Abstract
Methotrexate (MTX) is an immunosuppressor that is widely used to treat autoimmune diseases, including rheumatoid arthritis (RA). However, it can have serious adverse effects including a lymphoma: MTX-associated lymphoproliferative disorder (MTX-LPD). Extranodal lesions are common in MTX-LPD patients. However, MTX-LPD in the central nervous system (CNS) is extremely rare with few reported cases. Here, we describe a case of primary CNS MTX-LPD in a patient with RA, with a review of the literature. A 68-year-old woman who had received MTX for her RA for more than 10 years was referred to our hospital. Head magnetic resonance imaging (MRI) showed multiple lesions with heterogeneous contrast enhancement scattered throughout both hemispheres. As immunosuppression caused by MTX was suspected, MTX was discontinued, based on a working diagnosis of MTX-LPD. We performed an open biopsy of her right temporal lesion. Histopathologic examination showed atypical CD20+ lymphoid cells, leading to a definitive diagnosis of diffuse large B-cell lymphoma (DLBCL). In situ hybridization of an Epstein-Barr virus-encoded small RNA (EBER) was positive. Sanger sequencing confirmed that both MYD88 L265 and CD79B Y196 mutations were absent. The LPD regressed after stopping MTX. Follow-up head MRI at 8 months after surgery showed no evidence of recurrence. Although primary CNS MTX-LPD is extremely rare, it should be included in the differential diagnosis when a patient receiving MTX develops CNS lesions. Diagnosis by biopsy and MTX discontinuation are required as soon as possible.
Keywords: CD79B, Methotrexate, MTX-associated LPD, MYD88, Primary central nervous system lymphoma
Introduction
Methotrexate (MTX) is an effective immunosuppressant that is widely used as a standard therapy for rheumatoid arthritis (RA).1) Among its various adverse effects, lymphoma was reported for the first time in 1991 in a patient with RA who was taking weekly low-dose MTX.2) Since that time, lymphoproliferative disorder (LPD) as a result of immunosuppression caused by MTX (MTX-associated LPD; MTX-LPD) has been attracting attention.3) MTX-LPDs are categorized as “other iatrogenic immunodeficiency-associated LPDs” in the World Health Organization’s classification.4) Characteristically, MTX-LPD can regress spontaneously after stopping MTX.3,5) This suggests that the tumor cells proliferate because of MTX-induced immunosuppression. Additionally, MTX-LPD may be associated with Epstein-Barr virus (EBV); 30%–50% of patients with MTX-LPD are reportedly EBV+.3) Extranodal lesions are found in 40%–50% of MTX-LPD patients,3) commonly at the gastrointestinal tract, skin, liver, and lungs.3,5,6) However, MTX-LPD in the central nervous system (CNS) is extremely rare, with few reported cases.7–15) Here, we describe a rare case of primary CNS MTX-LPD in a patient with RA, and review the literature.
Case Report
Clinical history and presentation
A 68-year-old woman with a 2-day history of motor aphasia and right hemiparesis was referred to our hospital because of CNS lesions. She had been receiving MTX (8 mg/week) for her RA for more than 10 years.
Laboratory data showed a normal white blood cell count (8000/µL). Her serum soluble interleukin (IL)-2 receptor was slightly elevated (542 U/mL; normal: 145–519 U/mL). Her levels of serum lactate dehydrogenase level (189 IU/L; normal: 106–211 IU/L), C-reactive protein (0.2 mg/dL; normal: < 0.6 mg/dL) and serum β2-microglobulin (1.5 mg/L; normal: 0.9–1.9 mg/L) were normal. Serological tests for human immunodeficiency virus, human T-cell lymphotropic virus type 1, and cytomegalovirus were negative. EBV antibody showed a pattern indicative of previous infection (EB-VCA-IgG, 640 titer; EBV-VCA-IgM, negative; anti-EBNA, 40 titer). The cerebrospinal fluid protein content was slightly elevated at 46 mg/dL, but the cell count was normal.
