Abstract
Repair of injured skeletal muscle is a sophisticated process that uses immune, muscle, perivascular, and neural cells. In acute injury, the robust endogenous repair process can facilitate complete regeneration with little to no functional deficit. However, in severe injury, the damage is beyond the capacity for self-repair, often resulting in structural and functional deficits. Aside from the insufficiencies in muscle function, the aesthetic deficits can impact quality of life. Current clinical treatments are significantly limited in their capacity to structurally and functionally repair the damaged skeletal muscle. Therefore, alternative approaches are needed. Biomaterial therapies for skeletal muscle engineering have leveraged natural materials with sophisticated scaffold fabrication techniques to guide cell infiltration, alignment, and differentiation. Advances in biomaterials paired with a standardized and rigorous assessment of resulting tissue formation have greatly advanced the field of skeletal muscle engineering in the last several years. Herein, we discuss the current trends in biomaterials-based therapies for skeletal muscle regeneration and present the obstacles still to be overcome before clinical translation is possible. With millions of people affected by muscle trauma each year, the development of a therapy that can repair the structural and functional deficits after severe muscle injury is pivotal.
Keywords: Skeletal muscle regeneration, Scaffolds, Volumetric muscle loss, Tissue engineering
Graphical abstract
1. Introduction
Skeletal muscle comprises more than 40% of the adult human body by mass and is responsible for supporting the skeletal system and generating contractile forces responsible for movement. Owing to its relatively superficial location, it is prone to injury. Contusions and strains as a result of exercise and lacerations due to surgical procedures on underlying tissues are common sources of muscle injury. Fortunately, skeletal muscle has a high endogenous capacity for self-repair in acute injuries. Satellite cells are activated in response to injury and trigger a dynamic cell signaling cascade to repair minor muscle injuries with little to no functional deficit. In the case of severe injury, however, the signaling cascade is overwhelmed, leading to a persistent pro-inflammatory microenvironment and fibrosis or fatty muscle deposits with significant functional deficits [1,2]. Volumetric muscle loss (VML) after sport- or military-related trauma or surgical intervention results in significant long-term structural and functional deficits. These injuries often impair not only the injured muscle, but also the surrounding muscle and the underlying musculoskeletal system, resulting in a compound reduction in function. Musculoskeletal injuries constitute more than 50% of all Department of Defense disabilities and approximately 35–55% of all sports-induced injuries, resulting in approximately 4.5 million reconstructive surgeries per year [3].
Clinically, the treatment options for VML involve the transfer of an autologous muscle flap paired with physical therapy. The muscle flap contains vasculature to assist with integration into the defect site [4,5]. However, autografts are significantly limited by the amount of tissue that can be harvested and are often associated with donor site morbidity. As a result, autologous muscle flaps often produce limited structural repair and little to no increase in functional repair. Some biologics-based products have been FDA-regulated for soft tissue reinforcement, but no products have been approved for the reconstruction and repair of skeletal muscle. Because of these obstacles, tissue engineers have stepped in to bridge the gap. In the last several years, biomaterials for skeletal muscle engineering have taken large strides toward a therapy to repair skeletal muscle structure and function after severe injury. The recent advances in biomaterial-based strategies provide hope that the field is close to translating a product into the clinic for skeletal muscle regeneration.
Through advancements in biomaterials science, scaffold fabrication techniques, and adaptations of decellularized tissues, amazing translational research is ongoing and advancing the field of skeletal muscle engineering. Scaffold fabrication techniques such as electrospinning and 3D printing are being combined to create complex, multimaterial constructs with sophisticated architecture and physicochemical properties that were unachievable a decade ago. In addition, hybrid biomaterials and decellularized skeletal muscle have been utilized in new ways that further promote myogenesis as well as angiogenesis and neurogenesis, processes that have often been overlooked but are essential to muscle regeneration. Vasculature is needed to facilitate construct integration with the defect site and provide nutrient and waste exchange to the regenerating tissue, and formation of neuromuscular junctions (NMJs) is essential to reinnervate regrown tissue. In addition, it was discovered that pro-angiogenic and pro-neurogenic growth factors participate in myogenic, angiogenic, and neurogenic crosstalk, resulting in synergistic effects [[6], [7], [8]]. Therefore, it is extremely important that skeletal muscle therapies incorporate strategies to activate these pathways.
The surge in groundbreaking research in the field of biomaterials-based skeletal muscle research in the last decade has motivated this review. First, an overview of the natural response to skeletal muscle injury will be discussed. The material properties that are important for skeletal muscle biomaterial therapies and the ways in which tissue engineering commonly uses them will then be reviewed. Next, recent advances in biomaterial-based systems for skeletal muscle engineering will be examined, followed by a platform for assessing myogenesis and skeletal muscle regeneration. Finally, some of the obstacles still facing the field will be presented. Skeletal muscle regeneration is a complex process that relies on a number of cell types, signaling molecules, architectural cues, and physicochemical properties to be successful. However, the field has never been so close to translating therapies into the clinic to structurally and functionally repair muscle.
2. Skeletal muscle physiology
Skeletal muscle is a sophisticated system of muscle fibers acting together to produce a contractile force and support body movement. It relies heavily on a network of vasculature and peripheral nerves to provide nutrients and innervate contractions, respectively. Skeletal muscle is made up of muscle fascicles, which are individual bundles of myofibers that can be stimulated together via NMJs to contract [[9], [10], [11]]. Muscle fibers are the smallest skeletal muscle unit, and each one is innervated by axons from the nervous system to contract. During myogenesis, myoblasts, skeletal muscle precursor cells, break out of their normal cell cycle and begin to express muscle-specific genes [[12], [13], [14]]. Upon differentiation, the cells begin to fuse to form new myotubes or add to existing myotubes.
Skeletal muscle has a high potential for self-repair in acute injuries. Mononucleated, multipotent satellite cells reside in mature skeletal muscle fibers between the sarcolemma and the basement membrane [15]. Satellite cells exist in a quiescent state and are activated to proliferate in response to injury to replenish the satellite cell population or give rise to myoblasts to form new myotubes or fuse to existing myofibers [12,13,16,17]. Most acute injuries follow a similar pattern of repair—the degeneration/inflammatory phase, the repair phase, the remodeling phase.
Each phase of the repair process is marked by the infiltration of different cell types. At the occurrence of injury, damaged myofibers rupture and undergo necrosis, releasing cellular contents and chemokines initiating the degeneration/inflammatory phase. Fibroadipogenic progenitors are also activated to initiate muscle regeneration. However, in instances of VML, these cells can cause fibrosis and fatty tissue deposits, impeding muscle function [18,19]. Resident mast cells and neutrophils are then recruited to begin clearing the damaged tissue at the site of injury [14,20,21]. The resident immune cells in turn release cytokines and complement proteins, which activate the complement immune system [22]. The complement proteins recruit neutrophils and macrophages in the bloodstream to the site of injury to clear microbes and damaged tissue. Furthermore, the immune cells can activate a cascade of cell responses and release tumor necrosis factor-α (TNF-α) that can induce the quiescent satellite cells to enter the cell cycle, thus beginning the repair phase [23,24].
Macrophages and satellite cells play a critical role in the repair phase. It has been reported that macrophages begin to appear in the lesion site approximately 24 h after the onset of the injury [25,26]. The repair phase first involves the activation of M1 macrophages, which are pro-inflammatory cells primarily responsible for removing muscle cell debris and secreting cytokines. M1 macrophages also express nitric oxide synthase (iNOS), which in turn leads to the production of reactive free radical nitric oxide (NO). M1 macrophages can regulate the concentration of NO released, which can initiate apoptosis in damaged cells [14,27]. There are many important cytokines that are involved in the repair phase, among which are TNF-α and interleukin (IL)-6. TNF-α and IL-6 have been suggested to play important roles in the proliferation, differentiation, and regeneration of skeletal muscle cells. Once muscle cells have sufficiently proliferated, the cells begin to differentiate and fuse into myotubes. It is at this point that M2 macrophages are activated and become the dominant macrophage present. M2 macrophages express anti-inflammatory cytokines to decrease the inflammatory response at the site of injury. In addition, M2 macrophages have been thought to promote muscle cell fusion into myotubes [[27], [28], [29]]. As myotubes form and muscle begins to repair itself, it is also revascularized and reinnervated. The formation of new blood vessels begins almost immediately after muscle injury. The formation of NMJs, however, is a long process that is not completed until muscle fibers are repaired, a process that can take two to three weeks in acute injuries [17]. There have been many reports on the crosstalk between pro-myogenic, pro-angiogenic, and pro-neurogenic growth factors and their roles in muscle regeneration [[30], [31], [32], [33], [34]]. For example, vascular endothelial growth factor, a pro-angiogenic growth factor, has been shown to increase myogenesis and neurogenesis in addition promoting angiogenesis.
Once new myotubes are formed, they begin to fuse with existing myofibers, which marks the start of the remodeling phase. After injury, gaps exist between myofibers. Cells quickly deposit extracellular matrix (ECM), which is easily invaded by fibroblasts [35]. Fibrosis and resulting scar formation are then observed at the injury site. As the muscle fibers repair and begin to contract together, the scar is remodeled and depleted [36]. In acute injuries, damaged muscle will completely heal and begin to regain its contractile function. In severe injuries, such as VML, these endogenous mechanisms are not sufficient, and the signaling cascade is overwhelmed, resulting in a prolonged pro-inflammatory microenvironment that leads to excessive fibrosis and scar formation.
