Summary
Studies of patients with acute myeloid leukemia (AML) have led to the identification of mutations that affect different cellular pathways. Some of these have been classified as preleukemic, and a stepwise evolution program whereby cells acquire additional mutations has been proposed in the development of AML. How the timing of acquisition of these mutations and their impact on transformation and the bone marrow (BM) microenvironment occurs has only recently begun to be investigated. We show that constitutive and early loss of the epigenetic regulator, TET2, when combined with constitutive activation of FLT3, results in transformation of chronic myelomonocytic leukemia-like or myeloproliferative neoplasm-like phenotype to AML, which is more pronounced in double-mutant mice relative to mice carrying mutations in single genes. Furthermore, we show that in preleukemic and leukemic mice there are alterations in the BM niche and secreted cytokines, which creates a permissive environment for the growth of mutation-bearing cells relative to normal cells.
Keywords: TET2, Flt3ITD/ITD, bone marrow microenvironment, AML, CMML, MPN, cytokines
Highlights
-
•
Ubiquitous loss of Tet2 followed by expression of Flt3ITD/ITD results in lethal AML
-
•
Tet2−/− cells when exposed to leukemic environment manifest MPN-like features
-
•
Hyperproliferation of Flt3ITD donor cells in preleukemic Tet2−/− microenvironment
In this article, Kapur et al. studied how ubiquitous loss of Tet2 followed by expression of Flt3ITD/ITD results in lethal AML with shorter latency.
Introduction
Hematopoiesis is a tightly regulated process whereby all the blood cells arise from a pluripotent stem cell population. However, several mutations in hematopoietic stem cells have been identified that lead to deregulation of cellular differentiation and proliferation. In acute myeloid leukemia (AML), both chromosomal abnormalities and single gene mutations have been described to play a role in disease initiation, progression, and prognosis. Within the cytogenetically normal patients, mutations in genes that regulate DNA methylation including IDH, DNMT3A (20%–25%), and TET2 (∼20%) have been thought to occur early on during leukemogenesis (Abdel-Wahab and Levine, 2013, Cagnetta et al., 2014, Ley et al., 2010, Frohling et al., 2002). These loss-of-function mutations result in impaired differentiation and enhance the self-renewal of hematopoietic stem cells in mouse models can result in the development of chronic myelomonocytic leukemia (CMML)-like and myeloproliferative neoplasm (MPN)-like disease. Subsequent acquisition of additional mutations that provide proliferation and/or survival advantage to these preleukemic clones are required for their transformation to AML. One of the common mutation partners is Fms-like tyrosine kinase 3 (FLT3) with internal tandem duplication (ITD), leading to constitutive activation of this receptor tyrosine kinase. FLT3-ITD is present in ∼30% of AML patients and is considered an independent prognostic marker. AML patients with mutations in DNA-methylation regulators and FLT3-ITD constitute a poor prognosis group and often do not respond to conventional chemotherapy or kinase-targeted therapies (Kottaridis et al., 2001). Absence of relevant mouse models has hampered the progress in identifying the molecular approaches that will have better outcomes for these patients. To this end, we generated mice that co-express these mutations to find out whether they are sufficient for transformation from a CMML- and MPN-like disease to AML to obtain a better understanding of the earliest hematopoietic cells in the hierarchy to be affected.
TET methylcytosine dioxygenase 2 (TET2) plays an essential role in regulating the conversion of 5-methylcytosine to 5-hydroxymethylcytosine, which functions as an intermediate step for DNA demethylation. Loss of Tet2 function is associated with increased promoter methylation and aberrant expansion of myeloid cells in different mouse models. The myeloid progenitor cells in Tet2 mutant mice show impaired differentiation and demonstrate higher engraftment in transplant studies, indicating an increase in stem cell self-renewal abilities (Li et al., 2011). On the other hand, FLT3ITD knockin mice show increased proliferation of hematopoietic stem cells, leading to increased numbers of myeloid cells (Lee et al., 2007). In contrast to the Tet2−/− mice, Flt3ITD/ITD mice show defects in the competitive transplant setting, as stem cells derived from these mice are highly proliferative. Both of these mutations lead to expansion of the myeloid compartment, but the mice with single mutations do not develop AML. To mimic the co-occurrence and timing of expression of these two mutations in AML patients, we intercrossed these mice to bring the two mutations together and found that the double-mutant mice develop AML with complete penetrance and a short latency period. The transformation of CMML/MPN to AML occurred irrespective of the dosage of the two mutations.
While loss of Tet2 and presence of Flt3ITD in hematopoietic stem/progenitor cells (HSC/Ps) was capable of transforming hematopoietic stem cells in a cell-autonomous manner, we also found that the bone marrow (BM) niche plays an important role in regulating hematopoietic stem cell (HSC) behavior, which was significantly altered in the leukemic and preleukemic mice. Several recent studies (Duan et al., 2014, Forman and Rowe, 2013, Sanchez-Correa et al., 2013) have suggested that the BM microenvironment contributes to the clinical outcomes in MPN and AML patients by contributing to drug resistance. Alterations in the these BM niche cells results in changes in the milieu of soluble factors, and the newly acquired cellular composition can activate aberrant proliferation and migration of HSCs, leading to expansion of myeloid cells even in the absence of any mutations in HSCs (Schepers et al., 2013). Thus, the BM microenvironment not only protects leukemic cells from drugs but can also promote leukemogenesis in transplanted donor cells, accounting for disease relapse and donor-derived leukemia. Our mouse model shows that presence of germline mutations in Tet2 and FLT3ITD not only deregulates hematopoiesis at the earliest stem cell stage but also distorts the composition and soluble milieu of the BM niche to support the growth and survival of leukemic cells. Some of these changes in the BM niche become apparent in preleukemic mice that have loss of TET2 and are capable of promoting myeloid bias and expansion of even normal non-mutated hematopoietic cells. In summary, we describe physiologically relevant models of AML that are likely to have better predictive value for drug testing studies, and provide more information on essential features for transformation of CMML/MPN to AML.
Results
Loss of Tet2 Followed by Expression of Flt3ITD/ITD in BM Cells Leads to Development of Fatal AML
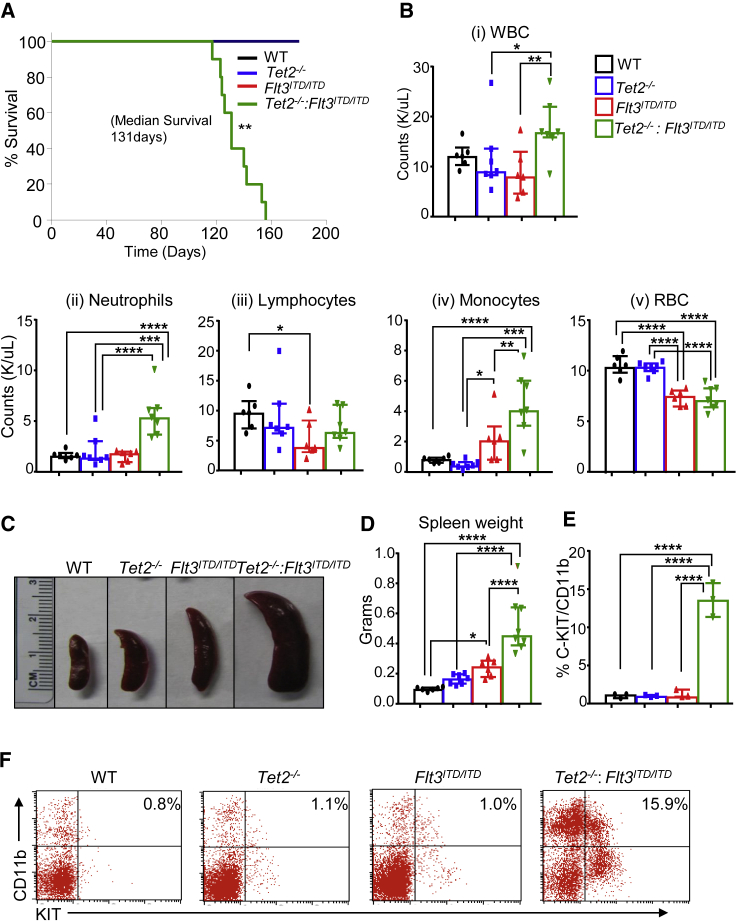
In humans, mutations in DNA-methylation regulator TET2 occur early on in preleukemic cells followed by acquisition of additional mutations, leading to the development of full-blown AML (Sato et al., 2016, Wakita et al., 2013, Weissmann et al., 2012). FLT3ITD/ITD is one such common mutation that occurs subsequent to the acquisition of TET2 mutations in humans (Tian et al., 2014, Wakita et al., 2013). However, it is unclear as to which cell-autonomous and non-autonomous changes take place when TET2 is lost early in development in H2SC/Ps prior to the acquisition of FLT3ITD/ITD. To study this, we intercrossed Tet2−/− (global knockout of Tet2) mice with Flt3ITD/ITD-expressing mice (specifically expressed in hematopoietic cells) to generate Tet2−/−:Flt3ITD/ITD compound mutant mice. Tet2−/−:Flt3ITD/ITD mice showed significantly shorter survival compared with wild type (WT) or the single mutants of Tet2−/− or Flt3ITD/ITD (Figure 1A). Although the survival data for the single-mutant mice is consistent with the published literature demonstrating development of CMML-like disease with a median survival of over a year (Li et al., 2011, Lee et al., 2007), the median survival of Tet2−/−:Flt3ITD/ITD mice was significantly reduced with all mice dead within 5 months post birth (Figure 1A). To further characterize Tet2−/−:Flt3ITD/ITD mutant mice, we analyzed hematopoietic tissues of these mice at 4 months post birth, as the majority of these mice began to succumb around this time. White blood cell (WBC) counts and spleen size were significantly elevated in Tet2−/−:Flt3ITD/ITD mutant mice compared with all the other groups, with neutrophils and monocytes being the major cell type responsible for elevated WBC counts (Figures 1B–1D). Importantly, only Tet2−/−:Flt3ITD/ITD compound mutant mice showed a significant increase in KIT+CD11b+ myeloid blasts, suggesting that early loss of Tet2 and acquisition of Flt3ITD/ITD rapidly transforms preleukemic cells into AML (Figures 1E and 1F). Histopathological examination of BM, spleen, and liver further confirmed these observations as reflected by an increase in myeloid cell infiltration and loss of tissue architecture (Figure S1). Neither Tet2−/− nor Flt3ITD/ITD mice by themselves led to such profound changes within this time span. Thus, early loss of Tet2 with subsequent acquisition of Flt3ITD/ITD is capable of transforming preleukemic HSC/Ps into AML with a short latency period.
