Abstract
Stem cell activity and cell differentiation is robustly influenced by the nutrient availability in the gonads. The signal that connects nutrient availability to gonadal stem cell activity remains largely unknown. In this study, we show that tumor necrosis factor Eiger (Egr) is upregulated in testicular smooth muscles as a response to prolonged protein starvation in Drosophila testis. While Egr is not essential for starvation-induced changes in germline and somatic stem cell numbers, Egr and its receptor Grindelwald influence the recovery dynamics of somatic cyst stem cells (CySCs) upon protein refeeding. Moreover, Egr is also involved in the refeeding-induced, ectopic expression of the CySC self-renewal protein and the accumulation of early germ cells. Egr primarily acts through the Jun N-terminal kinase (JNK) signaling in Drosophila. We show that inhibition of JNK signaling in cyst cells suppresses the refeeding-induced abnormality in both somatic and germ cells. In conclusion, our study reveals both beneficial and detrimental effects of Egr upregulation in the recovery of stem cells and spermatogenesis from prolonged protein starvation.
Subject terms: Developmental biology, Germline development
Introduction
Tissue homeostasis relies on precise regulation of stem cell activity and cellular differentiation. Identifying the signals that connect environmental stimuli to specific stem cells and their microenvironment is crucial in deciphering the mechanism for tissue homeostasis maintenance.
Germline is actively maintained by germline stem cells (GSCs). Germline and GSC homeostasis is robustly influenced by nutrient availability. In C .elegans, prolonged nutritional deprivation induces reproductive diapause, eliminating entire germline except for a small population of GSCs through apoptosis. Resumed nutrient uptake regenerates new germline from the protected GSCs1. In female Drosophila, egg production is dramatically reduced under the protein-poor diet2. This is partly due to the reduced division rate of both GSCs and follicle stem cells, and their progeny. In addition, cell death of germ cell cysts is also increased by low protein diet.
In male Drosophila under chronic protein starvation, GSC numbers are maintained by nutrients released from the dying spermatogonia. Starvation-induced spermatogonia death is triggered by the apoptosis of the surrounding somatic cyst cells3, which enclose the germ cells to regulate their self-renewal, proliferation and differentiation4–8. Protein resupply recovers GSC numbers and division, at least in part due to JNK-mediated de-differentiaion of spermatogonia to GSCs9. Somatic cyst cells are generated from division of cyst stem cells (CySCs). The population of early cyst cells, including CySCs, is reduced by half during prolonged protein starvation, and recovers upon protein refeeding10. However, the mechanism for the cyst lineage cell recovery through nutrient resupply remains largely unknown.
The Drosophila testis is surrounded by a sheath composed of an outer layer of pigment cells and the inner layer of smooth muscles. The smooth muscles lie immediately adjacent to cyst cells with a thin layer of extracellular matrix between them11. Recent studies have shown that testicular muscles produce tumor necrosis factor (TNF) to influence spermatogenesis12. Eiger (Egr) is the sole Drosophila TNF homologue. It was first discovered by its potent activity to induce apoptosis13–15. Although it is not essential for normal development, Egr plays critical role in the maintenance of Drosophila physiology and vitality in response to pathogen infection16, harmful environmental stimuli, and injury17–19. Egr induces cellular responses by binding to the TNF receptors (TNFRs), Wengen and Grindelwald (Grnd)20,21. Unlike mammalian TNFs that activate NF-κB, MAPK and JNK pathways, Egr-induced cellular responses are mainly mediated by JNK signaling activation only22.
Previously we have found that TNF-JNK signaling is hyper-activated by reproduction in testes, leading to ectopic expression of the CySC self-renewal protein Zfh-112. In this study, we show that prolonged protein starvation induces Egr upregulation in testis smooth muscle. Upon protein resupply, Egr and the receptor Grnd are required for the rapid recovery of CySC population. In addition, a novel phenotype in germline recovering from prolonged protein shortage is discovered; Zfh-1 is ectopically expressed in differentiating cyst cells away from the apical niche, resulting in overproduction of early germ cells. Both egr depletion in testis smooth muscles and JNK signaling inactivation in cyst cells suppress the abnormalities, indicating that too much TNF induced by prolonged protein starvation disrupts the germline recovery via JNK signaling in cyst cells. Moreover, we found that prolonged protein starvation impairs proteasome activity in testicular muscle. Inactivation of proteasome functions fails to increase Egr levels, suggesting that additional mechanism is required to upregulate TNF in smooth muscle during starvation.
