Highlights
-
•
CSF Amyloid- β is related to medial temporal and posterior brain atrophy in Lewy body dementia.
-
•
CSF p-Tau is associated with posterior brain atrophy in Lewy body dementia.
-
•
CSF AD-related biomarkers are not related to atrophy in the frontal lobes.
Keywords: Lewy body disease, Biomarkers, Atrophy, Alzheimer disease, Neuroimaging
Abstract
Background
Alzheimer’s disease (AD)-related pathology is frequently found in patients with dementia with Lewy bodies (DLB). However, it is unknown how amyloid-β and tau-related pathologies influence neurodegeneration in DLB. Understanding the mechanisms underlying brain atrophy in DLB can improve our knowledge about disease progression, differential diagnosis, drug development and testing of anti-amyloid and anti-tau therapies in DLB.
Objectives
We aimed at investigating the combined effect of CSF amyloid-β42, phosphorylated tau and total tau on regional brain atrophy in DLB in the European DLB (E-DLB) cohort.
Methods
86 probable DLB patients from the E-DLB cohort with CSF and MRI data were included. Random forest was used to analyze the association of CSF biomarkers (predictors) with visual rating scales for medial temporal lobe atrophy (MTA), posterior atrophy (PA) and global cortical atrophy scale-frontal subscale (GCA-F) (outcomes), including age, sex, education and disease duration as extra predictors.
Results
DLB patients with abnormal MTA scores had abnormal CSF Aβ42, shorter disease duration and older age. DLB patients with abnormal PA scores had abnormal levels of CSF Aβ42 and p-tau, older age, lower education and shorter disease duration. Abnormal GCA-F scores were associated with lower education, male sex, and older age, but not with any AD-related CSF biomarker.
Conclusions
This study shows preliminary data on the potential combined effect of amyloid-β and tau-related pathologies on the integrity of posterior brain cortices in DLB patients, whereas only amyloid-β seems to be related to MTA. Future availability of α-synuclein biomarkers will help us to understand the effect of α-synuclein and AD-related pathologies on brain integrity in DLB.
1. Introduction
Neuropathological studies have shown that many patients diagnosed with dementia with Lewy bodies (DLB) often have Alzheimer’s disease (AD)-related pathology (Gomez-Isla et al., 1999, Schneider et al., 2009, Halliday et al., 2011, Dugger et al., 2014, Sierra et al., 2016) In these mixed cases, it has been found that the degree of AD-related pathology is moderate or severe in more than 70% of patients (Kosaka, 1990, Marui et al., 2004, Barker et al., 2002, Irwin et al., 2017). The combination of these proteinopathies have implications in the clinical phenotype. Thus, postmortem studies have shown that the coexistence of amyloid-β and tau-related pathologies in addition to the defining alpha-synuclein pathology usually results in a less typical presentation of DLB core features: a lower frequency of recurrent visual hallucinations, parkinsonism, REM sleep behavior disorder (RBD) and fluctuating cognition (Merdes et al., 2003, Del Ser et al., 2001, Tiraboschi et al., 2015, Compta et al., 2013, Murray et al., 2013), and a more severe disease course (Irwin et al., 2017, Howlett et al., 2015, Kraybill et al., 2005, Williams et al., 2006).
Similar findings have been obtained in vivo (Di Censo et al., 2020), which is more relevant for the earlier disease stages compared to the end-stage diseases assessed at autopsy. In this regard, cerebrospinal fluid (CSF) and neuroimaging studies have shown concomitant AD-related biomarkers in a significant proportion of DLB patients who often are older, have shorter disease duration and worse cognitive performance (Van Steenoven et al., 2016, Gomperts et al., 2016, Abdelnour et al., 2016, Lemstra et al., 2017), mainly in orientation and memory (Andersson et al., 2011, Tagawa et al., 2015).
Nevertheless, how AD-related pathology influences the neurodegenerative process in DLB is less studied. In AD, amyloid-β and tau-related pathologies are hypothesized to lead to neuronal injury (Hyman et al., 2012, Braak et al., 2006). DLB patients with concomitant AD-related pathology have shown a faster rate of brain atrophy over time measured with MRI, mainly in the medial temporal lobe, when compared with DLB patients without concomitant AD-related pathology (Nedelska et al., 2015, Sarro et al., 2016, Blanc et al., 2017, Nelson et al., 2009). This finding suggests that AD pathology may contribute to medial temporal lobe atrophy (MTA) in DLB (Elder et al., 2017; Sarro et al., 2016). Similarly, when amyloid is present the patterns of deposition and atrophy resembles that observed in AD (Shimada et al., 2013, Donaghy et al., 2015, Irwin and Hurtig, 2018, Mak et al., 2019a) Understanding the underlying mechanisms of regional brain atrophy that could reflect distinct pathologies could have treatment implications. For example, a typical AD pattern of brain atrophy involving the medial temporal lobes and posterior cortices was associated with poorer response to acetylcholinesterase inhibitors in DLB patients (Graff-Radford et al., 2012). Also, amyloid or tau-targeted therapies might be effective in a subgroup of patients with DLB, i.e. it is important to improve our understanding in what degree AD pathologies may contribute towards personalized medicine approaches and improve differential diagnosis, disease prognosis, and treatment response in DLB.
Combining CSF biomarkers and structural MRI may inform about the mechanisms underlying regional atrophy, but few studies have been performed in DLB and results are inconsistent. A recent study reported an association between abnormal levels of CSF Aβ42 and MTA in DLB, as well as an association between abnormal levels of CSF total tau (T-tau) and global brain atrophy (van der Zande et al., 2018). By contrast, another study reported no differences between amyloid PET positive and negative DLB patients in hippocampal or gray matter volume (Donaghy et al., 2018). However, associations between regional atrophy and tau-related pathology have not been investigated yet. Importantly, in vitro studies have found cross-seeding of alpha-synuclein, amyloid and tau proteins (Spires-Jones et al., 2017), thus the combination of proteinopathies may have additional or even synergistic contributions to neurodegeneration. Hence, we aimed to explore this question by investigating the combined effect of CSF Aβ42, T-tau and p-tau on regional brain atrophy in the E-DLB cohort, a large multi-center study involving 19 centers from Europe (Oppedal et al., 2019). Our hypothesis was that DLB patients with abnormal levels of CSF Aβ42, T-tau and p-tau would have a higher level of brain atrophy, in particular, in the medial temporal lobes and posterior cortices, delineating the typical pattern of brain atrophy in AD (Oppedal et al., 2019).
2. Materials and methods
2.1. Participants population
A total of 86 DLB patients were selected from 6 centers of the E-DLB cohort. Inclusion criteria to enter the E-DLB cohort are reported in previous publications (Kramberger et al., 2017). For the current study, selection criteria were: 1) a diagnosis of probable DLB; 2) availability of CSF data; and 3) availability of MRI data. Detailed information about the centers that contributed to the current study is shown in Supplementary tables.
