Abstract
MicroRNA (miRNA) has been reported to exert important functions in papillary thyroid carcinomas (PTC). However, the role of miRNA in aggressive PTC (APTC) remains unclear. Here, we investigated the diagnostic potentials and mechanisms of miR-221/222 in APTC. Results showed that miR-221/222 were markedly up-regulated in PTC, compared with the adjacent normal tissue (ANT). A high expression of miR-221/222 were associated with a primary tumor, regional lymph node, and distant metastasis (TNM) stage, multicentricity, lymph node metastasis, and extra-thyroidal extension. Receiver operating characteristic (ROC) curve analysis indicated that miR-221/222 could be used as APTC diagnostic markers. Moreover, miR-221/222 tremendously promoted migration and invasion and inhibited apoptosis and autophagy in PTC cells. A luciferase assay showed that miR-221/222 inhibited the fluorescent activity of autophagy-related protein 10 (ATG10). Furthermore, miR-221/222 decreased ATG10 mRNA and protein levels. Silencing of ATG10 significantly abrogated the effect of miR-221/222 on apoptosis and autophagy. We suggested that miR-221/222 can promote migration and invasion, and inhibit autophagy and apoptosis by targeting ATG10 in APTC.
Keywords: microRNA-221/222, Receiver operating characteristic curve, Diagnostic markers, Aggressive papillary thyroid carcinoma, qRT-PCR
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid carcinoma, accounting for more than 90% of all thyroid cancers (Kitahara and Sosa 2016). The incidence of advanced-stage PTC is steadily increasing, which is largely driven by an increasing worldwide trend in thyroid cancer incidence (Wang and Sosa 2018; Zheng et al. 2009). Aggressive PTC (APTC) is an aggressive form of PTC with columnar variants and moderate differentiation (Costa et al. 2008). Thus, aggressiveness was the primary cause of progression and recurrence of PTC. PTC could be readily diagnosed using a cytological examination of a nodule aspirated using a fine-needle due to the fact that a solitary thyroid nodule usually appears in thyroid carcinoma patients (Sherman 2003). Fine needle aspiration cytology of the thyroid has inherent limitations, producing 20–30% of determinate results. However, this limitation might be overcome using a molecular diagnosis (Eszlinger et al. 2017). Therefore, the investigation and identification of diagnostic biomarkers could contribute to the exploration of effective schemes for APTC diagnosis and therapy.
MicroRNAs (miRNAs) are 20–25 nucleotides in length, which are small non-coding RNA strands that are evolutionary conserved (Lemcke and David 2018). Many miRNAs could derive from genetics and exert a coordinated expression and function; for example, miR-221 and miR-222 (miR-221/222) are expressed in the same single transcript, which was found to belong to the X chromosome (Jeong et al. 2017). Studies have reported that the dysregulation of miR-221/222 was ubiquitously detected, and that they could serve as oncogenes in all types of tumors, such as gastric cancer (Ning et al. 2017), prostate carcinoma (Galardi et al. 2011), glioblastoma (Galardi et al. 2011), breast cancer (Shah and Calin 2011), and cervical cancer (Yang et al. 2016). In a previous review, miR-221/222 have incurred broad attention as a targeting choice in cancer therapies and might be described as diagnostic, prognostic, and therapeutic biomarkers in various diseases, including cancer (Song et al. 2017; Di Martino et al. 2016; Sredni et al. 2010; Zhang et al. 2016). MiRNAs are also expected to identify mutation-negative malignant nodules, as part of the rule-in approach, and are considered to provide additional markers (Eszlinger et al. 2017). Studies have reported that miR-221 (Deng et al. 2017) and miR-222 (Lee et al. 2013) could serve as potential biomarkers in recurrent PTC. However, whether miR-221/222 could be considered diagnostic biomarkers of APTC has not been studied.
Autophagy is an evolutionarily conserved mechanism of lysosomal-mediated bulk degradation of cytoplasmic components in all eukaryotic cells, and plays an important role in cellular homeostasis (Karakaş and Gözüaçik 2014; Yu et al. 2019). Autophagy also plays an important role in maintaining cellular homeostasis (Gomes and Dikic 2014) and resisting foreign pathogens, together with the immune system (Zhao et al. 2017). In recent years, many laboratories have made tremendous contributions to the understanding and appreciation of physiologically significant molecules of the autophagy process (Nakatogawa et al. 2009; Levine and Kroemer 2008; Mizushima 2007; Xie and Klionsky 2007). It has been reported that miRNA-221 induces autophagy by inhibiting HDAC6 expression and promotes apoptosis of pancreatic cancer cells (Yang et al. 2018). MiR-221 targets TP53INP11 to inhibit autophagy of colorectal cancer (CRC) cells (Liao et al. 2018). However, whether miR-221/222 affect autophagy and apoptosis of PTC cells, remains unclear.
