Abstract
Background
Pulmonary Lymphangioleiomyomatosis (LAM) is an uncommon disease and may be associated with tuberous sclerosis complex (TSC). LAM is reported to occur exclusively in females of the premenopausal age group. Here we report a rare entity of lymphangioleiomyomatosis in a male patient of tuberous sclerosis, who developed pneumothorax following mechanical ventilation.
Case summary
A young adult presented to the emergency room with history of recurrent seizures since the 6th month of his age. He was intubated in the emergency room for protection of the airway and was initially maintained on manual ventilation using Bain's circuit. Neuroimaging revealed multiple calcified subcortical nodules and giant cell astrocytoma in left lateral ventricle. On the third day of hospitalization, he developed subcutaneous emphysema on his neck and anterior wall of chest. Contrast-enhanced CT chest revealed presence of subcentimetric thin walled cystic lesions in lungs, pneumomediastinum, right sided pneumothorax, and diffuse subcutaneous emphysema. Right sided pneumothorax was managed by intercostal chest tube drainage. CECT abdomen showed well defined heterogeneously enhancing lesions in right kidney suggestive of angiomyolipoma. A final diagnosis of Lymphangioleiomyomatosis (LAM) in tuberous sclerosis (TSC) was made. Considering the high recurrence of pneumothorax, pleurodesis was done and sirolimus (2 mg per oral OD) was initiated.
Conclusion
Cystic lung disease consistent with LAM is a rare entity in males with TSC, which can be missed easily in patients with extra-pulmonary manifestations. Treating clinician or intensivist should remain vigilant. Active follow-up, chest imaging and pulmonary function testing should be advised to screen the patients for coincidental finding of LAM.
Abbreviations: LAM, Lymphangioleiomyomatosis; TSC, Tuberous Sclerosis Complex
1. Case presentation
An 18-year-old boy presented to the emergency department with a history of seizures followed by loss of consciousness. He was intubated in the emergency room for the protection of the airway and was initially maintained on Bain's circuit ventilation. He received an initial intravenous loading dose of phenytoin (1 g) and lorazepam (2 mg) for control of seizure. His refractory convulsive state responded to injection valproate 1 g intravenously. Subsequently, he was shifted to the intensive care unit on mechanical ventilation and was put on CPAP/PSV settings of fractional inspired oxygen of 30%, pressure support of 10 and PEEP of 5 mmHg.
On repeated inquiry about his illness, the brother divulged a history of multiple episodes of generalized tonic-clonic seizures (GTCS) since the 6th month of age for which he was on Ayurvedic medication. But his seizures were poorly controlled. The boy had delayed developmental milestones. The antenatal and perinatal period was unremarkable. Two days before hospitalization, he had multiple episodes of GTCS (every 2–3 min) followed by post-ictal confusion with no regain of consciousness in between. Clinical examination revealed multiple angiofibromas over the forehead, and cheeks (Fig. 1A). Similar lesions were also present on the anterior and posterior aspects of the trunk. There were two well defined hyperpigmented plaques of sizes measuring 10 × 5cm and 12 × 10 cm over the left lower abdomen and posterior aspect of trunk suggestive of shagreen patches (Fig. 1B). Multiple periungual fibromas were also noted on the nails of both upper limbs (Fig. 1C). Oropharyngeal examination revealed dental pits (Fig. 1D).
Fig. 1.
A: Multiple angiofibromas over forehead, and cheeks. B: Well defined hyper pigmented plaques of sizes measuring 12 × 10 cm seen over the posterior aspect of trunk suggestive of shagreen patches. C: Multiple periungual fibromas noted on the nails of both upper limbs Fig. 1D: Oral cavity examination revealed dental pits (Black arrow head).