2.2. Diagnostic and clinical examination
Because the E-DLB cohort was assembled retrospectively, many patients had been diagnosed before 2017. Hence, The DLB diagnosis was made according to McKeith 2005 criteria (McKeith et al., 2005), but we were able to confirm the diagnosis of probable DLB according to McKeith 2017 criteria (McKeith et al., 2017) in 83 out of 86 patients. Diagnosis was made by the treating physician, a group of at least two expert clinicians, or a multidisciplinary team at a consensus diagnostic meeting on the basis of all available clinical and diagnostic test data as previously reported (Oppedal et al., 2019, Kramberger et al., 2017;57(3).).
Clinicians interviewed both patients and caregivers, recorded demographic information as well as medical and drug history. All centers included a detailed medical history, aside from physical, neurological, and psychiatric examinations using standardized scales such as the motor subscale of the Unified Parkinson’s Disease rating scale (Fahn et al., 1987) and the Neuropsychiatric Inventory (Cummings et al., 1994). Based on the clinical examination and/or the aforementioned scales the core diagnostic features fluctuating cognition, parkinsonism, and recurrent visual hallucinations were recorded as present or absent. Neuropsychological evaluation and complementary tests to rule out secondary causes of dementia (routine blood tests and brain imaging) were performed. 26 out of the total sample of 86 subjects had available DAT SPECT, 25 (96.15%) of which were abnormal.
2.3. Ethics
Local ethics committees at the individual centres approved the study. The patients gave their written consent to use the anonymised results of their clinical, instrumental and laboratory investigations for research purposes.
2.4. CSF procedures
CSF was obtained at all centers with the following procedures: 1) lumbar puncture at the L3-4 or L4-5 interspace; 2) collection in polypropylene tubes and centrifuged for 10 min at 4 °C; and 3) storage in aliquots of 0.5 mL at −80 °C or −70 °C until further analysis. Further details are summarized in Supplementary Table 1. CSF analyses were performed locally according to standard routines. INNOTEST enzyme-linked immunosorbent assays (ELISA) were used to analyze T-tau and p-tau (missing for 1 patient) in all samples and Aβ42 in 80 samples (Fujirebio, Ghent, Belgium). The remaining 6 samples were analyzed for Aβ42 using ELISA kits from Biosource Europe S.A. CSF values were dichotomized as normal or pathological based on well-established center-specific cut-off values for each biomarker as previously described (Abdelnour et al., 2016) (Supplementary Table A.1).
2.5. MRI analysis
Different neuroimaging acquisition protocols and MRI scanners were used as detailed in Supplementary Table A.2. The interval between MRI and CSF collection ranged from 0 to 3 months in the majority of the cases (73 out 86, which corresponds to 84.88%). In 13 patients the interval ranged from 3 to 12 moths (15.12%). We used visual rating of MRI scans, which is more feasible for clinical use than automated analysis, and is not influenced by between-center differences in acquisition protocols and MRI scanners. Ratings of all scans were performed by one expert radiologist (L. C.) as previously described (Ferreira et al., 2017), who has excellent intra-rater reliability - weighted κ of 0.94 and 0.89 for MTA in left and right hemispheres correspondingly, 0.88 for PA, and 0.83 for GCA-F in 120 random cases- (Ferreira et al., 2017) blinded to clinical data. T1-weigthed images were used to investigate regional brain atrophy by using three visual rating scales: the medial temporal lobe atrophy scale (MTA) (Scheltens et al., 1992), the posterior atrophy scale (PA) (Koedam et al., 2011) and the global cortical atrophy scale-frontal subscale (GCA-F) (Ferreira et al., 2016). Detailed information regarding the visual rating scales is provided elsewhere (Ferreira et al., 2017). The visual rating scores were dichotomized into normal and abnormal values in accordance with previously proposed cut-offs (Ferreira et al., 2015)
2.6. Statistics
The statistical analyses were done using R (www.R-project.org) version 3.2.4 and IBM SPSS version 26. Descriptive results are shown as mean ± SD for normally distributed continuous variables, and number and percentage for categorical variables.
The aim of this study was to investigate the combined effect of Aβ42, T-tau and p-tau (predictors) on regional brain atrophy as measured with visual rating scales (outcome variables). All these measures are dichotomous (0 normal, 1 abnormal). We also wanted to model the effects of age, sex, education and disease duration to investigate their possible added effect to the association between CSF biomarkers and regional brain atrophy. Age, education and disease duration are continuous variables while sex is dichotomous (0 males 1 females). Further, our interest was to investigate the predictive power of all these variables in combination as predictors of regional brain atrophy, rather than investigating their partial effects. Random forest (classification) (Breiman, 2001) was thus chosen given our aim, the nature of the variables, the number of predictors and the sample size. Random forest is an ensemble method in machine learning that involves growing of multiple decision trees via bootstrap aggregation (bagging). Each tree predicts a classification independently and votes for the corresponding class. The best model for each outcome variable is chosen from the majority of votes (Machado et al., 2018). Importantly, contrarily to other predictive methods such as multiple linear or logistic regression that investigate partial effects (competition among predictors in the prediction of the outcome), random forest investigates combined effects (the predictors do not compete with each other but “cooperate” in the prediction of the outcome) (Machado et al., 2018) Combined effects are closer to what we hypothesized in this study, i.e., amyloid-β (CSF Aβ42) and tau-related (CSF p-tau) pathologies have a synergistic deleterious effect on brain integrity. When CSF Aβ42 and CSF p-tau as predictors show a contribution to the prediction of brain atrophy, we can conclude that both pathologies have a combined effect on brain integrity, which may reflect their synergy at the pathological level (i.e. the “cooperation” between Aβ42 and CSF p-tau contributes to the prediction of brain atrophy). Further, random forest performs very similarly to other machine learning algorithms (Machado et al., 2018) but it was preferred in our current study due to the nature of our variables. We performed three random forest models: one for each atrophy scale (MTA, PA, and GCA-F) as the outcome variable. The random forest models were comprised of 5000 trees, providing an accurate estimation of the variables importance without introducing too much noise in the models due to the addition of redundant trees. Each of the trees was trained on randomly picked 70% of the data and subsequently tested on the unseen 30% of the data. Classification models (normal vs. abnormal) (Liaw and Wiener, 2002) were conducted, accounting for the fact that the outcome variable may present with an unbalanced amount of cases in its two levels (e.g. normal MTA n = 53, abnormal MTA n = 34). The classification error is reported as a measure of goodness of the model (out-of-the-bag estimated error rate, OOB-EER) (Breiman, 2001). When outcome variables are dichotomous, as it is our case, the error by chance is 50%. Therefore, a classification below 50% is better than chance, with values closest to 0% denoting better classification performance, hence good reliability of the model. We also report the importance (Imp) of the predictors as a measure of their contribution towards the prediction of the outcome variable (regional brain atrophy). Higher Imp values denote stronger contribution to the prediction. The random forest results were further complemented with the Pearson correlation coefficient to easily represent the magnitude and direction of the association between variables (bivariate association). P-values of Pearson correlation are reported for completeness of information.