Figure 1.
Loss of Tet2 with Concomitant Expression of Flt3ITD/ITD in Mice Leads to Development of Fatal AML with a Short Latency Period
(A) Kaplan-Meier survival curve for WT (n = 6), Tet2−/− (n = 7), Flt3ITD/ITD (n = 7), and Tet2−/−:Flt3ITD/ITD (n = 10) mice.
(B) Changes in peripheral blood counts of (i) WBCs, (ii) neutrophils, (iii) lymphocytes, (iv) monocytes, and (iv) RBCs (n = 6–7 mice per group).
(C–F) (C) Spleen pictures and (D) Quantification of spleen weight (in grams) of 4-month-old WT, Tet2−/−, Flt3ITD/ITD, and Tet2−/−:Flt3ITD/ITD mice (n = 6–7 mice per group). (E) Frequency of myeloid blasts in spleen (n = 3 mice per group). (F) Representative flow profiles of splenic myeloid blasts expressing C-KIT/CD11b.
Data were collected from two independent experiments. See also Figure S1.
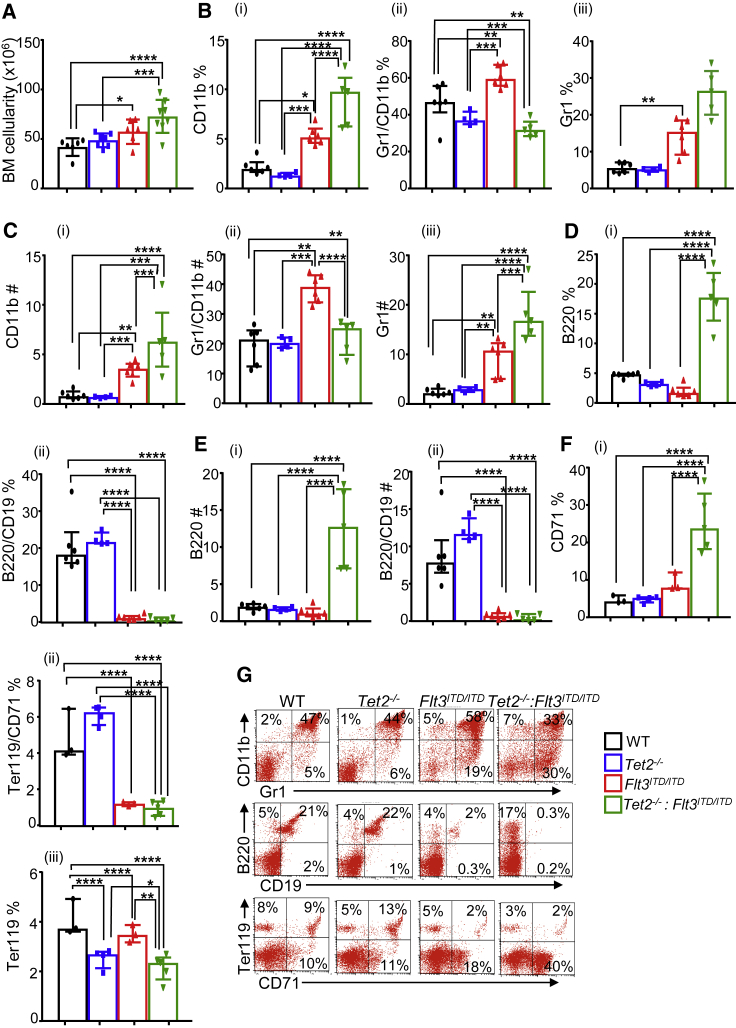
Tet2−/−:Flt3ITD/ITD Mice Show an Increase in BM Cellularity, Expansion of Myeloid Cell Compartment, and Defects in Maturation
Since various peripheral hematopoietic compartments showed a significant expansion of myeloid cells in Tet2−/−:Flt3ITD/ITD mice, we next examined whether this was observed in the BM as well. Consistent with the BM hypercellularity observed during the histopathological examination of Tet2−/−:Flt3ITD/ITD mice, the total number of mononuclear cells were also significantly higher in Flt3ITD/ITD and Tet2−/−:Flt3ITD/ITD mice compared with WT mice, with Tet2−/−:Flt3ITD/ITD mutant mice showing the greatest number of cells in the BM (Figure 2A). Increase in BM cellularity was accompanied with an increase in the frequency and absolute number of Gr-1 and CD11b single-positive myeloid cells (Figures 2B and 2C), with a concomitant reduction in B220/CD19 double-positive B cells (Figures 2D and 2E) in Tet2−/−:Flt3ITD/ITD mice relative to other genotypes. While in WT the majority of BM myeloid cells were of a mature CD11b+GR1+ phenotype, in the Flt3ITD/ITD and Tet2−/−:Flt3ITD/ITD mice there was a significant increase in both the absolute number and frequency of cells that expressed only CD11b or GR1, and this phenotype was more pronounced in Tet2−/−:Flt3ITD/ITD mice (Figures 2B, 2C, and 2G). Additionally, erythroid frequency of single-positive CD71 was found to be increased in Flt3ITD/ITD and Tet2−/−:Flt3ITD/ITD mice, with greater frequency in the Tet2−/−:Flt3ITD/ITD mutant mice compared with Flt3ITD/ITD mice (Figures 2F and 2G). While both of these groups showed a reduction in the development of mature Ter119+ erythroid cells (Figures 2G and 2F), accounting for the observed reduction in peripheral red blood cells (RBCs) (Figure 1), it was only in Tet2−/−:Flt3ITD/ITD mice that a significantly decreased at pro-erythroblast (CD71+) stage was observed (Figures 2F and 2G). Similarly, development of B-lineage cells was severely affected by the presence of Flt3ITD/ITD mutation, which is consistent with the published literature (Lee et al., 2007). These results demonstrate that loss of Tet2 followed by expression of Flt3ITD/ITD in BM cells results in significant alterations in the maturation of multiple lineages including myeloid, erythroid, and lymphoid.
Figure 2.
Tet2−/−:Flt3ITD/ITD Mice Show Significant Increase in Bone Marrow Cellularity and Expansion of Myeloid Cell Compartment
Bone marrow from 4-month-old WT, Tet2−/−, Flt3ITD/ITD, and Tet2−/−:Flt3ITD/ITD mice were analyzed for committed mature cells by flow cytometry as described in Experimental Procedures.
(A–C) (A) Bone marrow cellularity (n = 6–7 mice per group). (B) Frequency of (i) CD11b+, (ii) Gr1+/CD11b+, and (iii) Gr1+ myeloid cells in bone marrow (n = 4–6 mice per group). (C) Number of (i) CD11b+, (ii) Gr1+/CD11b+, and (iii) Gr1+ myeloid cells in bone marrow (n = 4–6 mice per group).
(D–F) (D) (i) Frequency of B220+ and (ii) B220+/CD19+ lymphoid cells in bone marrow (n = 4–6 mice per group). (E) (i) Number of B220+ and (ii) B220+/CD19+ lymphoid cells in bone marrow (n = 4–6 mice per group). (F) (i) Frequency of CD71+, (ii) Ter119+/CD71+, and (iii) Ter119+ cells in bone marrow (n = 4–6 mice per group).
(G) Representative flow profiles of erythroid, myeloid, and B cells in the bone marrow of indicated genotypes.