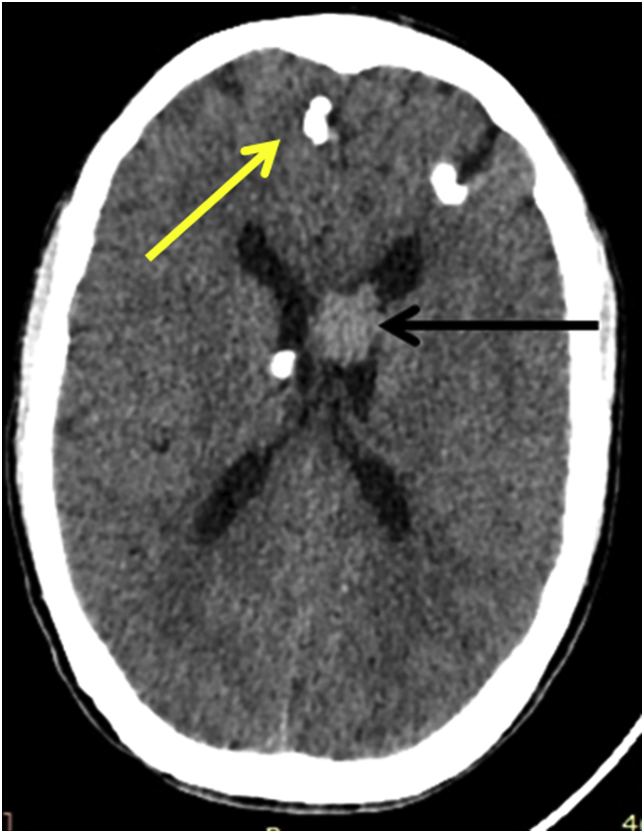
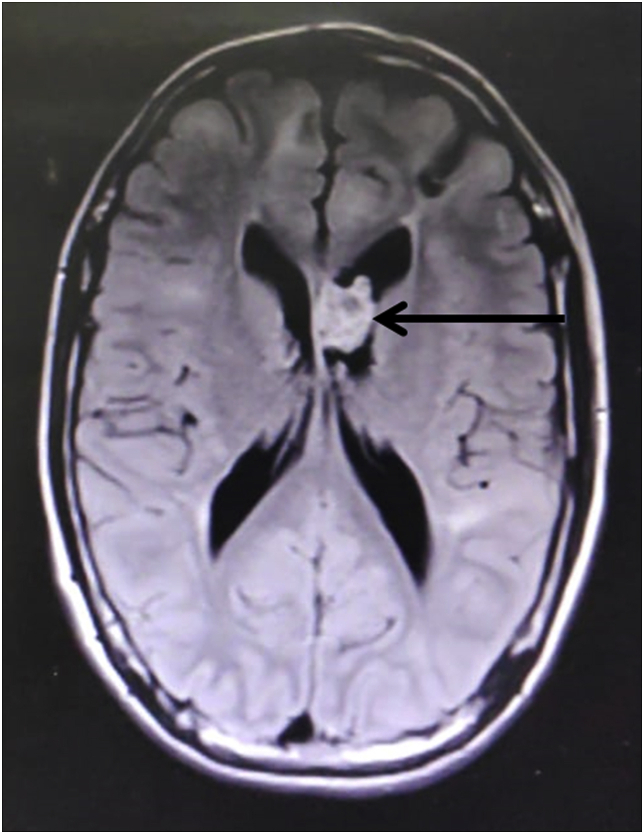
Laboratory examination revealed normal hemogram, liver function, renal function, and serum electrolyte. Non-contrast computed tomography (NCCT) scan of the brain showed multiple calcified subcortical nodules (yellow arrowhead, Fig. 2) and left lateral ventricle lesion (black arrowhead, Fig. 2). For a better characterization of the lesion, the MRI brain was done, which revealed a heterogenous contrast-enhancing lesion in the left lateral ventricle. The lesion was hypointense on T1, T2 sequences, and hyperintense on FLAIR sequences, suggestive of giant cell astrocytoma (Fig. 3).
Fig. 2.
Noncontract Computed tomography (NCCT) scan of the brain showed multiple calcified subcortical nodules (yellow arrowhead) and left lateral ventricle lesion (Black arrowhead).
Fig. 3.
MRI brain showed a heterogeneous contrast enhancing lesion in the left lateral ventricle, hyperintense on FLAIR sequences suggestive of giant cell astrocytoma.
On the third day of hospitalization, the patient was noticed to have subcutaneous emphysema on his neck and anterior wall of the chest. Chest skiagram revealed only heterogeneous opacities in the mid-zone of right hemithorax suspicious of ventilator associated pneumonia. Contrast-enhanced CT chest revealed the presence of subcentimetric thin-walled cystic lesions in the lungs (black arrowhead, Fig. 4), pneumomediastinum, right sided pneumothorax, and diffuse subcutaneous emphysema (Fig. 4). Right sided pneumothorax was managed by intercostal chest tube drainage.
Fig. 4.
Contrast-enhanced CT chest revealed the presence of subcentimetric thin walled cystic lesions in the lungs (Black arrowhead), pneumomediastinum, and right sided pneumothorax and diffuse subcutaneous emphysema.