3. Results
3.1. Sample features
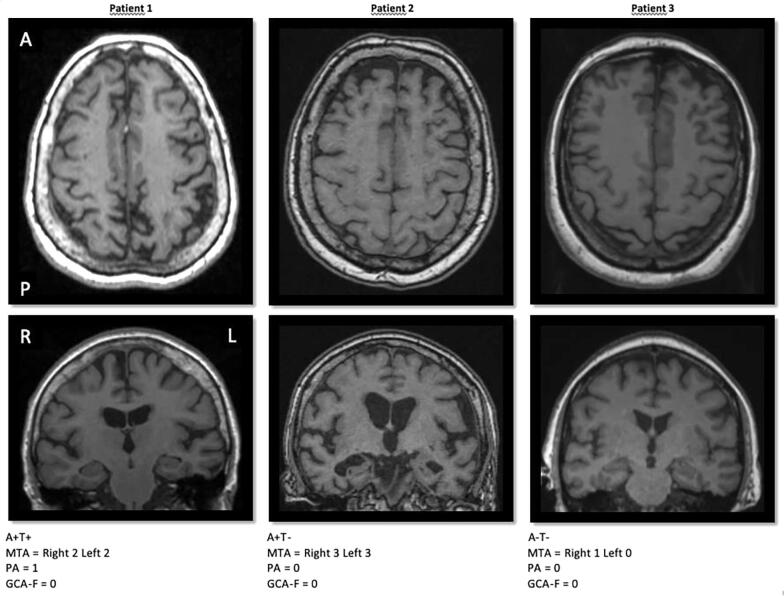
Clinical and demographic features of the sample are reported in Table 1. Of the 86 patients, the number of patients with pathological CSF values is 28 (32.56%) for Aβ42, 17 (19.77%) for Total-Tau and 24 (27.91%) for p-Tau. The number of patients with abnormal scores in the visual rating scales was: MTA: 33 (38.37%), GCA-F: 34 (39.53%) and PA: 45 (52.33%). Fig. 1 shows 3 examples of different combinations for CSF Aβ42, CSF p-Tau CSF and the visual rating scales.
Table 1.
Demographic and clinical data of the participants.
| Features | Mean (SD) | Range |
|---|---|---|
| Age at diagnosis | 69.36 (8.85) | 49–88 |
| Sex: Male N (%) | 49 (56.98) | |
| Years of education | 11.24 (4.08) | 5–22 |
| Disease duration (years) | 4.04 (3.10) | 0.5–14 |
| MMSE | 24.85 (3.72) | 15–30 |
| Parkinsonism (%) | 82.6 (N = 71) | |
| Visual hallucinations (%) | 58.1 (N = 50) | |
| Fluctuating cognition (%) | 75.6 (N = 65) |
N: number. MMSE: Minimental State Examination.
Fig. 1.
Normal and pathological CSF values of Aβ42 and p-Tau combined with visual rating scales. CSF levels of Aβ42 and p-Tau were dichotomized according the cut-offs of each center into normal or pathological values. MTA, PA and GCA-F visual rating scales were used to measure regional atrophy based on T1-weigthed images. A+= pathological CSF Aβ42; A− = normal CSF Aβ42; T+= pathological CSF p-Tau; T− = normal CSF p-Tau; MTA = medial temporal atrophy scale; PA = posterior atrophy scale; GCA-F = global cortical atrophy scale – frontal subscale; A = anterior part of the brain; P = posterior part of the brain; R = right; L = left.
3.2. Association between AD CSF biomarkers and visual rating scores measured with MRI
The distribution of abnormal scores in the visual rating scales in relation to normal or pathological CSF Aβ42, T-tau and p-tau is presented in Table 2.
Table 2.
Distribution of abnormal visual rating scores between normal and pathological AD CSF biomarkers groups.
| CSF Aβ42 |
CSF T-tau |
CSF p-tau |
||||
|---|---|---|---|---|---|---|
| Visual rating scales | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal |
| Abnormal MTA N (%) | 18 (54.55) | 15 (45.45) | 28 (84.85) | 5 (15.15) | 26 (78.79) | 7 (21.21) |
| Age (mean and SD) | 69.78 (8.37) | 73.93 (6.68) | 70.86 (8.01) | 76.20 (5.07) | 70.27 (8.13) | 76.86 (3.34) |
| Sex (Male N and %) | 11 (61.11) | 6 (40) | 18 (64.29) | 2 (40) | 17 (65.38) | 3 (42.86) |
| Disease duration (mean and SD) | 3.64 (2.91) | 2.43 (2.35) | 3.11 (2.80) | 3.00 (2.35) | 2.87 (2.54) | 3.93 (3.32) |
| Abnormal PA N (%) | 25 (55.56) | 20 (44.44) | 33 (73.33) | 12 (26.67) | 29 (64.44) | 16 (35.56) |
| Age (mean and SD) | 67.24 (9.40) | 75.70 (6.78) | 70.24 (8.22) | 73.08 (11.88) | 69.17 (8.07) | 74.31 (10.62) |
| Sex (Male N and %) | 15 (60) | 12 (60) | 20 (60.61) | 7 (58.33) | 18 (62.07) | 9 (56.25) |
| Disease duration (mean and SD) | 3.98 (2.69) | 2.48 (1.57) | 3.26 (2.24) | 3.46 (2.78) | 2.91 (1.91) | 4.03 (2.96) |
| Abnormal GCA-F N (%) | 19 (55.88) | 15 (44.12) | 27 (79.41) | 7 (20.59) | 20 (58.82) | 14 (41.18) |
| Age (mean and SD) | 70.68 (9) | 75.93 (7.06) | 71.30 (8.57) | 79.57 (6.05) | 70.95 (8.57) | 75.93 (8.36) |
| Sex (Male N and %) | 14 (73.68) | 11 (73.33) | 20 (74.07) | 5 (71.43) | 15 (75) | 10 (71.43) |
| Disease duration (mean and SD) | 4.61 (3.08) | 2.77 (2.15) | 3.63 (2.74) | 4.43 (3.31) | 3.18 (2.20) | 4.68 (3.44) |
N: number. CSF: cerebrospinal fluid. Aβ42: Amyloid-β42. T tau: Total tau. P tau: phosphorylated tau at threonine 181. MTA: medial temporal lobe atrophy. PA: posterior atrophy. GCA-F: global cortical atrophy scale-frontal subscale.
Classification performance in the three random forest models was better than chance: MTA, OOB-EER = 32.56%; PA, OOB-EER = 44.83%, GCA-F, OOB-EER = 24.14% (Table 3). The classification error for normal MTA was 24.52% and for abnormal MTA it was 45.45%. The classification error for normal PA was 46.34% and for abnormal PA it was 43.48%. The classification error for normal GCA-F scores was 19.23% while it was 31.43% for patients with abnormal values. Table 3 shows that the best predictors of MTA were disease duration, CSF Aβ42 and age, ordered by importance. We found a combined effect of CSF Aβ42 and CSF p-tau on PA. Age, education and disease duration also contributed to the prediction of PA. Finally, the best predictors of GCA-F were sex, education and age. AD CSF biomarkers did not contribute to the prediction of GCA-F. The same pattern of results was observed when adding the center as a predictor in the models (data not shown), thus suggesting that variability across-centers does not seem to affect our findings.
Table 3.
