Summary
Human pluripotent stem cells (PSCs) through somatic cell nuclear transfer (SCNT) may be an important source for regenerative medicine. The low derivation efficiency of stem cells and the accessibility of human oocytes are the main obstacles to their application. We previously reported that the efficiency of SCNT was increased by overexpression of H3K9me3 demethylase. Here, we applied a modified derivation method to the PSC line and first obtained human SCNT-PSC lines derived from both donated cryopreserved oocytes and cord blood cells with a homozygous human leukocyte antigen (HLA) type. The SCNT-PSCs have very similar characteristics with embryonic stem cells (ESCs) and additionally have shown immunocompatibility in an in vitro and in vivo humanized mouse with a matching HLA type. Our study demonstrates that SCNT technology using donated cryopreserved oocytes and cord blood cells with a known HLA type provides a promising method for establishing a human HLA-matched SCNT-PSC bank for regenerative medicine.
Keywords: somatic cell nuclear transfer, frozen oocytes, frozen cord blood cells, homozygous HLA, pluripotent stem cells
Highlights
-
•
Human normal SCNT-PSC line with homozygous HLA type is derived from both donated cryopreserved oocytes and cord blood cells
-
•
SCNT-PSC derivation is improved by modified method using ESC-conditioned medium
-
•
Differentiated functional cells from SCNT-PSC with homozygous HLA type have shown immunocompatibility in humanized mouse with a matching HLA type
-
•
This SCNT technology facilitates the establishment of the human HLA-matched SCNT-PSC bank
In this article, Lee and his colleagues reported the successful production of HLA-homozygous SCNT-PSCs using MNCs from frozen/thawed donated cord blood in a public bank and frozen (vitrified) human oocytes donated after storage for 5 years. Homozygous SCNT-PSC-derived cells are highly immunocompatible with allogeneic HLA-matched immune cells. Therefore, we may suggest that this SCNT technology facilitates the establishment of the human HLA-matched SCNT-PSC bank.
Introduction
Somatic cell nuclear transfer (SCNT) mediated by oocytes can produce cloned embryos in more than 20 species (Gurdon, 1962, Kato et al., 1998, Rodriguez-Osorio et al., 2012, Wakayama et al., 1998, Wilmut et al., 1997), and it has contributed to the establishment of pluripotent stem cells (PSCs) in various mammals, including humans (Chung et al., 2014, Tachibana et al., 2013, Yamada et al., 2014). In particular, PSCs may be an extremely important source for immunocompatible cell therapy and may enable a novel approach for regenerative medicine (Lanza et al., 1999). However, despite the value of SCNT-PSCs, several limitations, such as the low derivation efficiency of these stem cells due to poor development of cloned human eggs and the complexity of providing steady sources of human oocytes, make it difficult to apply these cells to cell therapy. Recently, our group evaluated a reprogramming barrier that is mediated by abnormally high activity of histone methylation (H3K9me3) during SCNT and partially overcome by mRNA injection of KDM4A (an H3K9me3 demethylase). In fact, the number of cloned eggs arrested at the eight-cell stage was greatly reduced, and the SCNT-PSC derivation rate also increased to 7.1% per donated mature oocyte (Chung et al., 2015). This technology has recently been applied to non-human primate cloning systems and successfully produced cloned macaque monkeys (Liu et al., 2018). Therefore, it appears that a major hurdle for the application of human SCNT-PSCs as sources for cell therapy has been partially overcome, although further research is still needed for better embryonic development.
The other major issue for the successful application of SCNT is securing ethical and steady sources of human oocytes. Recently, cryopreservation of human oocytes has become an integral part of the human assisted reproductive technology program since in vitro fertilization-embryo transfer (IVF-ET) using frozen/thawed oocytes has shown embryonic development and pregnancy rates similar to those achieved using fresh oocytes (Practice Committees of American Society for Reproductive Medicine and Society for Assisted Reproductive Techonology, 2013). Although variations in clinical outcome caused by the quality of frozen/thawed oocytes are still controversial, oocyte cryopreservation has been widely applied for fertility preservation in unmarried and married women. For this reason, cryopreserved human oocytes after the storage period may be a steady source of donor oocytes for SCNT-PSCs, and this approach could reduce the ethical dilemma caused by unnecessary ovarian hyperstimulation of women for research purposes. However, successful production of cloned embryos using cryopreserved human oocytes and the derivation of SCNT-PSC lines has still not been achieved until now. In a recent animal study, we found that cryopreserved mouse oocyte cytoplasm has a lower potential for SCNT-mediated reprogramming than fresh oocytes, possibly due to increased apoptosis and altered gene expression resulting from cryoinjury (Lee et al., 2019).
It is well known that immune rejection of transplanted cells from recipient targets should be overcome for the clinical application of PSCs in stem cell therapy. Although, autologous PSCs obtained from SCNT or induced PSC (iPSC) technologies can avoid immune rejection by the patient's immune system (Lanza et al., 1999, Mandai et al., 2017), it has been suggested that the use of autologous PSCs is not a good option for patients because it is a less economical and more time-consuming procedure. To overcome these obstacles, research groups have recently suggested another strategy using a homozygous HLA genotype-matched PSC bank that provides stem cells useful to allogeneic users (Lee et al., 2016, Turner et al., 2013). In fact, several reports from the UK and Japan have postulated that 150 and 140 HLA-homozygous iPSCs could match more than 90% of their populations (Okita et al., 2011, Taylor et al., 2012) and a modeling study also suggested that the construction of cell banks of top-ranked haplolines could match a majority of individuals in a multiethnic and admixed population, such as California (Pappas et al., 2015). In addition, the clinical significance of the HLA-homozygous iPSC bank is supported by recent reports showing a lack of T cell response to human iPSC-derived retinal pigment epithelial cells from HLA-homozygous donors and successful transplantation in the major histocompatibility complex (MHC)-matched monkey model (Sugita et al., 2016a, Sugita et al., 2016b). Based on these reports, several researchers have started establishing homozygous iPSC lines using fresh blood cells (Rim et al., 2018, Sugita et al., 2016b). Nucleated cells in fresh peripheral and cord blood would be suggested as a noninvasive cell source for the production of iPSCs, but this approach has shown low reprogramming efficiency compared with fibroblasts (Loh et al., 2009). However, despite several successful applications of nuclear donor cells from fresh blood for the production of HLA-homozygous PSCs, a highly labor-intensive process may be required to obtain proper blood cells from blood donors who do not know their HLA information. It was also suggested that frozen cord blood cells stored in a public cell bank could be a useful source to obtain nuclear donor cells with a known HLA type for SCNT, which requires a small number of mononucleated cells (MNCs) for reprogramming.