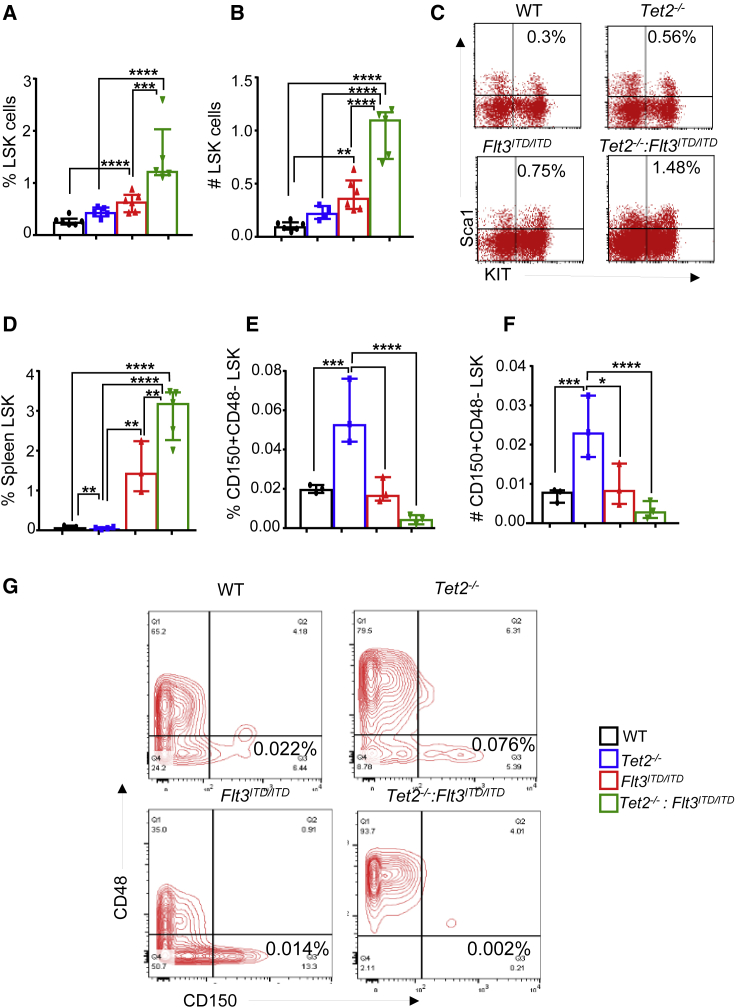
Tet2−/−:Flt3ITD/ITD Mice Show Alterations in the Composition of Primitive Stem and Progenitor Cells in the BM
In an effort to assess how loss of Tet2 followed by the expression of Flt3ITD/ITD affects the composition of more primitive stem and progenitor cells in the BM, we analyzed the BM of Tet2−/−:Flt3ITD/ITD mice. Both the absolute number and the frequency of Lin−Kit+sca1− (LSK) fraction of the BM cells, which is enriched in hematopoietic stem cells, was significantly expanded in all three mutant mouse groups compared with WT controls. However, the magnitude of expansion of LSK cells was most dramatically altered in the BM and spleen of Tet2−/−: Flt3ITD/ITD mice relative to other genotypes (Figures 3A–3D). The most profound changes were observed in Tet2−/−: Flt3ITD/ITD mice. The BM of these mice showed an almost complete loss of long-term (LT)-HSCs (CD48−CD150+LSK) accompanied by an increase in short-term HSCs (CD48+CD150−LSK) (Figures 3E–3G). Thus, differentiation blockade caused by the combination of these mutations possibly exerts its effects at different stages of hematopoiesis.
Figure 3.
Development of AML in Tet2−/−:Flt3ITD/ITD Mice Is Due to Expansion of LSK Cells
Bone marrow and spleens were harvested from 4-month-old WT, Tet2−/−, Flt3ITD/ITD, and Tet2−/−:Flt3ITD/ITD mice and analyzed for stem and progenitor cells by flow cytometry as described in Experimental Procedures.
(A and B) (A) Frequency and (B) absolute number of Lin-Sca1+KIT+ (LSK) cells in the bone marrow (n = 4–6 mice per group).
(C) Representative flow profiles of LSK cells in the bone marrow of indicated genotypes.
(D) Frequency of LSK cells in the spleen of indicated genotypes (n = 3–5 mice per group).
(E and F) (E) Frequency and (F) absolute number of CD48−CD150+LSK cells in the bone marrow of indicated genotypes (n = 3 mice per group).
(G) Representative flow profiles of CD48−CD150+LSK cells in the bone marrow of indicated genotypes (n = 3 mice per group).
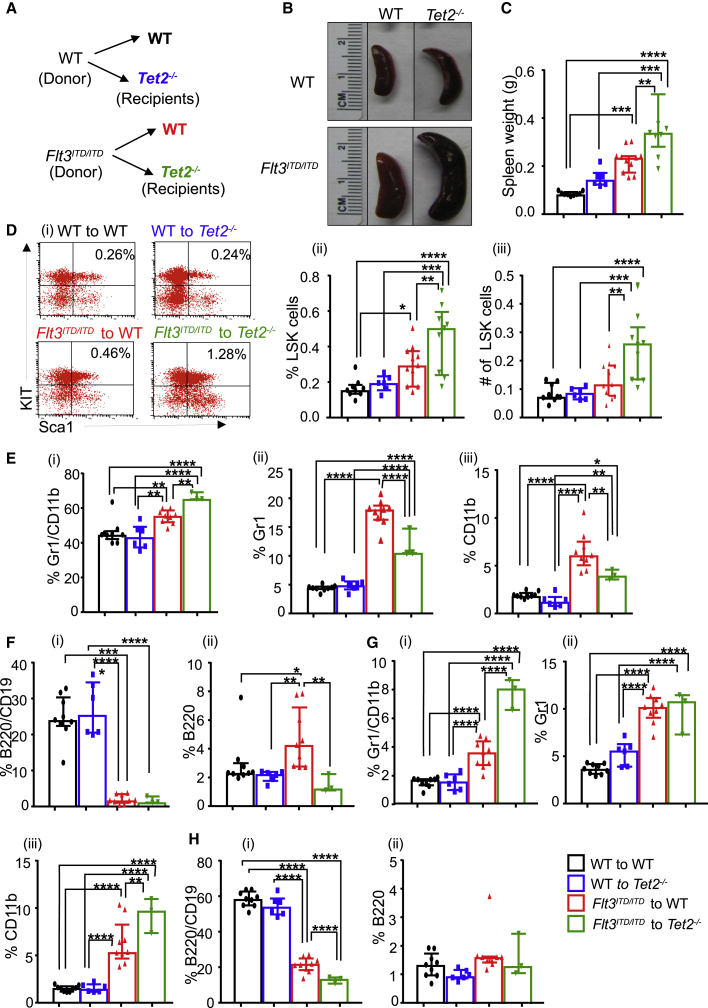
Heterozygous Loss of Tet2 and Expression of Heterozygous Flt3ITD/+ or Homozygous Flt3ITD/ITD in BM Stem and Progenitor Cells Results in the Development of AML but with Delayed Onset and Less Severity
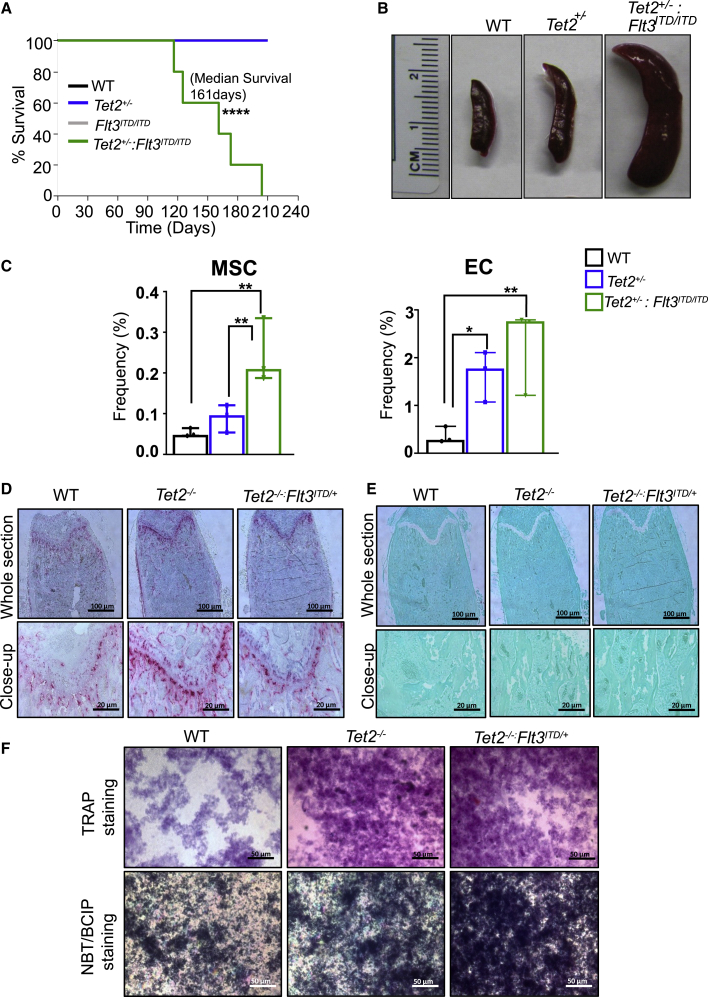
In a subset of AML patients, often heterozygosity of Tet2 along with heterozygous presence of Flt3ITD/+ is observed (Tefferi et al., 2009). How Tet2 heterozygosity as opposed to Tet2 homozygosity cooperates with the expression of Flt3ITD/+ or Flt3ITD/ITD in HSC/Ps was examined next. We generated mice of six different genotypes, namely WT, Tet2+/−, Flt3ITD/+, Flt3ITD/ITD, Tet2+/−:Flt3ITD/+, and Tet2+/−:Flt3ITD/ITD. At 4 months of age, we analyzed these mice for peripheral blood counts. As seen in Figure S2A, no significant differences were observed in peripheral blood parameters between any of the four groups of mice involving Tet2+/−:Flt3ITD/+. In contrast, Tet2+/−:Flt3ITD/ITD mice demonstrated a significant increase in both neutrophil and monocyte counts relative to rest of the genotypes in this group (Figure S2B). Both Flt3ITD/ITD and Tet2+/−:Flt3ITD/ITD mice demonstrated a significant reduction in peripheral RBC counts relative to other genotypes (Figure S2B). BM analysis revealed a significant increase in cellularity in Tet2+/−:Flt3ITD/+, Flt3ITD/ITD, and Tet2+/−:Flt3ITD/ITD mice compared with WT or Tet2+/− mice (Figures S2C and S2D). A significant increase in spleen weight in both Tet2+/−:Flt3ITD/+ and Tet2+/−:Flt3ITD/ITD mice was also observed relative to control mice (Figures S2E and S2F). We next analyzed the frequency and absolute number of LSK cells in the BM of all six genotypes. As seen in Figures S3A–S3D, the greatest increase in the absolute number of LSK cells was seen in Tet2+/−:Flt3ITD/+ and Tet2+/−:Flt3ITD/ITD mice relative to other groups. We also examined erythroid and myeloid cells in the BM of six genotypes and observed the greatest increase in the frequency of CD71-positive erythroid cells in Tet2+/−:Flt3ITD/ITD mice relative to other genotypes (Figure S4). Similar observations were made in the myeloid and B cell compartment. However, in every case the changes in the size and distribution of various HSC/Ps and mature cells in the BM, spleen, or peripheral blood were dose dependent and most impressive in Tet2−/−:Flt3ITD/ITD, mice followed by Tet2+/−:Flt3ITD/ITD mice and Tet2+/−:Flt3ITD/WT mice.