3. Properties that are desirable for skeletal muscle regenerative therapies
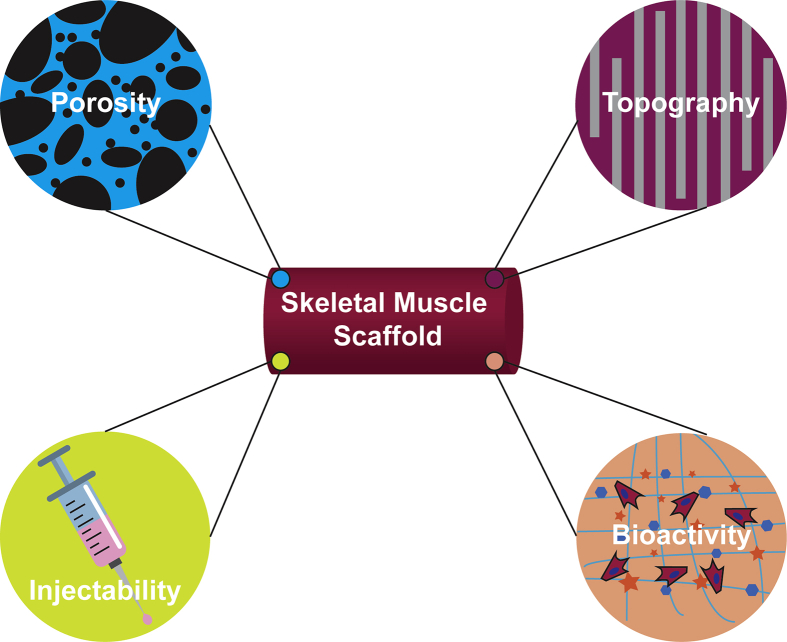
Due to the great need for therapies that adequately restore skeletal muscle structure and function after severe trauma and the clinical limitations of existing therapies, scientists have turned to tissue engineering efforts. Biomaterials for skeletal muscle tissue engineering should possess a number of favorable properties to effectively guide muscle regeneration. Among the most important properties are porosity, aligned architecture, and the presence of biochemical cues (Fig. 1). These material characteristics influence other physicochemical properties, including tensile modulus and degradation kinetics, which are important for supporting skeletal muscle regeneration. In an ideal system, the biomechanical cues of the engineered construct would mimic native tissue to avoid mechanical mismatch and support tissue function. Biodegradable scaffolds are also advantageous to allow for tissue reconstruction—the scaffold should degrade at approximately the same rate as new tissue is formed. In addition, many studies have shown the benefit of using injectable systems for the delivery of cells and biomaterials to the site of injury.
Fig. 1.
Key properties for skeletal muscle regenerative therapies. Scaffolds for skeletal muscle engineering should incorporate one or more key properties to successfully recruit, align, and promote differentiation of myogenic cells. Porosity is essential for proper nutrient and waste transport. Aligned topographical cues are important to promote myotube alignment. Bioactive molecules aid in cell recruitment, proliferation, and differentiation for muscle regeneration. In addition, injectable systems are minimally invasive and shorten recovery time after surgical intervention.
Fabricating a porous and interconnected scaffold is advantageous in several tissue engineering applications because it allows cells to migrate and further proliferate to fill the full depth of the construct. It also allows for the ingrowth of vasculature to support the delivery of essential nutrients and the removal of waste [37]. Traditional techniques for incorporating pores into a biomaterial often involve the use of leachable or gas porogens or freeze drying. Leachable porogens utilize salt [38,39] or sugar [40,41] that can dissolve in water or alcohol solvents after scaffold fabrication. The size and concentration of the porogen dictates the size of the pore and the overall porosity, respectively. Gas can also be an effective porogen and avoids the use of harsh solvents that may be needed to leach salt/sugar from a biomaterial. Unlike porogen leaching, pore fabricated by this method are incorporated into the system during scaffold fabrication through chemical or physical processes [42,43]. Finally, porous foams can be produced through freeze-drying techniques. Control of the freezing temperature and polymer concentration can be used to modulate the porosity of the resulting foams [3]. Furthermore, techniques have been developed to fabricate aligned structures by controlling the temperature gradient of the polymer scaffold during freezing [44]. In addition to techniques of incorporating pores into the biomaterial, many scaffold fabrication techniques exist to integrate pores into the architecture of the bulk scaffold. Electrospinning, 3D printing, and micromolding have been used to create porous scaffolds for tissue engineering applications [[45], [46], [47]].
Replicating the highly aligned architecture of skeletal muscle is also very important in guiding myotube alignment during myogenesis [48]. Many approaches have been used to fabricate aligned substrates for cells to grow along, the most common of which are electrospinning, 3D printing, and micromolding. Each of these techniques will be discussed in detail in later sections. However, ensuring proper substrate patterning to provide essential cues to promote the alignment of myotubes is critical for the effective regeneration of skeletal muscle. Skeletal muscle's highly aligned architecture is key to its production of contractile force and should be replicated in tissue engineering strategies [3].
Although many early strategies for muscle regeneration relied on poly(ε-caprolactone) (PCL) for its electrically conductive properties, recent strategies have turned to natural polymers to provide key biochemical cues necessary for cell attachment, proliferation, and myogenic differentiation. The incorporation of ECM components into biomaterial systems has shown great promise in the field. As the most abundant ECM component in skeletal muscle, collagen is widely used to improve biomaterial bioactivity and regulation of myogenic differentiation [[49], [50], [51]]. In addition, the use of decellularized tissues has also become more widely used to allow for better replication of the skeletal muscle microenvironment through the incorporation of native proteins and growth factors. These materials will be discussed further in later sections. However, there is a clear need for the incorporation of ECM components into skeletal muscle therapies. Many scientists are moving to naturally derived biomaterial systems or hybrid systems that incorporate a natural material to promote cell adhesion and differentiation and a synthetic material to control physicochemical properties.
Finally, many scientists have taken approaches to incorporate injectable systems into their regenerative strategies. Injectable systems are attractive because they are minimally invasive, allow for decreased patient recovery time, flow to fill the defect site, and reduce the risk of surgical site infection [52]. Many of the injectable strategies used for skeletal muscle engineering rely on a thermoresponsive hydrogel systems that gel at physiological temperatures, allowing cells/biomaterials to be dispensed through a syringe and deposit around the site of injury [[53], [54], [55]]. Although these strategies are limited in their ability to deliver some of the other key properties of a desirable skeletal muscle therapy, namely the highly aligned microarchitecture, an injectable system has many clinical advantages that can be applied in a number of skeletal muscle engineering applications.
4. Approaches to skeletal muscle tissue engineering
Several different approaches have been developed to regenerate skeletal muscle, but they can be classified into three main groups—in vitro, in vivo, and in situ muscle engineering (Fig. 2). Each of these approaches has advantages and limitations. In vitro skeletal muscle engineering typically involves growing and differentiating cells into myotubes, then further conditioning the tissue construct, often in a bioreactor, before implantation. The cells selected for these systems often include a coculture of cell types or multipotent cells to achieve blood vessel and NMJs formation [[56], [57], [58], [59]]. The advantages of this system are that functional skeletal muscle constructs should integrate into the host defect site and increase muscle healing and contractile function rapidly compared with other approaches. The main limitations of this strategy are the construct size and complexity that can be successfully achieved in vitro. Due to the high cell density required to induce differentiation into aligned myotubes, it is difficult to grow the large constructs needed to repair VML defects in vitro. Furthermore, successful vascularization is key to support nutrient delivery to the high number of metabolically active cells. Even when mechanical and electrical stimulation are applied to cells within a bioreactor, sufficient vascularization and regeneration of NMJs are very challenging processes to achieve in vitro [13]. Finally, the cells and biomaterials that are selected for in vitro skeletal muscle engineering should be compatible with the host into which the final construct will be implanted. This can be a challenge because many primary cells, such as satellite cells, can be difficult to differentiate in vitro or may behave differently in vitro than they would in vivo [60].
Fig. 2.
Tissue engineering approaches to regenerate skeletal muscle. In vitro engineering utilizes a preconditioned cell-laden construct to increase cell survival and promote greater graft integration and reinnervation. In vivo engineering leverages cells with other scaffold cues to promote host cell infiltration and increase the initiation of tissue regeneration. In situ engineering uses an acellular construct and relies on the topographical, biochemical, and physicochemical cues from the biomaterials to promote cell infiltration and tissue regeneration.
Due to the challenges of in vitro muscle engineering, many therapies turn to in vivo muscle engineering, which employs cell-laden biomaterials similar to in vitro engineering; however, constructs are implanted without extensive preconditioning and allowed to differentiate in vivo at the injury site. This strategy relies on the cell-laden construct to stimulate the complex local microenvironment to further infiltrate with appropriate cells and biochemical cues to induce regeneration of muscle, vasculature, and NMJs. The main advantages of this system are that the therapy is much less complicated and costly due to limited manipulation of cells before implantation and relies on the host local microenvironment to facilitate regeneration. In addition, many studies have shown that cell-laden biomaterials increased muscle regeneration compared to the biomaterial alone [[61], [62], [63]]. However, a limitation of this strategy is that the less densely packed and undifferentiated cells are more vulnerable rupture by the host immune response, leading to reduced viability of delivered cells.
The final strategy, in situ muscle engineering, relies on the physicochemical and biochemical cues of an acellular biomaterial to stimulate the infiltration of local cells from the injury site and induce muscle, vasculature, and NMJ regeneration. This strategy is becoming more attractive as advances are made in biomaterial science and scaffold fabrication techniques [64,65]. The main advantage of this strategy is that the fabricated construct can be used as an off-the-shelf product for physicians. However, the biomaterial requires greater complexity to guide tissue infiltration and differentiation compared to the other strategies, requiring significant research and understanding of the interactions between the biomaterial and the native healing response. For this reason, the key biomaterial properties for skeletal muscle engineering (Fig. 1) are of great importance to incorporate into in situ engineering systems.
Recent advances in biomaterials-based strategies for skeletal muscle tissue engineering are discussed below. Table 1 highlights select studies from the research discussed to provide examples of the types of materials used in each of the three skeletal muscle tissue engineering approaches. This table does not reflect an exhaustive literature search based on the three approaches and does not reflect the proportion of synthetic, natural, and hybrid materials utilized for each approach. In addition, it is worth mentioning that many scaffold-less strategies, such as spheroids and cell sheet therapies, also exist and have advanced the field of skeletal muscle tissue engineering. Previous reviews have explored scaffold-free strategies for skeletal muscle tissue engineering [13,66,67].
Table 1.
Examples of scaffold types used in each skeletal muscle tissue engineering approach.
5. Biomaterial therapies
5.1. Electrospun scaffolds
Electrospinning is an attractive platform for several tissue engineering applications because it is a high throughput method of fabricating micro- and nano-scale fibers that resemble the architecture of ECM (Table 2). Electrospinning works by creating an electrical field between a depositing needle and a collector. An electrically conductive and volatile solvent is advantageous and should be chosen based on polymer compatibility. In addition, fibers can be easily aligned by collecting onto a rotating mandrel, and the degree of alignment can be manipulated by adjusting the rotating speed of the collector. Under appropriate conditions, as the polymer solution is pushed through the needle, the electrostatic forces will overcome the surface tension in the needle, creating a polymer jet. As the jet moves toward the ground or negatively charged collector, a whipping action occurs, allowing the solvent to evaporate and polymer fibers to collect in individual layers. As biomaterials science has evolved, methods have adapted to allow more polymers to be compatible with electrospinning.