Results
Derivation of Human SCNT-PSCs Using Cryopreserved Human Oocytes and Its Characterization
To analyze the potential of cryopreserved human oocyte cytoplasm for SCNT-reprogramming, two types of nuclear donor cells were prepared for SCNT. One type was human dermal fibroblast (hDF) cells donated from a 42-year-old female patient with central areolar choroidal dystrophy (center spared, one of eye disease). The other type was MNCs from donated/cryopreserved cord blood with a homozygous human leukocyte antigen (HLA). First, to examine the efficiency of SCNT-mediated reprogramming in frozen/thawed oocytes, we used our recent SCNT protocol using histone demethylase after reconstruction of enucleated oocytes and nuclear donor cells (Chung et al., 2015). A total of 11 frozen/thawed oocytes were enucleated and reconstructed with hDF cells (Figures 1A and 1B). All reconstructed embryos were cleaved, but only three reconstructed embryos (27.3%) developed to the eight-cell stage. One of them was developed further to an expanded blastocyst at 6 days (Figure 1B). This expanded blastocyst was plated onto irradiated mouse embryonic fibroblast (MEF) cells and eventually resulted in a stable human SCNT-PSC line (CHA-FT-NT17, Figure 1B). In the next experiment, a total of 24 frozen/thawed oocytes were also enucleated and then reconstructed by piezo injection of MNCs obtained from frozen/thawed cord blood. Similarly, all the embryos were cleaved, but seven embryos (29.2%) developed to the eight-cell stage. Two of them (8.6%) became expanded blastocysts and then formed two stable human SCNT-PSC lines (CHA-FT-NT18 and CHA-FT-NT19, Figure 1B). When compared with our fresh SCNT, embryonic development from frozen SCNT was poor, but PSC derivation rate was similar to those using fresh oocytes (Figure S1).
Figure 1.
Human SCNT
(A) Photographs of the SCNT procedure using cryopreserved oocytes and MNCs from fetal cord blood cells (FCBCs), and embryonic results from SCNT experiments. Scale bars, 100 μm.
(B) Embryonic results from human SCNT experiment.
See also Figure S1.
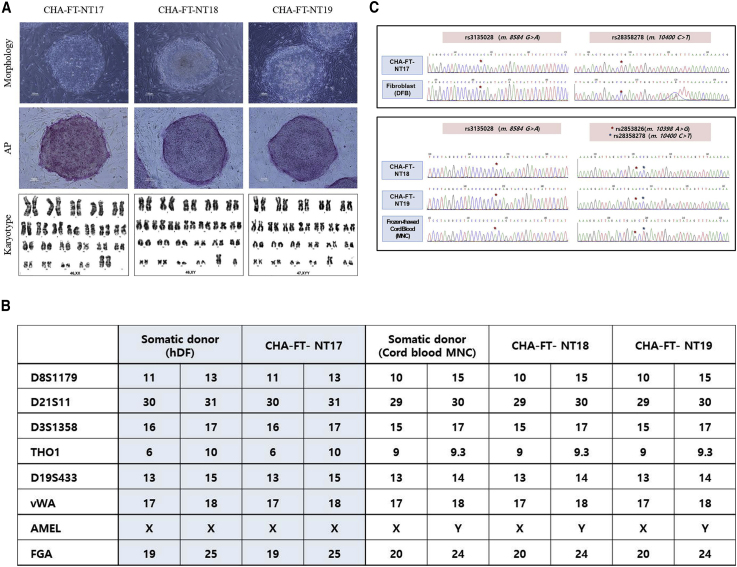
As shown in Figures 2 and S2, the morphology of the three SCNT-PSCs was very similar to that of conventional ESCs, and the cells had high alkaline phosphatase activity and high expression of pluripotency marker genes (POU5F1(OCT4), SOX2, and NANOG). In addition, immunostaining revealed that OCT4, SSEA4, TRA-1-60, and TRA-1-81 were expressed in all colonies of the three cell lines (Figure S2). In cytogenetic analysis, the karyotypes of CHA-FT-NT17 derived from hDFs and CHA-FT-NT18 derived from cord blood MNCs were normal (46, XX and 46, XY), while that of CHA-FT-NT19 from the same MNCs was abnormal (47, XYY) (Figure 2A). In addition, molecular genotyping showed that short tandem repeat markers of all SCNT-PSC lines perfectly matched those of their somatic (nuclear) donors (Figure 2B). The mitochondrial genomes of all SCNT-PSC lines were mismatched with those of their somatic (nuclear) donors (Figure 2C), which may suggest that their cytoplasm originated from donated cryopreserved oocytes. However, we did not confirm matching to the mitochondrial genotype of oocyte donors because we cannot secure cumulus cells for mitochondrial DNA genotyping from cryopreserved oocytes, which had been almost denuded before vitrification. Other characteristics of pluripotency, such as formation of three-germ layers in vitro and in vivo, are shown in Figure S2. These results clearly indicate that newly formed CHA-FT-NT17, CHA-FT-NT18, and CHA-FT-NT19 are reprogrammed PSC lines.
Figure 2.