At 8–10 weeks of age, 2 million BM cells from the six genotypes were injected into lethally irradiated recipients, and the mice were examined for survival. As seen in Figure 4A, starting on day 120 (4 months) post transplant, Tet2+/−:Flt3ITD/ITD mice began to succumb to AML and all mice died by 210 days or 7 months post transplant. Figure 4B shows the spleens of WT, Tet2−/−, and Tet2+/−:Flt3ITD/ITD. We analyzed for non-hematopoietic components, as shown in Figure 4C, and observed: (1) heterozygous loss of Tet2 along with co-occurrence of Flt3ITD/ITD mutation, showing greater expansion of mesenchymal stem cells (MSCs); and (2) significant expansion of endothelial cells (ECs) in Tet2+/− and a trend toward additive on co-occurrence with Flt3ITD/ITD mutation. To evaluate the functional impact of Tet2 loss alone or in combination with Flt3ITD (Tet2−/−:Flt3ITD/+) on osteoclasts, we performed tartrate-resistant acid phosphatase (TRAP) staining on femurs from WT, Tet2−/−, and Tet2−/−:Flt3ITD/+ primary mice. As shown in Figure 4D, presence of increased TRAP staining was observed in Tet2−/− and Tet2−/−:Flt3ITD/+ femurs compared with WT, which also supports the in vitro TRAP data (Figure 4F, upper panel) and suggests that osteoclasts potentially contribute to the leukemic phenotype. Next, we assessed the impact of Tet2−/− and Tet2−/−:Flt3ITD/+ mutations on the development of osteoblast cells (OBCs) by alkaline phosphatase assay on cultured cells derived from WT, Tet2−/−, and Tet2−/−:Flt3ITD/+ primary mice. Greater proliferating OB activity was found in Tet2−/− and Tet2−/−:Flt3ITD/+ cultures compared with WT controls, as shown in the lower panels of Figure 4F. A greater number of monocyte/macrophage precursors from Tet2−/− and Tet2−/−:Flt3ITD/+ mice might possibly be contributing to differentiation of cells into osteoclast bone-resorbing cells and greater bone nodule formation, as shown by Vonkossa staining on femurs, thereby inducing greater bone remodeling (Figure 4E).
Figure 4.
Heterozygosity or Homozygosity of Flt3ITD along with Concomitant Heterozygosity of Tet2 Results in AML Development with Similar Latency
(A) Primary bone marrow transplantation. Two million bone marrow mononuclear cells isolated from WT, Tet2+/−, Flt3ITD/ITD, and Tet2+/−:Flt3ITD/ITD mice were transplanted into lethally irradiated C57BL/6 mice through tail vein injection and monitored for disease progression, and Kaplan-Meier survival was established (n = 5 mice per group). See also Figure S3.
(B) Pictures of spleens from indicated genotypes. See also Figures S2 and S4.
(C) Mesenchymal stem cells and endothelial cells were analyzed in primary mice by flow cytometry (n = 3 mice). In brief, bones from WT, Tet2+/−, and Tet2+/−:Flt3ITD/ITD mice were subjected to collagenase digestion followed by staining for mesenchymal stem cells (CD45Neg, LinNeg, CD31Neg, Sca1Pos, CD51Pos), endothelial cells (CD45Neg, LinNeg, CD31Pos), and osteoblastic lineage cells (CD45Neg, LinNeg, CD31Neg, Sca1Neg, CD51Pos). See also Figure S5.
(D) Images of tartrate-resistant acid phosphatase (TRAP) staining on femurs from the primary mice of the indicated genotypes (n = 3 biological replicates).
(E) Images of Vonkossa staining on femurs from the primary mice of the indicated genotypes (n = 3 biological replicates).
(F) Representative images of TRAP staining (upper panels) and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) staining (lower panels) from in vitro cultures of indicated genotypes (n = 3 independent experiments).
Interestingly, mice transplanted with BM cells derived from Tet2+/−:Flt3ITD/+ mice also began to succumb around the same time as Tet2+/−:Flt3ITD/ITD mice, and all were dead by 7 months (Figure S3E). At 4 months post primary transplant, BM cells derived from the primary transplanted recipients were transplanted into lethally irradiated secondary hosts. As seen in Figures S3F and S3G, these recipients began to succumb around day 90 (3 months), and all mice were dead by 160 days (5 months). These results illustrate that haploinsufficiency or homozygosity of Flt3ITD cooperates equally well with Tet2 heterozygosity in the development of AML and overall survival.