Association between AD CSF biomarkers and visual rating scales (random forest models).
| Visual rating scales | Variables contribution | Pearson correlation | P value |
|---|---|---|---|
| MTA |
Overall model: OOB-EER = 32.56%
|
−0.244 0.217 0.207 |
0.024 0.045 0.056 |
| PA |
Overall model: OOB-EER = 44.83%
|
0.195 −0.166 0.179 0.266 −0.248 |
0.072 0.126 0.100 0.013 0.021 |
| GCA-F |
Overall model: OOB-EER = 24.14%
|
0.270 −0.231 0.334 |
0.012 0.033 0.002 |
N: number. CSF: cerebrospinal fluid. Aβ42: Amyloid-β42. T tau: Total tau. P tau: phosphorylated tau at threonine 181. MTA: medial temporal lobe atrophy. PA: posterior atrophy. GCA-F: global cortical atrophy scale-frontal subscale.
OOB-EER: out-of-the-bag estimated error rate (below 50% denotes good classification performance). Imp: importance (the contribution of a given variable in the random forest, with higher values indicating stronger contribution to the prediction). Pearson correlation indicates the direction of the association.
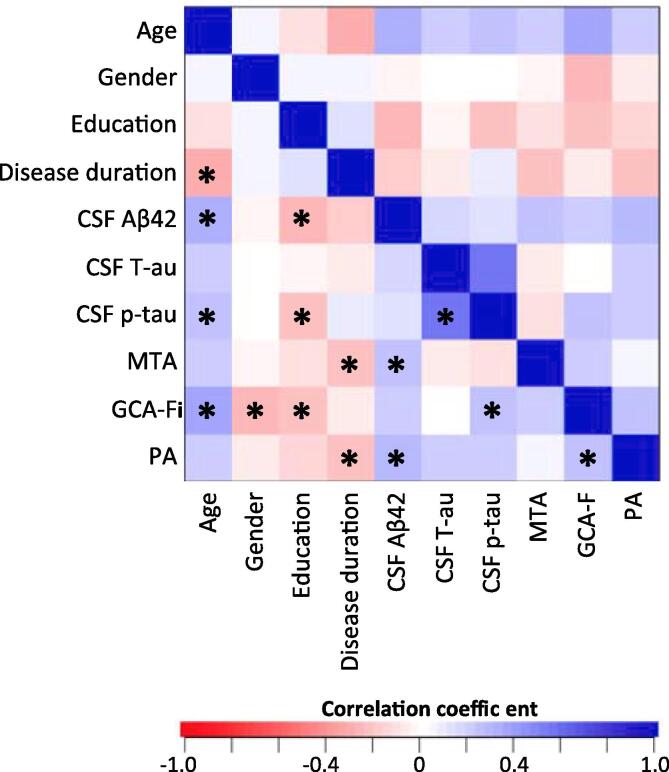
Pearson correlation coefficients show that abnormal scores in MTA were related to abnormal CSF Aβ42 levels, whereas abnormal values of PA were associated with both abnormal CSF Aβ42 and p-tau levels. Regarding the effect of age, sex, education and disease duration, abnormal scores in MTA were related to shorter disease duration and older age. Abnormal scores in PA were related to older age, less education and shorter disease duration. Abnormal scores in GCA-F were related to less education, male sex and older age (Table 3). Fig. 2 shows the correlation matrix between visual ratings and CSF biomarkers, as well as among all predictors in our random forest models (Breiman, 2001).
Fig. 2.
Correlation matrix between visual ratings, CSF biomarkers, and predictors in the random forest models. Asterisk symbols (*) denote p-values < 0.05. MTA = medial temporal atrophy scale; PA = posterior atrophy scale; GCA-F = global cortical atrophy scale – frontal subscale.
4. Discussion
We found that both amyloid-β and tau-related pathologies contribute in combination to atrophy in posterior brain cortex of probable DLB patients. In addition, amyloid β-related pathology was associated with atrophy in the medial temporal lobe. In contrast, atrophy in the frontal cortex was not associated with AD-related pathology, and no associations were found between regional brain atrophy and global unspecific neurodegeneration (CSF T-tau).
Our results are consistent with previous findings on the association between amyloid β-related pathology and atrophy in the medial temporal lobe in DLB (Sarro et al., 2016, van der Zande et al., 2018, Shimada et al., 2013, Mak et al., 2019b, Kantarci et al., 2012a, Kantarci et al., 2012b). Although MTA is less frequent in DLB when compared with AD (Shimada et al., 2013, Barber et al., 2000 Mar, Ballmaier et al., 2004), previous studies suggest that when MTA is present, it could reflect concomitant AD-related pathology (Sarro et al., 2016, van der Zande et al., 2018). Both MTA and abnormal CSF Aβ42 levels are associated with more rapid cognitive decline in DLB and Parkinson’s disease (Howlett et al., 2015 Jul 1, Siderowf et al., 2010, Stav et al., 2016, Caspell-Garcia et al., 2017). An interesting result of our study s that the classification error was higher for the prediction of patients with abnormal MTA scores as compared with the prediction of patients with normal MTA scores. This suggests that while in the absence of amyloid-beta pathology is unlikely to find MTA in probable DLB, the presence of MTA is not always associated with amyloid-beta pathology. Other factors than pathological CSF levels of Aβ42 may be involved in MTA in DLB. Our study shows that older age and shorter disease duration are associated with abnormal MTA scores. Future studies should also consider other pathologies potentially contributing to MTA, such as TDP-43 or hippocampal sclerosis.
Furthermore, we found that posterior brain atrophy was associated with both amyloid-β and tau-related pathologies. We acknowledge however that this finding should be considered as preliminary given the high classification error in our model (still under the threshold of error by chance). PET biomarkers are needed to confirm the collocation of amyloid and tau-related pathologies and neurodegeneration in the posterior cortex in DLB. Sarro et al showed that amyloid-β deposition is associated with greater atrophy rates in the posterior cingulate gyrus and the occipital lobe in addition to the temporal lobe (Sarro et al., 2016). Investigation of tau-related pathology with 18F-AV-1451 have found that DLB patients display increased uptake in the posterior and inferior temporoparietal, occipital (Kantarci et al., 2017) and parietal lobes (Smith et al., 2018). These findings indicate that tau-related pathology in DLB does not seem to follow AD Braak neurofibrillary tangle (NFT) distribution with the typical involvement of the medial temporal lobe (Braak and Braak, 1991), whereas amyloidosis presents with a diffuse cortical pattern similar to AD (Coughlin et al., 2019). Hence, the coexistence of DLB and AD pathologies could result in a distinctive pattern of regional brain atrophy in DLB. The novelty of our study is that we show that both amyloid-β and tau-related pathologies seem to be associated with level of atrophy in posterior cortex, while solely amyloid-β pathology appears to be related to atrophy in medial temporal lobes. This could be explained by a potential link between amyloid-β and tau-related pathologies in posterior brain areas, where tau pathology is primarily deposited in DLB (Kantarci et al., 2017).