Characterization of Human SCNT-PSC Lines
(A) Morphology, alkaline phosphatase (AP) staining, and karyotypes of SCNT-PSC lines from cryopreserved oocytes (CHA-FT-NT17, CHA-FT-NT18, and CHA-FT-NT19). Three PSC lines showed embryonic stem cell-like morphology and positive signals for AP. Cytogenetic G-banding analysis showed that CHA-FT-NT17 and CHA-FT-NT18 had normal karyotypes (46, XX and 46, XY, respectively), but CHA-FT-NT19 had an abnormal karyotype (47, XYY). Scale bars, 100 μm.
(B) Mitochondrial (mt) DNA genotyping of three single nucleotide polymorphic sites. m.8584 G > A (rs3135028), m.10400C > T (rs28358278), m.10398A > G (rs2853826), and m.10400C > T (rs28358278) demonstrate that the mtDNAs in the three SCNT-PSC lines were derived from the oocytes, not hDFs or cord blood MNCs.
(C) Nuclear DNA genotyping using eight short tandem repeat markers (seven at autosomal loci and one at the X/Y locus) showed that the nuclear DNAs in three SCNT-PSC lines were exclusively derived from somatic donor cells (hDFs or cord blood MNCs).
See also Figure S2.
Differentiation into Various Lineages Cells
To analyze the possibility of using the newly established SCNT-PSCs as sources for cell therapy, two normal SCNT-PSC lines from cryopreserved oocytes (CHA-FT-NT17 and CHA-FT-NT18) were differentiated into ectoderm lineage cells, retinal pigmented epithelial cells (RPEs), and neuronal progenitor cells (NPCs), using well-established protocols (Cho et al., 2008, Schwartz et al., 2012, Song et al., 2015, Zhang et al., 2001), and then compared with well-characterized ESC lines (MA09 and CHA-hES-15, respectively) (Figures S3 and S4). In the characterization of PSC-derived RPEs and NPCs, we did not find any difference in the differentiation capability between SCNT-PSCs derived from cryopreserved oocytes as a cytoplasm source and cryopreserved cord blood cells as nuclear donors for the reprogramming procedure. Generated NPCs successfully expressed MHC class I and II antigens, implying maturation (Figure S5).
Next, we explored whether CHA-FT-NT17 and CHA-FT-NT18 cells were also differentiated into mesoderm lineage cells, such as mesenchymal progenitor cells (MPCs) and osteoblasts, by using a previously established protocol (Jun et al., 2019). Clonally expanded MPCs displayed traditional markers for MPCs, such as the CD29+, CD44+, CD90+, CD105+, CD45−, and CD34− phenotypes and potential capacity for differentiation into adipocytes, osteocytes, and chondrocytes under differentiation conditions. In addition, the precursor genes for multilineage differentiated cells, CEBPA (C/EBPα), RUNX2, and SOX9, were clearly expressed in both CHA-FT-NT17- and CHA-FT-NT18-derived cells. The maturation-related genes PPARG (PPARγ, adipocytes), COL1A1 (COL1, osteocytes), and COMP (chondrocytes) were detected after differentiation. In addition, we measured the cumulative cell number of both SCNT-PSC-MPCs with different sources of donor cells and found that both cells were highly increased until 18 passages (Figure S6). These results may show that homozygous SCNT-PSCs derived using cryopreserved oocytes (CHA-FT-NT18) can be successfully differentiated into mesodermal and ectodermal lineage cells.
Immunocompatibility of Homozygous SCNT-PSC-Derived Somatic Cells In Vitro
To investigate the immune rejection response of SCNT-PSC-derived differentiated cells, allogeneic cells with different levels of MHC expression are needed. Osteoblasts from both SCNT-PSC-MPCs presented positive staining for alizarin red, osteocalcin, and alkaline phosphatase, suggesting differentiation into mature osteoblasts (Figures 3A–3D). In fact, SCNT-PSC-MPCs showed low expression of MHC class II, but further differentiated SCNT-PSC-osteoblasts expressed high levels of MHC class II molecules (Figure 4A). To analyze the immunocompetence of those cells, T cell activation against CHA-FT-NT18 was monitored after coculture with HLA-matched or HLA-mismatched CD4+ and CD8+ T cells. CHA-FT-NT18-derived somatic cells (MPCs and osteoblasts) can induce the proliferation of T cells according to the HLA allele. In this study, regardless of lineage cell type, the proliferation of HLA-matched T cells against homozygous CHA-FT-NT18 type (HLA-A:33:03,33:03; HLA-B:44:03,44:03; HLA-DRB1:13:02,13:02)-derived somatic cells was significantly lower than that of HLA-mismatched T cells against a heterozygous CHA-FT-NT17 type (HLA-A:01:01,02:01; HLA-B:35:01,37:01; HLA-DRB1:10:01,14:54)-derived somatic cells (HLA mismatched versus HLA matched; in MPCs, CD4, 1.5- ± 0.4-fold, CD8, 2.5- ± 0.3-fold; in osteoblasts, CD4, 1.8- ± 0.7-fold, CD8, 8.3- ± 1.3-fold) (Figure 4B). To further examine the immunogenicity by minor HLA-II, CD4 T cell proliferation was performed using mismatched group for HLA-II with HLA-I matching against SCNT-osteoblasts. First, we selected peripheral blood monocytes (PBMCs) having matched HLA-I (A, B, and C) and HLA-II (DR, DQ, and DP), which is referred to as a matched group, and having mismatched DP type of HLA-II, which is referred to as a mismatched group. This mismatched group means all matched HLA types except HLA-II DP. DNA sequencing data in this study are listed in Figure S7A. As expected, no significant difference was found by minor HLA-II DPB1 in SCNT-derived osteoblasts and it is similar to relative T cell proliferation of negative control, suggesting a negligible risk of immune rejection in therapeutic treatment (Figure S7B). Although osteoblasts having strong immune response displayed no significant difference by minor HLA DPB1 in immune rejection, we cannot still rule out that other alleles or DPB1 from HLA-II of lineage cells may occur strong immune rejection, such as graft-versus-host disease (GVHD) (Morishima et al., 2018). Consistent with previous literature (Chidgey et al., 2008), we found that SCNT cells have very low reaction with no significance in immune rejection, even minor HLA histocompatibility, suggesting the immunocompatibility of homozygous SCNT-PSC-derived somatic cells.