In our study, to explore the diagnostic potential of miR-221/222 in APTC, the correlation between the expression of miR-221/222 and APTC was analyzed using ROC curves. The role of miR-221/222 in autophagy and apoptosis was measured using western blot (WB) and FACS. Moreover, we used a luciferase assay to confirm the interaction of miR-221/222 with autophagy-related protein 10 (ATG10) to illustrate the miR-221/222 mechanism.
Methods
Clinical specimens
To study the expression of miR-221/222 in PTC and normal tissues, the tissues of PTC and their matched adjacent normal tumor tissues (ANT) were obtained from PTC patients (n = 10) undergoing curative-intent surgery at the Department of Surgery, Jing’An District Central Hospital, between July 2010 and May 2013. This study was approved by the ethics committee of Jing’An District Central Hospital (Shanghai, China). All patients provided a written informed consent. All tissues of PTC and their ANT were immediately separated during surgery, snap-frozen in liquid nitrogen, and preserved at − 80 °C. The clinical information for these patients is presented in Table 1.
Table 1.
Clinical information of patients with PTC
| Characteristics | Patient (n = 10) |
|---|---|
| Mean age (range), years | 59.1 ± 8.65 |
| Male:female | 7:3 |
| Differentiation degree | |
| High | 2 |
| Medium | 6 |
| Low | 2 |
| Proportion of tumor (%) | 50 ± 15.97 |
PTC papillary thyroid carcinoma
Additionally, to investigate the association of miR-221/222 with aggressive PTC, 47 APTC and 40 PTC patients were included. The inclusion criteria of APTC: (1) The PTC was confirmed by its pathology. (2) The tumors display extra-thyroidal extension and invasion of adjacent tissues or organs, which was confirmed using combined CT or Magnetic Resonance Imaging (MRI) manifestations, findings during operation, intraoperative frozen examination, and postoperative paraffin pathological results. The clinicopathologic variables were collected and included age, sex, tumor size, TNM stage, multicentricity, LN metastasis, and APTC. The clinicopathological information of patients with PTC is presented in Table 2.
Table 2.
Clinicopathological phenotypes of patients with PTC
| Characteristics | Patient (n = 87) |
|---|---|
| Mean age (range), years | 46 ± 0.88 |
| Male:female | 1:1.7 |
| Tumor size (cm) | 1.50 ± 0.88 |
| Extra-thyroidal extension | 54.02% (47/87) |
| Lymph node (LN) metastasis | 56.32% (49/87) |
| TNM stage (AJCC) | |
| I | 20 |
| II | 23 |
| III | 18 |
| IV | 26 |
PTC papillary thyroid carcinoma
Quantitative real-time polymerase chain reaction (qRT-PCR)
Trizol reagent (Invitrogen, MA, USA) was used to extract RNA from PTC tissues and their ANT. NanoDrop 1000 (Thermo Fisher Scientific, MA, USA) was used to evaluate the concentration and quality of the RNA. For the detection of mature miRNA, miRNAs were reverse transcribed into complementary DNA (cDNA) using Thermo Scientific™ RevertAidAM First Strand cDNA Synthesis kit (#K1622). qRT-PCR involved the appropriate TaqMan miRNA assay (Applied Biosystems Inc., Carlsbad, USA) and was performed using an ABI Q6 detection system (Applied Biosystems Inc., Carlsbad, USA). The comparative threshold (Ct) cycle method (2–ΔΔCt) was used to assess the relative expression of miRNA (Yang et al. 2016). Quantification of U6 served as an endogenous control. Table 3 shows the primer sequences, which were designed and synthesized (Yingbio, Shanghai, China).
Table 3.