Although both CSF T-tau and brain atrophy are considered markers of neurodegeneration, we did not find any association between the two. A possible explanation is that CSF T-tau is a marker of global unspecific neurodegeneration, while visual rating scales are markers of regional (local) neurodegeneration. AD studies show that the agreement between CSF T-tau and brain atrophy is limited (Alexopoulos et al., 2014). Moreover, we cannot exclude that this negative result is also explained by the small number of subjects with abnormal levels of CSF T-tau in our sample. Prior studies also observed that abnormal levels of CSF T-tau are less frequent than abnormal levels of CSF Aβ42 and p-tau in DLB patients (Mukaetova-Ladinska et al., 2010). Nevertheless, only one previous study analyzed CSF T-tau levels and regional brain atrophy in DLB, finding a correlation with posterior and global brain atrophy (van der Zande et al., 2018). Therefore, more studies are needed to determine the possible association between CSF T-tau -currently considered as a biomarker of neuronal damage- and brain atrophy in Lewy body dementia.
Similarly to what previously reported in AD, neither CSF Aβ42, T-tau nor p-tau were associated with atrophy in the frontal cortex in DLB (Ferreira et al., 2016). Previous research has demonstrated increased amyloid-β burden (Growdon et al., 2012) but not tau deposition (Kantarci et al., 2017) in frontal areas in patients with DLB. However, amyloid-β deposition in the frontal lobes has not been associated with grey matter atrophy in this region (Sarro et al., 2016). Frontal atrophy in DLB might be related to Lewy body pathology only, but more investigations are needed to elucidate the pathological mechanisms underlying the neurodegeneration of these areas.
Regarding the effect of age, sex, education and disease duration, we did not control for their effects but investigated to what extent they contribute to regional brain atrophy together with the CSF biomarkers. The decision to do so is because it is currently unknown whether these variables should be treated as confounding or contributing variables to regional brain atrophy in neurodegenerative disorders (Ferreira et al., 2020). For example, tau-related pathology is associated with increasing age, and it is currently unknown whether tau deposition in DLB is related to AD- or aging-, or both. Thus, including age, sex, education and disease duration in our models enabled us to investigate their combined effect together with the CSF biomarkers. We found that atrophy in medial temporal lobes and posterior cortices was associated with shorter disease duration and older age, whereas atrophy in the frontal cortex was associated with older age, male sex and less education. Further, our multivariate analyses exposed the effect of variables such as disease duration on the integrity of the brain, which traditional bivariate correlations could not capture in our study. In fact, multivariate models can capture effects of relevant variables masked by the effect of third variables that cannot be captured in univariate or bivariate models, and that are artificially removed in models testing for partial effects (Machado et al., 2018). This is therefore a strength of our multivariate statistical approach using random forest classification models. Another interesting finding is that the Pearson correlation between CSF p-tau and PA was not significant while CSF p-tau contributed to the prediction of PA in the multivariate random forest model. This suggests that the effect of tau-related pathology on the posterior cortex is not direct and instead emerges in combination with the effect of amyloid-β pathology. Hence, this dissociation between the results from our random forest and correlation analysis supports the potential synergistic effect of amyloid-β and tau-related pathologies when it comes to predict brain atrophy in the posterior cortex. Since age, education and disease duration were contributing variables in the random forest for PA, this suggests that AD-concomitant pathology in DLB may have a stronger impact on the posterior cortex in patients with lower education and older age, hence perhaps accelerating disease progression (i.e. shorter disease duration).
This study has some limitations. Firstly, we discuss on the observed association between CSF biomarkers and regional brain atrophy but our analyses are cross-sectional and we cannot assume causality. Still, we believe our findings may inform on potential underlying mechanisms of neurodegeneration in DLB, which need to be substantiated in future longitudinal studies. Secondly, the E-DLB cohort was assembled retrospectively using common registered variables and procedures across the participating centers. However, all variables and procedures are standard for clinical practice across centers and we carefully inspected the combinability of the data in order to exclude non-harmonized measures and cases. All centers have extensive clinical experience in the diagnosis of neurodegenerative diseases and align with international consensus diagnostic criteria, which we believe has contributed to minimize potential differences among centers. Further, we followed two more strategies to minimize methodological differences across-centers. We used cut-offs that were established at their respective center to dichotomize CSF biomarker results into normal or abnormal values, which is preferred in multi-center studies instead of continuous values. Similarly, due to the variability in the MRI protocols across centers, we used visual rating scales to investigate brain atrophy instead of more fine-grained automated methods or quantitative measures. Nonetheless, the use of visual rating scales substantially increases the clinical applicability of our current findings (Ferreira et al., 2017, Ferreira et al., 2015, Ferreira et al., 2020). On another hand, and connected to the retrospective nature of the cohort, it is worth mentioning that the interval between MRI and CSF collection was long in 15.11% of cases (ranged from 3 to 12 moths). Thirdly, there is no reliable in vivo biomarker of Lewy body pathology at present for diagnosis or analysis of the contribution of this pathology towards the neurodegenerative process. Thus, the diagnosis of probable DLB was based on clinical grounds with its known limitations (Rizzo et al., 2018, Huang and Halliday, 2013), although around one third of the cohort had a dopamine transporter SPECT scan. These limitations will be overcome in the prospective stage of E-DLB – we are currently collecting harmonized longitudinal data across many centers in Europe (Oppedal et al., 2019). Finally, although random forest is able to handle multicollinearity to some degree, it might lead to an underestimation of the contribution of multicollinear variables. The association among the predictors of the random forest models can be appreciated in Fig. 2. Our study has some important strengths. The study is a multicenter effort, which makes the generalization of the findings plausible through different clinical centers. Also, the use of modern multivariate models allowed us to investigate the combined effect of CSF biomarkers for the first time, in contrast to previous reports which investigated partial effects and could not investigate CSF p-tau in linear regression models due to collinearity (van der Zande et al., 2018).
5. Conclusions
This study shows preliminary data on the potential combined effect of amyloid-β and tau-related pathologies on posterior brain cortices in patients with DLB. Future research should confirm our current findings with more fine-grained automated methods for brain atrophy and, ideally, with amyloid and tau PET biomarkers in order to verify the potential collocation of these pathologies with neurodegeneration in posterior brain cortices. Likewise, future studies should also include alpha-synuclein biomarkers when available in order to advance our current understanding of the neurodegeneration process in DLB.
Funding sources of the study
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of interest
-
1.
Carla Abdelnour: Carla Abdelnour has received honoraria from Zambon and Schwabe.
-
2.
Daniel Ferreira: none.
-
3.
Ketil Oppedal: none.
-
4.
Lena Cavallin: none.
-
5.
Frédéric Blanc: none.
-
6.
Oliver Bousiges: none.
-
7.
Lars Olof Wahlund: none.
-
8.
Jakub Hort: none.
-
9.
Zuzana Nedelska: Dr. Nedelska has been supported by IBRO-ISN fellowship 2018.
-
10.
Alessandro Padovani: none.
-
11.
Andrea Pilotto: none.
-
12.
Laura Bonanni: none.
-
13.
Milica Kramberger: none.
-
14.
Mercè Boada: none.
-
15.
Eric Westman: none.
-
16.
Javier Pagonabarraga: none.
-
17.
Jaime Kulisevsky: none.
-
18.
Dag Aarsland: Dr Aarsland has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and served as paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura. Dag Aarsland is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation and the Royal Society for their support.
