Abstract
Colorectal cancer (CRC) is the third leading cause of cancer and second leading cause of cancer death in the United States. Recent evidence has linked a high fat and animal protein diet and microbial metabolism of host bile acids as environmental risk factors for CRC development. We hypothesize that the primary bile salt taurocholic acid (TCA) is a key, diet-controlled metabolite whose use by bacteria yields a carcinogen and tumor-promoter, respectively. The work is motivated by our published data indicating hydrogen sulfide (H2S) and secondary bile acid production by colonic bacteria, serve as environmental insults contributing to CRC risk. The central aim of this study is to test whether a diet high in animal protein and saturated fat increases abundance of bacteria that generate H2S and pro-inflammatory secondary bile acids in African Americans (AAs) at high risk for CRC. Our prospective, randomized, crossover feeding trial will examine two microbial mechanisms by which an animal-based diet may support the growth of TCA metabolizing bacteria. Each subject will receive two diets in a crossover design― an animal-based diet, rich in taurine and saturated fat, and a plant-based diet, low in taurine and saturated fat. A mediation model will be used to determine the extent to which diet (independent variable) and mucosal markers of CRC risk and DNA damage (dependent variables) are explained by colonic bacteria and their functions (mediator variables). This research will generate novel information targeted to develop effective dietary interventions that may reduce the unequal CRC burden in AAs.
Keywords: Colorectal cancer, Microbial sulfur metabolism, Colon cancer disparities, Prospective randomized crossover feeding trial, H2S
1. Introduction
Colorectal cancer (CRC) is the third leading cause of cancer in the United States (US), with an estimated 101,420 new CRC cases in 2019 [1]. Increased attention to CRC screening and diagnosis has led to a decline in incidence and mortality rates [1,2], however this has been attenuated in African Americans (AAs) [2]. Indeed, AAs have the highest risk of CRC in the US, with mortality rates 50% higher than non-Hispanic Whites [1]. While this difference likely reflects disparities in screening and treatment access [[2], [3], [4], [5], [6], [7], [8]], AAs have lower stage specific survival rates [2] and are diagnosed at earlier ages [3]. This points to genetic or environmental factors, in addition to socioeconomic inequalities, which may influence CRC incidence and mortality in AAs compared with other races.
As central components of a “western” diet, animal fat and protein have been implicated as environmental factors contributing to CRC risk. Early epidemiological data correlated populations consuming a high fat diet with increased CRC rates [9]. An analysis of Japanese-Americans observed increased CRC incidence to “western” rates within two generations of immigration [10,11]. Similarly, 1st and 2nd generation descendants of native Africans, who traditionally have negligible CRC incidence, developed incidence similar to “western” populations [12]. Vegetarian Seventh Day Adventists have lower CRC incidence than aged-matched and socioeconomically-similar cohorts who consume a “western” diet [13]. In addition, a 2011 meta-analysis revealed increased CRC risk in subjects who consumed abundant red and processed meat [14]. Together, these data provide compelling evidence that a “western” diet contributes to CRC risk, however, diet related mechanisms remain to be elucidated.
The taurine conjugated bile salt taurocholic acid (TCA) may be a key component linking diet and CRC risk [15]. Patients with CRC or adenomas have higher fecal bile acids, which correlate with a high animal fat and protein diet [16,17]. Two studies observed increased primary and secondary bile acid concentrations in feces of subjects consuming an animal protein-rich diet compared to a plant-based diet [18,19]. Taurine for bile acid conjugation is supplied by diet or synthesized in hepatocytes [15,20]. Humans are unable to metabolize taurine, and excess taurine is excreted through bile or urine [21,22]. Consequently, a high taurine diet increases tauro-conjugation of primary bile acids [23], providing an ideal niche for TCA metabolizing gut microbes [15].
There is compelling evidence that increased CRC risk associated with a “western” diet may be caused by microbial TCA metabolism. A diet exchange between higher CRC risk AA subjects and lower risk native African subjects, observed increased fecal bile acid concentrations and increased abundance of TCA metabolizing bacteria in subjects consuming a “western” diet [18]. In another study, a TCA metabolizing bacterium was observed to be relatively more abundant in subjects consuming a high fat and processed meat diet [19]. In accordance with this, our recently published data, revealed that AAs had a higher intake of dietary fat and protein than non-Hispanic White subjects, and that the TCA metabolizing bacterium Bilophila wadsworthia was a significant marker of AA CRC [24]. Thus, microbial TCA metabolism may be a key diet-controlled mechanism that partly explains higher CRC risk among AAs.
2. Aims and hypotheses
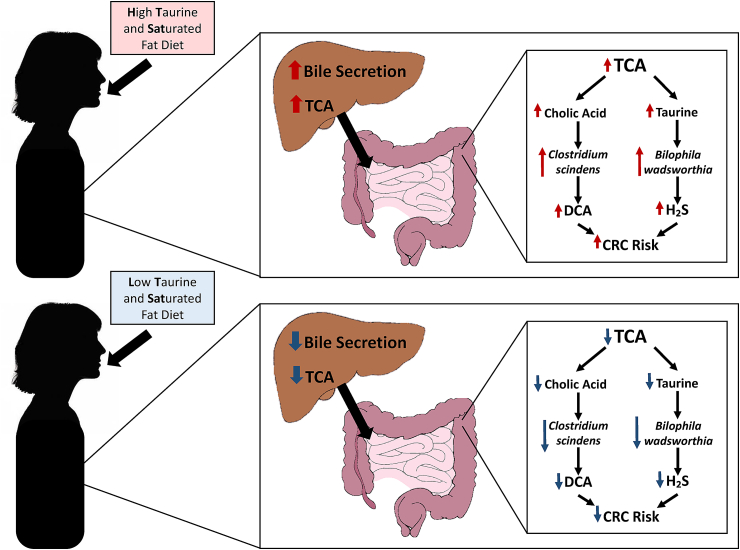
The overall aim of this study is to determine, in the context of a controlled diet-intervention trial, the role of TCA metabolism by gut bacteria in AA subjects at elevated risk for CRC. We hypothesize that TCA is a key diet-controlled metabolite whose metabolism by Bilophila wadsworthia (or related heretofore undiscovered taxa capable of converting taurine to H2S) and Clostridium scindens (or related taxa capable of converting cholic acid to deoxycholic acid (DCA)) yield a carcinogen and a tumor-promoter, respectively (Fig. 1) [15]. We also hypothesize that the colonic microbiota (including the latter two bacteria) can be specifically modulated by altering dietary intake of taurine and saturated fat (found in high red meat diets) in AA subjects at elevated risk for CRC.