Figure 3.
Production of MPCs and Osteoblasts from Human SCNT-PSCs with a Homozygous HLA Type
(A) Schematic diagram showing the experimental procedure in vitro.
(B) The representative morphology of SCNT-PSC (CHA-FT-NT17 and CHA-FT-NT18)-derived MPCs and osteoblasts (OS). Scale bars, 100 μm (×40).
(C and D) SCNT-PSC-OS were characterized by high alkaline phosphatase activity and osteocalcin expression. ALP activity and osteocalcin expression of SCNT-PSC-OS showed by ALP staining and immunocytochemistry (C, upper panel), FACS (C, lower panel), and quantitative RT-PCR (D). Scale bars, 100 μm (white) and 20 μm (yellow).
See also Figures S3 and S4.
Figure 4.
Immunological Characterization of MPCs and Osteoblasts from Human SCNT-PSCs with a Homozygous HLA Type
(A) Expression of MHC antigens by differentiation stage in human PSCs. MHC class I and II antigens were highly expressed in human fibroblasts used as controls. In SCNT-PSC-derived MPC, MHC class I also expressed, but MHC class II was not detected. However, in SCNT-PSC-derived osteoblasts (OS), both MHC class I and II antigens were highly expressed regardless of the cell line. Scale bars, 50 μm.
(B) When HLA-matched CD4+ and CD8+ T cells against homozygous CHA-FT-NT18-derived somatic cells were cocultured, the proliferation of T cells was not increased regardless of the lineage cell type (p > 0.05). However, HLA-mismatched CD4+ and CD8+ T cells were dramatically increased by osteoblasts highly expressing MHC molecules in a proliferation assay compared with MPC with relatively low expression of MHC molecules (∗p < 0.05, ∗∗p < 0.01).
See also Figure S6.
Immunocompatibility of Homozygous SCNT-PSC-Derived Somatic Cells In Vivo Humanized Mice
We next sought to examine whether host immune cells according to HLA types were affected by homozygous SCNT-PSC-derived somatic cells in vivo. To this end, we established a humanized mouse model that possesses human hematopoietic cells. Magnetic-activated cell sorting (MACS)-sorted cord blood-derived CD34+ (CB CD34+) cells were injected into irradiated 3-week-old NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl [NSG/SzJ]) mice (Figure 5). The HLA-matched group indicates that HLA-matched T cells reacted against homozygous CHA-FT-NT18-derived osteoblasts in humanized mice and displayed self-signals with all matched HLA-A, B, and DR alleles. In addition, the HLA-mismatched group is the group that has HLA-mismatched T cells against CHA-FT-NT18-derived osteoblasts; ultimately, it was defined as a non-self-signal when HLA-A, B, and DR were at least 75% mismatched.
Figure 5.
The Establishment of a Humanized Mouse Model Was Confirmed by a Human Pan Hematopoietic Cell Markers
(A) Although the frequencies of T lymphocytes were low, stable engraftment of human cells was revealed in tissues, including the PB, BM, and spleen.
(B) Immunocytochemistry showed that CD45 was detected in the PB. Scale bars, 20 μm.
(C) High engraftment of CD33+ myeloid lineage cells, compared with that of CD19+ and CD3+ lymphoid lineage cells, was revealed by FACS analysis.
(D) In T cells, CD4+CD8+ immature thymocyte precursors were regenerated in the mouse thymus and mature T cells, such as single CD4+ and CD8+ cells were detected in the PB, BM, and spleen at 18 weeks after transplantation of CD34+ cells.
The schematic plan for animal experiments is shown in Figure 6A. We first confirmed that CD45+ cells in the peripheral blood (PB) were similarly displayed over 20% at 8 weeks after CB CD34+ cell injection for both groups (humanized mice model for HLA-matched cells, 21.2% ± 9.8%; humanized mice model for HLA-mismatched cells, 20.8% ± 4.3%), suggesting no bias in human cell engraftment (Figure 6B). To investigate whether CD3+ lymphocytes from humanized mice were expanded by the recognition of SCNT-PSC-derived osteoblasts, we carried out fluorescence-activated cell sorting (FACS) analysis and found that homozygous CHA-FT-NT18-derived osteoblasts in the HLA-matched group had a weaker effect on the proliferation of mature T cells in the thymus than did those in the HLA-mismatched group. The same CHA-FT-NT18-derived osteoblasts in the HLA-mismatched group could significantly induce increased mature CD4+ lymphocytes at 15 days, mature CD8+ cells at 8 days, and thymocyte precursor CD4+CD8+ cells at 7 days (Figure 6C), suggesting effective suppression of homozygous CHA-FT-NT18-derived cells with HLA matching during the generation of T cells (Figure 6C). To further address the suppressive effects of homozygous CHA-FT-NT18-derived osteoblasts on T cell generation from other organs, humanized mice received a secondary osteoblast injection and were sacrificed at 4 weeks after cell transplantation. These data showed that homozygous SCNT-PSC-derived osteoblasts with HLA-mismatched alleles did not overall increase human immune cells in various organs from humanized mice. In particular, CD4+ in the PB, CD19+ in the spleen and bone marrow (BM), and thymocyte precursors in the thymus were significantly higher in the HLA-mismatched group than in the HLA-matched group. T cell receptor αβ (TCRαβ) was highly expressed in CD4+ and CD8+ T cells from the HLA-mismatched group, implying a role for SCNT-PSC-derived osteoblasts in the maturation of immune cells and reconstitution of the immune network. However, most human CD45+ cells were the naive phenotype, CD45RA rather than CD45RO (Figure 6D).