Table 2.
A comparison of the advantages and limitations of the main scaffold fabrication techniques utilized in the field of biomaterial-based skeletal muscle tissue engineering.
| Scaffold Fabrication technique | Advantage | Limitation |
|---|---|---|
| Electrospinning |
|
|
| Injectable Systems |
|
|
| Mold-cast Systems |
|
|
| Extrusion 3D Printing |
|
|
Synthetic materials with electrically conductive properties have been used widely as electrospun scaffolds for skeletal muscle engineering to promote formation of NMJs and restoration of contractile force. In addition, the tensile mechanical properties of synthetic materials can be more easily modulated than natural materials to mimic the native mechanical properties of skeletal muscle. Although nanofiber scaffolds are used in many skeletal muscle applications because of their high surface area, Narayanan et al. explored the effects of poly(lactide-co-glycolide) (PLGA) fiber diameter on myogenic differentiation in vitro and in vivo [68]. PLGA is an advantageous synthetic polymer because the ratio of lactide to glycolide can be modulated to control mechanical properties and degradation kinetics. In this study, an 85:15 (lactide:glycolide) was used to produce aligned fibers between 300 nm and 3 μm. Over the course of their study, they found that the larger fibers (3 μm) supported increased cell differentiation and alignment in vitro compared with smaller nano-scale fibers. They attributed these findings to the higher cell density that they were able to achieve via greater cell infiltration due to larger pore size. It should be noted that other studies have found that pores should remain smaller than 20 μm to keep cells growing together on the scaffold rather than allowing them to grow along the side of the fiber [68,69]. While the effects of fiber diameter were not evaluated in vivo, the authors did implant their largest fiber diameter scaffolds seeded with myoblasts into a dystrophin mouse model and observed cell differentiation and integration with the injury site after 3 weeks. In a similar way, Bloise et al. used poly(butylene1,4-cyclohexandicarboxylate- co-triethylene cyclohexanedicarboxylate) [P(BCE-co-TECE)] as a tunable synthetic material [70]. Increasing the concentration of ether linkages present in the copolymer impacted hydrophilicity, mechanical properties, and degradation kinetics. They also used this copolymer to study the effects of fiber diameter on myogenic differentiation in vitro and in vivo. The authors observed that the higher the TECE concentration in the copolymer, the lower the mechanical properties, and the greater the proliferation rate of C2C12 cells in vitro. While the tensile modulus was still much higher than values reported for skeletal muscle (~10 kPa), they were able to modulate the polymer stiffness within the range of soft tissues. In addition, they found that low micron-sized fibers promoted myogenic differentiation better than nano-sized fibers, which supports the findings discussed previously. Also similar to the aforementioned study, the authors implanted the micron-size, TECE-rich scaffolds into tibialis anterior (TA) defects within a mouse model. After 6 weeks, tissue explants were analyzed, and some neovascularization was observed. However, most of the infiltrating cells were immune cells. While both of these studies provided useful insight and demonstrated the effects of fiber diameter and mechanical properties on myogenic differentiation, the influence of pore size and fiber diameter still needs to be studied in more depth. Specifically, the fiber and pore size range that allows for effective infiltration, growth, and differentiation of myogenic, angiogenic, and neurogenic precursor cells in vivo should be more extensively characterized.
One of the big limitations of electrospun scaffolds is the ability for cells to infiltrate the full thickness of the scaffold [71]. Although electrospun scaffolds are typically very porous and interconnected, the pore size is often small, particularly for aligned scaffolds needed for muscle engineering. One of the ways that this is addressed is by creating meshes in the higher end of the nano-scale range, as mentioned previously, to decrease the fiber density and allow more space for cells to migrate. Another approach that can be taken to promote cell infiltration is incorporating biological cues into electrospun scaffolds. Natural polymer and hybrid scaffolds have gained a lot of popularity in recent years. Manchineella et al. utilized a silk fibroin/melanin composite to leverage the antioxidant and conductive properties of melanin with the bioactive properties of silk in a highly aligned electrospun scaffold to promote myoblast differentiation and assembly into aligned myotubes [72]. They found that the composite greatly outperformed the silk scaffolds in vitro, in part by reducing the oxidative stress experienced by myoblasts in culture, which has been shown to impair myogenesis [73]. Liu et al. also developed a composite to increase cell proliferation and differentiation [74]. They developed a hybrid electrospun composite from PCL and mussel-inspired poly(norepinephrine), which was shown to increase bioactivity compared to PCL alone and inhibit myostatin expression, thus increasing cell proliferation in vivo in a VML model. Through their studies, they found that poly(norepinephrine)-coated scaffolds induced greater myogenic differentiation in vitro and in vivo, and small micron-scale fibers (2 μm) performed better than larger fibers (10 μm), further supporting the importance of fiber diameter and resulting pore size in electrospun scaffolds.
In addition to the incorporation of bioactive factors, the incorporation of cells has been a promising area of research for skeletal muscle engineering. As mentioned previously, in vitro, in vivo, and in situ skeletal muscle engineering approaches have been applied with hopes of promoting muscle regeneration. Gilbert-Honick et al. compared these strategies using an acellular electrospun fibrin scaffold, a mouse C2C12 myoblast-laden fibrin composite, and a prevascularized fibrin construct conditioned with human adipose-derived stem cells [63]. To achieve these comparisons, an immunodeficient mouse VML model was used. Interestingly, the authors found that the contractile function was restored in the acellular and C2C12-laden groups after 2 weeks, whereas only the C2C12-laden group showed dense myofiber regeneration and restoration of muscle volume at this point. The recovery was expedited in this model compared with other literature surveyed, which may be due to the lack of immune cells and the variation from the normal injury response. However, this study provided insight into the different engineering approaches.
Another promising approach to increasing cell infiltration is cell electrospinning, which combines cells into the electrospun scaffold as it is fabricated. Yeo et al. developed a method to electrospin cells encapsulated in an alginate/poly(ethylene oxide) (PEO) copolymer [75]. The obstacles of this method are the selection of polymers, electrospinning solvents, and electrospinning parameters that are cell-compatible for the multiple hours that it takes to fabricate an electrospun scaffold. To aid in this process, the authors electrospun onto a PCL strut to provide robust mechanical properties to their system. The authors observed high cell viability after scaffold fabrication and found that their system effectively induced cell alignment and myogenic differentiation throughout their constructs. In a similar fashion, Guo et al. developed a cell electrospinning system that encapsulated cell aggregates into a fibrin/PEO copolymer to form highly aligned cell-laden microfiber bundles [76]. The authors used a wet electrospinning rotating collection bath filled with thrombin and CaCl2 to crosslink the cell-laden polymer fibers in situ. Although the authors found that encapsulating cell aggregates increased cell viability in the fabricated constructs compared with monodispersed cells, the regenerative capacity of the encapsulated cells was low compared with cells seeded postfabrication, resulting in low fusion index. While additional research needs to be conducted, combining cells into electrospun scaffolds during fabrication may overcome the limitation of cell infiltration into the full depth of electrospun scaffolds. Although other practical limitations exist in using electrospun systems to repair muscle, efforts are ongoing to overcome them, and electrospinning remains a viable option to mimic the muscle's sophisticated hierarchical structure.
5.2. Injectable hydrogels
Minimally invasive strategies for tissue regeneration have many important advantages, including lower risk of surgical site infection and lower surgical site recovery time. The use of injectable hydrogels, which often possess self-healing and/or thermoresponsive properties, allows for biomaterials to be delivered through a syringe and contour around the site of injury to fill in irregular defect volumes. Injectable systems have been developed extensively for cardiac muscle applications because of the high risk associated with invasive heart surgeries [[77], [78], [79]]. Although less focus has been given to injectable systems for skeletal muscle regeneration, the advantages are still worth investigating (Table 2). Guo et al. developed an injectable hydrogel composed of dextran grafted to tetraaniline and N-carboxyethyl chitosan (DEX-AT/CECS) for the minimally invasive repair of skeletal muscle [80]. The injected copolymer system formed a gel within 2 min after subcutaneous injection into a rat model, and dynamically crosslinked within 10 min. The dynamic Schiff base bonds derived from the tetraaniline and the N-carboxyethyl chitosan provided rapid self-healing properties to the hydrogel system, which is advantageous for injectable systems. In addition, the copolymer possessed inherent conductive properties, which are advantageous in skeletal muscle therapies. Within 1 week of implantation in a rat VML model, the authors observed the presence of myofibers in the DEX-AT/CECS hydrogels, while DEX/CECS and untreated groups showed minimal myogenic differentiation. Upon further examination after 4 weeks, the authors observed further myofiber generation in the DEX-AT/CECS hydrogel group and minimal generation in the DEX/CECS and the untreated groups. Although extensive analysis was not performed on the volumetric muscle regeneration, revascularization, or the contractile function, this preliminary study shows the potential for this system to be used as an injectable skeletal muscle therapy.
Another exciting direction for injectable therapies is their use in tandem with bioactive factors. Passipieri et al. conducted an extensive study on the use of an injectable keratin hydrogel alone and as a delivery vehicle for growth factors and skeletal muscle progenitor cells for the regeneration of skeletal muscle [61]. The authors compared their keratin groups to a no repair group (negative control) and a bladder acellular matrix group (positive control), which they have studied previously for muscle repair. Surprisingly, they found that keratin alone and keratin loaded with insulin-like growth factor 1 and basic fibroblast growth factor outperformed all other groups, including keratin loaded with cells. This was a very advantageous finding, as the successful constructs could have applications as an off-the-shelf, injectable therapy for skeletal muscle engineering. The authors used restoration of contractile function as their main metric, as this analysis encompasses muscle volume restoration as well as functional restoration of NMJs. The authors observed approximately 70% functional recovery in the keratin alone and keratin/growth factor groups, whereas the other groups resulted in less than 60% functional recovery after 12 weeks in vivo in a VML model. In addition, the authors found that keratin alone and keratin/growth factor groups produced an abundance of newly formed myofibers and blood vessels. Although there are still limitations in the tunability of injectable systems, this study provides new insights into the possibilities for in situ muscle engineering and the use of injectable hydrogels in skeletal muscle applications.