Results
Egr is induced in testicular smooth muscles during protein starvation
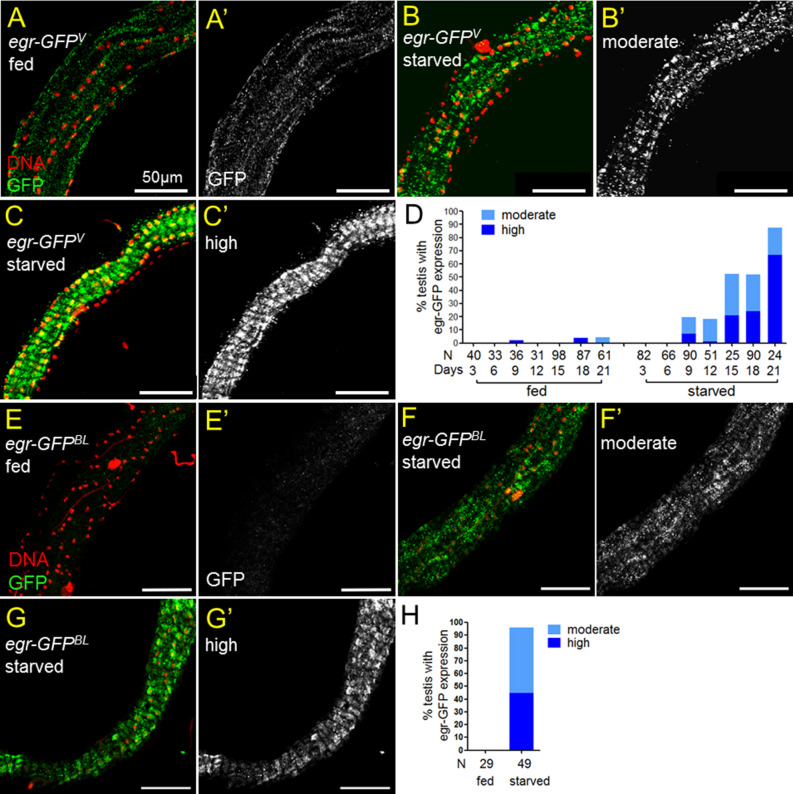
Previously we have shown that reproduction increases Egr levels in the testicular muscle to hyperactivate JNK signaling in somatic cyst cells12. Since JNK signaling is also activated in cyst cells in response to amino acid starvation3, we then asked if protein starvation induces Egr protein upregulation in testicular smooth muscles. By examining the levels of Egr-GFP expressed from a genomic fosmid clone (referred as Egr-GFPv)23, upregulation of GFP in smooth muscles was specifically observed in testes from flies suffered from prolonged protein starvation (starved) (Fig. 1B–D). Egr-GFP was not observed in the testicular muscles of males starved for 3 and 6 days. However, more than 50% and 80% of the testes from flies starved for 15 days and 21 days, respectively, had increased Egr levels in the testicular muscles (Fig. 1D). Egr-GFP was almost not detected in the testis muscle of 3- to 21-day old males fed with protein-containing food (fed) (Fig. 1A, D). To confirm the upregulation of Egr in response to protein starvation, we examined an Egr-GFP protein trap line in which GFP is inserted near the transmembrane domain of the endogenous Egr (referred as egr-GFPBL)24. As shown in Fig. 1E–H, GFP was highly expressed in the testicular smooth muscles of egr-GFPBL males starved for 18 days, but not in the testis muscles of the age-matched fed males. Together, we conclude that Egr protein level is upregulated in testicular smooth muscle in response to prolonged amino acid starvation.
Figure 1.
Protein starvation upregulates Egr levels in testicular muscles. (A–C’ and E–G’) Testes of egr-GFPv (A–C’) and egr-GFPBL (E–G’) immunostained for GFP (green in A–C and E–G, and white in A’–C’ and E’–G’). Nuclei are labeled by Hoechst 33342 (red). Flies were either raised on standard protein-rich food (fed) or protein-restrict diet (starved) for 18 days. Egr-GFP signals were detected in testicular muscles from starved males (B,C,F,G), but not from fed males (A,E). The grade of Egr-GFP expression was classified to moderate (B,B’ and F,F’) or high (C,C’ and G,G’) based on the signal intensity and the size of GFP-positive area (see “Methods”). Scale bars 50 μm. (D,H) Percentages of testes with detectable Egr-GFP expression in the testicular muscles. (D) The percentages of testes expressing Egr-GFP in smooth muscles were increasing over time in males starved from 9 to 21 days. (H) GFP accumulation in smooth muscles was detected in almost all testes from egr-GFPBL males starved for 18 days. GFP was not detected in the testicular muscles of males fed for 18 days.
Protein starvation impairs proteasome activity in testicular muscles
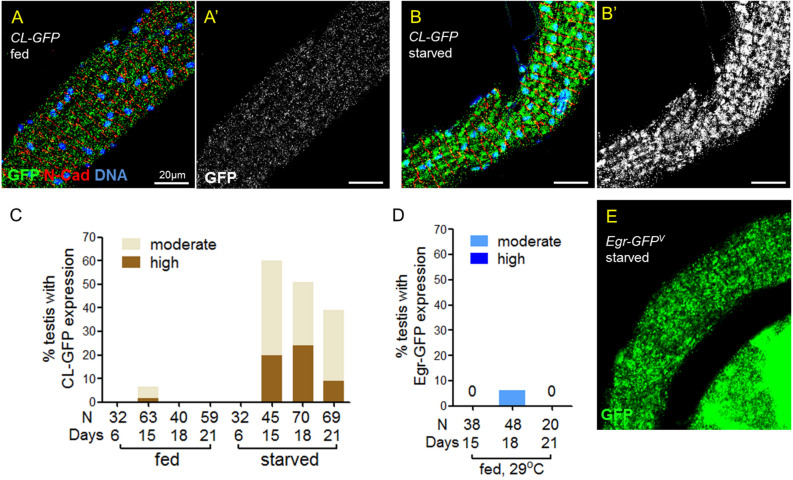
Proteasome-mediated degradation is the major mechanism for protein turnover. The Egr-GFP accumulation prompts us to ask whether protein starvation impairs proteasome functions in testicular muscles. To answer this question, we examined the abundance of the ubiquitin–proteasome system reporter CL1-GFP that carries the degron CL125. In contrast to almost undetectable GFP signal in animals fed with normal food for 6–21 days (Fig. 2A, C), CL1-GFP was detected abundantly in testicular muscle in males starved for 15–21 days (Fig. 2B, C), indicating that prolonged protein starvation impairs ubiquitin–proteasome system in the testis muscles.
Figure 2.
Protein starvation impairs ubiquitin–proteasome activity in testis muscle. (A–B’) Testes from mef2 > CL1-GFP flies immunostained for GFP (green), co-stained for N-cadherin (red) and DNA (blue). Anti-N-cadherin staining labels the muscle membrane. Flies were either fed (A,A’) or starved (B,B’) for proteins for 18 days. (C) The percentages of testes showing CL1-GFP accumulation in the testicular muscles. Testes were from males either fed or starved for 6, 15, 18 and 21 days. (D,E) GFP expressed from Egr-GFPv in testes of mef2 > DTS5 DTS7 males. (D) The percentage of testes showing GFP accumulation in the muscles. Fed flies were maintained at 29 °C to enhance DTS5 and DTS7 expression for 15, 18 or 21 days as indicated. (E) Testes immunostained for GFP. Upregulation of Egr-GFP was not detected in testicular muscle until starved for 9 days. Adult flies were maintained at 29C during the starvation phase.