Figure 6.
Immunological Characterization of Human SCNT-PSCs with a Homozygous HLA Type In Vivo (I)
(A) Schematic diagram showing the plan for animal studies using a humanized mouse model.
(B) FACS analysis showed the frequency of CD45+ human cells in a humanized mouse model at 8 weeks after CB CD34+ cells. To inject HLA-mismatched and HLA-matched osteoblasts with no bias, humanized mice with the CD45+ phenotype were equally divided into two groups.
(C) The thymus from each humanized mouse was collected at 7, 8, 15, 28, and 91 days and then subjected to FACS analysis. FACS analysis showed that CD3 lymphocytes in the thymus were significantly expanded by mismatched human SCNT-PSC-derived osteoblasts compared with matched osteoblasts.
(D) At 23 weeks, hematopoietic markers, including CD45, CD45RA, CD19, and TCR were overall increased in humanized tissues after two injections of mismatched osteoblasts. All results are presented as the means ± SEM.
See also Figures S6 and S7.
Next, to assess the graft survival of SCNT-PSC-derived osteoblasts in vivo, Beriplast-containing SCNT-PSC-derived osteoblasts were injected into the intradermal skin layer. To track the engrafted cells, skin tissues were harvested at 7 and 15 days after cell transplantation and were subjected to immunohistochemistry (IHC). Cells double-stained for human-specific markers (STEM121, Takara) and osteocalcin were counted in the skin. Robust engraftment of CHA-FT-NT18-derived osteoblasts was observed at 3 days (data not shown) and gradually decreased over 7–15 days. Against CHA-FT-NT18, the HLA-mismatched group displayed rapidly disappearing engraftment compared with that of the HLA-matched group (HLA matched versus HLA mismatched at 7 days, 2.33-fold; HLA matched versus HLA mismatched at 15 days, 1.88-fold) (Figure 7A). Intriguingly, immune CD3 T cells were sustained over 15 days, even after the grafted cells disappeared (Figures 6C and 7A). These data suggest that, even without having many remaining engrafted cells, the activity of human immune cells can still be sustained in the HLA-mismatched group. To confirm whether the infiltrated cells in the thymus were human CD3+ T cells generated via encouragement from human SCNT-PSC-derived osteoblasts, IHC was next carried out on the thymus with human-specific antibodies for CD4+ and CD8+ at 11 weeks after osteoblast transplantation. Gross examination and H&E staining results showed a larger thymus size and a higher frequency of thymocytes in the HLA-mismatched groups than in the HLA-matched group (Figure 7B). In addition, the data clearly showed a high level of human CD4+CD8+ thymocytes in humanized mice in the HLA-mismatched group (Figure 7C). From these data, we suggest that homozygous SCNT-PSC-derived osteoblasts could play a role as functional therapeutic cells without stimulating T cell generation.
Figure 7.
Immunological Characterization of Human SCNT-PSCs with a Homozygous HLA Type In Vivo (II)
(A) Engrafted HLA-mismatched human osteoblasts rapidly disappeared. These data are presented as the means ± SEM from at least three experiments. Asterisks depict statistically significant differences compared with HLA-matched human osteoblasts (∗∗∗p < 0.001 at 7 days, +++p < 0.001 at 15 days).
(B) Gross examination and H&E staining of the humanized thymus.
(C) Immunocytochemistry also showed that CD4+ and CD8+ cells were qualitatively increased in the thymus with mismatched osteoblasts. Scale bars, 20 μm.
Discussion
In this study, we have successfully established a human normal SCNT-PSC line with homozygous HLA type, which is derived from both donated cryopreserved oocytes and cord blood cells. At first, to examine the efficiency of frozen/thawed oocytes for SCNT-PSC production, we analyzed the SCNT results indirectly in comparison with our previous results obtained using fresh human oocytes (Chung et al., 2015). Although there was no difference in reconstruction or first cleavage after activation and KDM4A mRNA, the developmental rate after the eight-cell stage and the blastocyst formation rate were substantially decreased in this study using frozen/thawed oocytes. To increase the efficiency of PSC derivation, we applied a novel derivation medium (1:1 mixture of embryonic stem cell [ESC] derivation medium and ESC culture-conditioned medium) for the outgrowth of attached SCNT embryos. Remarkably, all three expanded blastocysts attached to MEFs were outgrown and established stable cell lines (Figures 1B and S1). These results suggest that frozen/thawed human oocytes could have a lower potency for reprogramming the donor nucleus than fresh oocytes, but reprogramming of the donor nucleus mediated by frozen/thawed oocytes can fully support further embryonic development. In addition, in this study, we suggest that a refined culture technique can contribute to increasing the efficiency of PSC derivation from SCNT embryos showing poor development.
Among many features of MPCs, a notable advantage of allogeneic MPCs is evasion from allorejection. Most studies have described human MPCs with MHC class I and rare MHC class II by showing evidence from in vitro experiments (Ryan et al., 2005). Although this evidence cannot fully explain the machinery for the evasion of alloreactivity in MPCs, low expression of MHC molecules in MPCs has hypoimmunogenic properties, which make MPCs promising therapeutic cell sources in regenerative medicine. As expected, HLA-mismatched CD4+ and CD8+ T cells were dramatically increased by osteoblasts with high expression of MHC molecules in a proliferation assay compared with MPCs with relatively low expression of MHC molecules. Because the response to immune rejection is affected by the high expression of MHC molecules in differentiated cells, the use of cell sources with matched HLA types could increase the efficiency of cell transplantation. In addition, SCNT-PSC-derived MPCs have a moderating effect on proliferating T cells when HLA-mismatched CD4+ and CD8+ T cells react, although this effect could induce significantly lower proliferation of T cells than more differentiated types of cells (SCNT-PSC-osteoblasts). This also suggests that a successful hypoimmunogenic response would be achieved by homozygous SCNT-PSC-derived somatic cells (even including MPCs) with matched HLA types. From these results, we propose that the hypoimmunogenic properties of homozygous SCNT-PSC-derived somatic cells will facilitate their use as cell therapeutic sources in regenerative medicine due to safety related to immune conflicts.