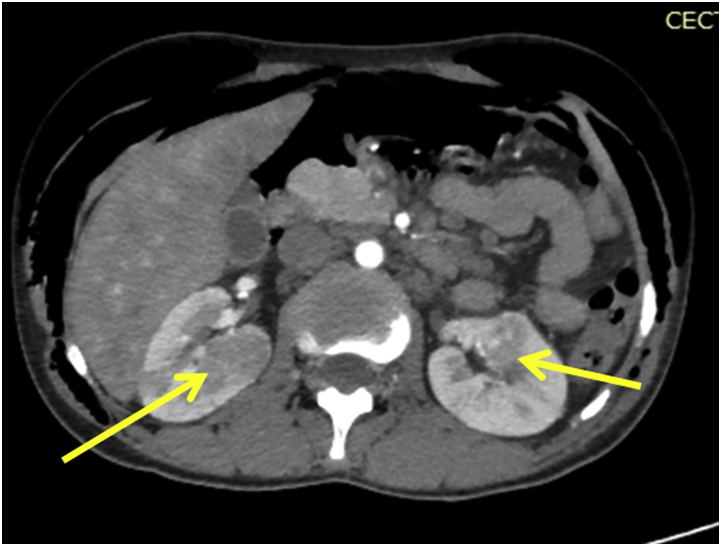
He was evaluated further. Fundoscopic examination of the eye revealed multiple astrocytomas. CECT abdomens showed well defined heterogeneously enhancing lesions in the right kidney suggestive of angiomyolipoma (Fig. 5).
Fig. 5.
CECT abdomens showed well defined heterogeneously enhancing lesions in the right kidney suggestive of angiomyolipoma.
A final diagnosis of Lymphangioleiomyomatosis (LAM) in tuberous sclerosis (TSC) was made. Considering the high recurrence of pneumothorax, pleurodesis was done and sirolimus (2 mg per oral OD) was initiated. He was managed in ICU for a period of 20days with antibiotics and antiepileptics. Tracheostomy was done considering the need for a prolonged duration of mechanical ventilation. Gradually the patient improved and was decannulated after a week. He was observed in ICU for further 2 days, got shifted to the ward, and finally discharged.
2. Discussion
Pulmonary lymphangioleiomyomatosis is characterised by the nonneoplastic, peribronchial, perivascular, and a perilymphatic proliferation of abnormal smooth muscle cells and cystic destruction of lung parenchyma. The most common presenting symptoms of LAM are dyspnea on exertion and pneumothorax. Other common signs and symptoms include non-productive chronic cough, hemoptysis, wheeze, chest pain, chylothorax. Pulmonary function tests vary from normal, obstructive to mixed pattern. An obstructive pattern seen initially is due to smooth muscle proliferation around the bronchi, gradually air trapping in the alveoli leads to the formation of cysts and bullae giving rise to a restrictive picture. When smooth muscle cell proliferation progressed from the airways to blood vessels resulting in cor-pulmonale and respiratory failure with reduction in diffusion capacity [1].
Lymphangioleiomyomatosis (LAM) occurs either as sporadic (S- LAM) or in association with tuberous sclerosis (TSC -LAM). Tuberous sclerosis is a genetic, autosomal dominant, disorder that can cause multisystem benign, non-invasive lesions [2]. The diagnostic criterion for TSC was revised in 2012 by International Tuberous Sclerosis Complex Consensus Conference diagnostic criteria (Table 1). This patient met 7 Major criteria. Definitive diagnostic criteria for LAM is the presence of characteristic and compatible lung HRCT and lung biopsy or characteristic findings on HRCT chest with anyone either angiomyolipoma (kidney) or TSC [3]. However, in our case, as the patient did not give consent, lung biopsy for histopathological confirmation of LAM could not be done. TSC-LAM patients usually present with gradual onset of dyspnoea, but S-LAM patients present more acutely often with pneumothorax. Our patient presented initially with central nervous system (CNS) manifestation of seizures. Subsequently, he developed pneumothorax and subcutaneous emphysema, mostly due to the rupture of pre-existing lung cysts on positive pressure ventilation. Both S-LAM and TSC-LAM are associated with mutations in tuberous sclerosis genes. In TSC-LAM, germline mutations in TSC genes are present in all cells of the body (i.e., first hit), and a when a second hit ‘somatic’ mutation of the remaining normal gene results in loss of heterozygosity it leads to the formation of neoplasm. Whereas double ‘somatic’ inactivation of both alleles of TSC1 or TSC2 gene without a germline mutation could explain the occurrence of S-LAM [4]. Most cases is caused by mutations in TSC2 and a few are by mutations in TSC1. [2]Alterations on the function of the protein product of TSC2 namely tuberin, promote the development of cellular proliferation and protein synthesis by activating ribosomal protein p70S6K, leading to the hyperphosphorylation of ribosomal protein S6. This step is mediated by multiple signalling pathways. One regulator is PI 3-kinase, which activates the phosphoinositide-dependent kinase-1 and phosphorylates p70S6K onThr-229. Another regulator of p70S6K is the mammalian target of rapamycin (mTOR) which also directly phosphorylate Thr-389 of p70S6K [5,6].
Table 1.
