Abstract
Tracheobronchopathia osteochondroplastica (TPO) is an idiopathic disease involving the cartilage rings of the large airway, characterized by submucosal calcified nodules. Localized tracheobronchial amyloidosis (TBA) is another rare disease with localized amyloid deposits in the tracheobronchial tree. The two diseases rarely coincide, and only a few case reports and series have been reported.
A patient with dyspnea was referred to our clinic for suspicion of TBA. Chest computed tomography (CT) scan showed marked thickening of the tracheobronchial wall with calcified endobronchial submucosal nodules. The nodules were resected with a Diode Laser under rigid bronchoscopy, and results from the biopsy showed both osteochondroid metaplasia on microscopy in Hematoxylin and Eosin staining and apple-green birefringence on polarized microscopy in Congo red staining. This is a rare case in which microscopic findings of both TPO and TBA were observed on one slide. These findings suggest that localized TBA could be a cause of TPO.
1. Introduction
Tracheobronchopathia osteochondroplastica (TPO) and primary tracheobronchial amyloidosis (TBA) are two rare diseases. Sometimes it is reported that the two diseases are diagnosed in one patient, but tissue confirmation is usually not done for both of the entities. This case report provides a pathology slide with both of the diseases.
2. Case presentation
A patient visited our hospital with dyspnea on exertion which had started three years previously and worsened over the previous three weeks. Three years ago when the symptom started, he had visited a local clinic and underwent bronchoscopy which showed bronchial narrowing. At that time, he received no treatment.
He had diabetes mellitus and took oral hyperglycemic agents. He was a 17.5-pack-year current smoker. He had undergone a cranial operation due to trauma. His father had a history of tuberculosis, and his mother had diabetes mellitus.
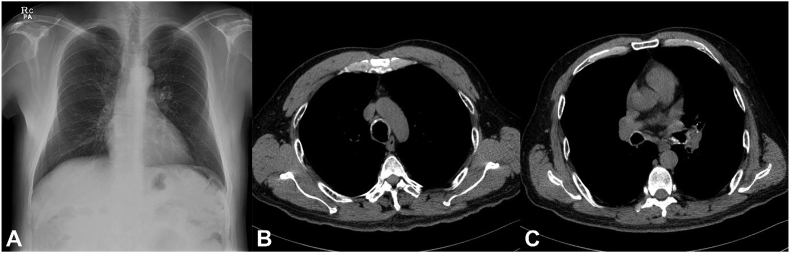
A chest radiograph revealed neither focal haziness in the lungs nor obstruction of the airways (Fig. 1A). A pulmonary function test showed a moderate obstructive pattern. A chest computed tomography (CT) scan showed tracheobronchial wall calcification from the trachea to the main bronchi and segmental bronchi (Fig. 1B and C). In the trachea, the nodules were prominent in the cartilaginous portion, while in the right and left main bronchi, luminal narrowing with calcification and mucus plugging were found.
Fig. 1.
Radiology. (A) Chest radiograph and (B, C) computed tomography (CT) scan showed no parenchymal lesion but airway calcifications. Irregular narrowing and thickening with calcification were found on (B) the upper trachea and (C) the both main bronchi.
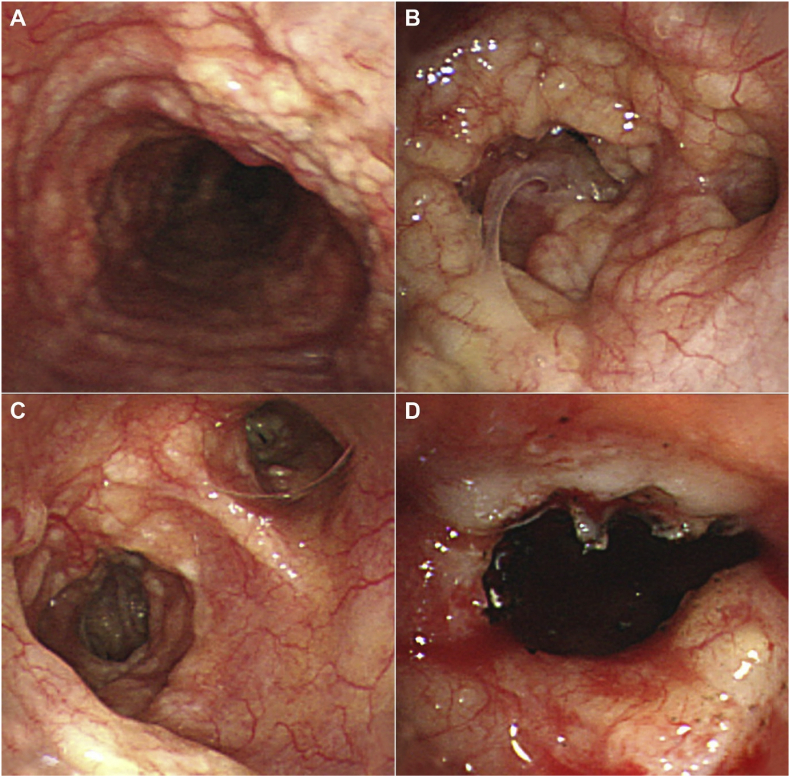
Under bronchoscopy, the whole trachea, the right middle and lower, and the left main and lingular bronchi were covered by submucosal whitish nodules with capillary dilatation (Fig. 2). Some bronchi were nearly obstructed by the nodules. The tissues were resected for pathology, and a biopsy sample was obtained from the right main bronchus.