Primers for the detection of miR221/222 by RT-qPCR
| Primer | Sequence |
|---|---|
| miR-221 | F: 5′ CGCAGCCTGGCATACAATG 3′ |
| R: 5′ AGTGCGTGTCGTGGAGTCG 3′ | |
| miR-222 | F: 5′ GCGGGAGCTACATCTGGCTA 3′ |
| R: 5′ AGTGCGTGTCGTGGAGTCG 3′ | |
| U6 | F: 5′ CGATACAGAGAAGATTAGCATGGC 3′ |
| R: 5′ AACGCTTCACGAATTTGCGT 3′ |
Cell culture and cell transfection
Human PTC cell line K1 and human renal epithelial cell line 293 T (tool cell) was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences in China. Cells were cultured in RPMI 1640 medium (Corning, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Sangon Biotech, Shanghai, China), 100 U/mL penicillin G, and 100 g/mL streptomycin (Sigma-Aldrich Co., St. Louis, USA) and maintained at 37 °C in a humidified cell incubator containing 5% CO2. The cells were selected for further experiments at the exponential phase. The cultured K1 cells (3 × 105) were uniformly seeded in six-well cultured plates. After cells were completely adherent, they were transiently transfected at 37 °C for 24 h with miR-221/222 mimics, mimics negative control (NC), miR-221/222 inhibitor, inhibitor NC, and miR-221/222 inhibitor + ATG10 siRNA (designed and synthesized by Invitrogen, MA, USA) with a final concentration of 50 nM, together with NC or ATG10 siRNA, using Lipofectamine™ 2000 (Invitrogen, MA, USA).
Flow cytometric analysis of apoptosis
K1 cells were sourced from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, and were transfected with miR-221/222 mimics, mimics NC, miR-221/222 inhibitor, and inhibitor NC. Then, they were seeded onto 6-well dishes and at 60–80% confluency. Cells were harvested with EDTA and fixed in 75% ethanol for 30 min at 4 °C, followed by a wash with phosphate-buffered saline (PBS, pH 8.0) (Corning, NY, USA) containing 2% FBS. Next, cells were treated with 1.8 ng/mL annexin-V-FITC (Roche Applied Science, Indianapolis, USA) for 15 min, under refrigeration (4 °C), and protected from light, and stained with propidium iodide (PI) (Becton Dickinson, CA, USA). Finally, the samples were rapidly analyzed using the FACS Calibur flow cytometer equipped with the FACS DIVA software.
Transwell assay
A transwell assay was used to estimate the effects of miR-221/222 on the migration and invasion abilities of K1 cells. After the cells were washed with PBS, 0.25% Trypsin–EDTA was added and digestion lasted for 3 min. The cells were resuspended in serum-free RPMI1640 (10–040-CVR) (Corning, NY, USA) medium. K1 cells (2 × 105/mL in serum-free medium) were placed in the top chamber of the transwell migration chamber (0.8 μm 24-well, FALCON) (BD Biosciences, CA, USA), whereas the lower chamber was loaded with 0.8 mL of medium containing 10% FBS. After 24 h, the K1 cells that had not migrated onto the transwell membrane surface were removed. Migrating cells on the lower membrane surface were fixed and stained using a crystal violet staining solution (C0121) (Beyotime Biotechnology Technology Co., Ltd, Shanghai, China). Six visual fields were randomly chosen for counting. In vitro invasion assays were done under the same conditions as the transwell migration assay, but in invasion chambers (BioCoat™ Matrigel® 0.8 μm 24-well, BioCoat, Tianjin, China) and Matrigel-coated transwells (BD Biosciences, CA, USA). The experiment was repeated three times.
Western blot analysis
The cells were washed three times using PBS. Then, they were lysed using radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing protease inhibitors (Pierce, USA). Total protein was quantified using a Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, USA). An equal amount of proteins was separated on an SDS-PAGE gel, and then transferred onto polyvinylidene difluoride membranes (Millipore, CA, USA). A blocking solution was added overnight at 4 °C. Membranes were incubated with a high-affinity anti-LC3II antibody (1:1000) (Abcam, Cambridge, USA), anti-ATG10 antibody (1:1000) (Abcam, Cambridge, USA), anti-GAPDH antibody (1:1000, 60,004–1-Lg) (Proteintech, Chicago, USA) at room temperature for 2 h, and then, with goat anti-rabbit HRP-IgG infrared-dye-conjugated secondary antibodies (1:10,000) (Abcam, Cambridge, USA) at 25 °C for 1 h. Anti-GAPDH antibody (1:1000, 60,004–1-Lg) (Proteintech, Chicago, USA) was used as a loading control. After washes, the immunoreactive bands were detected using the enhanced luminol-based chemiluminescent substrate (ECL, GE, USA) and analyzed using the Image Lab 6.0.1 software (Bio-Rad Laboratories, Hercules, USA).