Loss of Tet2 Alters the BM Microenvironment and Produces More Pro-inflammatory Cytokines
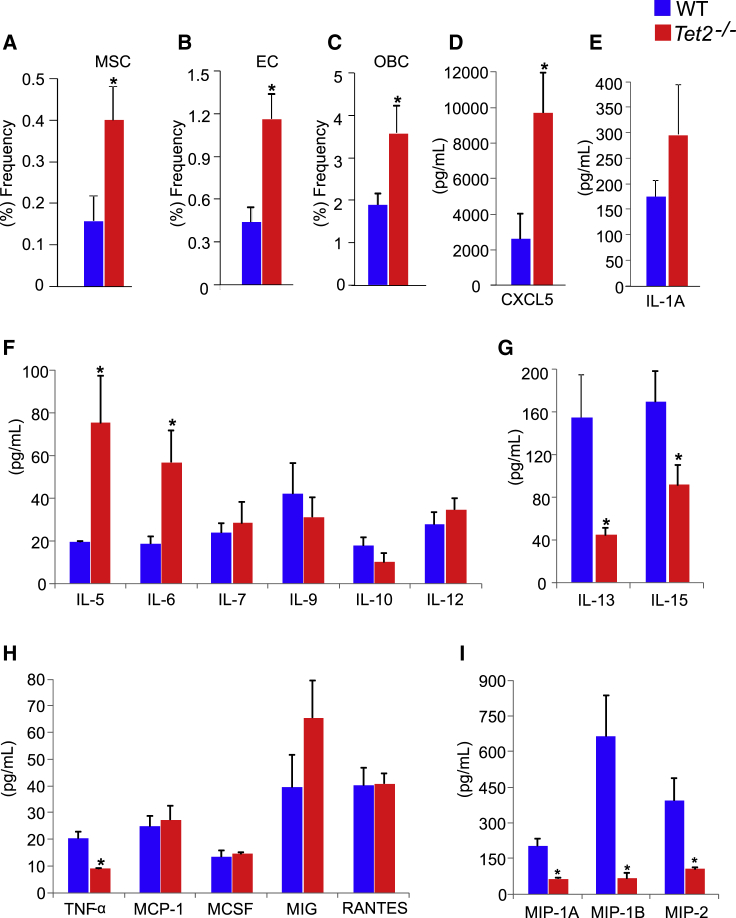
There is increasing evidence (Guarnerio et al., 2018, Schepers et al., 2013) of crosstalk between leukemic cells and their microenvironment whereby both can modulate each other to support the survival of leukemic cells and disease development. We next assessed the BM composition of Tet2−/− mice by examining the frequency of MSCs, ECs, and OBCs, three cell types that can influence the growth and survival of both normal and leukemic cells. As seen in Figures 5A–5C, a significant increase in the frequency of all three cell types was observed in Tet2−/− mice. Furthermore, loss of TET2 also resulted in significant alteration in the expression levels of various pro-inflammatory cytokines that have been implicated in regulating HSC/P functions including interleukin-5 (IL-5), IL-6 and CXCL5, which were all elevated in the absence of Tet2, and macrophage inflammatory protein 1A (MIP-1A), MIP-1B, MIP-2, tumor necrosis factor α (TNFα), IL-13, and IL-15, which were all downregulated in these mice compared with controls (Figures 5D–5I). High levels of TNFα are detrimental to the propagation of stem cells. A recent study in Flt3ITD mice (Mead et al., 2017) showed increased levels of TNFα and its association with stem cell exhaustion. Thus, less TNFα, as seen in our model, may contribute to better propagation and maintenance of overall hematopoiesis. IL-13 plays a critical role in B cell maturation and differentiation. IL-15 is a known mitogen and survival factor for T cells. In the absence or inhibition of IL-15, T cell immunity is likely to be affected. Studies (Devos et al., 2017, Yu et al., 2015, Harish and Schwartz, 2020, Ichiyama et al., 2015) demonstrate that TET2-mediated demethylation controls cytokine expression in T helper cells. Consistent with this notion, our AML mice are likely to have poor anti-tumor immunity due to the presence of possibly increased numbers of T suppressor cells.
Figure 5.
Loss of Tet2 Alters the Composition of the Bone Marrow Microenvironment and Cytokine Production
(A–C) Bones were harvested from 3-month-old WT and Tet2−/− mice (n = 4–5) and subjected to collagenase digestion followed by staining for (A) mesenchymal stem cells, (B) endothelial cells, and (C) osteoblastic lineage cells, and analyzed by flow cytometry. Cytokines and chemokines from serum of 3-month-old WT and Tet2−/− mice were assayed using mouse 32-plex cytokine/chemokine array.
(D–I) (D) Cxcl5, (E) IL-1α, and (F) IL-5 and IL-6 were increased and (G) IL-13 and IL-15, (H) TNFα, and (I) MIP1α, MIP1β, and MIP2 were decreased. Concentrations are presented in pg/mL (n = 3–5 mice per genotype).
Student’s unpaired t test was performed for statistical significance (∗p < 0.05, ∗∗p < 0.001).
Altered BM Microenvironment in Tet2−/− Mutant Mice Supports the Expansion of Leukemic Cells
Given the nature of changes in the composition of BM microenvironment in mice bearing loss of TET2, including enhanced frequency of MSCs, ECs, and OBCs in addition to cytokines, we asked whether transplanting normal BM cells or BM cells derived from mice bearing a key mutation associated with AML, such as Flt3ITD/ITD into Tet2-deficient mice, would support the growth of these cells or not. We took 2 million BM cells from WT or Flt3ITD/ITD mice and transplanted them into lethally irradiated WT or Tet2−/− mice, and monitored the engraftment (Figure 6A). At 5 months post transplant, mice were sacrificed and analyzed. As seen in Figures 6B and 6C, BM derived from Flt3ITD/ITD mice, when transplanted into Tet2−/− recipients, resulted in the development of largest spleen size and weight relative to all other experimental groups. In the BM, a profound increase in the frequency as well as in the absolute number of LSK cells was noted when Flt3ITD/ITD cells were transplanted into Tet2-deficient recipients (Figure 6D).
Figure 6.
Altered Bone Marrow Microenvironment in Preleukemic Tet2−/− Mice Supports the Expansion of Myeloid Malignant Flt3ITD/ITD-Bearing Cells
(A) Schematic showing transplantation of normal WT or Flt3ITD/ITD-bearing cells to normal WT or preleukemic Tet2−/− mice. Bone marrow cells (2 × 106) isolated from WT or Flt3ITD/ITD mice were transplanted into lethally irradiated WT or Tet2−/− mice through tail vein and monitored for disease progression. Reconstitution of hematopoietic stem and progenitor cells was analyzed 5 months after transplantation.
(B) Photos of representative spleens from transplanted mice.
(C) Spleen weights (6–11 mice in each group).
(D) (i) Representative flow profile of LSK cells in the bone marrow of indicated transplanted mice, (ii) Frequency and (iii) number of LSK cells in the bone marrow.
(E) Bone marrow frequency of indicated genotypes of (i) Gr1+/CD11b+, (ii) Gr1+, and (iii) CD11b+ myeloid cells.
(F) Frequency of (i) B220+/CD19+ and (ii) B220+ in bone marrow of indicated mice.
(G) Spleen frequency of indicated genotypes of (i) Gr1+/CD11b+, (ii) Gr1+, and (iii) CD11b+ myeloid cells.
(H) Frequency of (i) B220+/CD19+ and (ii) B220+ in spleen of indicated mice.
Data were collected from two independent experiments. See also Figure S6.
We next examined the impact of Tet2-deficient recipients transplanted with WT or Flt3ITD/ITD BM cells to give rise to more mature myeloid and lymphoid cells. In the BM, we found a significant increase in the presence of Gr-1/CD11b double-positive cells when Flt3ITD/ITD cells were transplanted into Tet2-deficient recipients relative to all other groups (Figure 6E). Consistent with our findings in the marrow, a much more pronounced expansion of Gr-1/CD11b double-positive as well as CD11b single-positive cells was noted in Tet2−/− mice transplanted with Flt3ITD/ITD BM cells in the spleen (Figure 6G). Representative flow profiles in the BM and spleen of transplanted mice in the four experimental groups are shown in Figures S6A and S6B. We also examined the contribution of B cells in the four transplanted groups. As seen in Figures 6F and 6H, a significant decrease in the production of B cells was noted in both the BM and the spleen of Tet2−/− recipient mice transplanted with Flt3ITD/ITD BM cells, as assessed by the decrease in frequency of B220/CD19 double-positive cells. A similar finding was observed when Flt3ITD/ITD BM cells were transplanted into WT recipients. These results suggest that the defects observed in B cell development and maturation associated with Flt3ITD/ITD-bearing HSC/Ps are mainly cell intrinsic and not dependent on the microenvironment, although a modest but significant further reduction in the B220/CD19 double-positive cells was noted in Tet2−/− recipients transplanted with Flt3ITD/ITD cells (Figure 6H).