To further test whether decreased proteasome activity contributes to Egr-GFP accumulation, proteasome activity was disrupted by overexpression of the dominant, temperature-sensitive proteasome mutants DTS5 and DTS726. The presence of DTS5 at non-permissive temperature (29 °C) for 24 h is sufficient to disrupt proteasome activity26. To our surprise, overexpression of DTS5 and DTS7 in muscles at 29 °C for 15–21 days did not lead to detectable Egr-GFP expression in testicular smooth muscles in fed males (Fig. 2D). In starved males expressing DTS5 and DTS7 at 29 °C in the muscles, Egr-GFP was not observed in testicular muscle until starvation for nine days (Fig. 2E), at that time when starved males without DTS5 and DTS7 expression also began to produce Egr-GFP in the muscle (Fig. 1D). Therefore, our results suggest that compromised ubiquitin–proteasome activity in testicular muscle by protein starvation is not the major mechanism for Egr upregulation.
Egr does not affect stem cell loss during protein starvation
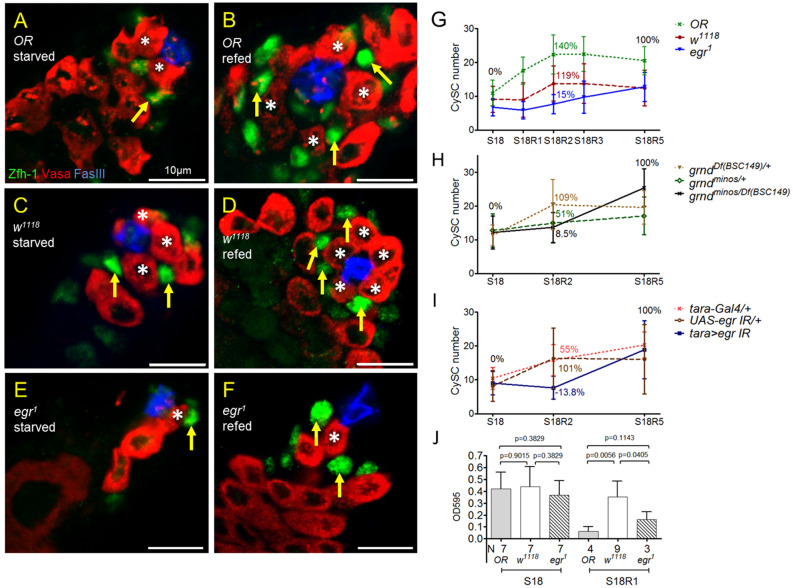
Since Egr-induced cellular responses are primarily mediated by JNK signaling, and JNK activation in cyst cells is required to maintain GSC numbers during protein starvation3, we first examined GSC numbers in egr mutants starved for amino acids. In testes of w1118 controls starved for 18 days, GSC number was reduced 33% (10.4 ± 2.6 vs. 6.9 ± 2.7) compared to the age-matched fed adults (Fig. 3A and upper panel in 3C)3,10. Starvation caused 40% decrease in GSC number in egr1 null mutants (6.9 ± 1.5 vs. 4.2 ± 1.9) (Fig. 3B and upper panel in 3C). The reduction of CySC number by amino acid starvation was also comparable between egr1 (53%, 14.1 ± 1.7 to 6.6 ± 2.7) and the control (47%, 18.1 ± 3.7 to 10.5 ± 2.3) (Fig. 3A, B, and bottom panel in 3C). Therefore, egr appears not critical for reduction or maintenance of somatic and germline stem cells by protein starvation.
Figure 3.
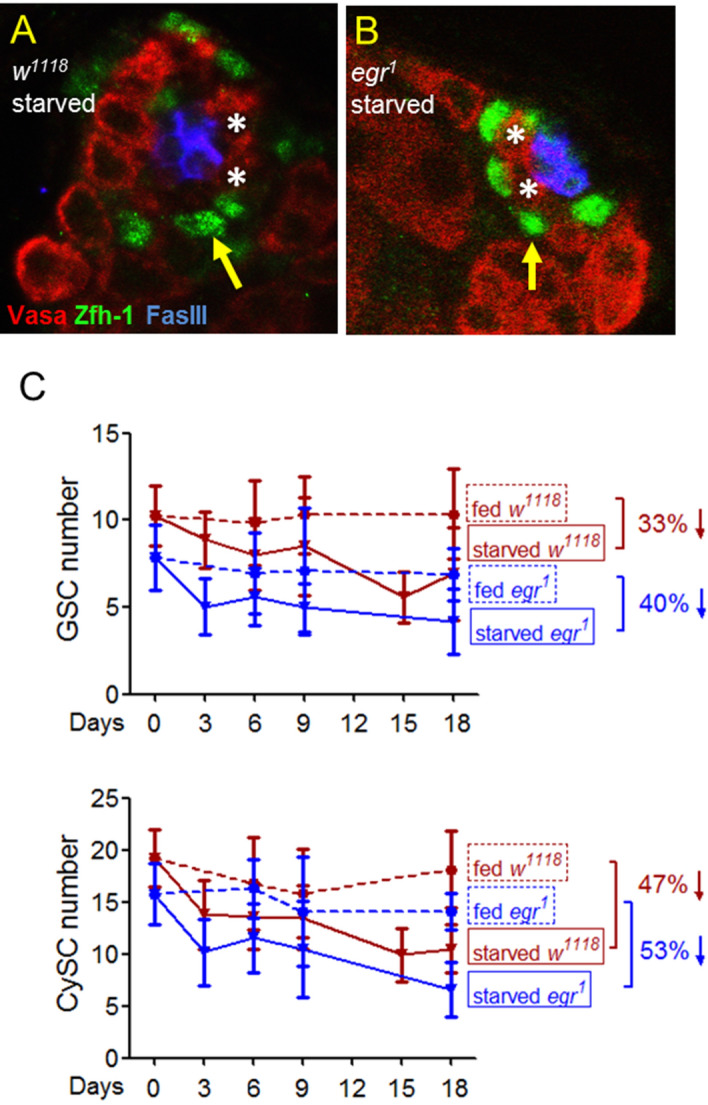
egr is not essential to regulate stem cell numbers in response to protein starvation. (A,B) Testes immunostained for Vasa (germ cells, red), co-stained for Zfh-1 (CySCs and early cyst cells, green) and FasIII (hub, blue). Asterisks and arrows indicate GSCs and CySCs, respectively. Flies were starved from 18 days. (C) The number of GSC and CySC after starvation for 0–18 days. Data is presented as mean ± SD. The number of testes in each time point is between 15 and 34.