Luciferase Reporter assay
For the luciferase assay, approximately 5 × 103 293 T cells were added into a six-well plate and incubated at 37 °C until the cells were 60–80% confluent. The cells were rinsed 2–3 times with PBS. 293 T cells were seeded in 24-well plates and co-transfected with the pGL-3 vector containing the wild-type or the mutant type of mimics NC, miR-221/222 mimics, inhibitor NC, and miR-221/222 inhibitor using Lipofectamine 2000 (Invitrogen, MA, USA). pRL-SV40 (Promega, WI, USA) was used as the control vector. The luciferase reporter assay system (Promega, WI, USA) was utilized to determine the relative luciferase activity of PTC cells, 48 h after transfection. The specific activity is expressed as the fold change of the experimental group compared to the miR-control group. The tests were repeated independently in triplicates.
Statistical analysis
Statistical analysis was carried out using the SPSS 12.0 and the data was visualized using the software GraphPad prism 8.0. The P values were used to evaluate the significance of miR-221/222 relative expression (2−ΔΔCT) using student’s t-test. All experiments were performed at least three times in triplicates for each group. For the multivariate analysis, risk scores of miR-221/222 were obtained by calculating the 2−ΔΔCT values after performing qRT-PCR. Receiver operating characteristic (ROC) curve was generated using SPSS 12.0 statistical software. The area under the ROC curve (AUC) was performed to evaluate survival prediction. The data were displayed as mean ± standard deviation (SD) and a difference with a P < 0.05 was considered statistically significant.
Results
Expression of miR-221/222 were significantly up-regulated in PTC
To study the characteristics of miR-221/222 in PTC malignancy, the expression levels of miR-221/222 in the tissue of PTC and their ANT were determined using qRT-PCR. The results (Fig. 1) showed that miR-221 expression increased about 35-fold in PTC, compared with their ANT (n = 10) (P = 0.006). For miR-222, there was a 23-fold increase in PTC compared with their ANT (n = 10) (P = 0.001).
Fig. 1.
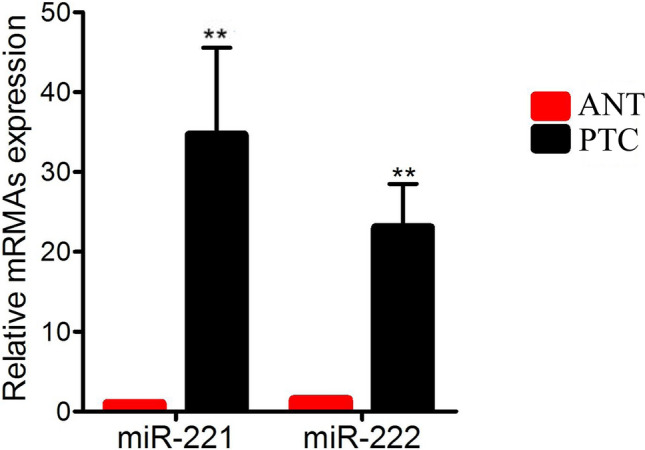
The different expression of miR-221/222 in papillary thyroid carcinoma (PTC) and their adjacent normal tissue (ANT). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect the relative expression of miR-221/222 in PTC tissues and their ANT of PTC patients, n = 10. The results showed that miR-221/222 were up-regulated in PTC tissues, compared with their ANT (the fold-change of miR-221 was about 35, and that of miR-222 was about 23). The data were analyzed using student’s t-test. Red and black histograms represent ANT and PTC groups, respectively. Error bars indicate mean ± SD (**P < 0.01)
MiR-221/222 were associated with clinicopathological aggressive phenotypes of PTC
To further confirm the correlation between miR-221/222 and clinicopathological phenotypes of PTC, the expression levels of miR-221/222 were determined under the different conditions of clinicopathological phenotypes of PTC, using qRT-PCR. Table 4 reveals that the expression of miR-221/222 does not significantly correlate with age. At the same time, expression of miR-221/222 was dramatically correlated with TNM stage, tumor size, multicentricity, LN metastasis, and extra-thyroidal extension. Interestingly, the highly expressed miR-221/222 was significantly positively correlated with PTC invasiveness.