5.3. Mold-cast scaffolds
While porosity can be incorporated into injectable hydrogels, control over architecture and mechanical properties can be difficult to modulate in injectable systems. Hydrogels are advantageous biomaterials in a number of tissue engineering applications because they are mostly water, similar to tissues, allowing them to integrate with the site of injury; they more easily fill large volumes than electrospun scaffolds; and their hydrophilic nature makes them excellent candidates as drug and cell delivery vehicles [81]. To better control architecture and physicochemical properties, many engineers use molds to forms their scaffolds, allowing flexibility to modify the constructs post-after fabrication before implantation. Shah et al. used a mold to encase parallel, aligned glass fibers in a collagen hydrogel containing cells [82]. The authors observed that the stiffer glass fibers promoted cell anchoring and alignment, whereas the compliant collagen hydrogel provided biochemical cues to support cell differentiation and degradation kinetics aligning with tissue replacement in vivo. In addition, they found that a more compliant hydrogel was necessary to support muscle contractions and recovery from the tensile forces experienced in the regenerating tissue.
As the field of 3D printing has advanced, molds for casting biomaterials have become more easily accessible and more complex configurations have become possible to overcome some of the limitations of mold-casting (Table 2). These sophisticated molds have allowed hydrogel formation to be combined with other scaffold fabrication techniques. Chen et al. used 3D printing to deposit water onto a cooled platform to form parallel strands of ice [50]. A collagen hydrogel was then cast on top of the ice mold to form microgrooves in the resulting biological hydrogel. The authors found that hydrogels with larger (200–300 μm), concave microgrooves promoted better cell alignment than smaller microgrooves (~100 μm). However, no difference was observed in myogenic gene expression. In a similar manner, Wang et al. utilized multiple scaffold fabrication techniques, using a sophisticated system of wet electrospinning paired with mold-casting to fabricate nanofiber cores encapsulated in a hydrogel shell [83]. By wet electrospinning a blend of PCL, silk fibroin, and polyaniline (PANI), the authors were able to lift the nanofibers with a rotating receptor, forming aligned copolymer yarn. The polymer strands were then fed into a mold containing PDMS patterned channels, into which a photocurable poly(ethylene glycol-co-glycerol sebacate) could be injected and crosslinked to form a shell around the electrospun core. Similar to the collagen encapsulated glass fiber study discussed previously, the authors observed that seeding cells on the electrospun yarn before applying the hydrogel shell allowed for cell attachment and alignment along the electrospun fibers, and that the compliant shell hydrogel further supported myogenic differentiation. Unfortunately, PANI is a nonbiodegradable polymer, and its use is not ideal for skeletal muscle engineering, where the biomaterial scaffold should degrade at approximately the same rate that new tissue grows to replace it. Although mold-casting techniques still have practical limitations, such as resolution and patterning of multiple materials that can be appropriately recovered from molds, the development of soft lithography systems has drastically improved the fidelity of constructs that can be fabricated with mold-casting.
5.4. 3D-printed scaffolds
Although advances in 3D printing have allowed for fabrication of more sophisticated molds to cast polymers, they have also facilitated the development of multimaterial and spaciotemporal patterning strategies to produce high-resolution constructs that cannot be fabricate by other means (Table 2). There are many different types of 3D printing—inkjet-based, extrusion-based, and light-based printing—that should be utilized based on material properties (viscosity, photosensitivity) and intended application. Each of these printing strategies has advantages and disadvantages. In short, inkjet-based printing is a low-cost, high-speed method of printing but is compatible with mostly low viscosity bioinks, which in turn limits the z-resolution achievable. Extrusion-based printing can be used to achieve large, freeform structures with the flexibility to incorporate multiple materials, cells, and bioactive molecules, but it requires relatively high-viscosity bioinks and results in higher shear stress and lower resolution compared with the other methods, which can have implications in cell printing. Higher shear stress on encapsulated cells leads to membrane stress and lower cell viability. Finally, light-based printing yields high resolution constructs with high shape fidelity and is compatible with a range of biomaterial viscosities but can be expensive compared with the alternative printing methods and is only compatible with photosensitive materials. Each of these printing methods has unique qualities and together, they can be leveraged to advance the field of tissue engineering. In addition, 3D printing muscle cells allows them to elongate and align in the direction they are printed, which expands possibilities for cell patterning and cell histoarchitecture for skeletal muscle engineering [84].
Extrusion-based 3D printing has been utilized in most scaffold printing for skeletal muscle tissue engineering applications. However, one of the big limitations of encapsulating cells in hydrogels through mold-cast or 3D-printing methods is the adequate nutrient transport to cells in the center of hydrogels/fibers. Kim et al. leveraged extrusion-based 3D cell printing with the native structural and biochemical cues from fibrin to promote muscle cell alignment and differentiation [85]. The authors aimed to demonstrate the effectiveness of cell printing in guiding cell alignment, so they conducted a comparative study of 3D-printed constructs and mold-casted constructs. By incorporating sacrificial gelatin hydrogels into their 3D-printed system, they produced microchannels to facilitate proper nutrient and waste transport and PCL support pillars to provide mechanical stability. The authors observed increased cell alignment in 3D-printed constructs compared with mold-casted hydrogels. They also found that printed cells were highly viable and differentiated by day 9 in culture, whereas casted cells were predominately dead by day 5. The constructs were further examined in vivo in a rat VML model. The authors found that the 3D-printed, cell-laden constructs had significantly increased regenerated muscle volume and restored contractile force (~85%) at 8 weeks compared with treatment with casted hydrogels with and without cells. Furthermore, the regenerated muscle formed in the 3D-printed groups showed evidence of vascular and neural integrity after 8 weeks. Although this study demonstrated the therapeutic advances that can be achieved through 3D printing, an immunodeficient model was used, and further research is needed to study healing in response to this therapy. Recently, advances have also been made towards leveraging 3D printing for personalized medicine. Russel et al. used a semi-automated portable 3D-printing system to extrude acellular gelatin methacryloyl directly into the defect site [86]. Through this in situ 3D-printing system, the authors were able to achieve effective integration of the scaffold with the host tissue and promote native cell infiltration, growth, and differentiation with 4 weeks. This technology opens many possibilities for delivering personalized medicine to these irregular defect types.
In addition to nutrient transport, another limitation of 3D printing for skeletal muscle engineering is the low resolution in guiding proper cell alignment, as most materials can achieve resolution on the order of hundreds of microns. To overcome this shortcoming, Arab et al. used printable, self-assembling peptides to create 3D hydrogel constructs to promote myogenic differentiation [87]. The authors found that cells within their peptide hydrogels aligned in response to the structural cues provided by their synthetic peptides, while cells grew in a random orientation in their alginate-gelatin controls. These findings indicate that synthetic peptides can be combined with 3D printing to provide a hierarchical system of alignment. In a similar manner, Yeo et al. combined 3D printing with electrospinning to provide micro-scale and nano-scale structure, respectively, to further guide cell alignment for myogenic differentiation [88]. The authors printed a PCL framework using extrusion-based printing, and electrospun PCL nanofibers on top. Interestingly, the collector voltage was altered to fabricate randomly oriented or aligned electrospun fibers on top of the PCL framework. Finally, myoblasts encapsulated in an alginate/PEO hydrogel were 3D printed atop the electrospun layers. PEO was used as a leaching material to create a porous hydrogel environment for the cells to facilitate nutrient transport. Furthermore, the authors rolled the fabricated cell-laden construct to mimic the geometry and hierarchical structure of muscle fibers. The authors observed increased myogenic differentiation in constructs containing electrospun nanofibers compared with constructs containing only the PCL framework and the cell-laden hydrogel, with the highest levels of myogenic gene expression observed in constructs containing aligned fibers. In addition, they found that constructs containing aligned electrospun fibers produced longer and more highly aligned myotubes on average compared with the other groups. This study demonstrated the potential of a hierarchical system of architectural cues to guide cell alignment and promote myogenic differentiation. ECM components can also be used to provide a natural hierarchical system of structural and biochemical cues to guide cell behavior. Kim et al. developed a method of 3D-printing cell-laden collagen hydrogels into a heated glycine/KCl bath to induce alignment of collagen fibrils within the 3D-printed fibers [89]. Similar to the synthetic peptide work described previously, the authors were able to induce alignment of the natural collagen molecules to further promote cell alignment within the lager constructs. The authors found that cells were much more uniformly aligned in the fibrillated collagen scaffolds compared with scaffolds that did not undergo the fibrillation process. In addition, they observed increased myogenic gene expression in fibrillated collagen constructs compared with the unfibrillated control, demonstrating the need for nano-scale structural cues to guide cell alignment and differentiation. Although 3D printing still has obstacles to overcome for soft tissue engineering, namely feature resolution, shear-induced cell viability, and reproducibility, measures are being taken to advance 3D printing and create more sophisticated biomimetic constructs for skeletal muscle engineering applications.
5.5. Decellularized ECM therapies
Therapies that utilize decellularized tissues (dECM) have become more and more attractive for tissue engineering applications because they retain biological cues that are difficult to recapitulate with synthetic and commercially available natural polymers. Although many dECM therapies have been translated into the clinic for repair of a number of tissues, decellularized skeletal muscle remains a challenge to translate because it is a relatively thick tissue without centralized vasculature. For this reason, thin-membrane dECM sources, such as small intestinal submucosa (SIS), have been used to regenerate skeletal muscle instead. Dziki et al. utilized SIS, urinary bladder, and dermal dECM therapies in a translational study involving 13 human patients suffering from VML injuries [65]. Due to the size limitations of SIS, the therapy was applied after several reconstructive surgeries and months of physical therapy in most cases. Metrics for contractile function and tissue remodeling at the defect site were compared before dECM implantation. Within 6 weeks of dECM implantation, myogenically differentiated cells were present throughout the dECM construct, demonstrating cell recruitment and differentiation. By the end of the study, dECM implantation paired with aggressive physical therapy resulted in increased muscle formation in all 13 patients, and biopsies revealed myogenic, angiogenic, and neurogenic differentiation by the conclusion of the 6-month study. In addition, 11 of 13 patients demonstrated increased contractile force production of 20–140%. Although many factors were uncontrolled because of limitations of early clinical testing, the addition of the dECM therapy elicited dramatic structural and functional restoration that was unachievable by the previous surgeries undergone by the patients. This study demonstrates the clear clinical applications of dECM therapies for skeletal muscle regeneration.