Lack of egr delays CySC recovery from starvation
The reduction in the number of stem cells by starvation is reversible upon refeeding10. We found that Egr is required for the fast recovery of CySCs induced by protein resupply. CySCs are defined as the Zfh-1 positive cells immediately adjacent to GSCs12,27. In wild-type Oregon-R (OR) flies, the number of CySC increased dramatically from 11 to 22 during the first two days of refeeding (R1 and R2) (compare Fig. 4B to 4A, and 4G). Because there was slight decrease in CySC number from refeeding day 3 to day 5 (R3–R5) (Fig. 4G), the CySC number at R2 was the highest during the five-day refeeding phase. The recovery rate was 140% at R2 compared to R5. In w1118, the number of CySCs also reached to the highest after two days of refeeding. The recovery rate at R2 was 119% compared to that at R5 (compare Fig. 4D to 4C and 4G). In egr1, however, CySC recovered much slower. The CySC recovery rate at R2 was 15% to that at R5 (compare Fig. 4F to 4E, and 4G). CySC number continued to increase gradually each day from R2 to R5 in egr1 adults. Egr acts on cyst lineage cells through the receptor Grnd12. Examination of CySC recovery in grnd null mutant (grndminos/Df) showed that the recovery rate was very low at R2 compared to R5 (9%, Fig. 4H). Together, our results reveal a late recovery of CySC population in the absence of Egr and Grnd.
Figure 4.
Egr is required for fast recovery of CySC number upon protein refeeding. (A–F) Testes immunostained for Zfh-1 (green), co-stained for Vasa (red) and FasIII (blue). (A,C,E) Testes from males starved for 18 days (S18). (B,D,F) Testes from males starved for 18 days and refed for two days (S18R2). (G–I) Average number of CySCs during refeeding phase. Data are presented as mean ± SD. The CySC recovery rate at S18 and S18R5 is set as 0% and 100%, respectively. The recovery rate at R2 is indicated (see “Methods”). (G) CySC recovery in Oregon R (OR), w1118 and egr1 adults upon refeeding. Number of testes for each time point is between 11 and 36. (H) Lack of Grnd in grndminos/Df (BSC149) mutant delayed CySC recovery upon refeeding. The number of testes for each point is between 25 and 38. (I) Tissue-specific depletion of egr in testicular muscle delayed CySC recovery upon refeeding. The number of testes for each point is between 19 and 37. (J) Quantification of food consumed by adult flies after starvation for 18 days (S18) and 1-day refeeding (S18R1). Statistics were generated by Student t test.
Since Egr was expressed in the testicular smooth muscles, we tested whether depletion of egr from the muscles influences the dynamics of CySC recovery upon refeeding. Tissue-specific knockdown of egr by RNAi driven via tara-Gal4, that is specifically expressed in the muscles but not the other cells in the testis12, markedly reduced the CySC recovery rate at R2 compared to R5 (− 14%, Fig. 4I). The CySC recovery rate at R2 was much higher in both Gal4 and UAS control flies (55% in Gal4 control, 101% in UAS control,) (Fig. 4I). Thus, our results suggest that Egr produced from testicular muscle is important for the rapid CySC recovery upon refeeding.
It has been shown that egr mutant adults ate less food compared to w1118 or several Gal4 lines when fed constantly with protein-containing food28. The slower recovery of CySC in egr1 flies during the refeeding period, thus, might be resulted from less food consumption. To answer the question whether CySC recovery rate upon refeeding positively correlates with the ingestion rate, we quantified the food intake. OR, w1118 and egr1 flies ingested comparable amount of protein-containing foods after 18 days of amino acid starvation (S18) (Fig. 4J). Interestingly, OR flies ingested the least amount of food after refeeding for one day (S18R1) despite the highest recovery rate during the first two days of refeeding (Fig. 4G). The amount of food consumed by OR flies was less than 20% of the food by w1118 adults. The egr1 flies consumed more food than OR but ingested less than w1118 at R1 (Fig. 4J). Our results suggest that the rate of ingestion does not positively correlate with the rate of CySC recovery from amino acid starvation. Thus, the slow recovery of CySC upon refeeding is unlikely resulted from eating too little in egr1 mutants.