Table 4.
Correlation of miR-221/222 with clinicopathological phenotypes of patients with PTC
| Clinicopathological feature | Number | miR-221 level (mean ± SD) | P value | miR-222 level (mean ± SD) | P value |
|---|---|---|---|---|---|
| Age(year) | |||||
| < 45 | 35 | 3.55 ± 0.63 | 0.625 | 2.79 ± 1.10 | 0.018 |
| ≥ 45 | 52 | 3.72 ± 1.98 | 3.17 ± 0.25 | ||
| Size(cm) | |||||
| ≤ 1 | 30 | 2.97 ± 0.18 | < 0.0001 | 2.36 ± 0.52 | < 0.0001 |
| > 1 | 57 | 3.94 ± 0.68 | 3.34 ± 0.73 | ||
| TNM stage (AJCC) | |||||
| I + II | 43 | 2.30 ± 0.96 | < 0.0001 | 2.36 ± 0.52 | < 0.0001 |
| III + IV | 44 | 3.30 ± 0.61 | 2.85 ± 0.44 | ||
| Mulifocality | |||||
| Yes | 51 | 3.53 ± 0.12 | < 0.0001 | 3.36 ± 0.50 | < 0.0001 |
| No | 36 | 3.04 ± 0.55 | 2.82 ± 0.62 | ||
| Lymph node (LN) metastasis | |||||
| Yes | 49 | 3.59 ± 0.63 | < 0.0001 | 3.86 ± 0.41 | < 0.0001 |
| No | 38 | 2.60 ± 0.63 | 2.78 ± 0.64 | ||
| Extra-thyroidal extension | |||||
| Yes | 47 | 4.88 ± 0.98 | < 0.0001 | 4.56 ± 0.76 | < 0.0001 |
| No | 40 | 2.57 ± 0.86 | 2.17 ± 0.75 | ||
MiR-221/222 act as diagnostic biomarkers for APTC
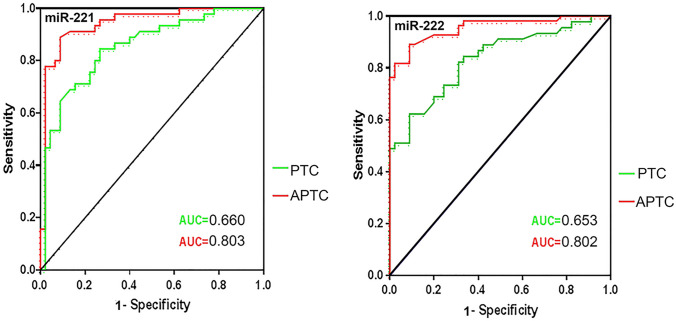
Clinicopathological diagnoses were the gold standard to assess the diagnostic accuracy of miRNAs using ROC curve analysis. To determine the diagnostic potential of miR-221/222 in APTC, ROC curves were constructed in PTC (n = 40) and APTC (n = 47) (Fig. 2). ROC curve analysis generated the AUC values of 0.660 for miR-221 (95% confidence interval (CI) 0.524–0.797), 0.653 for miR-222 (95% CI 0.512–0.794) in PTC, 0.803 for miR-221 (95% CI 0.735–0.871), and 0.802 for miR-222 (95% CI 0.737–0.868) in APTC. In addition, the 0.087 and 0.251 were the optimal cutoff values for miR-221 and miR-222, respectively, in APTC. With these cutoff values, the sensitivity and specificity values of miR-221 were 80.74 and 74.8%, respectively, and those of miR-222 were 78.3 and 73.2%, respectively.
Fig. 2.
The diagnosis potentials of miR-221/222 in aggressive PTC (APTC). The Receiver operating characteristic (ROC) curves were constructed using the expression of miR-221/222 to determine the diagnostic potential of miR-221/222 in PTC (n = 40) and APTC (n = 47). AUC for miR-221/222 demonstrates accuracy in terms of sensitivity and specificity in APTC. The ROC curve yielded a miR-221/222 area under the ROC curve (AUC) value higher in APTC, compared to PTC (the AUC value of miR-221 was 0.660, and that of miR-222 was 0.803). The optimal cutoff value of miR-221 was 0.0087 (sensitivity, 80.74%; specificity, 74.8%) and that of miR-222 was 0.251 (sensitivity, 78.3%; specificity, 73.2%) in APTC. (Red and green represent APTC and PTC, respectively.)