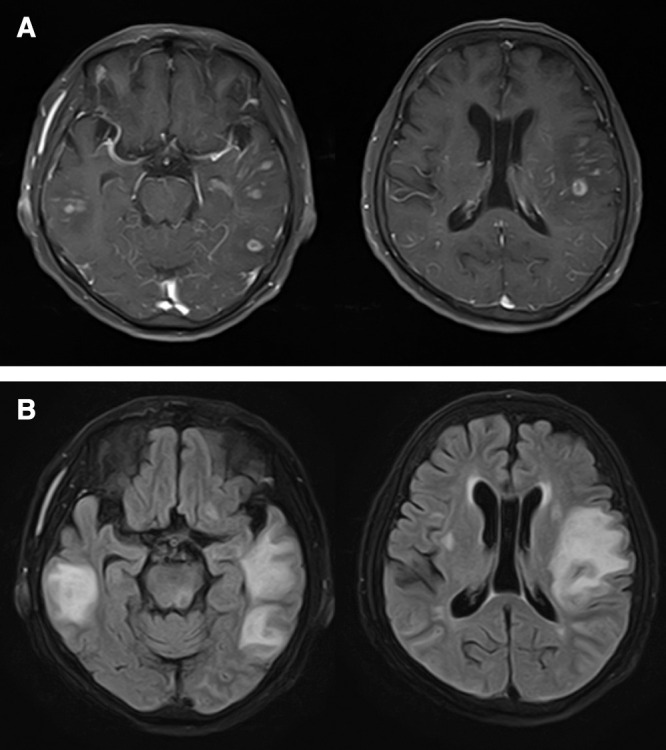
Head magnetic resonance imaging (MRI) showed multiple lesions scattered throughout both hemispheres with heterogeneous contrast enhancement on gadolinium contrast-enhanced T1-weighted images and peritumoral edema on fluid attenuation inversion recovery images (Fig. 1A and 1B). Whole-body computed tomography (CT) showed no apparent lymphadenopathies or other lesions suggestive of malignancy. The imaging-based differential diagnoses were metastasis, malignant lymphoma, or glioma.
Fig. 1. (A) Preoperative gadolinium-enhanced T1-weighted head magnetic resonance images shows multiple lesions with heterogeneous contrast enhancement. (B) Fluid attenuation inversion recovery images show surrounding high-intensity area.
Immunosuppression caused by MTX was also suspected and MTX was discontinued, based on a working diagnosis of MTX-LPD. However, on Day 4 after admission, the patient’s right hemiparesis worsened; a head CT showed expanded peritumoral edema. Therefore, we performed an open biopsy of the right temporal lesion for a definitive diagnosis on Day 9 after admission and initiated steroid therapy.
Pathological and molecular findings
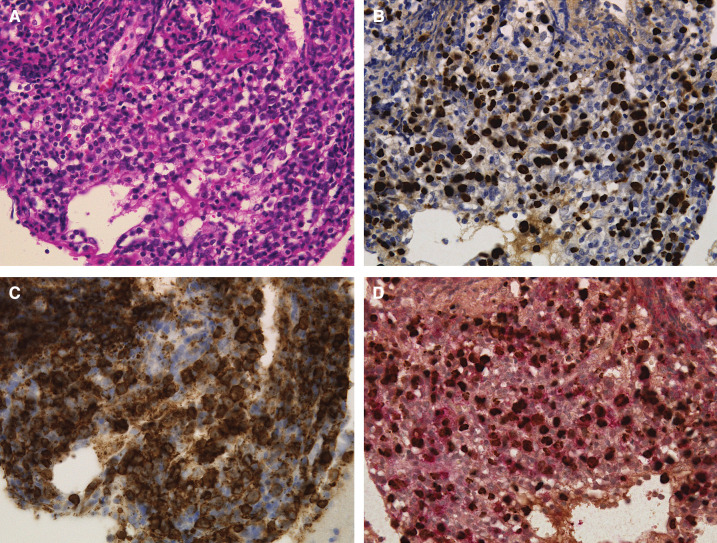
Microscopic histopathologic examination of the tumor specimen showed proliferation of atypical lymphoid cells (hematoxylin and eosin; Fig. 2A). These atypical lymphoid cells were CD20+ (Fig. 2B). In situ hybridization of an Epstein-Barr virus-encoded small RNA (EBER) was positive (Figs. 2C and 2D). A definitive diagnosis of diffuse large B-cell lymphoma (DLBCL) was established.