Protein refeeding leads to dysregulation of Zfh-1 in cyst cells via Egr
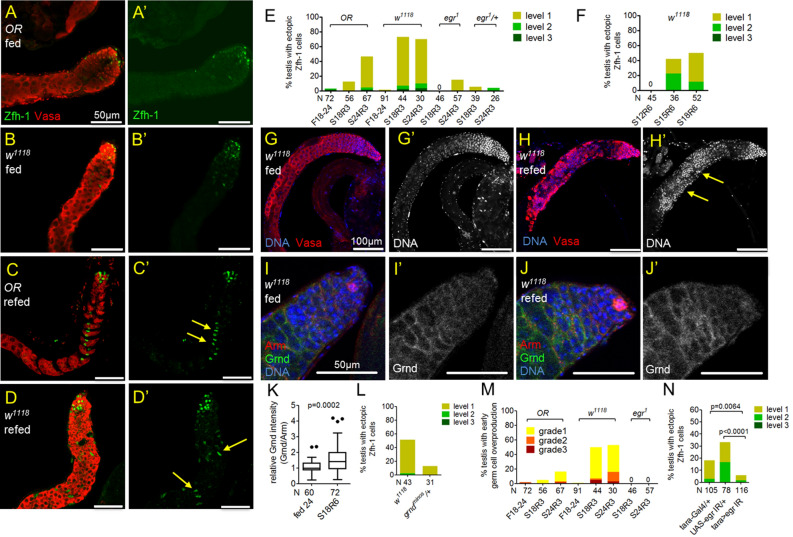
Zfh-1 maintains CySC self-renewal in somatic cyst cell lineage29. Its expression is normally activated in CySCs and early cyst cells in the apical region via signals from hub located in the testis tip27,29–32 (Fig. 5A, A’ and Fig. 5B, B’). Interestingly, Zfh-1 was ectopically expressed in cells away from the hub in wild-type OR and w1118 flies refed after prolonged protein starvation (Fig. 5C, C’, D, D’). In OR flies starved for 18 or 24 days and refed for 3 days, 13% and 46% testes, respectively, exhibited ectopic Zfh-1 expression away from the apical region (Fig. 5E). Ectopic Zfh-1 expression was also observed in more than 50% of the testes from w1118 males starved for 18 or 24 days and refed for 3 days (Fig. 5E). In contrast, ectopic Zfh-1 positive cells were rarely observed in OR and w1118 males fed with protein for 18–24 days (Fig. 5E). The dysregulation of Zfh-1 expression in testes from refed animals was only observed with prolonged starvation. For example, it was not observed in w1118 males starved for 12 days, but was found in 40% and 50% of testes from males starved for 15 days and 18 days, respectively (Fig. 5F).
Figure 5.
Egr and Grnd are essential for ectopic Zfh-1 expression by protein refeeding. (A–D’) Testes immunostained for Zfh-1 (green in A–D’), co-stained for Vasa (red). (A–B’) Zfh-1-positive cells were restricted to the apical region in testes of OR and w1118 flies fed for 24 days. (C–D’) Ectopic Zfh-1 expression (arrows) outside the apical region was found in testes from OR and w1118 males starved for 18 days and re-fed for 6 days. (E) Percentages of testes exhibiting ectopic Zfh-1 expression. Refeeding induced ectopic Zfh-1 expression in testes from OR and w1118. Ectopic Zfh-1 expression was rarely observed in testes from refed males of egr1 homozygous or heterozygous. Ectopic Zfh-1 expression was almost not observed in males fed for 18–24 days. (F) Percentages of testes showing ectopic Zfh-1 expression. Testes were form males starved for 12–18 days, followed by refeeding for 6 days. (G–H’) Testes immunostained for DNA (blue in G and H, white in G’ and H’), co-stained for Vasa (red). Early germ cells characterized by intense DNA staining were overproduced (arrows) in the testes from males starved for 18 days followed by refeeding for 6 days (H). Overproduction of early germ cells was not found in testes from males fed for 24 days (G). (I–J’) Testes immunostained for Grnd (green in I and J, white in I’ and J’), con-stained for Arm (cyst cell cytoplasm, red) and DNA (blue). Anti-Grnd staining was observed specifically on the cyst cells. (K) Box-and-whisker plots showing relative anti-Grnd/anti-Arm signal intensity in cyst cells, normalized to the median value of anti-Grnd/anti-Arm intensity in the testes from fed males. Significantly higher levels of anti-Grnd staining were observed in the re-fed males (S18R6) compared to the age-matched fed males (fed 24). P value was calculated by Mann–Whitney nonparametric test. (L) Percentages of testes exhibiting ectopic Zfh-1 expression. One copy of grnd null allele (grndminos) dominantly suppressed the ectopic Zfh-1 expression in the testes of the refed males (S18R6). (M) Percentages of the testes with overproduction of early germ cells. Early germ cell overproduction by refeeding was specifically observed in wild-type (OR and w1118) males, but not in egr1 mutants. (N) Knockdown of egr in testis muscles via tara-Gal4 suppressed ectopic Zfh-1 expression induced by refeeding. Testes are dissected from refed (S18R6) males. P value was calculated by Chi-squared test.
Cyst cells are critical to instruct germ cell differentiation5,7,33. Ectopic Zfh-1 expression in differentiating cyst cells non-autonomously blocks germ cell differentiation, leading to accumulation of early germ cells12. Consistently, we observed overproduction of early germ cells, which can be recognized by their smaller cell size and intense DNA staining, in testes from refed animals but not from fed animals (compare Fig. 5H’ to Fig. 5G’, and Fig. 5M).
Since the abnormal phenotypes in testes from refed males were reminiscent to the Egr-dependent Zfh-1 dysregulation by reproduction12, we then examined whether egr is also involved in the Zfh-1 abnormality induced by refeeding. Loss of egr almost completely suppressed the ectopic Zfh-1 expression and the early germ cell overproduction in egr1 mutants (Fig. 5E, M). We also found that refeeding significantly elevated the levels of the receptor Grnd on the cyst cells (Fig. 5I–K). Consistently, the percentages of testes exhibiting ectopic Zfh-1 expression was dominantly suppressed by lack of one copy of egr or grnd, as compared to the parental control w1118 (Fig. 5E, L). Tissue-specific depletion of egr by RNAi via tara-Gal4 significantly suppressed ectopic Zfh-1 expression in testes from re-fed animals (Fig. 5N). Together, our results strongly suggest that Egr and Grnd are essential for refeeding-induced Zfh-1 dysregulation.