Fig. 1.
Study Hypothesis. Intake of a high in taurine and saturated fat diet increases liver bile acid secretion and production of taurine conjugated bile acids. Metabolism of the taurine conjugated bile acid TCA by Bilophila wadsworthia and Clostridium scindens yield both a carcinogen (H₂S) and a tumor-promoter (DCA), respectively [15]. Altering dietary intake of taurine and saturated fat may modulate bile acid composition, abundance of TCA metabolizing bacteria, and consequently markers of CRC risk.
3. Study design
3.1. Overview
The study has been approved by the Institutional Review Board (IRB) at the University of Illinois at Chicago (UIC) (#2016-0495) and Rush University Medical Center (RUMC) (#13102201) and is registered at clinicaltrials.gov (NCT03550885). Prior to participation, all subjects will be informed of the study purpose and potential risks and will provide written informed consent. Forty-four AA subjects between the ages of 45–75 will be recruited from the Chicago metropolitan area to participate in a randomized, crossover, controlled feeding trial. Eligibility and exclusion criteria are presented in Table 1. Participation will be limited to individuals with increased risk of developing CRC (i.e., history of adenomatous colorectal polyps (AP) in the past 10 years, overweight/obesity (body mass index (BMI) 25.0–50.0 kg/m2) and evidence of systemic inflammation (as detected by high sensitivity C-reactive protein (CRP) > 3.0 mg/l) to improve the power to detect intervention effects on the outcome measures [25]. Two isocaloric experimental diets will be tested to determine the extent to which diet and markers of elevated CRC risk are explained by microbial TCA metabolism: 1) an animal-based diet, high in taurine and saturated fat (HT-HSAT) and 2) a plant-based diet, low in both taurine and saturated fat (LT-LSAT). Serving as their own control, subjects will consume each diet for 21 days followed by a minimum 21-day washout interval between diets [7]. Following baseline data collection, subjects will be randomized and blinded to their diet sequence (HT-HSAT diet → LT-LSAT diet or LT-LSAT diet → HT-HSAT diet). Measurements of outcome variables will be performed at baseline and following each diet regimen. Research staff performing the data collection measurements will be blinded to the diet sequence. The study design is described in Fig. 2.
Table 1.
Participant eligibility criteria.
| Inclusion criteria: |
|
|
|
|
|
|
|
|
|
| Exclusion criteria: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
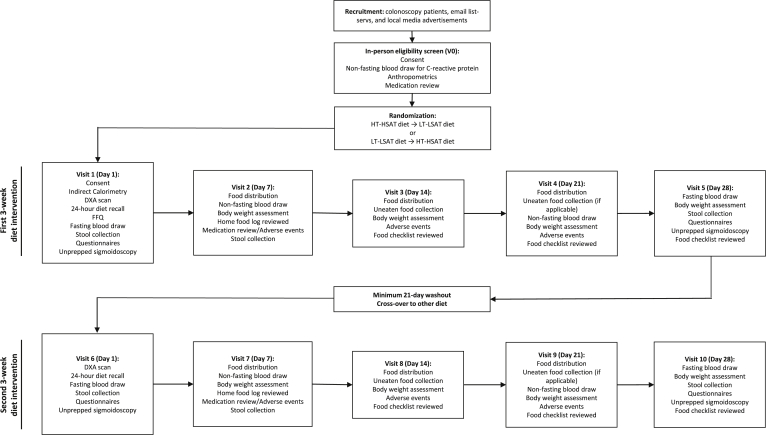
Fig. 2.
Trail Design. Forty-four AA subjects at elevated risk for CRC will be recruited to participate in a randomized, controlled, crossover feeding trial. Participation in the trial will be over the course of 10-weeks. After informed consent and eligibility screening, subjects will be randomized to one of two diet sequences (HT-HSAT diet → LT-LSAT diet or LT-LSAT diet → HT-HSAT diet). Each participant will receive the first assigned diet for 3-weeks, during which they will participate in weekly visits to receive intervention meals, drop off food logs and stool samples, and allow research personal to collect blood samples and measurements. After a 21-day washout period, participants will receive the second assigned diet for 3-weeks and participate in weekly visits as outlined above.
3.2. Diet design, standardization, and compliance
All subjects will be provided experimental diets that are isocaloric to their individual calorie needs. The research diets will be prepared at the UIC Metabolic Kitchen, by a ServSafe® certified trained chef and research assistants. The diets will be on a 7-day cycle and consist of three meals and 2–3 snacks daily. All beverages, including water, will be provided. Menus were developed at the 1600, 1800, 2000, 2200, 2400, and 2600 calorie levels. An example of a one-day menu at the 2000 kcal level for both diets is presented in Table 2 and Table 3. The diets were designed to be comparable in percentage of calories from fat and grams of dietary fiber per 1000 kcals but diverge in ratio of animal to vegetable protein (2:1 in HT-HSAT and 1:2 in LT-LSAT, respectively), percentage of calories from saturated fat, and the absolute amounts of taurine/sulfur amino acids. There will be a reciprocal difference between the diets in percentage of calories from total carbohydrate and percentage of calories from total protein with the HT-HSAT diet having a higher percentage of calories from protein and a lower percentage of calories from carbohydrate, compared to the LT-LSAT diet. Probiotic foods and beverages (e.g., yogurt) will be excluded from both diets. Moreover, for the LT-LSAT diet, special attention will be paid to avoid the food additive carrageenan, because it can be used by gut bacteria to produce H2S [26].