MiR-221/222 inhibit autophagy in PTC cells
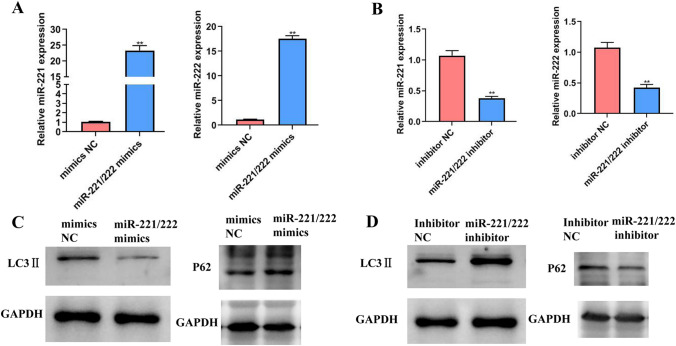
To verify the function of miR-221/222 in PTC cells, we transfected miR-221/222 mimics and inhibitor in K1 cells. The results showed that the expression of miR-221/222 was significantly increased in cells transfected with miR-221/222 mimics, but significantly decreased in cells transfected with miR-221/222 inhibitors, compared with the NC (Fig. 3a, b). WB results confirmed that the expression of autophagy protein LC3II was decreased and the protein level of autophagy-associated gene p62 (SQSTM1) was increased in miR-221/222 mimics, compared with the mimics NC; the expression of LC3II was increased and that of P62 was decreased in miR-221/222 inhibitors, compared with the inhibitor NC (Fig. 3c, d). This result indicated that miR-221/222 inhibit autophagy in PTC cells.
Fig. 3.
The effect of miR-221/222 on autophagy of PTC cells. a, b The expression levels of miR-221/222 were quantified using qRT-PCR, following transfection. c, d Western blot (WB) was used to verify the expression of autophagy protein LC3II and P62, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. (miR, microRNA; mimics, overexpression; inhibitor, interference; mimics NC, overexpression negative control; inhibitor NC, interference negative control. **P < 0.01 was considered statistically significant. Error bars indicate mean ± SD)
MiR-221/222 promote migration and invasion and inhibit apoptosis in PTC cells
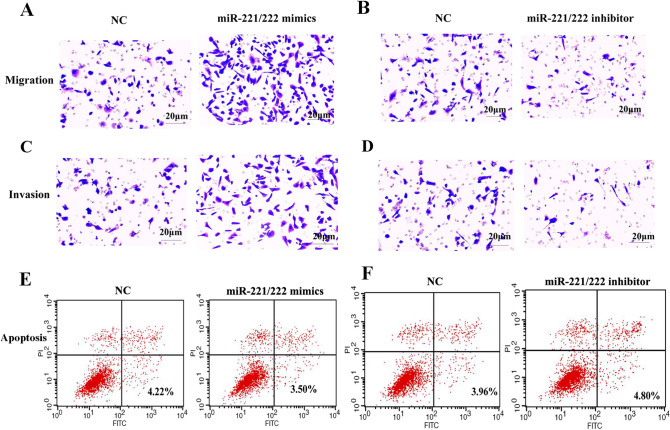
To investigate whether miR-221/222 can regulate migration and invasion in PTC cells, we did a further verification using a transwell assay. MiR-221/222 significantly promoted cellular migration and miR-221/222 inhibitor suppressed cell migration, compared with the control group (Fig. 4a, b). MiR-221/222 significantly promoted cellular invasion and miR-221/222 inhibitor suppressed cell invasion, compared with the control group (Fig. 4c, d). Flow cytometry was operated to evaluate apoptosis of PTC cells transfected with miR-221/222 mimics or miR-221/222 inhibitor. The results showed that miR-221/222 mimics suppressed cell apoptosis and that miR-221/222 inhibitor promoted cell apoptosis, compared with the control group (Fig. 4e, f). Hence, these data indicated that miR-221/222 promoted migration and invasion, and reduced apoptosis in PTC cells.
Fig. 4.