In this study, differentiated functional cells from SCNT-PSCs with homozygous HLA type have shown immunocompatibility in humanized mouse with a matching HLA type. However, after secondary osteoblast injection, heterozygous SCNT-PSC-derived osteoblasts did not overall increase human immune cells and produce fully mature immune cells in various organs from humanized mice (Figure 6D). In fact, the thymus is an important organ that produces lymphocytes via positive and negative selection of thymocyte precursors (Cohen et al., 2006). Thymic epithelial cells in the medulla express MHC class II, which can encourage the maturation of thymocytes; however, thymic epithelial cells in a humanized mouse model are mouse, not human, and may lead to the failure of full maturation in lymphocytes. Although several limitations of the humanized mouse, such as improper education of T cells in the murine thymus and inability of the reconstructed system to response against minor histocompatibility-mismatched cells still exist (Kooreman et al., 2017), immune response for major histocompatibility of homozygous SCNT-PSC-derived cells in our study was partly shown by showing a proliferation of human T cells against human cells with mismatched HLA in murine thymus (Figures 6C, 6D, and 7A–7C). We still cannot exclude the possibility that investigation for full immunity against entire human HLA failed due to immaturity of innate immune cells. To overcome this obstacle, further efforts to obtain an advanced humanized mouse model with a functional innate immune system will be required to generate functional T cells (Shultz et al., 2010).
Here, we have reported the successful production of HLA-homozygous SCNT-PSCs using MNCs from frozen/thawed donated cord blood in a public bank and frozen (vitrified) human oocytes donated after storage for 5 years. The homozygous PSCs are produced by frozen oocyte-mediated reprogramming and do not undergo an artificial genetic modulation. However, they have a differentiation capability very similar to that of conventional ESCs and SCNT-PSCs obtained from fibroblasts and/or fresh oocytes. In addition, homozygous SCNT-PSC-derived cells are highly immunocompatible with allogeneic HLA-matched immune cells. Therefore, we may suggest that this SCNT technology facilitates the establishment of the human HLA-matched SCNT-PSC bank.
Experimental Procedures
Ethics Statements
To confirm the potential of cryopreserved human oocytes on SCNT reprogramming, we performed the experimental protocol under approval by both the institutional review board (IRB) of CHA University (1044308-201511-SR-024-02) and the National Institutional Review Board of the Korean Government (no. 3 for SCNT study). Frozen oocytes were donated from the Fertility Center of CHA Gangnam Medical Center under informed consent of patients after 5 years of storing (by the law of the Bioethics and Safety of Republic of Korea). Also, the protocol for recruitment of skin cells was approved by the IRB of CHA Gangnam Medical Center (GCI 13-033) and by the IRB of Bundang CHA General Hospital (protocol 2013-07-077). Frozen cord blood cells were donated from the Public Cord Blood Bank of Bundang CHA General Hospital after the IRB of CHA Gangnam Medical Center approved the protocol for recruitment of donated frozen cord bloods (protocols GCI-13-032).
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of CHA University and all animal procedures were performed in accordance with approved guidelines and regulations. Donations of cord blood used for humanized mice study were approved by the IRB of CHA University (protocols 1044308-201702-BR-017-03, 04) and were obtained from the Public Cord Blood Bank of Bundang CHA General Hospital, Seongnam, Korea.
Preparation of Human Cryopreserved Oocytes
Ovarian stimulation for the oocytes was carried out using a commonly used clinical IVF-ET protocol. Most of the collected cumulus-oocytes complexes were used for conventional IVF-ET and the remaining were cryopreserved using a vitrification method until further use (Yoon et al., 2007).
Preparation of Fibroblasts, MNCs, and SCNT Using Cryopreserved Oocytes
hDF cells were obtained from an age-related macular degeneration patient. Small pieces of abdominal skin (0.5 × 0.3 cm) were biopsied under local anesthesia and cell preparation was performed as described previously (Chung et al., 2014). Human MNCs were prepared for another SCNT experiment using donated frozen/thawed cord blood with known HLA information for public use (with homozygous allele in HLA-A, B, and DRB1 loci). The MNCs from frozen cord blood were isolated using Ficoll-Paque PLUS (GE Healthcare) after thawing according to the manufacturer's instructions and cultured in vitro before use. The procedure of SCNT was performed by using our protocol as described previously (Chung et al., 2014, Matoba et al., 2014).
Derivation of Human SCNT-PSCs from Cloned Blastocysts
After removal of the zona pellucida using an acidic Tyrode solution (Merck), whole blastocysts were plated onto MEFs in a mixed medium (1:1 ratio): the conditioned hESC culture medium and the original hESC derivation medium (DMEM/F12 supplemented with 20% KnockOut Serum Replacement [KSR] [Gbico], 20 ng/mL human recombinant basic fibroblast growth factor [bFGF] [Giboc], and 2,000 units/mL recombinant human leukemia inhibitory factor [Giboc]). The new derivation medium was not changed for the next 3 days and then half of the media volume was replaced with fresh medium daily. After the second passage, PSC cells were maintained in DMEM/F12 medium supplemented with 20% KSR, 1% (v/v) non-essential amino acids (Gibco), 0.1mM β-mercaptoethanol (Gibco), and 4 ng/mL bFGF.
Expression of HLA Subtypes
To determine the expression of HLA subtypes on SCNT-PSC- and ESC-derived MPCs, cells were washed and fixed. After permeabilization, washing, and blocking, cells were incubated with primary antibodies (anti-human MHC class I [HLA-A + HLA-B, Abcam] and anti-human MHC class II [HLA DO, Abcam]) for 2 h at room temperature.