Diagnostic criteria 2012 International Tuberous Sclerosis Complex Consensus Conference.
| MAJOR CRITERIA | ||
| Facial angiofibromas (≥3) or fibrous cephalic plaque | Cortical tuber | Cardiac rhabdomyoma |
| Nontraumatic ungual or periungual fibroma (≥2) | Subependymal nodule | Lymphangioleiomyomatosis: |
| Hypomelanotic macules (>3) at least 5 mm diameter | Subependymal giant cell astrocytoma | Renal angiomyolipoma |
| Shagreen patch | Multiple retinal hamartoma | |
| MINOR CRITERIA | ||
| Multiple randomly distributed pits in dental enamel | Cerebral white matter radial migration lines | Retinal achromic patch |
| Hamartomatous rectal polyps | Intra oral fibroma | Confetti” skin lesions |
| Bone cysts | Nonrenal hamartoma | Multiple renal cysts |
Definite TSC - Two major features or one major feature plus two or more minor features.
TSC-LAM is described among the most gender restricted diseases predominantly affecting females of reproductive age and exacerbations usually occurs at times of hormonal fluctuation like pregnancy and use of the oral contraceptive pill is explained by the presence of hormone receptors in the lung tissue. The possible mechanism of gender restriction is an intersection of feedback inhibition Akt/mTOR pathway by oestrogen thereby releasing the tuberin or hamartin (tuberous sclerosis proteins) deficient cells in the presence of constitutive S6K activation [4].
There is no definitive treatment for pulmonary LAM. Bronchodilators are often used for symptomatic patients. Pneumothorax initially managed by intercostal chest tube drainage associated with a recurrence rate of two thirds, often requiring treatment with talc pleurodesis or sometime video-assisted thoracoscopic surgery (VATS) for lung bleb resection [7]. Hormonal therapy in terms of anti-oestrogen like Tamoxifen, oophorectomy, progesterone have been used anecdotally without much beneficial effect. Inhibitors of mTOR pathway by sirolimus is a promising therapy in LAM, the first study by Cincinnati Angiomyolipoma Sirolimus Trial (CAST) showed a significant decrease in angiomyolipoma volume; and improvement in the FEV1, forced vital capacity (FVC), and residual volume (RV) [8].
Human epidermal growth factor receptor (HER) is investigated in recent studies, which contribute to the progression of the disease and could be a target for effective treatment of pulmonary LAM [9].
To the best of our knowledge based on search in Medline and PubMed library, pulmonary LAM in men is extremely rare, and only six cases have been histologically diagnosed and reported in the literature. Here we report a rare entity of lymphangioleiomyomatosis in a male patient of tuberous sclerosis. He is a phenotypically normal male with normally developed external genitalia, normal sexual hair, and genotypically male (XY karyotype from buccal mucosa).
In conclusion, Cystic lung disease consistent with LAM is a rare entity in males with TSC, which can be missed easily in patients with extra-pulmonary manifestations. Treating clinicians or intensivists should remain vigilant. Active follow-up, chest imaging and pulmonary function testing should be advised to screen the patients for a coincidental finding of LAM. Various therapeutic strategies viz. modulation of oestrogen and progesterone levels and mTOR inhibitors are currently recommended for treatment.
Funding information
Nil.
Declaration of competing interest
All authors declared no conflict of interest.
Contributor Information
Ankita Kabi, Email: ankitakabi@yahoo.com.
Sagarika Panda, Email: sp.ruby@gmail.com.
Sonu Sama, Email: sonusama9287@gmail.com.
Subodh Kumar, Email: subodhgsvm@gmail.com.
Nidhi Kaeley, Email: drnidhi_kaeley@yahoo.com.
Sachin Sogal P, Email: sachinsogal07@gmail.com.
References
- 1.Castro M., Shepherd C.W., Gomez M.R. Pulmonary tuberous sclerosis. Chest. 1995;107:189–195. doi: 10.1378/chest.107.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Curatolo P., Bombardieri R., Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 3.Rhee J., Adial A., Gumpeni R., Iftikhar A. Lymphangioleiomyomatosis: a case report and review of literature. Cureus. 2019;11(1):e3938. doi: 10.7759/cureus.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mc Cormack F.X. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 5.Goncharova E.A., Goncharov D.A., Eszterhas A. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumour suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J. Biol. Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 6.Balendran A., Currie Armstrong C.G., Avruch J., Alessi D.R. J. Biol. Chem. 1999;274:37400–37406. doi: 10.1074/jbc.274.52.37400. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S.R., Tattersfield A.E. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000;55:1052–1057. doi: 10.1136/thorax.55.12.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissler J.J., McCormack F.X., Young L.R. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomotosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi K., Miki Y., Saito R., Adachi K., Seyama K., Okada Y., Sasano H. Roles of human epidermal growth factor receptor family in pulmonary lymphangioleiomyomatosis. Hum. Pathol. 2018;81:121–130. doi: 10.1016/j.humpath.2018.07.002. [DOI] [PubMed] [Google Scholar]