The effect of miR-221/222 on migration, invasion, and apoptosis of PTC cells. a, b The transwell assay shows the effect of interference or overexpression of miR-221/222 on the migration of PTC cells. c, d Transwell assay shows the effect of miR-221/222 on the invasion of PTC cells. e, f Flow cytometry shows the effect of miR-221/222 on the apoptosis of PTC cells. The test was repeated independently in triplicates. Two-sample and other variance t tests were used for data treatment. (miR, microRNA; mimics, overexpression; inhibitor, interference; mimics NC, overexpression negative control; inhibitor NC, interference negative control. The scale is 20 μm.)
ATG10 is targeted and regulated by miR-221/222
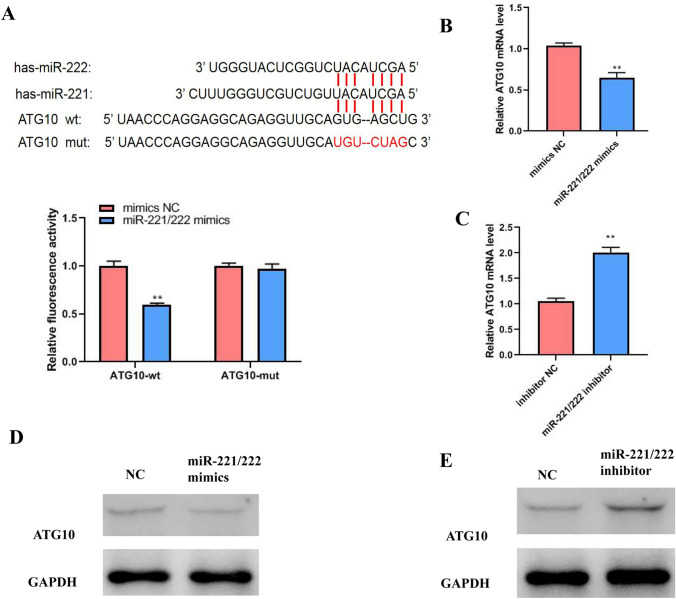
Bioinformatic analysis identified ATG10 as a potential miR-221/222 target. Further verification using a luciferase assay, showed that an overexpression of miR-221/222 inhibited luciferase activity of wild-type ATG10, while luciferase activity of mutant ATG10 in 293 T cells was not affected, compared with mimics NC (Fig. 5a). In addition, the levels of ATG10 expression were detected using qRT-PCR and WB analysis following transfection. These results show that miR-221/222 mimics down-regulated the expression of ATG10 mRNA and protein, while the expression of ATG10 protein was promoted by miR-221/222 inhibitor in K1 cells (Fig. 5b–e). The above data proved that miR-221/222 can target ATG10.
Fig. 5.
Autophagy-related protein 10 (ATG10) is a target of miR-221/222. a Luciferase reporter assay was used to detect the luciferase activity of wt and mut ATG10 in PTC cells. Wt and mut of the putative miR-221/222 targeting sequence in ATG10 mRNA. b, c qRT-PCR shows the expression of ATG10 protein in PTC cells following transfection. d, e WB analysis shows ATG10 protein levels, GAPDH was used as a control. The test was repeated independently in triplicates. Two-sample and other variance t-tests were used for data treatment. Error bars indicate mean ± SD. (**P < 0.01 was considered to be statistically significant; mimics, overexpression; inhibitor, interference; mimics NC, overexpression negative control; inhibitor NC, interference negative control; miR, microRNA; wt, wild-type; mut, mutant)
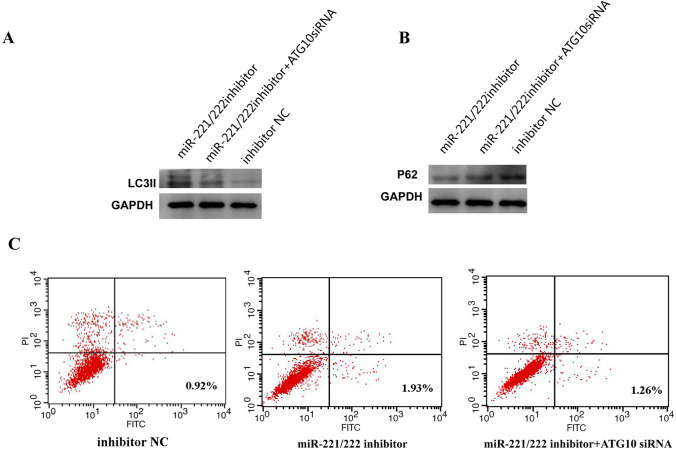
ATG10 siRNA could rescue miR-221/222 inhibitor mediated enhancement of autophagy and apoptosis
To further explore the role of miR-221/222, we examined LC3II and P62 protein levels in inhibitor NC, miR-221/222 inhibitor, miR-221/222 inhibitor, and ATG10 siRNA cells. WB confirmed that LC3II levels were enhanced by miR-221/222 inhibitor and then rescued by ATG10 siRNA (Fig. 6a). In addition, P62 level was down-regulated in miR-221/222 inhibitor cells, compared with the inhibitor control group, and this effect was abrogated by ATG10 siRNA, to promote autophagy (Fig. 6b). Flow cytometry analysis showed that miR-221/222 inhibitor promoted the apoptosis of PTC cells compared to the control group, while ATG10 siRNA suppressed this effect (Fig. 6c). Thus, our results demonstrated that miR-221/222 inhibits the autophagy process of PTC via inhibition of ATG10 activation.
