Buell et al. (2018)8) | NA/NA | H&H 3 | NA | NA | no | 6 days | 1.5 | Middle 1/3 | NA | End (stents) | No | None | 11 | NA | mRS 1 | |
| Buell et al. (2018)8) | NA/NA | H&H 1 | 3 | Diffuse | no | 5 days | 2.5 | Middle 1/3 | Proximal | End (stents) | No | None | 12 | 4 months | mRS 1 | |
| Buell et al. (2018)8) | NA/NA | H&H 4 | NA | NA | yes | 2 | Distal 1/3 | NA | End (failed) | NA | Rupture of perforator artery during microguidewire manipulation | NA | NA | mRS 6 | ||
| Chau et al. (2018)13) | 53/NA | WFNS I | 4 | Diffuse | yes | 1.8 | Distal 1/3 | Proximal | End (stents/coiling) | Yes | Vasospasm, recurrence of aneurysm | 6 | 6 months | mRS 0 | ||
| Chau et al. (2018)13) | 59/NA | WFNS I | 3 | Diffuse | No | 5 days | 1.5 | Distal 1/3 | NA | End (stents) | No | None | 6 | 6 months | mRS 0 |
End: endovascular, EVD: extraventricular drainage, F: female, FD: flow diverter, FU: follow-up, GOS: Glasgow Outcome Scale, H&H: Hunt and Hess, ICH: intracranial hemorrhage, M: male, mRS: modified Rankin Scale, NA: not available, pm: perimesencephalic, pp: prepontine, SAH: subarachnoid hemorrhage, VA: ventriculoatrial, VP: ventriculoperitoneal, WFNS: World Federation of Neurosurgical Societies.
In the conservative group, the aneurysm was occluded spontaneously without complications in 12 patients (52.2%). Two patients (8.7%) experienced rebleeding, five (21.7%) presented with a pontine stroke, three (13.0%) had symptomatic vasospasm, and four (17.4%) underwent VP shunt for hydrocephalus. The outcomes of 19 patients (82.6%) were excellent, with mRS scores of 0–1 (Glasgow Outcome Scale [GOS] score 5). The outcomes of 4 patients (17.4%) were poor, with mRS scores of 5–6; the poor prognostic factors in these patients were severe brain stem infarction in two cases, infarction due to vasospasm in one, and poor general condition in one. When conservative therapy was chosen for the usual ruptured saccular aneurysms, the rebleeding rate was 20%–30% within a month.22) Moreover, in ruptured dissecting aneurysms of the vertebrobasilar trunk, the possibility of rebleeding in the acute phase was much higher.23) On the other hand, BAPAs follow a more benign clinical course compared to these.
Endovascular treatment by coil or onyx was attempted in six cases and accomplished in three cases. In the failed cases, perioperative complications occurred and became unfavorable prognostic factors. Although aneurysms immediately disappeared in all three cases where endovascular treatment was accomplished, brain stem infarction developed in two (66.7%). Anastomoses among various perforating arteries of the basilar artery were found in 41.6%–66.6% of cases.7) Considering that BAPA is a dissecting aneurysm in the proximal part of the perforator arteries, treatments such as clipping or coil embolization that occlude the BAPA may pose a risk for brainstem ischemia.
Deployment of multiple stents or flow diverter (FD) in the basilar artery is a treatment that reduces blood flow into the BAPAs and promotes thrombosis while preserving the blood flow of the perforating arteries. In the Stents/FD group, the aneurysms disappeared without complications in seven patients (58.3%). In one case, the FD was placed after rebleeding during conservative treatment and there were no complications after FD deployment.3) Two cases (16.7%) required additional treatment due to recurrence or growth of the aneurysm.13,18) Other complications were intracranial hemorrhage other than rebleeding in one patient (8.3%), pontine infarction in three (25.0%), and symptomatic vasospasm in three (25.0%). No patient experienced rebleeding or underwent VP shunt placement. Ten patients (83.3%) had a mRS score of 0–1 (GOS score 5). Two patients (16.7%) had a mRS score of 2; the unfavorable prognostic factors in these patients were brainstem infarction due to in-stent thrombosis and intracranial hemorrhage around the ventricular drain. Thus, when comparing the conservative group and the Stents/FD group, the ratio of disappearance of the aneurysm without complications was 12/23 (52.2%) and 7/12 (58.3%), respectively. The ratio of good prognosis (mRS score 0–1 [GOS score 5]) in the two groups was 19/23 (82.6%) and 10/12 (83.3%), respectively. Therefore, conservative treatment for BAPAs is acceptable, and additional therapy such as multiple stents or FD should be considered when BAPAs increase in size or do not disappear after conservative treatment of the aneurysms. The possible interventional treatment may be desirable only for a mild grade case in young individuals because of a small risk of rebleeding in the conservative group. For the endovascular treatment to be considered as first-line therapy, it seems necessary to administer perioperative antithrombotic therapy for safety.
In our analysis, no aneurysm was detected on the initial angiogram in 27 cases. The median time until aneurysm detection was 8 days (interquartile range, 6–14). Therefore, DSA should be repeated within 8 days when the initial DSA is negative. In the conservative group, the rebleeding occurred 6 days and 20 days after the detection of the aneurysm. Although the number of cases used to conclude the above indication is limited, it seems necessary to repeat the DSA within a week after the detection.
Conclusion
SAH caused by ruptured BAPA is rare, and there may be many cases where BAPAs are not recognized in the acute phase. BAPAs may spontaneously disappear by conservative treatment. When BAPAs enlarge or do not disappear, additional therapy such as multiple stents or FD should be considered.