JNK signaling activation in cyst cells promotes Zfh-1 dysregulation upon refeeding
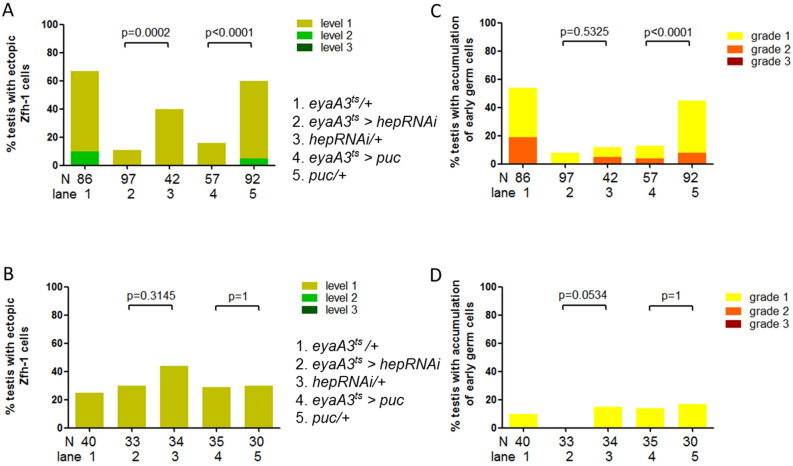
Protein refeeding activates JNK signaling in germline and cyst cell lineage9. While JNK signaling activation in germline is required for de-differentiation of spermatogonia to GSCs, the role of JNK signaling hyperactivation in cyst lineage is unknown. Because egr is critical for refeeding-induced Zfh-1 dysregulation, we tested whether JNK signaling is also involved in it via temperature-sensitive Gal80ts-mediated inhibition. It has been shown that depletion of JNKK encoding by hemipterous (hep) or forced expression of JNK phosphatase encoding by puckered (puc) suppresses JNK signaling responses34. JNK signaling was temporally inactivated in the refeeding phase by temperature shift from 25 to 29 °C three days before and during the entire refeeding phase. Knockdown of hep or overexpression of puc in cyst lineage cells by eyaA3-Gal4; tub-Gal80ts (eyaA3ts) markedly suppressed the percentages of testes with ectopic Zfh-1 expression when compared to the controls (Fig. 6A). In contrast, there was no significant suppression in the percentages of testes with extra Zfh-1 cells when JNK signaling inactivation was blocked at 25 °C during the entire starvation and refeeding phases (Fig. 6B). Overexpression of puc in cyst lineage also noticeably decreased the percentage of the testes exhibiting early germ cell overproduction at 29 °C but not at 25 °C (compare Fig. 6C and D). Together, our results indicate that protein refeeding induces aberrant Zfh-1 expression and excess early germ cells via JNK signaling activation in cyst lineage cells.
Figure 6.
JNK signaling activation is necessary for Zfh-1 dysregulation induced by refeeding. Percentages of testes exhibiting ectopic Zfh-1 expression (A, B) and early germ cell overproduction (C, D). (A) Ectopic Zfh-1 expression by refeeding was markedly suppressed by depletion of hep or overexpression of puc in cyst lineage cells at restrictive temperature 29 °C. (B) No suppression was observed when Gal80ts-mediated JNK signaling inactivation was blocked at permissive temperature 25 °C. (C) Refeeding-induced early germ cell overproduction was also noticeably suppressed by puc overexpression in cyst lineage cells at 29 °C. (D) No significant suppression of early germ cell accumulation was observed at permissive temperature 25 °C. Statistics were generated using Chi-squared test.
Discussion
Nutrient availability robustly influences animal physiology. In this study, we show that long-term protein starvation induces Egr production in the testis smooth muscles. While Egr is not essential for loss or maintenance of stem cells by protein starvation, it is required for the fast recovery of somatic cyst stem cells upon refeeding. Furthermore, we also observed that refeeding after chronic protein starvation leads to ectopic Zfh-1 expression and overproduction of early germ cells. The aberrant phenotypes upon refeeding are resulted from TNF-JNK signaling hyper-activation in the somatic cells. Since TNF-JNK signaling hyperactivation induces Zfh-1 expression in normal, nutrient-supply condition12, we propose that Egr induced by starvation “primes” cyst cells for Zfh-1 expression upon nutrient resupply, promoting CySC recovery and ectopic Zfh-1 expression in cyst lineage cells during protein refeeding.
We found that prolonged protein starvation promotes Egr upregulation in the testis muscle. Together with our previous observation that reproduction increases Egr levels in testis muscle in aged males, these results suggest that smooth muscle is the sensor tissue of the male gonads to sense the physiological changes. Indeed, many other studies have shown the critical roles of muscle to signal to other tissues for the adaptive responses. In Drosophila, Wingless (Wg) proteins from visceral muscle of the gut act non-autonomously on enterocytes to influence gut homeostasis35. In both mammals and flies, genetic alteration of signaling events or enzymatic activity in skeletal muscles could modulate stress resistance and lifespan of the organisms, via endocrine or paracrine mechanisms36.
It has been postulated that reproduction-induced Egr upregulation in smooth muscle might be resulted from muscle damage by chronic stress and aging37. While we observed severely impaired proteolysis in testicular smooth muscle in males suffered from chronic protein starvation, disruption of ubiquitin–proteasome pathway neither induced Egr upregulation in fed males nor enhanced Egr levels in starved males. These results suggest that proteasome-mediated protein turnover plays little role in regulation of Egr in testicular muscle. It is likely that other damages commonly observed in muscles such as accumulated DNA damage38,39 or compromised autophagy40 might also be required to modulate Egr levels. In Drosophila, stress- and nutrient-sensing pathways such as p38 kinase and mTOR signaling are required in skeletal muscles to modulate normal life span and stress responses41,42. Alternatively, Egr levels might be regulated by the nutrient- and stress-sensing pathways. Muscle is more sensitive to aging-induced apoptosis and DNA damage than the other tissues in Drosophila38,43. Interestingly, Egr upregulation in response to starvation and reproduction was only observed in older males. The changes in response to starvation and reproduction must couple with age-associated events to modulate Egr levels in testicular muscles.
Previous works have shown that JNK signaling is activated in cyst cells by protein starvation to promote cyst cell apoptosis, and is activated in germ cells upon protein refeeding to induce germ cell de-differentiation3,9. Together with our results, it shows that nutrient availability act through JNK signaling to modulate different tissue responses in adult testes in both starvation and refeeding phases, and in both somatic cells and germline. Our results, however, is the first to discover the mechanisms acting upstream of JNK signaling activation. Although protein starvation induced Egr upregulation and JNK-dependent cyst cell apoptosis, testis involution and comparable stem cell number were still observed in starved egr mutants. It suggests that Egr influences testis homeostasis in a context-dependent manner.