Table 2.
Low Taurine-Low Saturated Fat (LT-LSAT) sample menu at 2000 calories.
| Food item | Grams of Food | Energy (kcal) | Total Protein (g) | Animal Protein (g) | Vegetable Protein (g) | Carbohydrate (g) | Total Fat (g) | Saturated Fat (g) | Fiber (g) | Sodium (mg) | Taurine (mg) | Cystine (g) | Methionine (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast | |||||||||||||
| Breakfast burrito | 170.1 | 265 | 12 | 0 | 12 | 38.6 | 8.2 | 1.7 | 5.7 | 540 | ND | 0.19 | 0.206 |
| Orange juice | 187 | 92 | 1.3 | 0 | 1.3 | 21.6 | 0.22 | 0.026 | 0.56 | 4 | ND | 0.004 | 0.004 |
| Snack | |||||||||||||
| Chick pea hummus | 61.5 | 107 | 4.4 | 0 | 4.4 | 11.8 | 5.3 | 0.66 | 3.5 | 263 | ND | 0.068 | 0.083 |
| Olive oil | 13.5 | 119 | 0 | 0 | 0 | 13.5 | 1.86 | 0 | 0 | ND | 0 | 0 | |
| Pita chips | 37.2 | 167 | 3.5 | 0 | 3.5 | 25.4 | 5.7 | 0.53 | 1.4 | 318 | ND | 0.072 | 0.061 |
| Cucumber | 56.7 | 9 | 0.37 | 0 | 0.37 | 2.1 | 0.062 | 0.021 | 0.65 | 1 | ND | 0.002 | 0.003 |
| Lunch | |||||||||||||
| Pesto tortellini | 269.3 | 442 | 15.6 | 6.8 | 8.8 | 49.8 | 19.9 | 6.1 | 2.8 | 680 | ND | 0.2 | 0.281 |
| Chick pea salad | 48.3 | 75 | 2.1 | 0 | 2.1 | 7.2 | 4.5 | 0.58 | 2.1 | 88 | ND | 0.028 | 0.026 |
| Snack | |||||||||||||
| Almonds | 14.2 | 85 | 3 | 0 | 3 | 3 | 7.5 | 0.6 | 1.5 | 0 | ND | 0.03 | 0.022 |
| Dinner | |||||||||||||
| Pad Thai | 269 | 418 | 9.2 | 0 | 9.2 | 76 | 9 | 1.5 | 4.1 | 780 | ND | 0.169 | 0.181 |
| Dinner roll | 43 | 133 | 4.7 | 0 | 4.7 | 22.4 | 2.8 | 0.6 | 0.9 | 201 | ND | 0.098 | 0.062 |
| Margarine spread | 9.5 | 47 | 0.02 | 0.02 | 0 | 0.082 | 5.2 | 0.72 | 0 | 62 | 0.5a | 0 | 0 |
| Snack | |||||||||||||
| Strawberries | 110.5 | 39 | 0.48 | 0 | 0.48 | 10.1 | 0.122 | 0.007 | 2.3 | 2 | ND | 0.004 | 0.001 |
| Whipped topping | 4.5 | 10 | 0.14 | 0.14 | 0 | 1 | 0.6 | 0.5 | 0 | 3 | 0.5a | 0.001 | 0.004 |
| Other beverages | |||||||||||||
| Non-caloric, non-caffeinated soft drinks | 700 | 0 | 0.72 | 0 | 0.72 | 0 | 0 | 0 | 0 | 42 | ND | 0 | 0 |
| Bottled spring water | 1502 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | ND | 0 | 0 |
| Total | 3496.3 | 2008 | 57.53 | 6.96 | 50.57 | 269.082 | 82.604 | 15.404 | 25.51 | 2999 | 1 | 0.866 | 0.934 |
ND = not detected.
Based on estimates for milk (Laidlaw et al., 1990).
Table 3.
High Taurine-High Saturated Fat (HT-HSAT) sample menu at 2000 calories.