Fig. 2. (A) Hematoxylin and eosin staining shows proliferation of medium-sized atypical lymphoid cells (× 400). (B) Atypical lymphoid cells were positive for CD20 (× 400). (C) In situ hybridization of an EBER was positive (× 400). (D) Atypical lymphoid cells were doubly positive for double staining for CD20 and EBER (× 400). EBER: Epstein-Barr virus-encoded small RNA.
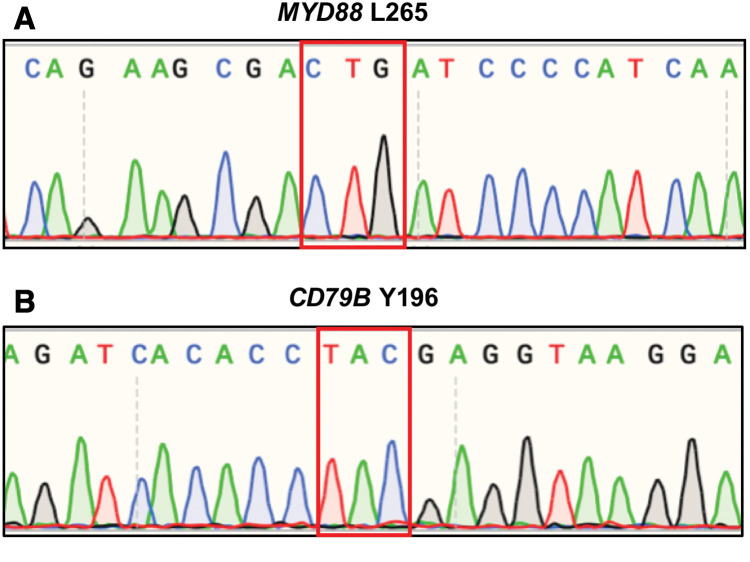
To detect MYD88 L265 and CD79B Y196 mutations, which are frequent mutations in primary CNS lymphoma (PCNSL), genomic DNA was extracted from formalin-fixed and paraffin-embedded specimens using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) and amplified by polymerase chain reaction. We used the following primer sequences: MYD88 L265: forward primer, 5'-GGGATGGCTGTTGTTAACCCT-3' and reverse primer, 5'-GGTGTAGTCGCAGACAGTGAT-3'.16) CD79B Y196: forward primer, 5'-TCTTGCAGAATGCACCTCAC-3' and reverse primer, 5'-GCAGCGTCACTATGTCCTCA-3'.17) The polymerase chain reaction products were then sequenced on a 3130xl genetic analyzer (Applied Biosystems, Foster City, CA, USA) with a Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems), in accordance with the manufacturer’s instructions. Sanger sequencing confirmed that both MYD88 L265 and CD79B Y196 mutations were absent (Figs. 3A and 3B).
Fig. 3. (A) MYD88 L265 mutation was absent. (B) CD79B Y196 mutation was absent.
Clinical outcome
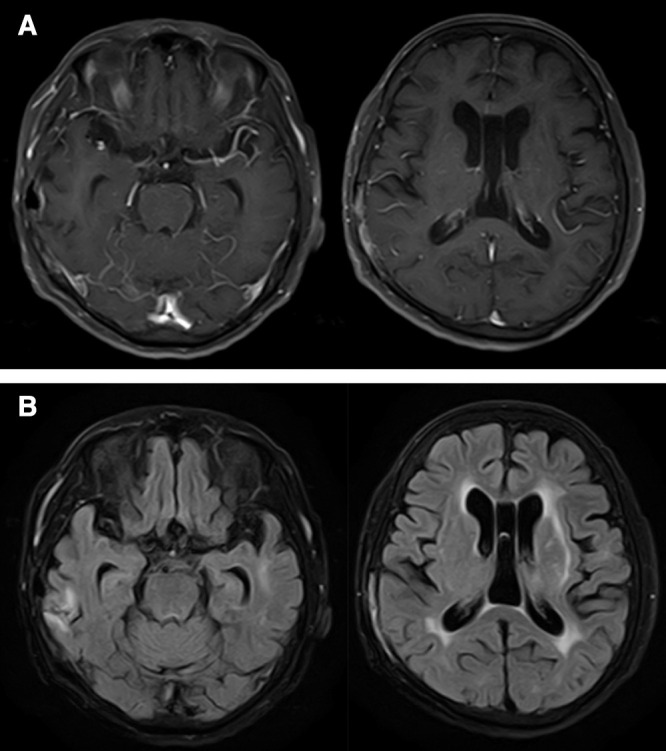
The patient’s postoperative course was uneventful and her neurological symptoms gradually improved. Three weeks after withdrawing MTX, a head MRI showed decreased high-intensity areas on fluid attenuation inversion recovery images and multiple contrast-enhanced lesions on gadolinium contrast-enhanced T1-weighted images. She was discharged without neurological symptoms, and her RA has remained stable with low-dose corticosteroids. A follow-up head MRI at 8 months after surgery showed no evidence of recurrence (Figs. 4A and 4B).