CRediT authorship contribution statement
Carla Abdelnour: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing - original draft, Writing - review & editing. Daniel Ferreira: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Ketil Oppedal: Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Lena Cavallin: Data curation, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing - review & editing. Olivier Bousiges: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Lars Olof Wahlund: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Jakub Hort: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Zuzana Nedelska: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Alessandro Padovani: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Andrea Pilotto: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Laura Bonanni: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Milica G. Kramberger: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Mercè Boada: Data curation, Investigation, Resources, Validation, Visualization, Writing - review & editing. Eric Westman: Conceptualization, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Javier Pagonabarraga: Validation, Visualization, Writing - review & editing. Jaime Kulisevsky: Validation, Visualization, Writing - review & editing. Frédéric Blanc: Data curation, Investigation, Resources, Supervision, Validation, Visualization, Writing - review & editing. Dag Aarsland: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Acknowledgements
The authors would like to express their thanks to all the members of the E-DLB consortium: Evelien Lemstra, Alzheimer Centre, Dept Neuro, VU Med Centre, Amsterdam, The Netherlands; Inger van Steenoven, Alzheimer Centre, Dept Neuro, VU Med Centre, Amsterdam, The Netherlands; Jan Booij, Amsterdam University, The Netherlands; Sevasti Bostantjopoulou, 3rd Department of Neurology, Medical School, Aristotle University of Thessaloniki, Greece; Zoe Katsarou, 3rd Department of Neurology, Medical School, Aristotle University of Thessaloniki, Greece; Irena Rektorova, Brain and mind research Program, Central EU Institute of technology, Masaryk Uni. Masaryk University, Brno Czech Republic; Angelo Antonini, Center for Parkinson's disease and Movement Disorder Venice-Lido, Italy; Roberta Biundo; Center for Parkinson's disease and Movement Disorder Venice-Lido, Italy; Elisabet Londos, Clinical Memory Research Unit, Dept. of Clinical Sciences, Lund University Malmo Sweden; Flavio Nobili, Clinical Neurology, Dept. of Neuroscience (DINOGMI) University of Genoa, Genova Italy; Josep Garre, ReDeGi, University of Girona, Spain; Cristian Falup-Pecurariu, Transilvania University of Brasov, Romania; Arvid Rongve, Haugesund Hospital, Norway; John O'Brien, Department of Psychiatry, University of Cambridge School of Clinical Medicine, UK; Dan Weintraub, Departments of Psychiatry and Neurology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA; Tormod Fladby, Dept of Neurology, Akershus University Hospital, Oslo, Norway; Richard Dodel, Dept of Neurology, University Hospital of Giessen and Marburg, Germany; Zuzana Walker, Division of Psychiatry, University College London and North Essex Partnership University NHS Foundation Trust, UK; Frank Jan de Jong, Erasmus MC Rotterdam, The Netherlands; Clive Ballard, Exeter University UK; Henrik Zetterberg, Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry,The Sahlgrenska Academy at the University of Gothenburg, Sweden; John Paul Taylor, Institute of Neuroscience, Campus for Aging and vitality, Newcastle University, UK; Ian McKeith, Institute of Neuroscience, Campus for Aging and vitality, Newcastle University, UK; Erika Stefanova, Intitute of Neurology, CCS, School of Medicine, University of Belgrade, Serbia; Per Svenningsson, Karolinska Institute Stockholm, Sweden; Sara Garcia-Pacek, Karolinska Insitutet Stockholm, Sweden, Bengt Winblad, Karolinska Insitutet Stockholm, Sweden; Richard Brown, Kings College of London, UK; Ray Choudhury, Kings College of London, UK; Oskar Hansson, Lund University Malmo, Sweden; Brad Boeve, Mayo Clinic Rochester, USA; Brit Mollenhauer, Department of Neurology, University Medicine Göttingen, Göttingen, Germany and Paracelsus-Elena Klinik, Center for Parkinsonism and Movement Disorders, Kassel, Germany; Jon Snaedal, Landspitali University Hospital, Reykjavik, Iceland; Gert J. Geurtsen, Department of Medical Psychology, Amsterdam University Medical Centers location Academic Medical Center, Amsterdam, The Netherlands; Maria Petrova, University Hospital “Alexandrovska”, Department of Neurology, Sofia, Bulgaria; Latchezar Traykov, University Hospital “Alexandrovska”, Department of Neurology, Sofia, Bulgaria; Leonidas Stefanis, University of Athens Medical School Athens, Greece; E. Kapaki, University of Athens Medical School Athens, Greece; Carlo Delena, Department of Physiology and Pharmacology “Vittorio Erspamer”, Sapienza University of Rome, Rome, Italy; Claudio Babiloni, Department of Physiology and Pharmacology “Vittorio Erspamer”, Sapienza University of Rome, Rome, Italy; Rik Vandenberghe, University Hospital Leuven, Leuven, Belgium; Vesna Jelic, Karolinska Institute Stockholm, Sweden; Maria Skaalum, The Faroese Hospital System and the University of Faroe Islands; Cristina Muscio, Vita-Salute San Raffaele University and In vivo human molecular and structural neuroimaging Unit, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy; Martha Therese Gjestsen, Centre for age-related medicine (SESAM), Stavanger University Hospital, Stavanger, Norway; Camille Carroll, Faculty of Medicine and Dentistry, Plymouth University, Plymouth, UK; Naji Tabet, Department of Social Work and Social Care, University of Sussex, Brighton, UK; Fabrizia D'Antonio, Department of Neurology and Psychiatry, Sapienza, University of Rome, Italy; Yvonne Freund-Levi, Karolinska Insitutet Stockholm, Sweden. This research study was performed as part of the Medicine doctoral program of Carla Abdelnour at Universitat Autònoma de Barcelona (Barcelona, Spain). Dr Aarsland has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and served as paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura. Dag Aarsland is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation and the Royal Society for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102333.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdelnour C., van Steenoven I., Londos E., Blanc F., Auestad B., Kramberger M.G. Alzheimer’s disease cerebrospinal fluid biomarkers predict cognitive decline in lewy body dementia. Mov. Disord. 2016;31(8):1203–1208. doi: 10.1002/mds.26668. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P., Kriett L., Haller B., Klupp E., Gray K., Grimmer T. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimers Dement. 2014;10(6):684–689. doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Andersson M., Zetterberg H., Minthon L., Blennow K., Londos E. The cognitive profile and CSF biomarkers in dementia with Lewy bodies and Parkinson’s disease dementia. Int. J. Geriatr. Psychiatry. 2011;26(1):100–105. doi: 10.1002/gps.2496. [DOI] [PubMed] [Google Scholar]
- Ballmaier M., O’Brien J.T., Burton E.J., Thompson P.M., Rex D.E., Narr K.L. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer’s disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004;23(1):325–335. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Barber R., Ballard C., McKeith I.G., Gholkar A., O’Brien J.T. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology. 2000;54(6):1304–1309. doi: 10.1212/wnl.54.6.1304. [DOI] [PubMed] [Google Scholar]