In Vitro T Cell Proliferation Assay Using SCNT-PSC-Derived MPCs and Osteocytes according to HLA Matching
Human PBMCs were isolated from blood samples obtained from healthy donors, and their HLA types were analyzed by the hematopoietic stem cell bank at the Catholic University, Seoul, Korea. PBMCs displaying HLA types equal to those in SCNT-PSC (CHA-FT-NT18)-derived cells were used for this study. In brief, PBMCs were maintained in AIM-V medium (Gibco) supplemented with DNase overnight; the next day, cells were harvested and incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Thermo Fisher Scientific) for 10 min. CFSE-incorporated PBMCs were plated in 24-well plates at a density of 1 × 106 cells/well. The test cells were harvested, washed three times with AIM-V medium, and cocultured with PBMCs. For the control, PBMCs were treated only with the AIM-V medium (without test cells). After a 7-day coculture, fresh test cells were added to the PBMCs for a second stimulation and cultured for an additional 7 days. Then, cells were harvested and stained with CD4 (BD) and CD8 (BD) antibodies to then measure T cell proliferation using FACSCanto II. To investigate immune rejection by mismatched HLA-II DPB1, full mismatched cells with cytokine were used as a positive control against CHA-FT-NT18 cells.
Establishment of Humanized Mice and In Vivo Assay
To establish a humanized mouse model, human CB CD34+ cells were isolated by MACS sorting; their purity after sorting was approximately 98%. A total of 1 × 105 CD34+ cells were resuspended in 100 μL of DPBS and were intravenously injected into NSG mice (Jackson Laboratory). Three-week-old mice were sub-lethally irradiated with 300 cGy of total body irradiation for 24 h before intravenous injection of CB CD34+ cells. At 8–12 weeks after the cell injection, FACS analysis was performed to confirm human cell engraftment using PB, BM, thymus, and spleen. Mice were monitored daily for symptoms of GVHD, including a ruffled coat, hunched back, weakness, and reduced motility. Once injected animals showed signs, they were sacrificed. In the absence of these signs of stress, mice were analyzed over 23 weeks after transplantation. All experiments were performed as described previously (Lee et al., 2014). Mice that did not show GVHD and showed a stable engraftment of human CD45 cells were used to monitor immune rejection by HLA types.
Flow Cytometry Analysis and IHC of Human Immune Cells in Reconstructed Humanized Mice
To analyze human hematopoietic cells, cells from the PB, BM, spleen, and thymus of reconstituted humanized mice were briefly resuspended in 100 μL of rinsing buffer and incubated with human antibodies. APC-conjugated mouse anti-human CD45 (BD), fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD45 (BD), PerCP-conjugated mouse anti-human CD45RA (BioLegend), FITC-conjugated mouse anti-human CD45RO (BioLegend), PE-conjugated mouse anti-human CD19 (BioLegend), mouse anti-human TCRαβ (BioLegend), APC-conjugated mouse anti-human CD4 (BD), PE-conjugated mouse anti-human CD8 (BD), FITC-conjugated mouse anti-human CD33 (BD), rabbit anti-human MHC class I (Abcam), and mouse anti-human MHC class II (Abcam) were used as a primary antibodies. Flow cytometry data were analyzed using appropriate controls with proper isotype-matched immunoglobulin G and unstained controls. After washing, the resulting cells were analyzed on a BD Accuri C6 Plus Flow cytometer (BD) and data were evaluated with the CFlow software. IHC in tissues, including Beriplast (Avin Darou) was conducted as described previously (Jun et al., 2019). Sections were blocked for endogenous peroxidase activity and incubated with mouse anti-human STEM121 (Takara) and anti-human osteocalcin (Abcam) antibodies. Also, to stain CD4 and CD8 cells in the thymus of the humanized mouse, anti-CD4 (Abcam) and anti-CD8 antibodies (Abcam) were used. For confirmation of human engraftment, BM was subjected to hematoxylin and eosin staining.
Statistical Analysis
All results are presented as the mean ± SEM. Statistical analyses were performed with the Mann-Whitney U test and chi-square test for comparisons between two groups. Values of p < 0.05 were considered to denote statistical significance. The GraphPad Prism v.4 software (GraphPad software) was used for statistical analysis.
Author Contributions
D.R.L. conceived the project. J.E.L., J.Y.L., C-H.P., and D.R.L. designed the experiments. J.H.E. performed SCNT experiments. J.E.L. performed human PSC derivation experiments. J.E.L., C-H.P., S.K.J., and J.S.L. conducted stem cell experiments. J.Y.L., A-R.H., H.S.S., J.H.I., D-W.S., T.C., K.H., and D.R.H. conducted humanized mice experiments and immune analysis. J.H.E., J.E.L., and T.K.Y. performed human oocyte experiments. J.E.L., J.Y.L., and D.R.L. wrote the manuscript.
Acknowledgments
We thank Drs. Jung-Il Lee and Seung-Young Go for technical help for humanized mouse project. This research is supported by the Bio & Medical Technology Development Program (2015M3A9C6028961, 2017M3A9F8072235, and 2017M3A9C6061284) and Basic Science Research Program (2019R1A2C2005681) of NRF and MSICT of Republic of Korea.
Published: June 4, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.05.005.