Fig. 4. (A) Follow-up gadolinium contrast-enhanced T1-weighted magnetic resonance images and (B) fluid attenuation inversion recovery images at 8 months after surgery show no evidence of recurrence.
Discussion
Here, we report a rare case of primary CNS MTX-LPD in a patient with RA, in whom DLBCL was definitively diagnosed by an open biopsy. The patient’s disease course was typical for EBV+ MTX-LPD. Her LPD spontaneously regressed after MTX cessation.
Characteristics of primary CNS MTX-LPD
In a literature search, we found nine cases of primary CNS MTX-LPD, which are summarized with the present case in Table 1.7–15) The primary disease was RA in all 10 patients. The affected primary CNS MTX-LPD sites were the cerebrum (8/10), medulla (1/10), and dura (1/10). Of the eight cases of cerebral MTX-LPD, seven (including our case) presented as multiple lesions; only one case presented as a solitary lesion.7,8,10,11,13–15) One case with medullary LPD presented with a solitary mass,9) and a case with hypertrophy of the dura mater presented as intravascular large B-cell lymphoma (IVLBCL).12)
Table 1. Reported cases of primary central nervous system methotrexate-associated lymphoproliferative disorder.
| Study | Age/ sex | MTX mg/week (yr) | Other immune suppressors | Neurological symptoms | LPD site/properties | Biopsy site | Histopathology | EBER | Regression after MTX withdrawal | Additional LPD treatment | Outcome | Follow-up duration | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kleinschmidt 2008.7) | 78/F | Standard dose (>10) | NA | NA | Cerebrum, multiple lesions | Cerebrum | P/L LPD | Yes | Yes | No | Died 3 mo later; multi-organ failure attributable to RA | 3 mo |
| 2 | Fukushima 2013.8) | 64/F | 6 (4) | No | Headache, dementia | Cerebrum, multiple lesions | Cerebrum | PTCL-NOS | Yes | Yes (transient) | High-dose MTX with leucovorin rescue | Good | 1 yr |
| 3 | Migita 2013.9) | 53/F | 8 (1) | Bucillamine, PRD | Nausea, vomiting, vertigo, double vision | Medulla, single lesion | Medulla | DLBCL | Yes | Yes | Resection of mass | Good | 8 yr |
| 4 | Liu 2015.10) | 58/M | 10 (2) | No | Depression, nausea, vomiting | Cerebrum, multiple lesions | Cerebrum | DLBCL | No | NA | Radiotherapy | Good | 1 yr |
| 5 | Shimada 2015.11) | 60s/F | 14 (7) | ETN, ADL, PRD | Convulsion | Cerebrum, multiple lesions | Cervical lymph node | NC (dispersed Hodgkin-like large cells with CD20+ lymphocytes) | Yes | Yes | No | Good | >1 yr |
| 6 | Kikuchi 2016.12) | 50/F | 8 (3) | ETN, ADL | No | Hypertrophy of frontal dura mater | Skin, subcutaneous | IVLBCL | No | Partial improvement | R-CHOP + intrathecal CT (PRD+ MTX + cytarabine) | Good | 2 yr |
| 7 | Matsuda 2018.13) | 76/F | NA (2) | No | Staggering gait | Cerebrum, multiple lesions | Cerebrum | NC (perivascular proliferation of CD20+ lymphoid cells) | Yes | Yes | NA | NA | NA |
| 8 | Uchida 2018.14) | 52/F | 8 (4) | ETN | Dementia, hemiparesis | Cerebrum, multiple lesions | Cerebrum | DLBCL | No | No | Rituxan + high-dose MTX + cytarabine, RT | Died 17 mo after onset | 17 mo |
| 9 | Miyaza 2019.15) | 68/F | 16 (5) | No | Hemiparesis, dysgraphia, speech disturbance | Cerebrum, single lesion | Cerebrum | NC (dense proliferation of CD20+ lymphocytes with atypical nuclei in perivascular lesion) | Yes | Yes | No | Good | >3 yrs |
| 10 | Present case | 68/F | 8 (>10) | No | Motor aphasia, hemiparesis | Cerebrum, multiple lesions | Cerebrum | DLBCL | Yes | Yes | No | Good | 8 mo |
All patients’ primary disease was RA.