- Barker, W.W., Luis, C.A., Kashuba, A., Luis, M., Harwood, D.G., Loewenstein, D., et al. Relative Frequencies of Alzheimer Disease, Lewy Body, Vascular and Frontotemporal Dementia, and Hippocampal Sclerosis in the State of Florida Brain Bank. 2002;16(4):203–212. [DOI] [PubMed]
- Blanc F., Mahmoudi R., Jonveaux T., Galmiche J., Chopard G., Cretin B. Long-term cognitive outcome of Alzheimer’s disease and dementia with Lewy bodies: Dual disease is worse Alzheimer’s. Res. Ther. 2017;9(1) doi: 10.1186/s13195-017-0272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- Caspell-Garcia C., Simuni T., Tosun-Turgut D., Wu I.-W., Zhang Y., Nalls M. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS ONE. 2017;12(5) doi: 10.1371/journal.pone.0175674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y., Parkkinen L., Kempster P., Selikhova M., Lashley T., Holton J.L. The Significance of α-synuclein, amyloid-β and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener. Dis. 2013;13:154–156. doi: 10.1159/000354670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin D.G., Xie S.X., Liang M., Williams A., Peterson C., Weintraub D. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann. Neurol. 2019;85(2):259–271. doi: 10.1002/ana.25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopatlhology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Del Ser T., Hachinski V., Merskey H., Munoz D.G. Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: effect of coexisting Alzheimer-type lesion load. Alzheimer Dis. Assoc. Disord. 2001;15(1):31–44. doi: 10.1097/00002093-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Di Censo R., Abdelnour C., Blanc F., Bousiges O., Lemstra A.W., van Steenoven I. CSF tau proteins correlate with an atypical clinical presentation in dementia with Lewy bodies. J. J. Neurol. Neurosurg Psychiatry. 2020;91(1):109–110. doi: 10.1136/jnnp-2019-320980. [DOI] [PubMed] [Google Scholar]
- Donaghy P.C., Michael J., Thomas A.J., Lloyd J., Petrides G., Barnett N. Clinical and imaging correlates of amyloid deposition in dementia with Lewy bodies. Mov. Disord. 2018;33(07):1130–1138. doi: 10.1002/mds.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy P., Thomas A.J., O’Brien J.T. Amyloid PET imaging in lewy body disorders. Am. J. Geriatr. Psychiatry. 2015;23(1):23–37. doi: 10.1016/j.jagp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Dugger B.N., Adler C.H., Shill H.A., Caviness J., Jacobson S., Driver-Dunckley E. Concomitant pathologies among a spectrum of parkinsonian disorders. Park Relat. Disord. 2014;20(5):525–529. doi: 10.1016/j.parkreldis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G.J., Mactier K., Colloby S.J., Watson R., Blamire A.M., O’Brien J.T. The influence of hippocampal atrophy on the cognitive phenotype of dementia with Lewy bodies. Int. J. Geriatr. Psychiatry. 2017;32(11):1182–1189. doi: 10.1002/gps.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn, S., Marsden, C., Calne, D., Goldstein, M., (Eds.), Unified Parkinson’s Disease Rating Scale. Vol. 2, Recent Developments in Parkinson’s Disease. Florham Park, NJ: MacMillan Healthcare; 1987. 153–163 p.
- Ferreira D., Cavallin L., Larsson E.M., Muehlboeck J.S., Mecocci P., Vellas B. Practical cut-offs for visual rating scales of medial temporal, frontal and posterior atrophy in Alzheimer’s disease and mild cognitive impairment. J. Intern. Med. 2015;278(3):277–290. doi: 10.1111/joim.12358. [DOI] [PubMed] [Google Scholar]
- Ferreira D., Cavallin L., Granberg T., Lindberg O., Aguilar C., Mecocci P. Quantitative validation of a visual rating scale for frontal atrophy: associations with clinical status, APOE e4, CSF biomarkers and cognition. Eur. Radiol. 2016;26(8):2597–2610. doi: 10.1007/s00330-015-4101-9. [DOI] [PubMed] [Google Scholar]
- Ferreira, D., Nordberg, A., Westman, E., Biological subtypes of Alzheimer disease. Neurology. 2020 F;10.1212/WNL.0000000000009058. [DOI] [PMC free article] [PubMed]
- Ferreira D., Verhagen C., Hernández-Cabrera J.A., Cavallin L., Guo C.J., Ekman U. Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci. Rep. 2017;7:46263. doi: 10.1038/srep46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T., Growdon W.B., McNamara M., Newell K., Gomez-Tortosa E., Hedley-Whyte E.T. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53(9):2003–2009. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- Gomperts S., Locascio J.J., Makaretz S.J., Schultz A., Caso C., Vasdev N. Tau PET imaging in the Lewy body diseases. J. AMA Neurol. 2016;73(11):1334–1341. doi: 10.1001/jamaneurol.2016.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Boeve B.F., Pedraza O., Ferman T.J., Przybelski S., Lesnick T.G. Imaging and acetylcholinesterase inhibitor response in dementia with Lewy bodies. Brain. 2012;135:2470–2477. doi: 10.1093/brain/aws173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growdon J.H., Locascio J.J., Rentz D.M., Johnson K.A., Santarlasci A.L., Marquie M. Brain amyloid and cognition in Lewy body diseases. Mov. Disord. 2012;27(8):965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G.M., Holton J.L., Revesz T., Dickson D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122(2):187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- Howlett D.R., Whitfield D., Johnson M., Attems J., O’Brien J.T., Aarsland D. Regional multiple pathology scores are associated with cognitive decline in Lewy body dementias. Brain Pathol. 2015;25(4):401–408. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett D.R., Whitfield D., Johnson M., Attems J., O’Brien J.T., Aarsland D. Regional multiple pathology scores are associated with cognitive decline in lewy body dementias. Brain Pathol. 2015;25(4):401–408. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Halliday G. Can we clinically diagnose dementia with Lewy bodies yet? Transl. Neurodegener. 2013;2(1):1–9. doi: 10.1186/2047-9158-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease for the neuropathologic assessment of Alzheimer ’ s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D.J., Hurtig H.I. The contribution of Tau, Amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders HHS public access. J. Alzheimers Dis. Park. 2018;8(4) doi: 10.4172/2161-0460.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D.J., Grossman M., Weintraub D., Hurtig H.I., Duda J.E., Xie S.X. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55. doi: 10.1016/S1474-4422(16)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K., Ferman T.J., Boeve B.F., Weigand S.D., Przybelski S., Vemuri P. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012;79(6):553–560. doi: 10.1212/WNL.0b013e31826357a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K., Lowe V.J., Boeve B.F., Weigand S.D., Senjem M.L., Przybelski S.A. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol. Aging. 2012;33(9):2091–2105. doi: 10.1016/j.neurobiolaging.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K., Lowe V.J., Boeve B.F., Senjem M.L., Tosakulwong N., Lesnick T.G. AV-1451 Tau and b-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 2017;81(1):58–67. doi: 10.1002/ana.24825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam E.L.G.E., Lehmann M., Van Der Flier W.M., Scheltens P., Pijnenburg Y.A.L., Fox N. Visual assessment of posterior atrophy development of a MRI rating scale. Eur. Radiol. 2011;21(12):2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K. Diffuse lewy body disease in Japan. J. Neurol. 1990;237(3):197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- Kramberger M.G., Auestad B., Garcia-Ptacek S., Abdelnour C., Olmo J.G., Walker Z. Long-term cognitive decline in dementia with Lewy bodies in a large multicenter. Int. Cohort. J. Alzheimer’s Dis. 2017;57(3) doi: 10.3233/JAD-161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill M.L., Larson E.B., Tsuang D.W., Teri L., McCormick W.C., Bowen J.D. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64(12):2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra A.W., De Beer M.H., Teunissen C.E., Schreuder C., Scheltens P., Van Der Flier W.M. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J. Neurol. Neurosurg Psychiatry. 2017;88(2):113–118. doi: 10.1136/jnnp-2016-313775. [DOI] [PubMed] [Google Scholar]