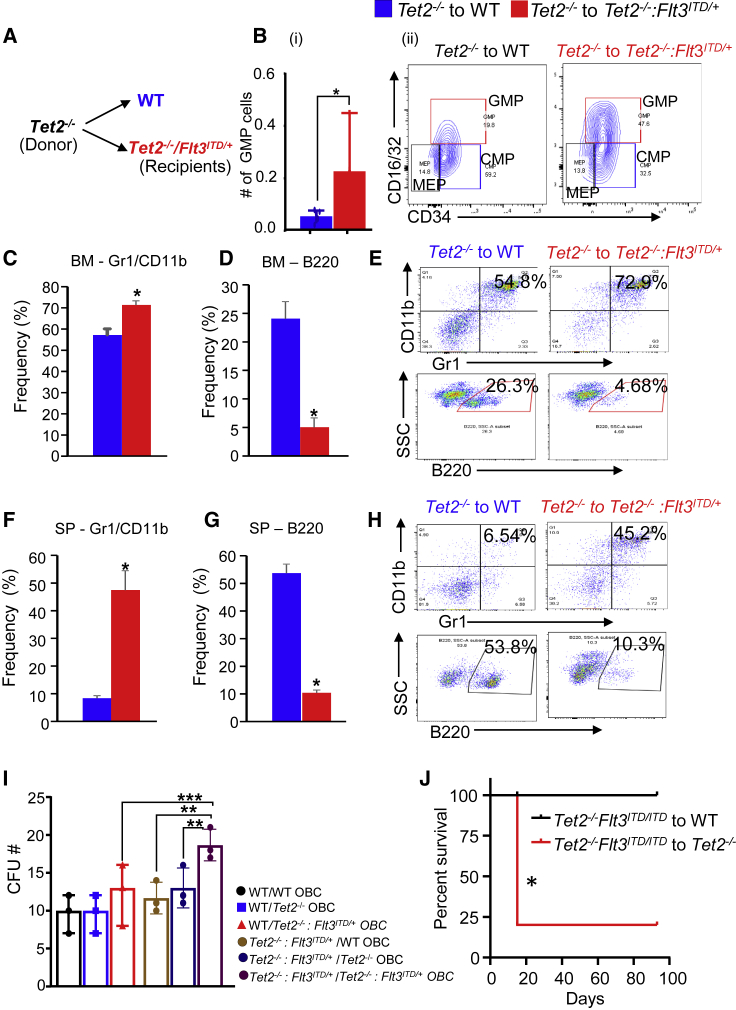
Tet2-Deficient Myeloid Progenitors Expand in Recipient Mice Lacking a Combination of Tet2 and Expressing Flt3ITD/+
To assess how Tet2−/− preleukemic BM cells grow in a microenvironment with AML driven by a combination of Tet2−/−:Flt3ITD/+ mutations, we transplanted 2 million BM cells from 7 to 8 month-old Tet2−/− mice into lethally irradiated age-matched WT or Tet2−/−:Flt3ITD/+ mice and monitored the engraftment of donor cells. Six weeks after transplantation, mice were sacrificed on account of moribund features. As shown in Figure 7B, phenotypic analysis of BM cells derived from Tet2−/−:Flt3ITD/+ recipients showed an elevated number of myeloid progenitor (GMP) cells relative to controls. The frequency of mature myeloid Gr1/CD11b was also increased in the BM (Figure 7C) and spleen (Figure 7F) of Tet2−/−:Flt3ITD/+ recipients compared with controls; whereas the frequency of lymphoid cells (B220) was reduced (Figures 7D and 7G). Figures 7E and 7H show representative flow profiles of myeloid and lymphoid cells in Tet2−/−:Flt3ITD/+-recipient mice transplanted with Tet2−/− cells. Next, we performed coculture assay to assess the relative contribution of OBCs in supporting the growth of Tet2−/−:Flt3ITD/+ cells. As shown in Figure 7I, an increase in colony-forming units (CFU) was observed when purified lineage-negative Tet2−/−:Flt3ITD/+ cells were cocultured with OBCs derived from Tet2−/−:Flt3ITD/+ mice compared with OBCs derived from Tet2−/− and/or WT OBCs. Furthermore, to test whether Tet2−/−:Flt3ITD/ITD BM supports the survival of WT cells better than preleukemic Tet2−/− cells, we transplanted Tet2−/−:Flt3ITD/ITD cells into WT and Tet2−/− recipients and followed their survival. Four Tet2−/− recipients out of five that were transplanted with Tet2−/−:Flt3ITD/ITD cells died 15 days post transplantation and one was moribund after 93 days. None of the WT recipients transplanted with Tet2−/−:Flt3ITD/ITD cells were found dead (Figure 7J), suggesting that Tet2−/−:Flt3ITD/ITD leukemic cells survive better in the normal WT microenvironment but die when exposed to the preleukemic Tet2−/− microenvironment. Significantly elevated peripheral WBCs, neutrophils, lymphocytes, and monocyte counts and percentages were found in one of the surviving Tet2−/− recipients compared with four of the WT recipients, as presented in Figure S7E (table). To rule out the possibility of age-associated leukemic transformation contributing to pro-leukemogenic phenotype, we transplanted 2 million BM cells from 2- to 3-month-old Tet2−/− mice to lethally irradiated age-matched WT and Tet2−/−:Flt3ITD/+ recipients and analyzed them for hematopoietic and non-hematopoietic stromal components at 36 weeks after transplantation. As shown in Figures S7A–S7D, peripheral blood platelet counts are decreased with significantly increased frequency of CD11b BM cells, ECs, and OBCs cells in the Tet2−/−:Flt3ITD/+ recipients relative to WT controls, suggesting that CD11b, ECs, and OBCs may all contribute to the malignant phenotype. Taken together, these data show that when preleukemic Tet2-deficient BM cells are exposed to leukemic microenvironment driven by Tet2−/−:Flt3ITD/+ mutations, MPN-like features are manifested.
Figure 7.
Expansion of Tet2−/− Myeloid Cells but Not B Cells in the Bone Marrow of Tet2−/−:Flt3ITD/+ Mice
(A) Schematic showing preleukemic Tet2 cells exposed to normal WT or leukemic Tet2−/−:Flt3ITD/+ microenvironment. Two million bone marrow cells isolated from Tet2−/− mice were transplanted into lethally irradiated WT or Tet2−/−:Flt3ITD/+ mice through tail vein and monitored for disease progression. Mice were analyzed 6 weeks after transplantation.
(B) (i) Absolute number of granulocyte/macrophage progenitor cells (GMP) in the bone marrow of indicated recipients. (ii) Representative flow profile of various bone marrow progenitors in the indicated recipients. CMP, common myeloid progenitor cells. MEP, megakaryocyte erythroid progenitor cells.
(C–E) (C) Frequency of Gr1+/CD11b+ myeloid cells in the bone marrow of indicated recipients and (D) frequency of B220+ lymphoid cells in bone marrow of indicated recipients. (E) Representative flow profile of myeloid and lymphoid cells in the bone marrow of recipient mice.
(F and G) (F) Frequency of Gr1+/CD11b+ myeloid cells in the spleen of indicated recipients and (G) frequency of B220+ lymphoid cells in spleen of indicated recipients.
(H) Representative flow profile of myeloid and lymphoid cells in the spleen of recipient mice. Student’s t test was performed for statistical significance (∗p < 0.05, n = 3–4 mice in each group).
(I and J) (I) Coculture assay. In brief, bones from WT and Tet2−/−:Flt3ITD/+ after flushing marrow were subjected to collagenase digestion. Lineage-negative, CD45-negative stromal cells were sorted (4,000 cells/well) directly into 12-well plates containing DMEM, 15% FBS, 100 U penicillin/100 μg streptomycin, and 50 mM 2-mercaptoethanol. OBCs were induced by adding 3 mM β-glycerophosphate and 10mg/mL L-ascorbic acid phosphate. After 24 h, lineage-negative bone marrow cells from WT and Tet2−/−:Flt3ITD/+ mice were added to the pre-established OBCs and cocultured for another 4 days in the presence of SCF (25 mg/mL), Flt3 ligand (25 mg/mL), and IL-11 (25 mg/mL). After 4 days, CFU activity in methylcellulose was assessed from all the cells including adherent OBCs, and colonies were counted after 7 days. Statistical analysis was performed by one-way ANOVA with uncorrected Fisher's test. ∗∗p < 0.001, ∗∗∗p < 0.0001. (J) Two million bone marrow mononuclear cells from Tet2−/−: Flt3ITD/ITD mice was transplanted to lethally irradiated 5-month-old WT (n = 4) and Tet2−/− (n = 5) mice, and their survival was monitored. Peripheral blood counts from the surviving WT recipients and moribund Tet2−/− recipients are presented in Figure S7E.
Discussion
In addition to genetic and epigenetic changes in BM progenitors, accumulating evidence suggests a role for the BM microenvironment in the development of MPN and AML (Lane et al., 2009). Using a transgenic BCR/ABL mouse model of chronic myeloid leukemia (CML), Schepers et al. (2013) investigated the role of MPN in the regulation of the endosteal BM niche and found that leukemic myeloid cells induce the expansion of OBCs from MSCs and contribute to BM fibrosis. These studies suggest a contribution of the BM microenvironment to MPN development, and therapeutic intervention of both leukemic cells and the BM microenvironment may be necessary for the complete elimination of leukemia-initiating cells. Thus, understanding the role of BM microenvironment cells in the development of MPN and AML is necessary for developing better therapies to treat these diseases. Although genetic and in vivo studies on the role of Tet2 loss in cooperation with Flt3ITD/ITD and in inducing AML have been conducted (Shih et al., 2015), the in vivo effects of early and ubiquitous loss of Tet2 followed by expression of Flt3ITD/ITD in these same cells and their impact on the function of the BM microenvironment has not previously been described. First, we found that loss of Tet2 and constituent activation of Flt3ITD/ITD in BM cells causes a lethal AML phenotype with a latency of less than 200 days. Utilizing a VavCre Tet2−/−/Flt3ITD, Shih et al. (2015) reported a long survival of 600 days. Second, we observed altered BM microenvironment cell composition including in the ECs and MSCs in Tet2+/−:Flt3ITD/ITD mice. Third, we observed expansion of pro-inflammatory cytokines and hyperproliferation of Flt3ITD/ITD donor cells in the preleukemic microenvironment.