ADL: adalimumab, CT: chemotherapy, DLBCL: diffuse large B-cell lymphoma, EBER: Epstein-Barr virus-encoded small RNA, ETN: Etanercept, F: female, HL LPD: Hodgkin’s lymphoma–like lymphoproliferative disorder, IVLBCL: intravascular large B-cell lymphoma, LPD: lymphoproliferative disorder, M: Male, mo: months, MTX: Methotrexate, NA: not available, NC: non-classifiable, P/L LPD: polymorphic/lymphoplasmacytic lymphoproliferative disorder, PRD: Prednisolone, PTCL-NOS: peripheral T-cell lymphoma, not otherwise specified, RA: rheumatic arthritis, R-CHOP: rituximab + cyclophosphamide + vincristine + adriamycin + prednisolone, RT: radiotherapy, yr: years.
The age range at diagnosis of primary CNS MTX-LPD was 50–78 years, which is similar to that reported for all MTX-LPDs, including other sites (all-site; median: 67 years, range: 34–87 years).3) Although most primary CNS MTX-LPD occurred in women, as reported for all-site MTX-LPDs, the ratio of women to men for primary CNS MTX-LPD is higher than for all-site MTX-LPDs (9:1 vs 2:1).3)
The median dose of MTX treatment in primary CNS MTX-LPD is 8 mg/week (range: 6–16 mg/week); and the median duration of MTX treatment until primary CNS MTX-LPD diagnosis is 4 years (range, 1 year to >10 years). These values are similar to those for all-site MTX-LPD cases (8.4 [range, 5.9–10.0] mg/week, 4–6 years).3,18,19) A higher MTX dose is associated with LPD development in RA patients. Other immune suppressors, such as adalimumab, bucillamine, etanercept, and prednisolone, were used in four primary CNS MTX-LPD cases.9,11,12,14)
Neurological symptoms depend on the location of primary CNS MTX-LPD (Table 1). Patients with cerebral lesions presented with headache, dementia, dysgraphia, convulsion, speech disturbance, motor aphasia, hemiparesis, depression, nausea, and vomiting.7,8,10,11,13–15) A patient with a medullary lesion presented with nausea, vomiting, vertigo, and double vision.9) A patient with hypertrophy of the dura mater presented as IVLBCL had no neurological symptoms.12)
Biopsy for exact diagnosis of CNS MTX-LPD
Biopsy is an important method to determine diagnosis and treatment strategy in these patients. In the present case, we performed an open biopsy of the right temporal lesion. Biopsy sites of primary CNS MTX-LPD cases including the present case were the cerebrum (7/10), medulla (1/10), skin (1/10), and cervical lymph node (1/8; Table 1) In two cases (Cases 5 and 6), cervical lymph node or skin were biopsied rather than the CNS lesion11,12); the authors concluded that the CNS lesions and biopsy sites were from the same origins, because after stopping MTX, these lesions regressed.