- Liaw A., Wiener M. Classification and regression by random forest. R News. 2002;2(3):18–22. [Google Scholar]
- Machado A., Barroso J., Molina Y., Nieto A., Díaz-Flores L., Westman E. Proposal for a hierarchical, multidimensional, and multivariate approach to investigate cognitive aging. Neurobiol Aging. 2018;71:179–188. doi: 10.1016/j.neurobiolaging.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Mak E., Donaghy P.C., McKiernan E., Firbank M.J., Lloyd J., Petrides G.S. Beta amyloid deposition maps onto hippocampal and subiculum atrophy in dementia with Lewy bodies. Neurobiol Aging. 2019;1(73):74–81. doi: 10.1016/j.neurobiolaging.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Mak E., Donaghy P.C., McKiernan E., Firbank M.J., Lloyd J., Petrides G.S. Beta amyloid deposition maps onto hippocampal and subiculum atrophy in dementia with Lewy bodies. Neurobiol. Aging. 2019;73:74–81. doi: 10.1016/j.neurobiolaging.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Marui W., Iseki E., Kato M., Akatsu H., Kosaka K. Pathological entity of dementia with Lewy bodies and its differentiation from Alzheimer’s disease. Acta Neuropathol. 2004;108(2):121–128. doi: 10.1007/s00401-004-0869-4. [DOI] [PubMed] [Google Scholar]
- McKeith I., Boeve B., Dickson D., Halliday G., Taylor J., Weintraub D. Diagnosis and management of dementia with Lewy bodies Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I.G., Dickson D.W., Lowe J., Emre M., O’Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Merdes A.R., Hansen L.A., Jeste D.V., Galasko D., Hofstetter C.R., Ho G.J. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska E.B., Monteith R., Perry E.K. Cerebrospinal fluid biomarkers for dementia with Lewy bodies. Int. J. Alzheimers Dis. 2010;2010:1–17. doi: 10.4061/2010/536538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M.E., Ferman T.J., Boeve B.F., Przybelski S.A., Lesnick T.G., Liesinger A.M. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81(19):1681–1689. doi: 10.1212/01.wnl.0000435299.57153.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z., Ferman T.J., Boeve B.F., Przybelski S.A., Lesnick T.G., Murray M.E. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol. Aging. 2015;36(1):452–461. doi: 10.1016/j.neurobiolaging.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.T., Kryscio R.J., Jicha G.A., Abner E.L., Schmitt F.A., Xu L.O. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73(14):1127–1133. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppedal K., Ferreira D., Cavallin L., Lemstra A., ten Kate M., Padovani A. A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from The European DLB Consortium. Alzheimers Dement. 2019;15(3):400–409. doi: 10.1016/j.jalz.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Oppedal K., Borda M.G., Ferreira D., Westman E., Aarsland D., European T. European DLB consortium: diagnostic and prognostic biomarkers in dementia with Lewy bodies, a multicenter international initiative. Neurodegener. Dis. Manag. 2019;9(5):247–250. doi: 10.2217/nmt-2019-0016. [DOI] [PubMed] [Google Scholar]
- Rizzo G., Arcuti S., Copetti M., Alessandria M., Savica R., Fontana A. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2018;89(4):358–366. doi: 10.1136/jnnp-2017-316844. [DOI] [PubMed] [Google Scholar]
- Sarro L., Senjem M.L., Lundt E.S., Przybelski S.A., Lesnick T.G., Graff-Radford J. Amyloid-b deposition and regional grey matter atrophy rates in dementia with Lewy bodies. BRAIN. 2016;139:2740–2750. doi: 10.1093/brain/aww193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P. Atrophy of medial temporal lobes on MRI in “Probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J. Neurol Neurosurgery, Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J.A., Aggarwal N.T., Barnes L., Boyle P., Bennett D.A. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J. Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Shinotoh H., Hirano S., Miyoshi M., Sato K., Tanaka N. β-amyloid in lewy body disease is related to Alzheimer’s disease-like atrophy. Mov. Disord. 2013;28(2):169–175. doi: 10.1002/mds.25286. [DOI] [PubMed] [Google Scholar]
- Shimada H., Shinotoh H., Hirano S., Miyoshi M. b-Amyloid in Lewy body disease is related to Alzheimer’s disease-like atrophy. Mov. Disord. 2013;28(2):169–175. doi: 10.1002/mds.25286. [DOI] [PubMed] [Google Scholar]
- Siderowf A., Xie S.X., Hurtig H., Weintraub D., Duda J., Chen-Plotkin A. CSF amyloid beta 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M., Gelpi E., Martí M.J., Compta Y. Lewy- and Alzheimer-type pathologies in midbrain and cerebellum across the Lewy body disorders spectrum. Neuropathol. Appl. Neurobiol. 2016;42(5):451–462. doi: 10.1111/nan.12308. [DOI] [PubMed] [Google Scholar]
- Smith R., Schöll M., Londos E., Ohlsson T., Hansson O. F-AV-1451 in Parkinson’s disease with and without dementia and in Dementia with Lewy bodies. Sci. Rep. 2018;8(1):4717. doi: 10.1038/s41598-018-23041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T.L., Attems J., Thal D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134(2):187–205. doi: 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stav A.L., Johansen K.K., Auning E., Kalheim L.F., Selnes P., Bjørnerud A. Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: a cross-sectional study. Park Dis. 2016;2(1) doi: 10.1038/npjparkd.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa R., Hashimoto H., Nakanishi A., Kawarada Y., Muramatsu T., Matsuda Y. The relationship between medial temporal lobe atrophy and cognitive impairment in patients with dementia with Lewy bodies. J. Geriatr. Psychiatry Neurol. 2015;28(4):249–254. doi: 10.1177/0891988715590210. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P., Attems J., Thomas A., Brown A., Jaros E., Lett D.J., Ossola M., Perry R.H., Ramsay L., Walker L.M.I. Clinicians’ ability to diagnose dementia with Lewy bodies is not affected by β-amyloid load. Neurology. 2015;84(5):496–499. doi: 10.1212/WNL.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zande J.J., Steenwijk M.D., ten Kate M., Wattjes M.P., Scheltens P., Lemstra A.W. Gray matter atrophy in dementia with Lewy bodies with and without concomitant Alzheimer’s disease pathology. Neurobiol Aging. 2018;71:171–178. doi: 10.1016/j.neurobiolaging.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Van Steenoven I., Aarsland D., Weintraub D., Londos E., Blanc F., Van Der Flier W.M. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of Lewy body diseases: results from a large multicenter cohort on behalf of the European DLB consortium. J. Alzheimers Dis. 2016;54(1):287–295. doi: 10.3233/JAD-160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.M., Xiong C., Morris J.C., Galvin J.E. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67(11):1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.