| Food item | Grams | Energy (kcal) | Total Protein (g) | Animal Protein (g) | Vegetable Protein (g) | Carbohydrate (g) | Total Fat (g) | Saturated fat (g) | Fiber (g) | Sodium (mg) | Taurine (mg) | Cystine (g) | Methionine (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breakfast | |||||||||||||
| Scrambled egg substitute | 123 | 59 | 11.5 | 11.3 | 0.2 | 2.6 | 0.21 | 0.005 | 0.83 | 333 | NR | 0.302 | 0.417 |
| Bacon | 15 | 70 | 6.3 | 6.3 | 0 | 0.13 | 4.7 | 1.6 | 0 | 251 | 7.5b | 0.075 | 0.17 |
| Wheat bread | 58 | 159 | 6.2 | 0 | 6.2 | 27.6 | 2.6 | 0.03 | 2.3 | 274 | ND | 0.075 | 0.052 |
| Butter | 4.7 | 34 | 0.04 | 0.04 | 0 | 0.003 | 3.8 | 2.4 | 0 | 30 | 1a | 0 | 0.001 |
| Orange juice | 187 | 92 | 1.3 | 0 | 1.3 | 21.6 | 0.22 | 0.026 | 0.56 | 4 | ND | 0.004 | 0.004 |
| Snack | |||||||||||||
| Mozzarella cheese | 28.4 | 84 | 6.7 | 6.7 | 0 | 1.6 | 5.6 | 3.2 | 0 | 189 | ND | 0.035 | 0.16 |
| Whole wheat crackers | 18.7 | 2.7 | 1.8 | 0 | 1.8 | 12.8 | 2.7 | 0.43 | 2.16 | 131 | ND | 0.052 | 0.03 |
| Lunch | |||||||||||||
| Chicken salad | 71.7 | 147 | 16.8 | 16.7 | 0.1 | 0.6 | 8.15 | 1.7 | 0.12 | 63 | 120 | 0.19 | 0.45 |
| Wheat bread | 58 | 159 | 6.2 | 0 | 6.2 | 27.6 | 2.6 | 0.03 | 2.3 | 274 | ND | 0.075 | 0.052 |
| Potato chips | 28.4 | 153 | 1.3 | 0 | 1.3 | 15 | 10.1 | 1.04 | 1.5 | 170 | NR | 0.017 | 0.02 |
| Baby carrots | 56.7 | 23 | 0.53 | 0 | 0.53 | 5.4 | 0.14 | 0.02 | 1.6 | 39 | ND | 0.47 | 0.11 |
| Ranch dressing, low-fat | 15.3 | 30 | 0.19 | 0.09 | 0.1 | 3.3 | 1.9 | 0.19 | 0.17 | 172 | NR | 0.003 | 0.003 |
| Dinner | |||||||||||||
| Pork tenderloin | 85 | 145 | 24 | 24 | 0 | 0 | 4.7 | 1.5 | 0 | 55 | 48.5 | 0.27 | 0.66 |
| Gravy | 29 | 15 | 1.1 | 0.8 | 0.3 | 1.4 | 0.69 | 0.34 | 0.12 | 163 | NR | 0.015 | 0.017 |
| Macacroni and cheese | 350 | 591 | 26.8 | 17 | 9.8 | 58.3 | 29.8 | 16.2 | 6.2 | 477 | 1.8c | 0.3 | 0.58 |
| Broccoli | 170 | 48 | 5.3 | 0 | 5.3 | 9.1 | 0.2 | 0.03 | 5.1 | 19 | ND | 0.037 | 0.063 |
| Snack | |||||||||||||
| Chocolate pudding | 98 | 139 | 2 | 1.4 | 0.6 | 22.6 | 4.5 | 1.2 | 0 | 149 | 1.2a | 0.017 | 0.043 |
| Whipped topping | 9 | 23 | 0.16 | 0.15 | 0.01 | 2.3 | 1.7 | 1.37 | 0 | 1 | 0.5a | 0.001 | 0.004 |
| Other beverages | |||||||||||||
| Non-caloric, non-caffienated soft drinks | 700 | 0 | 0.72 | 0 | 0.72 | 0 | 0 | 0 | 0 | 42 | 0 | 0 | 0 |
| Bottled spring water | 1502 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 |
| Total | 3607.9 | 1973.7 | 118.94 | 84.48 | 34.46 | 211.933 | 84.31 | 31.311 | 22.96 | 2851 | 180.5 | 1.938 | 2.836 |
ND = not detected; NR = not reported.
Based on estimates for milk (Laidlaw et al., 1990).
Based on estimates for ham (Laidlaw et al., 1990).
Based on estimates for 2% milk (Laidlaw et al., 1990).
The menus were developed by registered dietitians (RDs). Food items readily available through a local grocery delivery service (i.e., Peapod®) and local grocery stores were considered when developing the study menus. Nutrition Data Systems for Research (NDSR) software and the taurine content of foods reported by Laidlaw et al. [27,28] were used to design the menus to obtain detailed nutrient information. Study recipes and daily food preparation forms were developed for each day of the controlled diets that included preparation instructions and volumetric/gravimetric quantities for the metabolic kitchen chef.
Individual energy needs will be determined from Resting Energy Expenditure obtained via indirect calorimetry using the TrueOne 2400 Canopy System (ParvoMedics, Sandy, Utah) and multiplied by an activity factor based on self-reported activity at baseline [29]. Foods and beverages will be distributed weekly for each controlled diet. Study food consumption will not be supervised by the research staff. Subjects receive the foods and beverages, food record checklists to track daily consumption, and heating/storing instructions. Subjects will be instructed not to consume other foods and beverages (except water), outside of the study provided meals, and will be asked to return any uneaten foods or beverages during their weekly food pick-up. The uneaten foods and beverages will be weighed by the research team and data regarding uneaten amounts will be recorded on the subject's daily food checklist. Subjects who fail to consume 100% of study foods ≥5 days per week will be removed from the study. Body weight will be checked at each weekly food pick-up. Body weight change more than ±2% from the previous week will be addressed by adding or subtracting 100–250 calories daily while maintaining the intended nutrient composition of the diets. Subjects starting antibiotics during the study period will no longer qualify for the study. To further monitor compliance with the diets, subjects will participate in unannounced phone-based 24-h dietary recalls during each 3-week dietary intervention period.
3.3. Participant recruitment and screening
Recruitment will take place over a 3-year period. Subjects will be recruited from patients completing screening or surveillance colonoscopies at UIC and RUMC and passively recruited citywide through local email list-servs and media advertisements. Interested individuals will contact the study coordinator by phone or email and respond to basic eligibility questions related to race/ethnicity, age, presence of APs in the past 10 years, and body weight and height to calculate BMI. If the basic eligibility criteria are met, an in-person screening visit will be scheduled. For those outside of the UIC and RUMC health systems, a request will be made for proof of previous AP prior to the in-person screening. During the in-person screening visit, written informed consent will be obtained, and the study procedures will be explained in detail. Following this, a venous blood sample will be obtained to assess CRP, height and weight will be measured to verify BMI, and an interview will also be conducted to assess the individual's ability to adhere to the study procedures (e.g., vacation plans, food storage capabilities). Those meeting eligibility criteria will be invited to a baseline visit. Baseline testing will take place within 5–30 days of the in-person screening. Subjects will be instructed to keep a 3-day food record and collect a stool sample 24 h prior to their baseline research visit.