Histopathology of CNS MTX-LPD
Several histologic patterns were reported in CNS MTX-LPD, with DLBCL as the most common (4/10); polymorphic/lymphoplasmacytic lymphoproliferative disorder (P/L LPD; 1/10), peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS; 1/10), Hodgkin’s lymphoma–like lymphoproliferative disorder (HL LPD; 1/10), and non-classifiable patterns (3/10) were also reported. These results are similar to those for all-site MTX-LPDs, in which DLBCL was the most common histologic patterns (35–50%), followed by Hodgkin lymphoma (10–30%).4,18,20)
Genomic alterations in CNS MTX-LPD and PCNSL
In our patient, both MYD88 L265 and CD79B Y196 mutations were absent. MTX-LPD is presumed to have a distinct molecular profile compared with PCNSL; however, to date, genomic alterations in MTX-LPD, including CNS MTX-LPD, remain unconfirmed because of the rarity of the disease. In PCNSL, B-cell receptor/nuclear factor-κB (NF-κB) signaling is the core pathway.21–23) Additionally, MYD88 L265 and CD79B Y196 mutations may play a key role in up-regulating NF-κB.21–23) MYD88 encodes a signaling adaptor protein that induces activation of NF-κB and the JAK/STAT3 pathway after stimulating Toll-like receptors, interferon-β production, and IL-1/IL-18 receptors.24) The CD79B gene encodes a B-cell antigen receptor (BCR) subunit that is essential for BCR signaling, resulting in NF-κB activation.24) An MYD88 L265 mutation was reported to occur in 76%–85% of patients with PCNSL, and a CD79B Y196 mutation has been reported in 83% of patients.22,23) The prevalence of MYD88 and CD79B mutations in PCNSL was considerably higher than that reported for systemic DLBCL.22,23)
EBER expression and regression after MTX withdrawal in CNS MTX-LPD
EBV is an oncogenic virus associated with several malignant diseases, including Burkitt’s lymphoma and non-Hodgkin lymphoma. Although EBV+ MTX-LPD cases often spontaneously regress after withdrawal of MTX, this is less common in EBV− cases.3) This suggests that MTX has possible effects on the regulation of EBV gene expression, which reactivates EBV. In EBV+ MTX-LPD cases, MTX activates EBV promoters and stimulates viral replication.25) Then, excessive proliferation and survival of EBV-infected lymphoid cells are induced, leading to the development of LPD.25) Withdrawal of MTX abrogates signals for proliferation and/or survival, leading to spontaneous regression without additional chemotherapy.25) The low expression of the antiapoptotic protein, Bcl-2 protein, and the low hypermethylation of apoptosis-related genes in EBV+ MTX-LPD cases may explain the rapid regression of LPD after withdrawing MTX.25)
We found that 7/10 (70%) of the primary CNS MTX-LPD cases to be EBER+, which is a little higher than reported for all-site MTX-LPDs (30–50%).3,4)
Withdrawal of MTX is the initial treatment for MTX-LPD. Regression of LPD after stopping MTX was reported in eight (89%) of the nine cases of primary CNS MTX-LPD including the present case, and the remaining case had no information about this point (Table 1). This rate of the spontaneous regression after MTX withdrawal only is higher than reported for all-site MTX-LPDs (25–60%).3,4,18,26) All seven EBER+ cases including the present case showed regression after stopping MTX, whereas the two EBER− cases (Cases 6 and 8) showed partial or no regression after stopping MTX.12,14) These results are consistent with results reported for all-site MTX-LPDs; patients with spontaneous regression after MTX withdrawal had a significantly higher rate of EBV positivity (85% vs 50%).18)
In some CNS MTX-LPD cases, including the present case, steroid therapy combined with MTX withdrawal was used to alleviate the symptoms.11,15) Steroid therapy for PCNSL works rapidly to cause tumor regression and decrease peritumoral edema27); therefore, it is the treatment of choice for short-term palliation. However, because steroid therapy obscures the histological diagnosis of PCNSL, steroid therapy before biopsy should be avoided as much as possible.28)
Regrowth following regression after MTX withdrawal and additional treatments for CNS MTX-LPD