It has been shown that amino acid starvation promotes Egr secretion from fat body to activate JNK signaling in brain, that in turn reduces insulin peptide production and inhibits larval growth44. However, our studies of adult testis showed that TNF-JNK signaling act locally to modulate cyst cell and germ cell homeostasis upon refeeding. TNFR Grnd level was significantly upregulated in cyst cells in the testes from re-fed males, and reduction of JNK signaling in cyst cells suppressed Zfh-1 dysregulation and overproduction of early germ cells. Protein starvation rapidly induces TACE expression to release soluble Egr from fat body during larval growth44. Instead, our results showed that both Egr upregulation in testicular muscles and ectopic Zfh-1 expression in cyst cells were only observed after prolonged starvation. Together, these results suggest diverse and distinct functions of TNF-JNK signaling in modulating systemic body growth of larvae and homeostasis of one particular adult tissue in response to protein starvation.
Methods
Stocks and fly husbandry
Oregon-R (OR) and w1118 were used as wild-type, control strains. egr1 and grndminos (BL43677) are null alleles13, 21. Df (BSC149) is the deletion line for grnd21. egr-GFPv (v318615) and egr-GFPBL (BL66381)24 are for detection of Egr proteins. UAS-CL1-GFP was used as a reporter for proteome activity as described45. UAS-DTS5 and UAS-DTS7 are used to disrupt proteasome activity26. eyaA3-Gal4;tub-Gal80ts (referred as eyaA3ts)29, tara-Gal4 (BL-63905)46 and mef2-Gal4 (BL25765)47,48 are for tissu-specific expression. UAS-egr IR (BL58993)13 and UAS-hep RNAi (VDRC109277)49 are for gene knockdown. UAS-puc (BL20627)50 is for overexpression.
To starve flies on the protein-restrict diet, the 0–3-day old males were transferred onto protein deficient food (16% sucrose/0.7% argar)3 at a density of 20–30 flies per vial. Flies were transferred to fresh vials every 2–3 days. For protein refeeding, starved flies were transferred onto the regular food for one to five days. All flies were reared at 25 °C unless otherwise noted.
Temperature shift scheme for Gal4/UAS-mediated experiment
For co-overexpression of DTS5 and DTS7 by mef2-Gal4 and knockdown of egr by tara-Gal4, flies were raised at 18 °C during development and the 0–3-day old adult males were maintained at 29 °C throughout the entire fed or starved/refed stages. To inactivate JNK signaling in cyst lineage, eyaA3ts > UAS-hep RNAi and eyaA3ts > UAS-puc flies were raised at 25 °C during development. Adult flies were maintained at 25 °C during starvation and moved to 29 °C three days before protein refeeding in order to inactivate JNK signaling in the refeeding phase.
Immunostaining
Immunofluorescence staining of testes was performed as described previously12. The following primary antibodies were used: mouse anti-Arm (DHSB, 1:100), mouse anti-Fasciclin III (DSHB, 1:100), rabbit anti-GFP (Invitrogen A11122, 1:250), rat anti-DN-cadherin (DSHB, 1:20), goat anti-Vasa (Santa Cruz dc-13, 1:250), rabbit anti-Vasa (Santa Cruz d-260, 1:500), Rabbit anti-Zfh-1 (1:5,000)29. Secondary antibodies conjugated to Alexa 488- (Molecular Probes), Cy3 or Cy5 (Jackson ImmunoResearch) were used at 1:250. Hoechst 33342 was used to stain DNA at 1:500 for 15 min.
Classification of testis phenotypes
The grading of ectopic Zfh-1 expression and excess early germ cells was described previously12. Ectopic Zfh-1 cells are defined as the Zfh-1 positive cells located over 200 μm away from the hub. Level 1, 2, and 3 are defined by the number of ectopic cells less than 50 (level 1), between 50 and 200 (level 2) and more than 200 (level 3). The grade of excess early germ cells were classified into grade 1, grade 2, and grade 3 based on the range of early germ cell accumulation at 200–400 μm, 400–600 μm, and more than 600 μm away from the hub, respectively.
The classification of Egr-GFP levels is based on the specific GFP signal strength and the size of GFP-positive area in the testis sheath. Clear GFP signals in muscle cell cytoplasm throughout the whole testis was classified as “high.” The testes showing Egr-GFP in only some of the muscles or showing relatively weaker Egr-GFP signals were classified as “moderate”.
GSCs are defined as the Vasa-positive cells in contact with hub. CySCs are the Zfh-1 positive cells immediately adjacent to GSCs.
Feeding assay
Quantification of food intake was based on the uptake of a blue dye (Brilliant Blue R, sigma B7920-10G). A batch of four flies of each genotype was transferred onto fresh food containing blue dye (2.5% w/v) or not at 10 AM for 360 min. Flies were homogenized in 150 μL methanol. Homogenates were centrifuged at 13,000 rpm for 15 min. 140 μL of the clear supernatant were diluted with same volume of distilled water and the absorbance was measured at OD595 nm using spectrophotometer (SpectraMax M2). The flies exposed to regular non-dyed food were used as the baseline for the spectrophotometry.
CySC recovery rate during refeeding
During the 5-day refeeding period (R1–R5), the CySC recovery rate at R2 is defined as the percentage of CySC increase at R2 relative to R5: (CySC number at R2 − CySC number at S18)/(CySC number at R5 − CySC number at S18).
Acknowledgements
We are grateful to Shu Yuan Yang for critical reading of the manuscript. We thank Bloomington stock Center, Vienna Drosophila Resource Center, NIG-FLY Center and Developmental Studies Hybridoma Bank for fly stocks and antibodies. This study is supported by grants from Chang Gung Memorial Hospital (CMRPD1F0371-73) and Ministry of Science and Technology of Taiwan (MOST 108-2311-B-182-003) to H.P.