As encouraging as the use of acellular dECM tissue has been in preclinical and clinical work to date, there are still obstacles that should be addressed. Unmanipulated dECM tissue used for severe muscle injury is derived from thin tissues (dermis, SIS) and does not have the capacity to fill and provide support to large defects, which leads to inadequate formation of muscle tissue [90]. Therefore, it is advantageous to take a multidisciplinary approach by combining dECM with established tissue engineering scaffold fabrication techniques. SIS and dermis have been shown to provide a number of biological cues that can promote cell infiltration in a wide range of tissue types, but it is likely that muscle-specific growth factors, structural proteins, and architecture would further improve cell infiltration and differentiation within muscle defects. For this reason, many efforts have been made to utilize skeletal muscle dECM in a form that is appropriate for regenerative therapies. In addition to difficulty in decellularizing skeletal muscle while maintaining a clinically relevant size and shape, the resulting constructs often do not retain the mechanical properties which are essential for functional restoration of skeletal muscle. Therefore, the scaffold fabrication techniques discussed previously have been leveraged with skeletal muscle dECM to create tunable, bioactive scaffolds for tissue engineering. Although many therapies have been developed, only a short overview of studies since 2015 will be discussed here. Patel et al. utilized an electrospun blend of PCL and muscle dECM for the regeneration of muscle in a mouse VML model [91]. The authors compared this treatment to electrospun PCL alone and found that the addition of dECM promoted the presence of M2 macrophages and increased myogenic differentiation. However, restoration of muscle structure and function were not supported within the short, 4-week study. To further leverage the biochemical cues provided by skeletal muscle dECM with the tunable fiber diameter and alignment afforded through electrospinning, our laboratory developed a method of fabricating electrospun scaffolds completely derived from skeletal muscle dECM with tunable physicochemical properties [92]. Unlike other natural polymers, the electrospun dECM system demonstrated enhanced versatility in its innate physiologically relevant mechanical properties and degradation kinetics in the absence of a crosslinking agent. Although in vitro assessment is still ongoing, previous studies utilizing electrospinning and dECM give us confidence that the electrospun dECM therapy with support cell recruitment, alignment, and myogenic regeneration.
Hydrogels derived from skeletal muscle dECM have also been widely used because of their thermoresponsive behavior and intrinsic gelation properties driven by collagen self-assembly. Injectable hydrogels derived from skeletal muscle dECM have been developed for regenerative applications in muscle ischemia and muscle defects. Ungerleider et al. developed an injectable hydrogel system derived from skeletal muscle that retains many important native matrix components and possesses a storage modulus in the 5–10 kPa range [93]. In addition, the material is shear thinning, allowing for the possibility of the hydrogel system to be injected into the defect site. Rao et al. further expanded on this system by using it as a delivery vehicle for cells in a mouse ischemia model [94]. The authors demonstrated the feasibility of the dECM hydrogel as an injectable system, and their results suggested that dECM protects cells from shear-induced apoptosis and hypoxic conditions in the initial ischemic environment delivering muscle-specific cues to aid in muscle and blood vessel repair. Increased perfusion was observed in the dECM/cell groups by day 35, indicating synergistic interplay between the muscle-specific cells and the muscle-specific hydrogel. Although an acellular dECM hydrogel was not included in this study, other studies have found that skeletal muscle dECM alone increases perfusion in an ischemia model [95]. For muscle regeneration applications, Fu et al. developed a method of producing a stable skeletal muscle dECM hydrogel that can be used as an injectable or mold-cast system [96]. Utilizing the same thermoresponsive properties as the studies aforementioned, the authors were able to demonstrate that their system formed a stable hydrogel within 30 min of injection into a rat subcutaneous model. They also found that they can cast the solution into a mold to produce a stable yet malleable hydrogel within 30 min at physiological temperatures. The authors then demonstrated that the hydrogel could maintain shape fidelity when cast into different geometric molds. Although further research still needs to be conducted, dECM hydrogels have been shown to exhibit mechanical properties within the range of native muscle, which provides hope that this system may be useful as a therapy for muscle regeneration.
As discussed previously, injectable and mold-cast hydrogel systems have many advantages but are also limited in some ways that can be overcome by 3D printing efforts such as precision architecture and porosity. Amazing strides have been made in 3D printing dECM in the last several years driven by insight on the gelation mechanisms of dECM and the development of more sophisticated printing methods. Choi et al. developed a skeletal muscle dECM bioink compatible with extrusion 3D cell printing without the need for a copolymer support [97]. The authors were able to demonstrate the control of cell alignment within their dECM bioinks through modulation of print geometry and fiber diameter; the smaller the fiber width, the more aligned the cells grew. In addition, it was observed that dECM bioinks supported greater cell proliferation and myogenic differentiation than collagen bioinks, indicating that the bioactivity of matrix components and growth factors was retained throughout decellularization and bioink preparation. Of particular interest was the retention of agrin within the dECM bioink, which promoted greater formation of acetylcholine receptors (AChR) within myotubes compared with collagen. AChR are essential for the development of NMJs and successful reinnervation of skeletal muscle. Finally, the authors examined the mechanical properties of their cell-laden constructs after 14 days in culture and found that the matrix stiffness was within the range of native muscle. Encouraged by their findings, the group went on to conduct further studies to assess the 3D-printed system in a rat VML model [98]. To improve feature resolution for their relatively low viscosity bioinks, the authors adapted a version of the freeform reversible embedding of suspended hydrogels method [99] by printing their bioink into a bath of gelatin granules. As mentioned previously, limited nutrient transport in hydrogels can lead to hypoxic conditions in structures greater than 200 μm. Therefore, the authors utilized a coaxial printing method to fabricate constructs containing a cell-laden skeletal muscle dECM core surrounded by a cell-laden vascular dECM shell. The construct was then preconditioned to form a prevascularized muscle construct before implantation into a rat VML model. The authors compared 3D-printed, cell-laden muscle dECM constructs with cell-laden muscle dECM sponges, which were cut from the initial decellularized skeletal muscle before bioink preparation. After 4 weeks, muscle regeneration was observed in both dECM treatments, with the highest muscle volume and contractile force restoration (~71%) observed in the 3D-printed group. The authors then compared the prevascularized group with the cell-laden dECM bioink group. With prevascularization, even greater muscle regeneration (~79%) and contractile force restoration (~85%) were observed. In addition, NMJ formation and host neural integration were observed in the prevascularized group. The results of these studies epitomize the advances that are being made in the field of skeletal muscle engineering, and further highlight the benefits of tissue-specific dECM materials for the structural and functional repair of skeletal muscle. Although the use of skeletal muscle dECM is still a new area of research, the innate properties of skeletal muscle and the advances made in scaffold fabrication techniques have allowed for rapid advances in fabricating biomaterials with tunable physicochemical properties that retain key matrix components to support cell recruitment, proliferation, and differentiation.
6. Methods for assessing skeletal muscle regeneration
6.1. In vitro myogenic differentiation
When developing a new biomaterial for skeletal muscle engineering, it is first essential to test the biomaterial for cytocompatibility and its potential to promote myogenic differentiation within an in vitro cell culture system. Myogenic culture can be performed in static conditions or in bioreactor systems using a number of different cell types ranging from myogenic precursor cells (C2C12 mouse myoblasts or L6 rat myoblasts) to primary multipotent cells (satellite cells or bone marrow-derived stem cells). When precursor cells overcome the difficulty of isolating and maintaining primary cells in vivo, they are often associated with low population differentiation [100]. Assessment of myogenic differentiation can be performed in several different ways (Table 3), but the most common analysis is cell morphology and myotube formation via immunofluorescent (IF) labeling. Unlike many other musculoskeletal systems, an effective assay does not exist to easily and accurately quantify myogenic differentiation. Therefore, imaging is considered the most efficient preliminary tool to assess cell alignment and myotube formation. A number markers can be used, but the most common myogenic marker for IF is myosin heavy chain (MHC) [63,68,70,76,83,85,88,89,97,101]. Once myotubes are labeled with MHC, myotubes can be imaged, and fusion index (percent differentiation) can be calculated by dividing the number of nuclei (stained with a nuclear stain) colocated in MHC+ myotubes by the total number of nuclei in the image field. Fusion index along with myotube width, length, and alignment are often quantified through image software, such as ImageJ or Photoshop. However, recent research has focused on developing open-source software or code to more easily and accurately quantify myotube formation parameters without incorporating human error or bias [102]. Other myogenic markers that are commonly used to visualize and assess myotube formation, include desmin [82,103] and α-sarcomeric actin [82,85,88,89,101]. In addition, a cytoskeletal actin stain can be utilized to assess overall biomaterial-guided cell alignment [50,70,72,76,84,87,98].
Table 3.
Methods of assessing myogenic differentiation within in vitro culture systems.
| Myogenic outcome | Marker assessed | In vitro analysis method | Time after induction |
|---|---|---|---|
| Myotube formation |
|
Immunofluorescence | 3–7 days |
| Myogenic gene expression |
|
Polymerase chain reaction | 3–21 days |
| Myogenic protein production |
|
Western blotting ELISA |
7–14 days |
| Force production |
|
Electrical stimulation | 7–21 days |
| NMJs formation |
|
Immunofluorescence | 14 days |
Myotube formation in vitro often involves growing cells to confluency in growth media (DMEM + ~10% FBS) before switching the culture to differentiation media, which often contains reduced mitogens (DMEM + ~2% horse serum or FBS). Myogenic markers tend to be expressed within the first 3 days of culture in differentiation media, and myotube formation can be observed within the first 7 days. Imaging is often executed as a first step in the evaluation of myotube formation. Additional analyses can then be performed, including polymerase chain reaction (PCR) to assess myogenic gene expression, western blotting or enzyme-linked immunosorbent assay (ELISA) to assess myogenic protein production, and contraction production in response to electrical stimulation to assess development of NMJs and myotube maturation. Common markers of myogenic gene expression that are quantified via PCR include MHC [50,70,82,88,89,94,97,101], myogenic factor 5 (Myf5) [89,97,101], myogenin [50,70,82,84,88,89,97,101], MyoD [70,82,84,88,89,94,97,101], and sarcomeric actin [84]. Although not as common, western blots and ELISAs have been used to assess production of early-stage myogenic proteins [68,70], which can provide a clearer assessment of myotube maturation. Finally, formation of NMJs is often assessed through contractile response to electrical stimulation [63,89,97] or staining for AChrR [97], which can further indicate myotube maturation. Although in vitro studies do not provide a complete correlation to the behavior of the biomaterial system in vivo, analyzing growth and myogenic differentiation can provide an initial assessment of the biocompatibility and myogenic potential of a skeletal muscle therapy. Screening for the induction of proper cell growth, alignment, and myogenic differentiation may shed light on potential problems that can be addressed prior to testing muscle regeneration in a complex animal model.