Supplemental Information
References
- Chidgey A.P., Layton D., Trounson A., Boyd R.L. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330–337. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- Cho M.S., Lee Y.E., Kim J.Y., Chung S., Cho Y.H., Kim D.S., Kang S.M., Lee H., Kim M.H., Kim J.H. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.G., Eum J.H., Lee J.E., Shim S.H., Sepilian V., Hong S.W., Lee Y., Treff N.R., Choi Y.H., Kimbrel E.A. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14:777–780. doi: 10.1016/j.stem.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Chung Y.G., Matoba S., Liu Y., Eum J.H., Lu F., Jiang W., Lee J.E., Sepilian V., Cha K.Y., Lee D.R. Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell. 2015;17:758–766. doi: 10.1016/j.stem.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen C.J., Zhao Y., Zheng Z., Rosenberg S.A., Morgan R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- Jun S.M., Park M., Lee J.Y., Jung S., Lee J.E., Shim S.H., Song H., Lee D.R. Single cell-derived clonally expanded mesenchymal progenitor cells from somatic cell nuclear transfer-derived pluripotent stem cells ameliorate the endometrial function in the uterus of a murine model with Asherman's syndrome. Cell Prolif. 2019;52:e12597. doi: 10.1111/cpr.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Tani T., Sotomaru Y., Kurokawa K., Kato J., Doguchi H., Yasue H., Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Kooreman N.G., de Almeida P.E., Stack J.P., Nelakanti R.V., Diecke S., Shao N.Y., Swijnenburg R.J., Sanchez-Freire V., Matsa E., Liu C. Alloimmune responses of humanized mice to human pluripotent stem cell therapeutics. Cell Rep. 2017;20:1978–1990. doi: 10.1016/j.celrep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza R.P., Cibelli J.B., West M.D. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 1999;17:1171–1174. doi: 10.1038/70709. [DOI] [PubMed] [Google Scholar]
- Lee A.R., Hong K., Choi S.H., Park C., Park J.K., Lee J.I., Bang J.I., Seol D.W., Lee J.E., Lee D.R. Anti-apoptotic regulation contributes to the successful nuclear reprogramming using cryopreserved oocytes. Stem Cell Reports. 2019;12:545–556. doi: 10.1016/j.stemcr.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.E., Chung Y.G., Eum J.H., Lee Y., Lee D.R. An efficient SCNT technology for the establishment of personalized and public human pluripotent stem cell banks. BMB Rep. 2016;49:197–198. doi: 10.5483/BMBRep.2016.49.4.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Park S., Han A.R., Lim J., Min W.S., Kim H.J. High ALDHdim-expressing CD34+CD38− cells in leukapheresed peripheral blood is a reliable guide for a successful leukemic xenograft model of acute myeloid leukemia. Oncol. Rep. 2014;32:1638–1646. doi: 10.3892/or.2014.3359. [DOI] [PubMed] [Google Scholar]
- Liu Z., Cai Y., Wang Y., Nie Y., Zhang C., Xu Y., Zhang X., Lu Y., Wang Z., Poo M. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell. 2018;172:881–887.e7. doi: 10.1016/j.cell.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Loh Y.H., Agarwal S., Park I.H., Urbach A., Huo H., Heffner G.C., Kim K., Miller J.D., Ng K., Daley G.Q. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Matoba S., Liu Y., Lu F., Iwabuchi K.A., Shen L., Inoue A., Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell. 2014;159:884–895. doi: 10.1016/j.cell.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima S., Shiina T., Suzuki S., Ogawa S., Sato-Otsubo A., Kashiwase K., Azuma F., Yabe T., Satake M., Kato S. Evolutionary basis of HLA-DPB1 alleles affects acute GVHD in unrelated donor stem cell transplantation. Blood. 2018;131:808–817. doi: 10.1182/blood-2017-08-801449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Pappas D.J., Gourraud P.A., Le Gall C., Laurent J., Trounson A., DeWitt N., Talib S. Proceedings: human leukocyte antigen haplo-homozygous induced pluripotent stem cell haplobank modeled after the California population: evaluating matching in a multiethnic and admixed population. Stem Cells Transl. Med. 2015;4:413–418. doi: 10.5966/sctm.2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Techonology Mature oocyte cryopreservation: a guideline. Fertil. Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Rim Y.A., Park N., Nam Y., Ham D.S., Kim J.W., Ha H.Y., Jung J.W., Jung S.M., Baek I.C., Kim S.Y. Recent progress of national banking project on homozygous HLA-typed induced pluripotent stem cells in South Korea. J. Tissue Eng. Regen. Med. 2018;12:e1531–e1536. doi: 10.1002/term.2578. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Osorio N., Urrego R., Cibelli J.B., Eilertsen K., Memili E. Reprogramming mammalian somatic cells. Theriogenology. 2012;78:1869–1886. doi: 10.1016/j.theriogenology.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Ryan J.M., Barry F.P., Murphy J.M., Mahon B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Saito Y., Najima Y., Tanaka S., Ochi T., Tomizawa M., Doi T., Sone A., Suzuki N., Fujiwara H. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.K., Park K.M., Kim H.J., Lee J.H., Choi J., Chong S.Y., Shim S.H., Del Priore L.V., Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kamao H., Mandai M., Shiina T., Ogasawara K., Hirami Y., Kurimoto Y., Takahashi M. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Reports. 2016;7:635–648. doi: 10.1016/j.stemcr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kimura T., Futagami T., Suegami S., Takahashi M. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7:619–634. doi: 10.1016/j.stemcr.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Amato P., Sparman M., Gutierrez N.M., Tippner-Hedges R., Ma H., Kang E., Fulati A., Lee H.S., Sritanaudomchai H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Turner M., Leslie S., Martin N.G., Peschanski M., Rao M., Taylor C.J., Trounson A., Turner D., Yamanaka S., Wilmut I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Wakayama T., Perry A.C., Zuccotti M., Johnson K.R., Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., Campbell K.H. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Johannesson B., Sagi I., Burnett L.C., Kort D.H., Prosser R.W., Paull D., Nestor M.W., Freeby M., Greenberg E. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- Yoon T.K., Lee D.R., Cha S.K., Chung H.M., Lee W.S., Cha K.Y. Survival rate of human oocytes and pregnancy outcome after vitrification using slush nitrogen in assisted reproductive technologies. Fertil. Steril. 2007;88:952–956. doi: 10.1016/j.fertnstert.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Zhang S.C., Wernig M., Duncan I.D., Brustle O., Thomson J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.