In VavCre-driven Tet2−/−:Flt3ITD/ITD mice, TET2 is deleted in HSCs specifically and partly on ECs whereas in our model system, TET2 is deleted globally including MSCs and OBCs, which play a critical role in the expansion of leukemic cells and disease progression, thus contributing to a shorter latency. Our results in regard to the composition of microenvironment cell composition shows an increase in the frequency of endothelial, mesenchymal, and osteoblastic lineage cells in preleukemic Tet2−/− mice compared with WT mice, suggesting that the loss of TET2 in these cell types is a prerequisite for an aggressive lethal AML phenotype. Our data are consistent with those of Shih et al. (2015) with respect to cell-autonomous features including increase in WBCs, neutrophils, and monocytes, and a reduction in RBCs. Increase in BM cellularity, expansion of LSK cells in the BM and spleen, reduction in LT-HSCs, increase in c-kit/CD11b double-positive myeloid blasts, and accumulation of CD71, Gr1, and CD11b single-positive cells in the BM are all features consistent with those observed by Shih et al. (2015) utilizing a different model of Tet2−/−:Flt3ITD/ITD. While loss of Tet2 and expression of Flt3ITD in BM stem/progenitor cells might be required for disease initiation in a cell-autonomous manner; AML blasts might occupy and alter the BM niche composition leading to secretion of several pro-inflammatory cytokines, which are likely to function as growth-promoting factors for the growth of leukemic cells while repressing normal hematopoiesis. Li et al. (2018) showed that conditional deletion of Tet2 in mesenchymal cells results in accelerated malignant progression and shortened survival. Other studies (Dong et al., 2016, Schepers et al., 2013) in CML and MPN model systems have shown that leukemic myeloid cells induce the expansion of OBCs from MSCs and secretion of several pro-inflammatory factors.
While screening AML patients' plasma Sanchez-Correa et al. (2013) observed higher levels of IL-5, IL-6, and IL-10, including an inverse correlation between survival and plasma levels of IL-6 and IL-10. Several additional studies have shown that pro-inflammatory cytokines and growth factors play a crucial role in myeloid malignances by supporting the growth and survival of leukemic cells (Reynaud et al., 2011, Zhang et al., 2012). In our studies, loss of Tet2 resulted in significant increase in the expression of IL-5 and IL-6 and a reduction in IL-10 levels, implicating a role for these cytokines in the pathogenesis of AML driven by loss of Tet2 and expression of Flt3ITD. IL-5 produced by mast cells, T cells, and eosinophils and has been shown to play an important role in eosinophilia (Campbell et al., 1987, Sanderson, 1992). Our peripheral blood counts in Tet2−/−:Flt3ITD/ITD mice also showed increased levels of eosinophils, correlating with an increase in IL-5 levels, which could be a consequence of cell-mediated immunity against progression of lethal AML. Furthermore, elevated frequency of microenvironmental cells observed in Tet2−/− might also contribute to more cytokine production, thereby contributing to the aggressive AML phenotype seen in the current study. Several studies (Bochev et al., 2008, Corcione et al., 2006) have shown that MSCs inhibit B cell proliferation, differentiation, and immunoglobulin production. Our data on phenotypic analysis of Tet2−/−:Flt3ITD/ITD show reduced frequency of B220/CD19 double-positive cells but not single B220-positive cells in the BM, suggesting that the elevated numbers of MSCs/OBCs in the stroma of these animals might be critical for the maturation of B cells.
Tet2 mutations are shown to be present in both hematopoietic and non-hematopoietic malignancies. It is not clear whether the coexistence of hematopoietic and non-hematopoietic cancers occurs in the same individual because of Tet2 mutations. Given that cellular alterations are interrelated and occur simultaneously, the extent to which monocytes, ECs, or OBCs play an important role over another is not clear. Fuster et al. (2017), and Jaiswal et al. (2017) showed that loss of Tet2 results in enhanced recruitment of monocytes, which has been linked to accelerated atherosclerosis through increased production of cytokines and chemokines. Cull et al. (2017) showed that TET2 restrains inflammatory gene expression in macrophages; suggesting the possibility of altered microenvironment. ECs play a critical role in HSC maintenance. Duarte et al. (2018) have shown an increased number of ECs in AML spleens and that AML progression leads to differential remodeling of vasculature by producing pro-inflammatory and anti-angiogenic cytokines. Cogle et al. (2014) showed that functional integration of AML cells can either differentiate into endothelial-like cells or fuse with established endothelium. Osteoblasts play a critical role in the maintenance and expansion of HSC/Ps as well as oncogenic transformation (Dong et al., 2016, Kode et al., 2014, Kode et al., 2016).
In summary, our mouse genetics studies show that loss of function of Tet2 in cooperation with Flt3ITD/ITD expression have pathogenic effects on the BM microenvironment and on resident HSCs in promoting leukemogenesis. Our coculture data and transplantation data correlate with enhanced CD11b, EC, and OBC activity and/or numbers in Tet2−/−:Flt3ITD/+ mice (Figures 7I and S7B–S7D) suggesting that CD11b, ECs, and OBCs also contribute to the malignant phenotype.
Overall, our data support the notion that leukemic cells alter the BM niche by altering the microenvironmental cell composition and secretion of pro-inflammatory cytokines, which can promote the growth and survival of leukemic cells. Therapeutic strategies including reversing the damage caused to the niche by leukemia cells should be considered as part of the treatment process, while considering elimination of fertile ground for disease recurrence may be equally essential.
Experimental Procedures
Mice
All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine. The mice were housed in pathogen-free conditions at the Indiana University Laboratory Animal Research Center (Indianapolis, IN, USA). Flt3ITD/ITD mice (Lee et al., 2007) were crossed with Tet2−/− mice(Li et al., 2011) to generate the double-mutant mice. WT (C57BL/6) mice were procured from the In Vivo Core facility at Indiana University.
Flow-Cytometry Analysis
Immunophenotyping was performed as described previously (Ghosh et al., 2016). Preparation of single-cell suspension and staining is described in Supplemental Information.
Preparation of Stroma and Microenvironmental Cell Staining
Mesenchymal cells, ECs, and osteoblastic lineage cells from stroma were stained for flow cytometry following collagenase digestion of bone fragments as described earlier (Winkler et al., 2010, Schepers et al., 2013).
Acid and Alkaline Phosphatase Assay
TRAP and Vonkossa staining were done on femurs from 22-week-old from WT, Tet2−/−, and Tet2−/−:Flt3ITD/+ mice following the methods of Erlebacher and Derynck (1996) and Schenk (1984) at Indiana University School of Medicine Histology Core Facility. For in vitro experiments CD45-negative, lineage-negative stromal cells from collagenase digested bone cells were sorted directly into 12-well plates containing DMEM, 15% fetal bovine serum (FBS), 100 U penicillin/100 μg streptomycin, and 50 mM β-mercaptoethanol. Osteoblasts were induced by addition of 3 mM β-glycerophosphate and 10 mg/mL L-ascorbic acid-2-phosphate, and cultured for 2 weeks before TRAP staining was performed as per manufacturer's instructions (Sigma-Aldrich Kit, cat. #387A). Osteoclasts were cultured for 3 weeks and BCIP/NBT (5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium) staining was performed for alkaline phosphatase activity following manufacturer’s instructions (Thermo Scientific, cat. #34042).
BM Transplantation
Two million whole BM mononuclear cells isolated from WT, Tet2+/−, Flt3ITD/WT, Flt3ITD/ITD, Tet2+/−:Flt3ITD/WT, and Tet2+/−:Flt3ITD/ITD were transplanted into lethally irradiated C57BL/6 mice through the tail vein and monitored for disease progression.
Serum Cytokine Profiling
Serum was separated from peripheral blood and subjected to serum cytokine bioplex analysis through Eve Technologies (Canada). Results are expressed as pg/mL of serum.
Statistical Analysis
Median values of each group are shown with interquartile range. Each data point represents the value from individual mice in their respective groups in Figures 1, 2, 3, 4, and 6. Error bars indicate the standard error of the mean in Figures 5 and 7. Statistical analysis was performed using GraphPad version 7 by one-way ANOVA with uncorrected Fisher's test for multiple groups and Student's t test between two groups. p values in figures are denoted by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Author Contributions
B.R. and R.K. conceived the study, designed experiments, and wrote the manuscript. B.R., R.S.M., L.R.P., R.P., S.K.P., S.S.B., and Z.C. carried out the experiments and data analysis. All authors read and approved the manuscript.
Acknowledgments
We thank Riley Children's Foundation for their support to R.K. B.R. was supported by funds from the Showalter Foundation. This work was supported by grants from Leukemia and Lymphoma Society and National Institute of Health, USA.: (R01CA173852, R01CA134777, R01HL146137, and R01HL140961) to R.K. We gratefully thank our lab managing scientist Dr. Ping Hu and Tracy Winkle for their excellent administrative assistance. None of the authors listed have any financial interest related to this work.
Published: June 4, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.05.002.
Contributor Information
Baskar Ramdas, Email: ramdasb@iupui.edu.
Reuben Kapur, Email: rkapur@iupui.edu.