In one EBER+ case (Case 2), the lesions regrew after initially regressing when MTX was withdrawn and additional chemotherapy was performed.8) The 2 EBER− patients (Cases 6 and 8) showed only partial or no regression after stopping MTX, and received additional chemotherapy.12,14) In these three patients, MTX was included in additional chemotherapy regimens.8,12,14) However, according to the 2016 update of the Japan College of Rheumatology recommendations for the use of MTX in patients with RA, LPD within the most recent 5 years is included in the contraindications for MTX.29) Therefore, using MTX as additional LPD treatment should be avoided. In Case 3, the mass was completely resected when the biopsy was performed, and the authors suggested that perfect surgical resection contributed to non-recurrence of LPD in their case.9) According to the reports of all-site MTX-LPDs, subsequent regrowth or relapse occurred in 18%–45% of patients whose MTX-LPD spontaneously regressed.3,4,18,26) After spontaneous MTX-LPD regression, patients should be followed up and rigorously monitored, even when they show no sign of recurrence. If remission does not occur after stopping MTX or relapse occurs after spontaneous regression, chemotherapy or radiotherapy should be considered. However, there are no standardized chemotherapy regimens across institutions; various regimens are used to treat LPD in patients with RA. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) was the most frequently reported regimen, but various other regimens have been reported.29) The treatment regimen should be selected in consultation with hematologists according to histopathology and the anatomical stages of LPD.29)
Treatment of RA after MTX withdrawal
According to the 2016 update of the Japan College of Rheumatology recommendations for the use of MTX in patients with RA, the treatment of RA after LPD regression should not include immunosuppressive drugs, as much as possible, and MTX rechallenge and the use of tumor necrosis factor inhibitors should be avoided.19) However, withdrawing MTX in RA patients with LPD may result in enhanced RA activity and requires treatment intensification. Similar to the treatment of RA in general, corticosteroids, conventional synthetic disease-modifying antirheumatic drugs (DMARDs), and biological DMARDs have been used for such cases.25) In patients with low to moderate RA activity, the use of low-dose corticosteroids is a reasonable option, but treating RA with high disease activity is a difficult challenge, clinically.25)
In patients with CNS MTX-LPD, some reports have described RA treatment after MTX withdrawal. In Case 3, prednisolone plus salazosulfapyridine was used after CNS MTX-LPD resection.9) However, the patient’s RA disease activity was high; therefore, the authors introduced abatacept, and the patient’s RA improved remarkably. In Case 9 and the present case, RA activity remained stable with low-dose corticosteroids.14) Follow-up of RA activity after MTX withdrawal and adjusting the treatment according to the patient’s RA activity are necessary.
Conclusions
In conclusion, we report a rare case of primary CNS MTX-LPD in a patient with RA. Use of MTX has increased because MTX is widely used as a standard therapy for RA. Although CNS MTX-LPD is extremely rare, it should be considered in the differential diagnosis when a patient who receives MTX develops CNS lesions. An early diagnosis by biopsy and MTX discontinuation as soon as possible are required. Follow-up is necessary, even for patients who show no sign of recurrence.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed Consent
Informed consent was obtained from the patient included in the study.
Acknowledgment
We thank Marla Brunker and Jane Charbonneau, DVM, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Footnotes
Conflicts of Interest Disclosure
The authors declare that they have no conflict of interest.
References
- 1).Singh JA, Saag KG, Bridges SL, Jr, et al. : 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68: 1–26, 2016 [DOI] [PubMed] [Google Scholar]
- 2).Ellman MH, Hurwitz H, Thomas C, Kozloff M: Lymphoma developing in a patient with rheumatoid arthritis taking low dose weekly methotrexate. J Rheumatol 18: 1741–1743, 1991 [PubMed] [Google Scholar]
- 3).Hoshida Y, Xu JX, Fujita S, et al. : Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 34: 322–331, 2007 [PubMed] [Google Scholar]
- 4).Gaulard P, Swerdlow SH, Harris NL, Sundstrom C, Jaffe ES: Other iatrogenic immunodeficiency associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al., (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed., Lyon, IARC, 462–464, 2017 [Google Scholar]
- 5).Salloum E, Cooper DL, Howe G, et al. : Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol 14: 1943–1949, 1996 [DOI] [PubMed] [Google Scholar]
- 6).Makihara S, Kariya S, Noujima-Harada M, et al. : Methotrexate- associated lymphoproliferative disorder with multiple pulmonary nodules and bilateral cervical lymphadenopathy. Auris Nasus Larynx 46: 927–933, 2019 [DOI] [PubMed] [Google Scholar]