Footnotes
Conflicts of Interest Disclosure
All authors have no conflicts of interest regarding this article. All authors who are members of The Japan Neurosurgical Society (JNS) have registered with the online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Ghogawala Z, Shumacher JM, Ogilvy CS: Distal basilar perforator artery aneurysm: case report. Neurosurgery 39: 393–396, 1996 [DOI] [PubMed] [Google Scholar]
- 2).Aboukais R, Zairi F, Estrade L, Quidet M, Leclerc X, Lejeune JP: A dissecting aneurysm of a basilar perforating artery. Neurochirurgie 62: 263–265, 2016 [DOI] [PubMed] [Google Scholar]
- 3).Finitsis S, Derelle AL, Tonnelet R, Anxionnat R, Bracard S: Basilar perforator aneurysms: presentation of 4 cases and review of the literature. World Neurosurg 97: 366–373, 2017 [DOI] [PubMed] [Google Scholar]
- 4).Chavent A, Lefevre PH, Thouant P, et al. : Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg 121: 1107–1111, 2014 [DOI] [PubMed] [Google Scholar]
- 5).Sanchez-Mejia RO, Lawton MT: Distal aneurysms of basilar perforating and circumferential arteries. Report of three cases. J Neurosurg 107: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 6).Mizutani T, Kojima H, Miki Y: Arterial dissections of penetrating cerebral arteries causing hypertension-induced cerebral hemorrhage. J Neurosurg 93: 859–862, 2000 [DOI] [PubMed] [Google Scholar]
- 7).Marinković SV, Gibo H: The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery 33: 80–87, 1993 [DOI] [PubMed] [Google Scholar]
- 8).Buell TJ, Ding D, Raper DMS, et al. : Posterior circulation perforator aneurysms: a proposed management algorithm. J Neurointerv Surg 10: 55–59, 2018 [DOI] [PubMed] [Google Scholar]
- 9).Park SQ, Kwon OK, Kim SH, Oh CW, Han MH: Pre-mesencephalic subarachnoid hemorrhage: rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien) 151: 1639–1646, 2009 [DOI] [PubMed] [Google Scholar]
- 10).Ding D, Starke RM, Jensen ME, Evans AJ, Kassell NF, Liu KC: Perforator aneurysms of the posterior circulation: case series and review of the literature. J Neurointerv Surg 5: 546–551, 2013 [DOI] [PubMed] [Google Scholar]
- 11).Forbrig R, Eckert B, Ertl L, et al.: Ruptured basilar artery perforator aneurysms--treatment regimen and long-term follow-up in eight cases. Neuroradiology 58: 285–291, 2016 [DOI] [PubMed] [Google Scholar]
- 12).Daruwalla VJ, Syed FH, Elmokadem AH, Hurley MC, Shaibani A, Ansari SA: Large basilar perforator pseudoaneurysm: a case report. Interv Neuroradiol 22: 662–665, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Chau Y, Sachet M, Sédat J: Should we treat aneurysms in perforator arteries from the basilar trunk? Review of 49 cases published in the literature and presentation of three personal cases. Interv Neuroradiol 24: 22–28, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Chen L, Chen E, Chotai S, Tian X: An endovascular approach to ruptured aneurysms of the circumferential branch of the basilar artery. J Clin Neurosci 19: 527–531, 2012 [DOI] [PubMed] [Google Scholar]
- 15).Nyberg EM, Chaudry MI, Turk AS, Spiotta AM, Fiorella D, Turner RD: Report of two cases of a rare cause of subarachnoid hemorrhage including unusual presentation and an emerging and effective treatment option. J Neurointerv Surg 5: e30, 2013 [DOI] [PubMed] [Google Scholar]
- 16).Chalouhi N, Jabbour P, Starke RM, et al. : Treatment of a basilar trunk perforator aneurysm with the pipeline embolization device: case report. Neurosurgery 74: E697–701; discussion 701, 2014 [DOI] [PubMed] [Google Scholar]
- 17).Peschillo S, Caporlingua A, Cannizzaro D, et al.: Flow diverter stent treatment for ruptured basilar trunk perforator aneurysms. J Neurointerv Surg 8: 190–196, 2016 [DOI] [PubMed] [Google Scholar]
- 18).Satti SR, Vance AZ, Fowler D, Farmah AV, Sivapatham T: Basilar artery perforator aneurysms (BAPAs): review of the literature and classification. J Neurointerv Surg 9: 669–673, 2017 [DOI] [PubMed] [Google Scholar]
- 19).Hamel W, Grzyska U, Westphal M, Kehler U: Surgical treatment of a basilar perforator aneurysm not accessible to endovascular treatment. Acta Neurochir (Wien) 147: 1283–1286, 2005 [DOI] [PubMed] [Google Scholar]
- 20).Mathieson CS, Barlow P, Jenkins S, Hanzely Z: An unusual case of spontaneous subarachnoid haemorrhage - a ruptured aneurysm of a basilar perforator artery. Br J Neurosurg 24: 291–293, 2010 [DOI] [PubMed] [Google Scholar]
- 21).Gross BA, Puri AS, Du R: Basilar trunk perforator artery aneurysms. Case report and literature review. Neurosurg Rev 36: 163–168; discussion 168, 2013 [DOI] [PubMed] [Google Scholar]
- 22).Mayberg MR, Batjer HH, Dacey R, et al. : Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 25: 2315–2328, 1994 [DOI] [PubMed] [Google Scholar]
- 23).Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T: Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36: 905–911; discussion 912-913, 1995 [DOI] [PubMed] [Google Scholar]