Supplemental Information
References
- Abdel-Wahab O., Levine R.L. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121:3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochev I., Elmadjian G., Kyurkchiev D., Tzvetanov L., Altankova I., Tivchev P., Kyurkchiev S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol. Int. 2008;32:384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Cagnetta A., Adamia S., Acharya C., Patrone F., Miglino M., Nencioni A., Gobbi M., Cea M. Role of genotype-based approach in the clinical management of adult acute myeloid leukemia with normal cytogenetics. Leuk. Res. 2014;38:649–659. doi: 10.1016/j.leukres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Campbell H.D., Tucker W.Q., Hort Y., Martinson M.E., Mayo G., Clutterbuck E.J., Sanderson C.J., Young I.G. Molecular cloning, nucleotide sequence, and expression of the gene encoding human eosinophil differentiation factor (interleukin 5) Proc. Natl. Acad. Sci. U S A. 1987;84:6629–6633. doi: 10.1073/pnas.84.19.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogle C.R., Goldman D.C., Madlambayan G.J., Leon R.P., Masri A.A., Clark H.A., Asbaghi S.A., Tyner J.W., Dunlap J., Fan G. Functional integration of acute myeloid leukemia into the vascular niche. Leukemia. 2014;28:1978–1987. doi: 10.1038/leu.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A., Benvenuto F., Ferretti E., Giunti D., Cappiello V., Cazzanti F., Risso M., Gualandi F., Mancardi G.L., Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Cull A.H., Snetsinger B., Buckstein R., Wells R.A., Rauh M.J. Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 2017;55:56–70.e13. doi: 10.1016/j.exphem.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Devos F.C., Pollaris L., Cremer J., Seys S., Hoshino T., Ceuppens J., Talavera K., Nemery B., Hoet P.H.M., Vanoirbeek J.A.J. IL-13 is a central mediator of chemical-induced airway hyperreactivity in mice. PLoS One. 2017;12:e0180690. doi: 10.1371/journal.pone.0180690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Yu W.M., Zheng H., Loh M.L., Bunting S.T., Pauly M., Huang G., Zhou M., Broxmeyer H.E., Scadden D.T. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539:304–308. doi: 10.1038/nature20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C.W., Shi J., Chen J., Wang B., Yu Y.H., Qin X., Zhou X.C., Cai Y.J., Li Z.Q., Zhang F. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014;25:778–793. doi: 10.1016/j.ccr.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Duarte D., Hawkins E.D., Akinduro O., Ang H., De Filippo K., Kong I.Y., Haltalli M., Ruivo N., Straszkowski L., Vervoort S.J. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell. 2018;22:64–77.e6. doi: 10.1016/j.stem.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A., Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S.J., Rowe J.M. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077–1082. doi: 10.1182/blood-2012-08-234492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohling S., Schlenk R.F., Breitruck J., Benner A., Kreitmeier S., Tobis K., Dohner H., Dohner K. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- Fuster J.J., MacLauchlan S., Zuriaga M.A., Polackal M.N., Ostriker A.C., Chakraborty R., Wu C.L., Sano S., Muralidharan S., Rius C. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J., Kobayashi M., Ramdas B., Chatterjee A., Ma P., Mali R.S., Carlesso N., Liu Y., Plas D.R., Chan R.J. S6K1 regulates hematopoietic stem cell self-renewal and leukemia maintenance. J. Clin. Invest. 2016;126:2621–2625. doi: 10.1172/JCI84565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnerio J., Mendez L.M., Asada N., Menon A.V., Fung J., Berry K., Frenette P.S., Ito K., Pandolfi P.P. A non-cell-autonomous role for Pml in the maintenance of leukemia from the niche. Nat. Commun. 2018;9:66. doi: 10.1038/s41467-017-02427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish A., Schwartz S.A. Targeted anti-IL-5 therapies and future therapeutics for hypereosinophilic syndrome and rare eosinophilic conditions. Clin. Rev. Allergy Immunol. 2020 doi: 10.1007/s12016-019-08775-4. [DOI] [PubMed] [Google Scholar]
- Ichiyama K., Chen T., Wang X., Yan X., Kim B.S., Tanaka S., Ndiaye-Lobry D., Deng Y., Zou Y., Zheng P. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 2015;42:613–626. doi: 10.1016/j.immuni.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Natarajan P., Silver A.J., Gibson C.J., Bick A.G., Shvartz E., McConkey M., Gupta N., Gabriel S., Ardissino D. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A., Manavalan J.S., Mosialou I., Bhagat G., Rathinam C.V., Luo N., Khiabanian H., Lee A., Murty V.V., Friedman R. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A., Mosialou I., Manavalan S.J., Rathinam C.V., Friedman R.A., Teruya-Feldstein J., Bhagat G., Berman E., Kousteni S. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia. 2016;30:1–13. doi: 10.1038/leu.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottaridis P.D., Gale R.E., Frew M.E., Harrison G., Langabeer S.E., Belton A.A., Walker H., Wheatley K., Bowen D.T., Burnett A.K. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- Lane S.W., Scadden D.T., Gilliland D.G. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., Tothova Z., Levine R.L., Anderson K., Buza-Vidas N., Cullen D.E., McDowell E.P., Adelsperger J., Frohling S., Huntly B.J. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T.J., Ding L., Walter M.J., McLellan M.D., Lamprecht T., Larson D.E., Kandoth C., Payton J.E., Baty J., Welch J. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhou Y., Cao Z., Liu L., Wang J., Chen Z., Xing W., Chen S., Bai J., Yuan W. TET2 loss dysregulates the behavior of bone marrow mesenchymal stromal cells and accelerates Tet2(-/-)-driven myeloid malignancy progression. Stem Cell Reports. 2018;10:166–179. doi: 10.1016/j.stemcr.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cai X., Cai C.L., Wang J., Zhang W., Petersen B.E., Yang F.C., Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead A.J., Neo W.H., Barkas N., Matsuoka S., Giustacchini A., Facchini R., Thongjuea S., Jamieson L., Booth C.A.G., Fordham N. Niche-mediated depletion of the normal hematopoietic stem cell reservoir by Flt3-ITD-induced myeloproliferation. J. Exp. Med. 2017;214:2005–2021. doi: 10.1084/jem.20161418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud D., Pietras E., Barry-Holson K., Mir A., Binnewies M., Jeanne M., Sala-Torra O., Radich J.P., Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Correa B., Bergua J.M., Campos C., Gayoso I., Arcos M.J., Banas H., Morgado S., Casado J.G., Solana R., Tarazona R. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–891. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Sanderson C.J. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- Sato H., Wheat J.C., Steidl U., Ito K. DNMT3A and TET2 in the pre-leukemic phase of hematopoietic disorders. Front. Oncol. 2016;6:187. doi: 10.3389/fonc.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk R. Preparation of calcified tissue for light microscopy. In: Dickson G.R., editor. Methods of Calcified Tissue Preparation. Elsevier; 1984. pp. 1–56. [Google Scholar]
- Schepers K., Pietras E.M., Reynaud D., Flach J., Binnewies M., Garg T., Wagers A.J., Hsiao E.C., Passegue E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A.H., Jiang Y., Meydan C., Shank K., Pandey S., Barreyro L., Antony-Debre I., Viale A., Socci N., Sun Y. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell. 2015;27:502–515. doi: 10.1016/j.ccell.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A., Lim K.H., Abdel-Wahab O., Lasho T.L., Patel J., Patnaik M.M., Hanson C.A., Pardanani A., Gilliland D.G., Levine R.L. Detection of mutant TET2 in myeloid malignancies other than myeloproliferative neoplasms: CMML, MDS, MDS/MPN and AML. Leukemia. 2009;23:1343–1345. doi: 10.1038/leu.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Xu Y., Yin J., Tian H., Chen S., Wu D., Sun A. TET2 gene mutation is unfavorable prognostic factor in cytogenetically normal acute myeloid leukemia patients with NPM1+ and FLT3-ITD- mutations. Int. J. Hematol. 2014;100:96–104. doi: 10.1007/s12185-014-1595-x. [DOI] [PubMed] [Google Scholar]
- Wakita S., Yamaguchi H., Omori I., Terada K., Ueda T., Manabe E., Kurosawa S., Iida S., Ibaraki T., Sato Y. Mutations of the epigenetics-modifying gene (DNMT3a, TET2, IDH1/2) at diagnosis may induce FLT3-ITD at relapse in de novo acute myeloid leukemia. Leukemia. 2013;27:1044–1052. doi: 10.1038/leu.2012.317. [DOI] [PubMed] [Google Scholar]
- Weissmann S., Alpermann T., Grossmann V., Kowarsch A., Nadarajah N., Eder C., Dicker F., Fasan A., Haferlach C., Haferlach T. Landscape of TET2 mutations in acute myeloid leukemia. Leukemia. 2012;26:934–942. doi: 10.1038/leu.2011.326. [DOI] [PubMed] [Google Scholar]
- Winkler I.G., Sims N.A., Pettit A.R., Barbier V., Nowlan B., Helwani F., Poulton I.J., van Rooijen N., Alexander K.A., Raggatt L.J. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Yu H., Sui Y., Wang Y., Sato N., Frey B., Xia Z., Waldmann T.A., Berzofsky J. Interleukin-15 constrains mucosal T helper 17 cell generation: influence of mononuclear phagocytes. PLoS One. 2015;10:e0143001. doi: 10.1371/journal.pone.0143001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Ho Y.W., Huang Q., Maeda T., Lin A., Lee S.U., Hair A., Holyoake T.L., Huettner C., Bhatia R. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21:577–592. doi: 10.1016/j.ccr.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.