3.4. Data collection procedures
Subjects will be instructed to arrive after a minimum 12-h fast (water is allowed) and having performed no vigorous physical activities for 48 h. In addition, subjects will report no antibiotic use, or infection or illness since the in-person screening. Participants will have eight laboratory-related visits in total while enrolled in the study (Fig. 2).
3.4.1. Body mass and composition analysis
Subjects will be weighed to the nearest 0.1 kg using a digital scale (Tanita BWB-800, Arlington Heights IL). Height is measured to the nearest 0.1 cm during the in-person screening visit using a portable stadiometer (seca, UK). At the baseline for each diet, whole body composition will be assessed via Dual Energy X-ray absorptiometry (DXA) (GE iLUNAR), by a trained research assistant.
3.4.2. Dietary intake assessments
To improve the accuracy of self-reported dietary data, a combination of dietary assessment methods will be used. Prior to baseline for each diet, subjects will be asked to keep a 3-day food record. Subjects will be instructed how to keep food records by a study RD and will be provided with an example. Food records will be reviewed for legibility and completeness and will be entered into NDSR software [27] to calculate macro- and micronutrient intake. At the baseline for each diet, the research staff will conduct a 24-h diet recall, capturing the data in NDSR. At the first baseline visit, subjects will also complete the full Block food frequency questionnaire (FFQ) detailing their food and beverage intake over the past 12 months.
3.4.3. Other relevant subject data
Questionnaires will be administered regarding self-reported engagement in physical activity over the previous 12 months (using the Paffenbarger physical activity questionnaire) [30], current tobacco use and alcohol consumption, medication and supplement use, health history (including past medical and surgeries histories), family medical history, and recent (past week) gastrointestinal symptoms (if any) (using a structured gastrointestinal symptom checklist) [31].
3.4.4. Colonic and fecal microbiota sampling and testing
To examine baseline and dietary induced changes in microbial markers of colonic TCA metabolism, stool samples will be collected and stored. Subjects will be given a stool collection kit (including an ice pack, a water-proof gas-proof bag with glycerol, and instructions), and instructed to collect a stool sample 24 h prior to their research visit. Stool samples will be collected twice at baseline for each diet, and once at post-intervention of each diet, and stored at −80 °C until use.
To examine changes to mucosally adherent bacteria due to the controlled diets, unprepped flexible sigmoidoscopies will be completed at the start and completion of each experimental diet. During the scope, 7 samples of endoscopically healthy appearing colonic mucosa will be collected using a standard 2.2 mm sterile biopsy forceps at the distal sigmoid colon at about 20 cm from the anal verge.
Quantitative PCR will be performed with a Light Cycler 480 Real-Time PCR System (Roche Diagnostics, Risch-Rotkreuz, Switzerland) using stool and mucosa DNA isolated with a Qiagen DNeasy PowerLyzer Powersoil Kit. Functional gene targets for TCA metabolism, including production of genotoxic H2S and pro-inflammatory DCA will be quantified. Targets for H2S metabolism include degenerate dissimilatory sulfite reductase A (pan-dsrA) [32] which detects sulfate reducing bacteria (SRB) [33] and B. wadsworthia-specific (dsrA-Bw) [34] which is used during the final step of taurine respiration [15]. Primers targeting 16S rRNA genes will be used to measure total bacterial abundance as well as Desulfovibrio spp., [35], which is the most abundant SRB found in the human colon [36]. The bile acid 7α-dehydratase (baiE) gene from DCA producing Clostridium scindens will also be quantified by forward primer 5′-TGGTATTCCATAGCCCGAAG-3′ and reverse primer 5′TAGCCGTAGTCTCGCTGTCA-3'.
3.4.5. Bile acid measurement
To measure baseline and dietary induced changes in circulating and fecal bile acid concentration and composition, a non-fasting blood sample will be taken at beginning and during each 3-week dietary intervention period, and stool collection will take place as described above to measure fecal bile acids. Measurement of serum bile acids by electrospray-ionization mass spectrometry will be performed to determine the extent of taurine-conjugation of bile acids, the ratio of conjugated:unconjugated bile acids, and the concentration of secondary bile acids absorbed from the gut. Fecal bile acid analysis by HPLC will focus on dietary-induced changes in bile acids in fecal water (soluble bile acids interacting with epithelium) and total bile acids (insoluble bile acids in fecal pellet + fecal water).
3.4.6. Colonic proliferation, DNA damage, and immunohistochemistry
Conventional approaches for tissue fixation and immunohistochemistry will be used to quantify Ki-67+ cells, a marker for cellular proliferation, with the primary antibody Ki-67 (# MIB-1, 1:100, mouse monoclonal, Dako). Secondary detection will be accomplished via the Immpress universal antibody Polymer detection kit (# MP-7500, Vector Labs). Proportions of positive staining cells will be counted in well-oriented crypts (minimum 8/slide) using light microscopy at x400 magnification by an investigator, under blinded conditions.
Base excision repair protein localization will be assayed at sites of DNA damage, tissue sections will be stained with primary [anti-Ogg1 (ab91421); anti-APE1 (ab2717); anti-XRCC1 33-2-5 (ab1838)] and appropriate secondary reagents from Abcam. Proteins will be colocalized to spots of oxidative DNA damage via immunohistochemistry staining for oxoguanine 8 with anti-oxoguanine 8 2Q2311 (ab64548). To assess inter-observer variability, 40 randomly selected slides will be recounted by a 2nd senior pathologist. The expression of 84 key genes encoding the enzymes involved in base-excision, nucleotide excision, mismatch, double-strand break, and other repair processes will be quantified using the Human DNA Repair RT [2] Profiler™ PCR Array (PAHS-042Z; SABioscience). In addition, expression of the two isoenzymes (thiosulfate sulfurtransferase and mercaptopyruvate sulfurtransferase) of the H2S detoxifying protein Rhodanase will be measured via RT-qPCR to estimate presence of volatile H2S.