- 7).Kleinschmidt-DeMasters BK, Damek DM, Lillehei KO, Dogan A, Giannini C: Epstein Barr virus-associated primary CNS lymphomas in elderly patients on immunosuppressive medications. J Neuropathol Exp Neurol 67: 1103–1111, 2008 [DOI] [PubMed] [Google Scholar]
- 8).Fukushima M, Katayama Y, Yokose N, et al. : Primary central nervous system malignant lymphoma in a patient with rheumatoid arthritis receiving low-dose methotrexate treatment. Br J Neurosurg 27: 824–826, 2013 [DOI] [PubMed] [Google Scholar]
- 9).Migita K, Miyashita T, Mijin T, et al. : Epstein-Barr virus and methotrexate-related CNS lymphoma in a patient with rheumatoid arthritis. Mod Rheumatol 23: 832–836, 2013 [DOI] [PubMed] [Google Scholar]
- 10).Liu W, Xue J, Yu S, Chen Q, Li X, Yu R: Primary central nervous system lymphoma mimicking recurrent depressive disorder: a case report. Oncol Lett 9: 1819–1821, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Shimada H, Dobashi H, Morimoto H, et al. : Primary central nervous system lymphoma in a rheumatoid arthritis patient treated with methotrexate: a case report. BMC Res Notes 8: 88, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kikuchi J, Kaneko Y, Kasahara H, et al. : Methotrexate-associated intravascular large B-cell lymphoma in a patient with rheumatoid arthritis. Intern Med 55: 1661–1665, 2016 [DOI] [PubMed] [Google Scholar]
- 13).Matsuda I, Hirota S: Methotrexate-associated lymphoproliferative disorder masquerading as multiple cerebral metastases. Br J Haematol 180: 628, 2018 [DOI] [PubMed] [Google Scholar]
- 14).Uchida Y, Hokkoku K, Hatanaka Y, Kikuchi Y, Tashiro H, Sonoo M: [Primary central nervous system methotrexate associated lymphoproliferative disorders in a patient with rheumatoid arthritis]. Rinsho Shinkeigaku 58: 485–491, 2018. (Japanese) [DOI] [PubMed] [Google Scholar]
- 15).Miyaza S, Matsuda R, Nakamura M, Nakagawa I, Motoyama Y, Nakase H: Intracranial methotrexate-associated lymphoproliferative disorder in rheumatoid arthritis. World Neurosurg 130: 138–141, 2019 [DOI] [PubMed] [Google Scholar]
- 16).Takano S, Hattori K, Ishikawa E, et al. : MyD88 mutation in elderly predicts poor prognosis in primary central nervous system lymphoma: multi-institutional analysis. World Neurosurg 112: e69–e73, 2018 [DOI] [PubMed] [Google Scholar]
- 17).Kraan W, Horlings HM, van Keimpema M, et al. : High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 3: e139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ichikawa A, Arakawa F, Kiyasu J, et al. : Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 91: 20–28, 2013 [DOI] [PubMed] [Google Scholar]
- 19).Kameda T, Dobashi H, Miyatake N, et al. : Association of higher methotrexate dose with lymphoproliferative disease onset in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 66: 1302–1309, 2014 [DOI] [PubMed] [Google Scholar]
- 20).Tokuhira M, Saito S, Okuyama A, et al. : Clinicopathologic investigation of methotrexate-induced lymphoproliferative disorders, with a focus on regression. Leuk Lymphoma 59: 1143–1152, 2018 [DOI] [PubMed] [Google Scholar]
- 21).Braggio E, Van Wier S, Ojha J, et al. : Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res 21: 3986–3994, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Fukumura K, Kawazu M, Kojima S, et al. : Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol 131: 865–875, 2016 [DOI] [PubMed] [Google Scholar]
- 23).Nakamura T, Tateishi K, Niwa T, et al. : Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 42: 279–290, 2016 [DOI] [PubMed] [Google Scholar]
- 24).Cai Q, Fang Y, Young KH: Primary central nervous system lymphoma: molecular pathogenesis and advances in treatment. Transl Oncol 12: 523–538, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Harigai M: Lymphoproliferative disorders in patients with rheumatoid arthritis in the era of widespread use of methotrexate: a review of the literature and current perspective. Mod Rheumatol 28: 1–8, 2018 [DOI] [PubMed] [Google Scholar]
- 26).Gion Y, Iwaki N, Takata K, et al. : Clinicopathological analysis of methotrexate-associated lymphoproliferative disorders: comparison of diffuse large B-cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci 108: 1271–1280, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G: Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology 40: 80–86, 1990 [DOI] [PubMed] [Google Scholar]
- 28).Weller M: Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol 43: 237–239, 1999 [DOI] [PubMed] [Google Scholar]
- 29).Kameda H, Fujii T, Nakajima A, et al. : Japan College of Rheumatology subcommittee on the guideline for the use of methotrexate in patients with rheumatoid arthritis: Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol 29: 31–40, 2019 [DOI] [PubMed] [Google Scholar]