6.2. In vivo muscle regeneration
As research into skeletal muscle engineering has progressed, in vivo models for muscle regeneration have become fairly standardized. Initial studies are often performed in rats, although mouse [104] and rabbit [105] small animal VML models have also been used. Wu et al. developed a reproducible rat VML model to provide a standardized platform for testing skeletal muscle engineering therapies [106]. The TA muscle is the most common target for VML defects in small animals [63,85,98]. For preclinical testing in large animals, pigs are commonly used and the peroneus tertius muscle is targeted for modeling VML defects [[107], [108], [109]]. Regardless of the animal used, the assessment of muscle regeneration in VML models evaluates several central components (Table 4). Because reduced force production is a hallmark of VML, isometric torque production is often evaluated to assess muscle regeneration. This analysis often entails securing the animal's foot to a force plate and inserting sterile electrodes to stimulate the peroneal nerve [61,63,65,85,91,98]. By comparing isometric torque before injury and at set time points, often 4- and 8-weeks after treatment, information can be gathered on the extent of muscle repair and regeneration of NMJs. Although force production is a very important parameter, it should be combined with other analyses to gain a clearer picture of the effects of the skeletal muscle therapy on repair.
Table 4.
Methods of assessing muscle regeneration within in vivo systems.
| Outcome | Marker assessed | Analysis method |
|---|---|---|
| Force production |
|
Electrical stimulation of peroneal nerve |
| Muscle regrowth |
|
Weighing target muscle |
| Cell infiltration |
|
H&E histology Immunofluorescence |
| Fibrosis |
|
Masson's Trichrome histology |
| Myotube formation |
|
Immunofluorescence |
| Blood vessel formation |
|
Immunofluorescence |
| NMJs formation |
|
Immunofluorescence |
| Immunogenic inflammation |
|
Immunofluorescence |
| Pro-inflammatory macrophage |
|
Western blotting |
| Ant-inflammatory macrophage |
|
Western blotting |
Upon completion of predetermined time points, target muscles are harvested, and wet muscle weight is measured, allowing comparisons to be drawn between no-treatment groups, therapy groups, and contralateral uninjured muscles. From there, histology and IF staining can be performed. Hematoxylin and eosin (H&E) paired with Masson's trichrome histological stain can be used to identify the types of cells that have infiltrated the explant and assess the extent of muscle fiber formation versus fibrosis at the defect site [61,63,70,91,98]. IF labeling can be used to evaluate myogenic, angiogenic, and neurogenic differentiation within the defect site. Similar to in vitro assessment of myogenesis using IF, MHC [61,63,70,85,91,98], desmin [65], and dystrophin [68] can be utilized. Pax7 has also been utilized to evaluate the restoration of muscle satellite cells within newly formed tissue [110,111]. To assess angiogenesis, CD31 [63,91], von Willebrand factor (vWF) [70,85], α-smooth muscle actin [70,85], and isolectin [70] can be utilized. In addition, α-bungarotoxin (AChR) [85,98] and β III tubulin [65,98] can be utilized to assess neurogenesis, whereas F4/80 [70,91] and Mac-3 [70] immunogenic markers can determine if inflammation is occurring in the defect site because of the presence of macrophages. Although less common, western blotting can be performed in conjunction with IF to assess the production of myogenic and immunogenic proteins. MyoD and myogenin can be correlated to myogenesis, while heat shock protein-70 (cell stress), iNOS (pro-inflammatory), and arginase (anti-inflammatory) can be used to assess the immune response at the defect site. Although other methods of evaluating skeletal muscle regeneration in vivo exist, the methods discussed have been highlighted because they have been utilized in research that is paving the way in the field of biomaterials-based skeletal muscle engineering.
7. Conclusions and obstacles still to overcome
The development of a skeletal muscle therapy that promotes host cell infiltration, muscle regeneration, blood vessel formation, and reinnervation would address a global unmet need. Although current clinical practices have shown some success in structurally restoring lost muscle, patients are left with large functional deficits to the injured muscle as well as surrounding musculoskeletal tissues. The field of biomaterials-based skeletal muscle engineering has taken large strides in the last several years to develop sophisticated strategies to regenerate muscle, blood vessels, and NMJs, with a special focus on restoration of force production. While structural regeneration should not be discounted as it plays a large role in psychological healing after trauma, restoration of functional deficits has been a focus recently after being overlooked for so long. Advances in 3D printing technology and the use of ECM-derived polymers are shaping the future of skeletal muscle engineering. With encouraging results being reported from ongoing preclinical and clinical research, the door may open for more therapies to enter the regulatory pathway in the coming years.
Although skeletal muscle engineering was built on a foundation of synthetic polymer systems, the future of skeletal muscle engineering may increasingly rely on combining ECM-derived polymers with scaffold fabrication techniques that allow for tunable physicochemical properties and modulation of construct size and architecture to fill the defect site and support integration with the host tissue. In an ideal scenario, the biochemical cues from skeletal muscle dECM can be leveraged with the versatility afforded through modern scaffold fabrication techniques to create a tunable, protein-rich construct that can provide the necessary mechanical, topographical, and biochemical cues to promote muscle regeneration, blood vessel infiltration, and NMJ formation. Most of the research into these sophisticated biomaterial systems is still in early small animal stages of research, but the results have created optimism for the field. A final obstacle to address is the use of cells in preclinical models. Cell delivery has been used widely in the clinic with mixed outcomes. The incorporation of cells should not be disregarded, but the flexibility afforded by an acellular therapy that can be used as an off-the-shelf product should be striven for. Such a therapy would allow for quicker surgical intervention and may limit scar formation. Although many years of research remain before these therapies reach the clinic, the field has never been closer to developing a strategy to regenerate skeletal muscle after severe trauma.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
The authors acknowledge support toward the development of biomaterials for tissue engineering applications from the National Institutes of Health (P41 EB023833). The authors also acknowledge support from a National Science Foundation Graduate Research Fellowship and a Ford Foundation Pre-Doctoral Research Fellowship.
References
- 1.Mann C.J., Perdiguero E., Kharraz Y., Aguilar S., Pessina P., Serrano A.L., Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahdy M.A.A. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375:575–588. doi: 10.1007/s00441-018-2955-2. [DOI] [PubMed] [Google Scholar]
- 3.Grasman J.M., Zayas M.J., Page R.L., Pins G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015;25:2–15. doi: 10.1016/j.actbio.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurtgen B.J., Ward C.L., Leopold Wager C.M., Garg K., Goldman S.M., Henderson B.E.P., McKinley T.O., Greising S.M., Wenke J.C., Corona B.T. Autologous minced muscle grafts improve endogenous fracture healing and muscle strength after musculoskeletal trauma. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M.T.A., Willett N.J., Uhrig B.A., Guldberg R.E., Warren G.L. Functional analysis of limb recovery following autograft treatment of volumetric muscle loss in the quadriceps femoris. J. Biomech. 2014;47:2013–2021. doi: 10.1016/j.jbiomech.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarovici P., Marcinkiewicz C., Lelkes P.I. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr. Pharmaceut. Des. 2006;12:2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- 7.Kikuno N., Kawamoto K., Hirata H., Vejdani K., Kawakami K., Fandel T., Nunes L., Urakami S., Shiina H., Igawa M., Tanagho E., Dahiya R. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2009;103:1424–1428. doi: 10.1111/j.1464-410X.2008.08129.x. [DOI] [PubMed] [Google Scholar]
- 8.Nico B., Mangieri D., Benagiano V., Crivellato E., Ribatti D. vol. 75. 2008. pp. 135–141. (Nerve Growth Factor as an Angiogenic Factor). [DOI] [PubMed] [Google Scholar]
- 9.OpenStax . 2013. 10.2 Skeletal Muscle. [Google Scholar]
- 10.Damjanov I. Pathology Secrets. Elsevier Inc.; 2009. Skeletal muscles; pp. 434–447. [DOI] [Google Scholar]
- 11.Radák Z. The Physiology of Physical Training. Elsevier; 2018. Skeletal muscle, function, and muscle fiber types; pp. 15–31. [DOI] [Google Scholar]
- 12.Bianco P., Robey P.G. vol. 2. Elsevier Inc.; 2004. Skeletal stem cells; pp. 415–424. (Handbook of Stem Cells). [Google Scholar]
- 13.Qazi T.H., Mooney D.J., Pumberger M., Geißler S., Duda G.N. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- 14.Yang W., Hu P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018;13:25–32. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan J.E., Partridge T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 16.Fishman J.M., Tyraskis A., Maghsoudlou P., Urbani L., Totonelli G., Birchall M.A., De Coppi P. Skeletal muscle tissue engineering: which cell to use? Tissue Eng. B Rev. 2013;19:503–515. doi: 10.1089/ten.TEB.2013.0120. [DOI] [PubMed] [Google Scholar]
- 17.Laumonier T., Menetrey J. 2016. Muscle Injuries and Strategies for Improving Their Repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogarth M.W., Defour A., Lazarski C., Gallardo E., Manera J.D., Partridge T.A., Nagaraju K., Jaiswal J.K. Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biferali B., Proietti D., Mozzetta C., Madaro L. Fibro–adipogenic progenitors cross-talk in skeletal muscle: the social network. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C., Kruger M.J., Smith R.M., Myburgh K.H. The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med. 2008;38:947–969. doi: 10.2165/00007256-200838110-00005. [DOI] [PubMed] [Google Scholar]