Fig. 2.
Bronchoscopy. Irregular submucosal nodules were found on (A) the whole trachea, (B) the right lower lobar bronchi, and (C) the left lingular and lower lobar bronchi. (D) The pin-point narrowing of the left lingular bronchus was relieved by tissue resection using a Diode Laser.
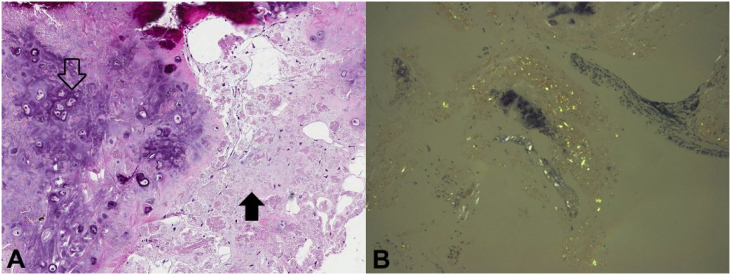
The pathology showed pinkish amorphous deposition in the bronchial wall with multifocal osteochondroid metaplasia (Fig. 3A). Using Congo-red staining, apple-green birefringence was observed under the polarized microscope (Fig. 3B).
Fig. 3.
Pathology. (A) Hematoxylin and Eosin staining showed osteochondroid metaplasia (open arrow) and pink amorphous material (closed arrow). (B) Polarized microscopy revealed apple-green birefringence. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Tracheobronchopathia osteochondroplastica (TPO) with primary tracheobronchial amyloidosis (TBA) is very rare. One study of 41 patients with TPO demonstrated that TBA confirmed by Congo red staining was observed in only 2/16 specimens [1]. The coincidence of these two rare diseases occurring simultaneously was first documented in 1968 [2]. The necropsy of a patient showed extensive amyloid infiltration in the submucosa and islands of bony deposits along cartilaginous rings on microscopy as well as a “cobblestone appearance” of the trachea on bronchoscopy. The authors postulated that the advanced ossified stage of primary TBA may be TPO.
There are only a few reports successfully demonstrated the coexistence of TPO and TBA with Hematoxylin and Eosin (H&E) and Congo red staining [3,4]. In some studies, amyloidosis was confirmed by Congo red staining, but TPO was not proved by pathology because the lesion spared the posterior membrane of the trachea. However, some cases of localized amyloidosis could involve only the anteromedial aspects of trachea. Therefore, pathologic confirmation is needed for diagnosis of TPO and TBA.
Regarding pathogenesis, amyloid fibrils have affinity for calcium, and amyloid deposits frequently accompany calcification and ossification [5]. It could be suggested that TBA is one of the causes of TPO, which is characterized by calcification and ossification in the submucosa.
In conclusion, chest CT and bronchoscopy are helpful for diagnosis of TPO and TBA, although definitive diagnosis is a pathological diagnosis. The present case demonstrated the coexistence of osteochondrosis and amyloidosis in the submucosa, suggesting their association in the pathogenesis.
Declaration of competing interest
The authors have nothing to disclose.
Contributor Information
Ju Yeun Song, Email: idanegos@gmail.com.
Bo-Guen Kim, Email: kbg1q2w3e@gmail.com.
Sungmin Zo, Email: aboutsweets@gmail.com.
Sujin Park, Email: sujin423.park@samsung.com.
Joung Ho Han, Email: joungho.han@samsung.com.
Hojoong Kim, Email: hjk3425@skku.edu, hjk3425@skku.edu.
References
- 1.Leske V., Lazor R., Coetmeur D. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine. 2001;80(6):378–390. doi: 10.1097/00005792-200111000-00004. [published Online First: 2001/11/13] [DOI] [PubMed] [Google Scholar]
- 2.Sakula A. Tracheobronchopathia osteoplastica: its relationship to primary tracheobronchial amyloidosis. Thorax. 1968;23(1):105–110. doi: 10.1136/thx.23.1.105. [published Online First: 1968/01/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyoda M., Ebihara Y., Kato H. Tracheobronchial AL amyloidosis: histologic, immunohistochemical, ultrastructural, and immunoelectron microscopic observations. Hum. Pathol. 1993;24(9):970–976. doi: 10.1016/0046-8177(93)90110-3. [published Online First: 1993/09/01] [DOI] [PubMed] [Google Scholar]
- 4.Kirbas G., Dagli C.E., Tanrikulu A.C. Unusual combination of tracheobronchopathia osteochondroplastica and AA amyloidosis. Yonsei Med. J. 2009;50(5):721–724. doi: 10.3349/ymj.2009.50.5.721. [published Online First: 2009/11/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan E.D., Morales D.V., Welsh C.H. Calcium deposition with or without bone formation in the lung. Am. J. Respir. Crit. Care Med. 2002;165(12):1654–1669. doi: 10.1164/rccm.2108054. [published Online First: 2002/06/19] [DOI] [PubMed] [Google Scholar]