Biomarkers of colonic inflammation [CD3 (lymphocytes) & CD68 (macrophages)], will be measured using immunohistochemistry of colonic biopsies. Inflammatory genes induced by secondary bile acids will observed though quantification of TNF-α, IL-11, IL-6, and COX-2 mRNA using our published protocols. Biopsy RNA will be isolated using standard approaches; protocols for PCR gene arrays will be as previously performed [[37], [38], [39], [40]].
3.4.7. Side effect monitoring
Subjects will be asked to report any side effects they experience during the intervention. Following the flexible sigmoidoscopy, subjects will be called within 24–48 h to assess any adverse effects related to the procedure. Subjects will have 24-h, 7 day per week contact with study staff while consuming the diets and will be encouraged to contact study staff immediately regarding health changes or diet concerns. During weekly food pick up, subjects will complete a structured gastrointestinal symptom checklist and will be interviewed regarding any other adverse health effects.
4. Data analysis
4.1. Power analysis
Our power calculations are based on effect sizes generated from, B. wadsworthia and dsrA-Bw (bacterial gene markers for saturated fat exposure) in our previous feeding trial of a high animal protein diet and high saturated fat exposure (similar to the proposed HT-HSAT diet) in native Africans that normally consume a diet very low in animal protein and saturated fat [18]. The 14-day intervention resulted in a mean effect size for B. wadsworthia (16S rDNA), and dsrA-Bw of 1.11 (SD 1.0) and 0.63 (SD 1.0), respectively. Based on these results and a significance level of 5%, a sample of 40 subjects will provide >97% power to detect effect sizes at these levels using a two-sided one-sample t-test. Assuming a 10% loss to follow-up, an initial recruitment sample of 44 will yield 40 participants that complete the trial. Moreover, our previous trial substantiates that even 20 subjects provide enough power to detect diet effects for many of the proposed study endpoints (bacterial functional gene targets, Ki67, CD3, CD68, DCA and other secondary bile acids). However, the current trial is being implemented under “real-world” conditions whereas the previous study was tightly controlled with participants under 24-h, 7 day per week surveillance.
4.2. Statistical analysis
The main independent variable of interest, diet composition, is incorporated into the crossover design. Mediator variables and response variables, all continuous, will be measured three times for each subject – at baseline, after the HT-HSAT diet, and after the LT-LSAT diet. Three change scores will be computed for each subject which are HT-HSAT versus baseline, LT-LSAT versus baseline, and HT-HSAT versus LT-LSAT. Descriptive statistics will be tabulated for each of the three measurements, as well as each of the three change scores. Distributions of change scores will be evaluated for normality and transformations will be applied if needed. In case of extremely non-normal distributions that are not improved with transformation, ranks will be calculated and used for analysis resulting in nonparametric tests. Paired t-tests will be used to test the effect of each diet on response variables and to compare the two diets; response variables include A) Mucosal markers of CRC risk: number of positive cells in each biopsy tissue section; epithelial proliferation (Ki-67); Inflammation markers by immunohistochemistry (CD3, CD68) and qRT-PCR (TNF-α, IL-11, IL-6, and COX-2). B) DNA damage- 8-oxodG and BER protein localization; and C) Systemic inflammation marker CRP. Pearson correlation coefficients will be tested against zero to see if changes in mediator variables are associated with changes in response variables; mediator variables include A) Microbial genes associated with H2S and DCA production: B. wadsworthia-specific dissimilatory sulfite reductase gene (dsrA- Bw); SRB dissimilatory sulfite reductase gene (pan-dsrA); C. scindens bile acid 7α-dehydratase (baiE), which encodes the rate-limiting enzyme in DCA production; and 16S rRNA genes for Desulfovibrio spp., C. scindens, C. hylemonae, C. sordellii, and C. hiranonas; B) Fecal and serum DCA concentrations. Multiple linear regressions predicting change in response as a function of more than one mediator simultaneously will be used to explore independent effects of mediators and test their interactions.
4.3. Data management and quality control
The Principal Investigator team (LTH and EAM) are responsible for the quality of the clinical data. An operations manual with all study related procedures was developed by the UIC and RUMC investigators and is strictly followed at both sites. Training sessions for all study staff cover all aspects of the study including recruitment, consent, data collection, assessment procedures (e.g., DXA, RMR), and specimen processing and handling. The UIC and RUMC project coordinators are responsible for handling and processing data and specimens, entering data into our Research Electronic Data Capture (REDCap) [41,42] (Vanderbilt University, Nashville, TN) data structure, specimen sample entry in FreezerWorks (Seattle, WA), backing up DXA and dietary data collected on stand-alone desktops and laptops, and securing data files. Food records and food checklists are reviewed carefully by study RDs for legibility and completeness.