Author contributions
H.P. conceived and designed the project. Y.C.C., H.T., T-W.H. and B-W.X. performed research. Y.C.C., H.T., and H.P analyzed data. H.P., Y.C.C. and H.T. contributed to the writing of the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi Chieh Chang and Hsin Tu.
References
- 1.Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- 2.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Yamashita YM. The regulated elimination of transit-amplifying cells preserves tissue homeostasis during protein starvation in Drosophila testis. Development. 2015;142:1756–1766. doi: 10.1242/dev.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JG, Fuller MT. Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc Natl Acad Sci U S A. 2012;109:18477–18481. doi: 10.1073/pnas.1215516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 7.Matunis E, Tran J, Gonczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- 8.Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 9.Herrera SC, Bach EA. JNK signaling triggers spermatogonial dedifferentiation during chronic stress to maintain the germline stem cell pool in the Drosophila testis. Elife. 2018;7:1. doi: 10.7554/eLife.36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller, M. Spermatogenesis. in The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, pp. 71–147 (1993).
- 12.Chang YC, et al. Reproduction disrupts stem cell homeostasis in testes of aged male Drosophila via an induced microenvironment. PLoS Genet. 2019;15:e1008062. doi: 10.1371/journal.pgen.1008062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igaki T, et al. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. Embo J. 2002;21:3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauppila S, et al. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene. 2003;22:4860–4867. doi: 10.1038/sj.onc.1206715. [DOI] [PubMed] [Google Scholar]
- 15.Moreno E, Yan M, Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol. 2002;12:1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 16.Schneider DS, et al. Drosophila eiger mutants are sensitive to extracellular pathogens. PLoS Pathog. 2007;3:e41. doi: 10.1371/journal.ppat.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato K, Awasaki T, Ito K. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development. 2009;136:51–59. doi: 10.1242/dev.023366. [DOI] [PubMed] [Google Scholar]
- 19.Jo J, et al. Drosophila caspase activity is required independently of apoptosis to produce active TNF/Eiger during nociceptive sensitization. Cell Death Dis. 2017;8:e2786. doi: 10.1038/cddis.2016.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- 21.Andersen DS, et al. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature. 2015;522:482–486. doi: 10.1038/nature14298. [DOI] [PubMed] [Google Scholar]
- 22.Igaki T, Miura M. The Drosophila TNF ortholog Eiger: emerging physiological roles and evolution of the TNF system. Semin Immunol. 2014;26:267–274. doi: 10.1016/j.smim.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Sarov M, et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife. 2016;5:e12068. doi: 10.7554/eLife.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doupé DP, Marshall OJ, Dayton H, Brand AH, Perrimon N. Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. Proc. Natl. Acad. Sci. 2018;115:12218. doi: 10.1073/pnas.1719169115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 26.Schweisguth F. Dominant-negative mutation in the beta2 and beta6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proc Natl Acad Sci USA. 1999;96:11382–11386. doi: 10.1073/pnas.96.20.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140:56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabery EM, Schneider DS. The Drosophila TNF ortholog eiger is required in the fat body for a robust immune response. J Innate Immun. 2010;2:371–378. doi: 10.1159/000315050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 31.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 32.Michel M, Kupinski AP, Raabe I, Bokel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–2669. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- 33.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 34.Noselli S, Agnes F. Roles of the JNK signaling pathway in Drosophila morphogenesis. Curr Opin Genet Dev. 1999;9:466–472. doi: 10.1016/S0959-437X(99)80071-9. [DOI] [PubMed] [Google Scholar]
- 35.Tian A, Benchabane H, Wang Z, Ahmed Y. Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries. PLoS Genet. 2016;12:e1005822. doi: 10.1371/journal.pgen.1005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013;12:943–949. doi: 10.1111/acel.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Reilly AM. Irreversible effects of youthful choices in aged adults. PLoS Genet. 2019;15:e1008218. doi: 10.1371/journal.pgen.1008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yui R, Ohno Y, Matsuura ET. Accumulation of deleted mitochondrial DNA in aging Drosophila melanogaster. Genes Genet Syst. 2003;78:245–251. doi: 10.1266/ggs.78.245. [DOI] [PubMed] [Google Scholar]
- 39.Garcia AM, et al. Age- and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet. 2010;6:e1000950. doi: 10.1371/journal.pgen.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrailas-Mortimer A, et al. A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila. Dev Cell. 2011;21:783–795. doi: 10.1016/j.devcel.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel PH, Tamanoi F. Increased Rheb-TOR signaling enhances sensitivity of the whole organism to oxidative stress. J Cell Sci. 2006;119:4285–4292. doi: 10.1242/jcs.03199. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, et al. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci USA. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal N, et al. The Drosophila TNF Eiger Is an Adipokine that Acts on Insulin-Producing Cells to Mediate Nutrient Response. Cell Metab. 2016;23:675–684. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 46.Bunt SM, Monk AC, Siddall NA, Johnston NL, Hime GR. GAL4 enhancer traps that can be used to drive gene expression in developing Drosophila spermatocytes. Genesis. 2012;50:914–920. doi: 10.1002/dvg.22341. [DOI] [PubMed] [Google Scholar]
- 47.Ranganayakulu G, Elliott DA, Harvey RP, Olson EN. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development. 1998;125:3037–3048. doi: 10.1242/dev.125.16.3037. [DOI] [PubMed] [Google Scholar]
- 48.Kuckwa J, Fritzen K, Buttgereit D, Rothenbusch-Fender S, Renkawitz-Pohl R. A new level of plasticity: Drosophila smooth-like testes muscles compensate failure of myoblast fusion. Development. 2016;143:329–338. doi: 10.1242/dev.126730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demay Y, Perochon J, Szuplewski S, Mignotte B, Gaumer S. The PERK pathway independently triggers apoptosis and a Rac1/Slpr/JNK/Dilp8 signaling favoring tissue homeostasis in a chronic ER stress Drosophila model. Cell Death Dis. 2014;5:e1452. doi: 10.1038/cddis.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen J, Curtis C, Tavare S, Tower J. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging (Albany NY) 2009;1:191–211. doi: 10.18632/aging.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]