- 21.Tidball J.G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 22.Philippou A., Maridaki M., Theos A., Koutsilieris M. vol. 58. Academic Press Inc.; 2012. Cytokines in muscle damage; pp. 49–87. (Advances in Clinical Chemistry). [DOI] [PubMed] [Google Scholar]
- 23.Warren G.L., Hulderman T., Jensen N., McKinstry M., Mishra M., Luster M.I., Simeonova P.P. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002;16:1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- 24.Chen S.E., Gerken E., Zhang Y., Zhan M., Mohan R.K., Li A.S., Reid M.B., Li Y.P. Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. Cell Physiol. 2005;289 doi: 10.1152/ajpcell.00062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabe Y., Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Zhao W., Ransohoff R.M., Zhou L. Infiltrating macrophages are broadly activated at the early stage to support acute skeletal muscle injury repair. J. Neuroimmunol. 2018;317:55–66. doi: 10.1016/j.jneuroim.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak M.L., Weinheimer-Haus E.M., Koh T.J. Macrophage activation and skeletal muscle healing following traumatic injury. J. Pathol. 2014;232:344–355. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigamonti E., Zordan P., Sciorati C., Rovere-Querini P., Brunelli S. 2014. Macrophage Plasticity in Skeletal Muscle Repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey S.P., Jansen H., Raschke M.J., Meffert R.H. 2012. BASIC RESEARCH VEGF Improves Skeletal Muscle Regeneration After Acute Trauma and Reconstruction of the Limb in a Rabbit Model; pp. 3607–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levenberg S., Rouwkema J., Macdonald M., Garfein E.S., Kohane D.S., Darland D.C., Marini R., Van Blitterswijk C.A., Mulligan R.C., D'Amore P.A., Langer R. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 32.Loss M., Dziki J.L., Sicari B.M., Wolf M.T., Cramer M.C., Badylak S.F. Immunomodulation and mobilization of progenitor cells by extracellular matrix bioscaffolds for volumetric muscle loss treatment. Tissue Eng. A. 2016;22:1129–1139. doi: 10.1089/ten.TEA.2016.0340. [DOI] [PubMed] [Google Scholar]
- 33.Dziki J., Badylak S., Yabroudi M., Sicari B., Ambrosio F., Stearns K., Turner N., Wyse A., Boninger M.L., Brown E.H.P., Rubin J.P. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen. Med. 2016;1:16008. doi: 10.1038/npjregenmed.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziki J.L., Giglio R.M., Sicari B.M., Wang D.S., Gandhi R.M., Londono R., Dearth C.L., Badylak S.F. The effect of mechanical loading upon extracellular matrix bioscaffold-mediated skeletal muscle remodeling. Tissue Eng. A. 2018;24:34–46. doi: 10.1089/ten.tea.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darby I.A., Zakuan N., Billet F., Desmoulière A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell. Mol. Life Sci. 2016;73:1145–1157. doi: 10.1007/s00018-015-2110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Järvinen T.A.H., Järvinen M., Kalimo H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2013;3:337–345. [PMC free article] [PubMed] [Google Scholar]
- 37.Loh Q.L., Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prieto E.M., Guelcher S.A. Biomedical Foams for Tissue Engineering Applications. Elsevier Ltd.; 2014. Tailoring properties of polymeric biomedical foams; pp. 129–162. [DOI] [Google Scholar]
- 39.Murphy W.L., Dennis R.G., Kileny J.L., Mooney D.J. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8 doi: 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 40.Smith B.T., Lu A., Watson E., Santoro M., Melchiorri A.J., Grosfeld E.C., van den Beucken J.J.J.P., Jansen J.A., Scott D.W., Fisher J.P., Mikos A.G. Incorporation of fast dissolving glucose porogens and poly(lactic-co-glycolic acid) microparticles within calcium phosphate cements for bone tissue regeneration. Acta Biomater. 2018;78:341–350. doi: 10.1016/j.actbio.2018.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodoso-Torrecilla I., Grosfeld E., Marra A., Smith B.T., Mikos A.G., Ulrich D.J., Jansen J.A., van den Beucken J.J. Multimodal porogen platforms for calcium phosphate cement degradation. J. Biomed. Mater. Res. A. 2019;107 doi: 10.1002/jbm.a.36686. jbm.a.36686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moglia R.S., Holm J.L., Sears N.A., Wilson C.J., Harrison D.M., Cosgriff-Hernandez E. Injectable PolyHIPEs as high-porosity bone grafts. Biomacromolecules. 2011;12:3621–3628. doi: 10.1021/bm2008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C., Garber L., Smoak M., Fargason C., Scherr T., Blackburn C., Bacchus S., Lopez M.J., Pojman J.A., Piero F.D., Hayes D.J. In vitro and in vivo characterization of pentaerythritol triacrylate-co-trimethylolpropane nanocomposite scaffolds as potential bone augments and grafts. Tissue Eng. A. 2015;21:320–331. doi: 10.1089/ten.tea.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroehne V., Heschel I., Schügner F., Lasrich D., Bartsch J.W., Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J. Cell Mol. Med. 2008;12:1640–1648. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwee B.J., Mooney D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017;47:16–22. doi: 10.1016/j.copbio.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayanan N., Jiang C., Uzunalli G., Thankappan S.K., Laurencin C.T., Deng M., Eng R. Polymeric electrospinning for musculoskeletal regenerative engineering. Regen. Eng. Transl. Med. 2016;2:69–84. [Google Scholar]
- 47.Jammalamadaka U., Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018;9 doi: 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt C., Tahtinen M., Zhao F. Tissue Engineering and Nanotheranostics. World Scientific Publishing Co. Pte Ltd; 2017. Engineering approaches for creating skeletal muscle; pp. 1–27. [DOI] [Google Scholar]
- 49.Ryan A.J., Kearney C.J., Shen N., Khan U., Kelly A.G., Probst C., Brauchle E., Biccai S., Garciarena C.D., Vega-Mayoral V., Loskill P., Kerrigan S.W., Kelly D.J., Schenke-Layland K., Coleman J.N., O'Brien F.J. Electroconductive biohybrid collagen/pristine graphene composite biomaterials with enhanced biological activity. Adv. Mater. 2018;30:1706442. doi: 10.1002/adma.201706442. [DOI] [PubMed] [Google Scholar]
- 50.Chen S., Nakamoto T., Kawazoe N., Chen G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials. 2015;73:23–31. doi: 10.1016/j.biomaterials.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Choi J.S., Lee S.J., Christ G.J., Atala A., Yoo J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 52.Guyot C., Lerouge S. Can we achieve the perfect injectable scaffold for cell therapy? Future Sci. OA. 2018;4 doi: 10.4155/fsoa-2017-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeQuach J.A., Lin J.E., Cam C., Hu D., Salvatore M.A., Sheikh F., Christman K.L. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur. Cell. Mater. 2012;23:400–412. doi: 10.22203/ecm.v023a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinnaird T., Stabile E., Burnett M.S., Shou M., Lee C.W., Barr S., Fuchs S., Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 55.Qazi T.H., Duda G.N., Ort M.J., Perka C., Geissler S., Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle. 2019;10:501–516. doi: 10.1002/jcsm.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacHingal M.A., Corona B.T., Walters T.J., Kesireddy V., Koval C.N., Dannahower A., Zhao W., Yoo J.J., Christ G.J. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng. A. 2011;17:2291–2303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao L., Qian Y., Khodabukus A., Ribar T., Bursac N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018;9 doi: 10.1038/s41467-017-02636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandusen K.W., Syverud B.C., Williams M.L., Lee J.D., Larkin L.M. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng. A. 2014;20:2920–2930. doi: 10.1089/ten.tea.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J.H., Kim I., Seol Y.J., Ko I.K., Yoo J.J., Atala A., Lee S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-14930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cornelison D.D.W. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J. Cell. Biochem. 2008;105:663–669. doi: 10.1002/jcb.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passipieri J.A., Baker H.B., Siriwardane M., Ellenburg M.D., Vadhavkar M., Saul J.M., Tomblyn S., Burnett L., Christ G.J. Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng. A. 2017;23:556–571. doi: 10.1089/ten.tea.2016.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C., Vitiello L., Elvassore N., De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25:2296–2304. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert-Honick J., Iyer S.R., Somers S.M., Lovering R.M., Wagner K., Mao H.Q., Grayson W.L. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials. 2018;164:70–79. doi: 10.1016/j.biomaterials.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Ge J., Liu K., Niu W., Chen M., Wang M., Xue Y., Gao C., Ma P.X., Lei B. Gold and gold-silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through p38 MAPK signaling pathway and promote in vivo skeletal muscle regeneration. Biomaterials. 2018;175:19–29. doi: 10.1016/j.biomaterials.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 65.Dziki J., Badylak S., Yabroudi M., Sicari B., Ambrosio F., Stearns K., Turner N., Wyse A., Boninger M.L., Brown E.H.P., Rubin J.P. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen. Med. 2016;1:1–12. doi: 10.1038/npjregenmed.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mertens J.P., Sugg K.B., Lee J.D., Larkin L.M. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen. Med. 2014;9:89–100. doi: 10.2217/rme.13.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Saul D., Böker K.O., Ernst J., Lehman W., Schilling A.F. 2018. Review Article Current Methods for Skeletal Muscle Tissue Repair and Regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narayanan N., Jiang C., Wang C., Uzunalli G., Whittern N., Chen D., Jones O.G., Kuang S., Deng M. Harnessing fiber diameter-dependent effects of myoblasts toward biomimetic scaffold-based skeletal muscle regeneration. Front. Bioeng. Biotechnol. 2020;8:203. doi: 10.3389/fbioe.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badami A.S., Kreke M.R., Thompson M.S., Riffle J.S., Goldstein A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 70.Bloise N., Berardi E., Gualandi C., Zaghi E., Gigli M., Duelen R., Ceccarelli G., Cortesi E., Costamagna D., Bruni G., Lotti N., Focarete M., Visai L., Sampaolesi M. Ether-oxygen containing electrospun microfibrous and sub-microfibrous scaffolds based on poly(butylene 1,4-cyclohexanedicarboxylate) for skeletal muscle tissue engineering. Int. J. Mol. Sci. 2018;19:3212. doi: 10.3390/ijms19103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khorshidi S., Solouk A., Mirzadeh H., Mazinani S., Lagaron J.M., Sharifi S., Ramakrishna S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016;10:715–738. doi: 10.1002/term.1978. [DOI] [PubMed] [Google Scholar]