5. Discussion
The metabolites H2S and DCA are formed endogenously in the colon as a consequence of microbial TCA metabolism [15]. Hydrogen sulfide has been shown in a series of publications to cause DNA damage, induce proinflammatory pathways, and be genotoxic in concentrations less than normally found in the colon [[37], [38], [39], [40]]. Deoxycholic acid is a tumor promoting secondary bile acid, which causes degradation of tumor suppressor genes, induces COX-2 expression, and activates β-catenin resulting in cancer cell proliferation and invasiveness [[43], [44], [45], [46]]. Previous studies have observed an increase of bacteria that form these metabolites with intake of a diet high in saturated fat and animal protein [18,19]. Moreover, mice fed a diet high in milk-fat had a greater abundance of H2S-producing B. wadsworthia compared with those fed a low-fat or polyunsaturated fat diet [34]. This observation was reproduced in mice gavaged with TCA, demonstrating that B. wadsworthia abundance was a direct consequence of TCA metabolism [34]. Similarly, several human studies have observed an increase in “bile tolerant” bacteria with intake of a “western” type diet, correlating with an increase in primary and secondary bile acids including DCA [18,19]. While it is clear that TCA-metabolizing bacteria may be more abundant with intake of a “western” diet, the impact of diet on B. wadsworthia abundance has been minimally studied in humans. In addition, it is unclear whether the abundance of TCA metabolizing bacteria is stimulated in tandem, and whether this coordinated effect increases markers of CRC risk in humans. This study is designed to investigate the biological basis of increased CRC risk independently associated with consuming a high animal protein and saturated fat diet. Our results will provide novel information regarding in vivo interactions between diet and gut microbes that heretofore have not been explored in humans, particularly AAs. Food taurine content is not currently provided in either NDSR [27] or the USDA Standard Reference (USDA SR) [47] nutrient databases, which are the standard sources for the nutrient content of foods. Evidence that a high fat diet containing sulfur amino acids, typical of a "western" diet, promotes growth of bacteria whose metabolites are capable of inducing biomarkers of CRC risk, would be an important novel observation justifying concomitant evaluation of fat content and sulfur amino acids in nutrient analyses and databases. If our hypothesis is substantiated, simple vigilance of fatty acid type and amino acid intake might diminish susceptibility to CRC in all individuals, especially AAs at elevated risk. Further, if our hypothesis is upheld, in the future we can design similar studies that can manipulate the microbiota in ways beyond diet (potentially through pre-, pro- or synbiotics). Finally, if our study reveals particular modes of bacterial sulfur or bile acid metabolism correlating with epithelial proliferation or inflammation in AAs, the endpoints identified can potentially be used to predict high-risk individuals who should be: a) advised on specific dietary interventions (those investigated herein); b) offered pharmaceutical or targeted probiotic therapy to reduce risk; and/or c) additionally targeted for intense counseling about the importance of colon screening.
6. Conclusions
This study will test the central hypothesis that dietary interventions can alter the bile salt TCA whose metabolism by specific colonic bacteria yields both a carcinogen and a tumor-promoter. The key outcome will be novel understanding of diet-nutrient microbiome interactions that can be used to develop effective cancer prevention interventions that may contribute to a reduction in the unequal colon cancer burden in AA men and women.
Funding
RO1CA204808 (H.RG, J.M.R., E.M, L.TH), 5R01CA204808-03 (S.GP), KL2TR002002 (C.Y.) T32CA057699 (P.G.W.) American Cancer Society MRSG 14-025-01-CNE (L.TH), U54MD012523-03 Sub-Project ID: 7086 (L.TH, E.M., H.R.G., J.M.R.).
Contributor Information
Ece Mutlu, Email: Ece_Mutlu@rush.edu.
Lisa Tussing-Humphreys, Email: ltussing@uic.edu.
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2019. Cancer Facts & Figures 2019. [Google Scholar]
- 2.Irby K., Anderson W.F., Henson D.E., Devesa S.S. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002) Canc. Epidemiol. Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 3.Lansdorp-Vogelaar I., Kuntz K.M., Knudsen A.B., van Ballegooijen M., Zauber A.G., Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Canc. Epidemiol. Biomarkers Prev. 2012;21:728–736. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler E.N., Chawla N., Lund J., Harlan L.C., Warren J.L., Yabroff K.R. Patterns of colorectal cancer care in the United States and Canada: a systematic review. J. Natl. Cancer Inst. Monogr. 2013;2013:13–35. doi: 10.1093/jncimonographs/lgt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X.L., Fang S., Vernon S.W., El-Serag H., Shih Y.T., Davila J., Rasmus M.L. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 6.Gross C.P., Smith B.D., Wolf E., Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potosky A.L., Harlan L.C., Kaplan R.S., Johnson K.A., Lynch C.F. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J. Clin. Oncol. 2002;20:1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 8.Troisi R.J., Freedman A.N., Devesa S.S. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975-1994. Cancer. 1999;85:1670–1676. [PubMed] [Google Scholar]
- 9.Wynder E.L. The epidemiology of large bowel cancer. Canc. Res. 1975;35(11 Pt.2):3388–3394. [PubMed] [Google Scholar]
- 10.Wynder E.L., Kajitani T., Ishikawa S., Dodo H., Takano A. Environmental factors of cancer of the colon and rectum. II. Japanese epidemiological data. Cancer. 1969;23(5):1210–1220. doi: 10.1002/1097-0142(196905)23:5<1210::aid-cncr2820230530>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Haenszel W., Berg J.W., Segi M., Kurihara M., Locke F.B. Large-bowel cancer in Hawaiian Japanese. J. Natl. Canc. Inst. 1973;51(6):1765–1779. doi: 10.1093/jnci/51.6.1765. [DOI] [PubMed] [Google Scholar]
- 12.Berg A. Nutrition, development, and population growth. Popul. Bull. 1973;29(1):3–37. Epub 1973/01/01. [PubMed] [Google Scholar]
- 13.Wynder E.L., Lemon F.R., Bross I.J. Cancer and coronary artery disease among Seventh-Day Adventists. Cancer. 1959;12:1016–1028. doi: 10.1002/1097-0142(195909/10)12:5<1016::aid-cncr2820120523>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Chan D.S., Lau R., Aune D., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridlon J.M., Wolf P.G., Gaskins H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microb. 2016;22:1–15. doi: 10.1080/19490976.2016.1150414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy B.S., Wynder E.L. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J. Natl. Canc. Inst. 1973;50(6):1437–1442. doi: 10.1093/jnci/50.6.1437. [DOI] [PubMed] [Google Scholar]
- 17.Reddy B.S., Wynder E.L. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39(6):2533–2539. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe S.J., Li J.V., Lahti L., Ou J., Carbonero F., Mohammed K., Posma J.M., Kinross J., Wahl E., Ruder E., Vipperla K., Naidoo V., Mtshali L., Tims S., Puylaert P.G., DeLany J., Krasinskas A., Benefiel A.C., Kaseb H.O., Newton K., Nicholson J.K., de Vos W.M., Gaskins H.R., Zoetendal E.G. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015;6:6342. doi: 10.1038/ncomms7342. Cancer 1977; 39(6):2533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., Biddinger S.B., Dutton R.J., Turnbaugh P.J. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann A.F., Hagey L.R., Krasowski M.D. Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 2010;51(2):226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert I.H., Kristensen D.M., Holm J.B., Mortensen O.H. Physiological role of taurine-from organism to organelle. Acta Physiol. 2015;213:191–212. doi: 10.1111/apha.12365. [DOI] [PubMed] [Google Scholar]
- 22.Reissig C.J., Strain E.C., Griffiths R.R. Caffeinated energy drinks-a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöval J. Dietary glycine and taurine on bile acid conjugation in man. Bile acids and steroids 75. Proc. Soc. Exp. Biol. Med. 1959;100(4):676–678. doi: 10.3181/00379727-100-24741. [DOI] [PubMed] [Google Scholar]
- 24.Yazici C., Wolf P.G., Kim H., Cross T.L., Vermillion K., Carroll T., Augustus G.J., Mutlu E., Tussing-Humphreys L., Braunschweig C., Xicola R.M., Jung B., Llor X., Ellis N.A., Gaskins H.R. Race dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman D., Rex D., Winawer S., Giardiello F., Johnson D., Levin T. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Zanardo R.C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb. J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 27.Nutrient Coordinating Center . University of Minnesota; Minneapolis, MN: 1992. Nutrientdatasystem, Release 2.4. [Google Scholar]
- 28.Laidlaw S.A., Grosvenor M., Kopple J.D. The taurine content of common foodstuffs. J. Parenter. Enteral Nutr. 1990 Mar-Apr;14(2):183–188. doi: 10.1177/0148607190014002183. Erratum in: J Parenter Enteral Nutr 1990 Jul-Aug;14(4):380. [DOI] [PubMed] [Google Scholar]
- 29.Compher C., Frankenfield D., Keim N., Roth-Yousey L. Evidence Analysis Working Group. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J. Am. Diet Assoc. 2006 Jun;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Paffenbarger R.S., Jr., Blair S.N., Lee I.M., Hyde R.T. Measurement of physical activity to assess health effects in free-living populations. Med. Sci. Sports Exerc. 1993;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Koloski N.A., Jones M., Hammer J., von Wulffen M., Shah A., Hoelz H., Kutyla M., Burger D., Martin N., Gurusamy S.R., Talley N.J., Holtmann G. The validity of a new structured assessment of gastrointestinal symptoms scale (SAGIS) for evaluating symptoms in the clinical setting. Dig. Dis. Sci. 2017;62(8):1913–1922. doi: 10.1007/s10620-017-4599-6. [DOI] [PubMed] [Google Scholar]
- 32.Daly K., Sharp R.J., McCarthy A.J. Development of oligonucleotide probes and PCR primers for detecting phylogenetic subgroups of sulfate-reducing bacteria. Microbiol. 2000;146(7):1693–1705. doi: 10.1099/00221287-146-7-1693. [DOI] [PubMed] [Google Scholar]
- 33.Klein M., Friedrich M., Roger A.J. Multiple lateral transfers of dissimilatory sulfite reductase genes between major lineages of sulfate-reducing prokaryotes. J. Bacteriol. 2001;183(20):6028–6035. doi: 10.1128/JB.183.20.6028-6035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen W., Wolf P.G., Carbonero F. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male C57BL/6J mice. J. Nutr. 2014;144:1181–1187. doi: 10.3945/jn.114.194332. [DOI] [PubMed] [Google Scholar]
- 36.Gibson G.R., Macfarlane G.T., Cummings J.H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 1988;65:103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 37.Deplancke B., Gaskins H.R. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. Faseb. J. 2003;17:1310–1312. doi: 10.1096/fj.02-0883fje. [DOI] [PubMed] [Google Scholar]
- 38.Attene-Ramos M.S., Nava G.M., Muellner M.G., Wagner E.D., Plewa M.J., Gaskins H.R. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ. Mol. Mutagen. 2010;51:304–314. doi: 10.1002/em.20546. [DOI] [PubMed] [Google Scholar]
- 39.Attene-Ramos M.S., Wagner E.D., Plewa M.J., Gaskins H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Canc. Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 40.Attene-Ramos M.S., Wagner E.D., Gaskins H.R., Plewa M.J. Hydrogen sulfide induces direct radical-associated DNA damage. Mol. Canc. Res. 2007;5(5):455–459. doi: 10.1158/1541-7786.MCR-06-0439. [DOI] [PubMed] [Google Scholar]
- 41.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N., REDCap Consortium The REDCap consortium: building an international community of software partners. J. Biomed. Inf. 2019 May 9 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng K., Raufman J.P. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem. Pharmacol. 2005;70(7):1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 44.Brown J.R., DuBois R.N. COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 45.Qiao L., Studer E., Leach K., McKinstry R., Gupta S., Decker R., Kukreja R., Valerie K., Nagarkatti P., El Deiry W. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol. Biol. Cell. 2001;12(9):2629–2645. doi: 10.1091/mbc.12.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pai R., Tarnawski A.S., Tran T. Deoxycholic acid activates b-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol. Biol. Cell. 2004;15:2156–2163. doi: 10.1091/mbc.E03-12-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Department of Agriculture, Agricultural Research Service . 2016. Nutrient Data Laboratory.http://www.ars.usda.gov/nea/bhnrc/mafcl USDA National Nutrient Database for Standard Reference, Release 28 (Slightly revised). Version Current: May 2016. [Google Scholar]