- 72.Manchineella S., Thrivikraman G., Khanum K.K., Ramamurthy P.C., Basu B., Govindaraju T. Pigmented silk nanofibrous composite for skeletal muscle tissue engineering. Adv. Healthc. Mater. 2016;5:1222–1232. doi: 10.1002/adhm.201501066. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda Y., Satoh A., Horinouchi Y., Hamano H., Watanabe H., Imao M., Imanishi M., Zamami Y., Takechi K., Izawa-Ishizawa Y., Miyamoto L., Hirayama T., Nagasawa H., Ishizawa K., Aihara K., Tsuchiya K., Tamaki T. Iron accumulation causes impaired myogenesis correlated with MAPK signaling pathway inhibition by oxidative stress. FASEB J. 2019;33:9551–9564. doi: 10.1096/fj.201802724RR. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Zhou G., Liu Z., Guo M., Jiang X., Taskin M.B., Zhang Z., Liu J., Tang J., Bai R., Besenbacher F., Chen M., Chen C. Mussel inspired polynorepinephrine functionalized electrospun polycaprolactone microfibers for muscle regeneration. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-08572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeo M., Kim G.H. Anisotropically aligned cell-laden nanofibrous bundle fabricated via cell electrospinning to regenerate skeletal muscle tissue. Small. 2018;14:1803491. doi: 10.1002/smll.201803491. [DOI] [PubMed] [Google Scholar]
- 76.Guo Y., Gilbert-Honick J., Somers S.M., Mao H.Q., Grayson W.L. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem. Biophys. Res. Commun. 2019;516:558–564. doi: 10.1016/j.bbrc.2019.06.082. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen M.M., Gianneschi N.C., Christman K.L. Developing injectable nanomaterials to repair the heart. Curr. Opin. Biotechnol. 2015;34:225–231. doi: 10.1016/j.copbio.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hernandez M.J., Christman K.L. Designing acellular injectable biomaterial therapeutics for treating myocardial infarction and peripheral artery disease. JACC (J. Am. Coll. Cardiol.): Basic Transl. Sci. 2017;2:212–226. doi: 10.1016/j.jacbts.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaetani R., Ungerleider J., Christman K.L. Stem Cell and Gene Therapy for Cardiovascular Disease. Elsevier Science Ltd.; 2015. Acellular injectable biomaterials for treating cardiovascular disease; pp. 309–325. [DOI] [Google Scholar]
- 80.Guo B., Qu J., Zhao X., Zhang M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019;84:180–193. doi: 10.1016/j.actbio.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 81.El-Sherbiny I.M., Yacoub M.H. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob. Cardiol. Sci. Pract. 2013;2013:38. doi: 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah R., Knowles J.C., Hunt N.P., Lewis M.P. Development of a novel smart scaffold for human skeletal muscle regeneration. J. Tissue Eng. Regen. Med. 2016;10:162–171. doi: 10.1002/term.1780. [DOI] [PubMed] [Google Scholar]
- 83.Wang L., Wu Y., Guo B., Ma P.X. Nanofiber yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano. 2015;9:9167–9179. doi: 10.1021/acsnano.5b03644. [DOI] [PubMed] [Google Scholar]
- 84.Mozetic P., Giannitelli S.M., Gori M., Trombetta M., Rainer A. Engineering muscle cell alignment through 3D bioprinting. J. Biomed. Mater. Res. A. 2017;105:2582–2588. doi: 10.1002/jbm.a.36117. [DOI] [PubMed] [Google Scholar]
- 85.Kim J.H., Seol Y.J., Ko I.K., Kang H.W., Lee Y.K., Yoo J.J., Atala A., Lee S.J. 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-29968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russell C.S., Mostafavi A., Quint J.P., Panayi A.C., Baldino K., Williams T.J., Daubendiek J.G., Hugo Sánchez V., Bonick Z., Trujillo-Miranda M., Shin S.R., Pourquie O., Salehi S., Sinha I., Tamayol A. In situ printing of adhesive hydrogel scaffolds for the treatment of skeletal muscle injuries. ACS Appl. Bio Mater. 2020 doi: 10.1021/acsabm.9b01176. [DOI] [PubMed] [Google Scholar]
- 87.Arab W., Rauf S., Al-Harbi O., Hauser C. Novel ultrashort self-assembling peptide bioinks for 3D culture of muscle myoblast cells. Int. J. Bioprinting. 2018;4 doi: 10.18063/IJB.v4i2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yeo M., Lee H., Kim G.H. Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration. Biofabrication. 2016;8:35021. doi: 10.1088/1758-5090/8/3/035021. [DOI] [PubMed] [Google Scholar]
- 89.Kim W.J., Kim G.H. A functional bioink and its application in myoblast alignment and differentiation. Chem. Eng. J. 2019;366:150–162. [Google Scholar]
- 90.Corona B.T., Greising S.M. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials. 2016;104:238–246. doi: 10.1016/j.biomaterials.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Patel K.H., Talovic M., Dunn A.J., Patel A., Vendrell S., Schwartz M., Garg K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res. B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34584. jbm.b.34584. [DOI] [PubMed] [Google Scholar]
- 92.Smoak M.M., Han A., Watson E., Kishan A., Grande-Allen K.J., Cosgriff-Hernandez E., Mikos A.G. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng. C Methods. 2019;25:276–287. doi: 10.1089/ten.tec.2018.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ungerleider J.L., Johnson T.D., Rao N., Christman K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods. 2015;84:53–59. doi: 10.1016/j.ymeth.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rao N., Agmon G., Tierney M.T., Ungerleider J.L., Braden R.L., Sacco A., Christman K.L. Engineering an injectable muscle-specific microenvironment for improved cell delivery using a nanofibrous extracellular matrix hydrogel. ACS Nano. 2017;11:3851–3859. doi: 10.1021/acsnano.7b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ungerleider J.L., Johnson T.D., Hernandez M.J., Elhag D.I., Braden R.L., Dzieciatkowska M., Osborn K.G., Hansen K.C., Mahmud E., Christman K.L. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. JACC (J. Am. Coll. Cardiol.): Basic Transl. Sci. 2016;1:32–44. doi: 10.1016/j.jacbts.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y., Fan X., Tian C., Luo J., Zhang Y., Deng L., Qin T., Lv Q. Decellularization of porcine skeletal muscle extracellular matrix for the formulation of a matrix hydrogel: a preliminary study. J. Cell Mol. Med. 2016;20:740–749. doi: 10.1111/jcmm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi Y.-J., Kim T.G., Jeong J., Yi H.-G., Park J.W., Hwang W., Cho D.-W. 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv. Healthc. Mater. 2016;5:2636–2645. doi: 10.1002/adhm.201600483. [DOI] [PubMed] [Google Scholar]
- 98.Choi Y.J., Jun Y.J., Kim D.Y., Yi H.G., Chae S.H., Kang J., Lee J., Gao G., Kong J.S., Jang J., Chung W.K., Rhie J.W., Cho D.W. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 2019;206:160–169. doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 99.Hinton T.J., Jallerat Q., Palchesko R.N., Park J.H., Grodzicki M.S., Shue H.J., Ramadan M.H., Hudson A.R., Feinberg A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyake T., McDermott J.C., Gramolini A.O. A method for the direct identification of differentiating muscle cells by a fluorescent mitochondrial dye. PloS One. 2011;6 doi: 10.1371/journal.pone.0028628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim W., Kim M., Kim G.H. 3D-Printed biomimetic scaffold simulating microfibril muscle structure. Adv. Funct. Mater. 2018;28:1800405. [Google Scholar]
- 102.Murphy D.P., Nicholson T., Jones S.W., O'Leary M.F. MyoCount: a software tool for the automated quantification of myotube surface area and nuclear fusion index [version 1; referees: 2 approved] Wellcome Open Res. 2019;4 doi: 10.12688/wellcomeopenres.15055.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seyedmahmoud R., Çelebi-Saltik B., Barros N., Nasiri R., Banton E., Shamloo A., Ashammakhi N., Dokmeci M.R., Ahadian S. Three-Dimensional bioprinting of functional skeletal muscle tissue using GelatinMethacryloyl-alginate bioinks. Micromachines. 2019;10:679. doi: 10.3390/mi10100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quarta M., Cromie M., Chacon R., Blonigan J., Garcia V., Akimenko I., Hamer M., Paine P., Stok M., Shrager J.B., Rando T.A. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017;8:1–17. doi: 10.1038/ncomms15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee H., Ju Y.M., Kim I., Elsangeedy E., Lee J.H., Yoo J.J., Atala A., Lee S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods. 2020;171:77–85. doi: 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 106.Wu X., Corona B.T., Chen X., Walters T.J. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. BioRes. Open Access. 2012;1:280–290. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pollot B.E., Corona B.T. Volumetric muscle loss. Methods Mol. Biol. 2016;1460:19–31. doi: 10.1007/978-1-4939-3810-0_2. [DOI] [PubMed] [Google Scholar]
- 108.Greising S.M., Rivera J.C., Goldman S.M., Watts A., Aguilar C.A., Corona B.T. Unwavering pathobiology of volumetric muscle loss injury. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chao T., Burmeister D.M., Corona B.T., Greising S.M. Oxidative pathophysiology following volumetric muscle loss injury in a porcine model. J. Appl. Physiol. 2019;126:1541–1549. doi: 10.1152/japplphysiol.00026.2019. [DOI] [PubMed] [Google Scholar]
- 110.Matthias N., Hunt S.D., Wu J., Lo J., Smith Callahan L.A., Li Y., Huard J., Darabi R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs) Stem Cell Res. 2018;27:65–73. doi: 10.1016/j.scr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuoco C., Rizzi R., Biondo A., Longa E., Mascaro A., Shapira-Schweitzer K., Kossovar O., Benedetti S., Salvatori M.L., Santoleri S., Testa S., Bernardini S., Bottinelli R., Bearzi C., Cannata S.M., Seliktar D., Cossu G., Gargioli C. In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol. Med. 2015;7:411–422. doi: 10.15252/emmm.201404062. [DOI] [PMC free article] [PubMed] [Google Scholar]