Fig. 6.
MiR-221/222 inhibits autophagy and apoptosis by ATG10. a, b WB was used to verify the effects of miR-221/222 inhibitor or miR-221/222 inhibitor and ATG10 siRNA on autophagy protein LC3II and P62. c Flow cytometry shows the effect of miR-221/222 inhibitor or miR-221/222 inhibitor and ATG10 siRNA on the apoptosis of PTC cells. The test was repeated independently in triplicates. Two-sample and other variance t-tests were used for treatment. (miR, microRNA; mimics, overexpression; inhibitor, interference; mimics NC, overexpression negative control; inhibitor NC, interference negative control)
Discussion
For most PTC patients, the prognosis is excellent. However, an aggressive behavior is seen in some cases and approximately 25% of patients have presented established or developing distant metastases (Danysh et al. 2016). Aggressiveness is the primary cause of progression and recurrence of PTC. A recent study has revealed that miR-221/222 could serve as diagnostic biomarkers in PTC recurrence (Lee et al. 2013; Dai et al. 2017). However, the diagnostic biomarkers and mechanism of miR-221/222 in APTC have not yet been studied. In this study, we revealed that high-expressed miR-221/222 were significantly correlated with aggressive clinicopathological phenotypes of PTC, and miR-221/222 might be considered potential diagnostic biomarkers of APTC. We verified that miR-221/222 stimulate the migration and invasion and inhibits autophagy and apoptosis by targeting and down-regulating ATG10 in PTC cells.
Some clinicopathologic factors have been shown to be associated with the characteristics of PTC, which include progression and a poor prognosis, including age, tumor size, TNM stage, multifocality, LN metastasis, and extra-thyroidal extension (Kim et al. 2012; Jeon et al. 2012). MiR-221 might improve PTC cell proliferation and invasion by suppressing the expression of TIMP3 (Diao et al. 2017). Our research found that the expression of miR-221/222 tended to be increased in different aggressive clinicopathological phenotypes of PTC. The expression of miR-221/222 was significantly correlated with TNM stage, multicentricity, LN metastasis, and extra-thyroidal extension. The expression of miR-221/222 was also dramatically high-regulated in PTC compared with their ANT, which further emphasized the high-expression trend of miR-221/222 in PTC. Some studies have described that miR-221/222 may exert predictive roles in cancer (Di Martino et al. 2016). For example, AUC values of miR-221 (AUC 0.84) and of miR-222 (AUC 0.92) were yielded using ROC curve analysis in glioma and demonstrated that miR-221/222 were associated with a poor survival rate of glioma patients (Zhang et al. 2016). In this study, ROC results clearly showed that miR-221/222 was identified as a predictive diagnostic biomarker for APTC. MiR-221/222 are the most sensitive miRNAs for PTC (He et al. 2005). The investigation and identification of diagnostic biomarkers could contribute to the exploration of effective schemes of APTC diagnosis and therapy. All reports suggested that miR-221/222 might play a pivotal role in the occurrence and progression of PTC. Thus, we expect that miR-221/222 might be an indicator and target for APTC.
Autophagy is a dual function participant in catabolic degradation processes by eliminating damaged organelles, misfolded or aggregated proteins, and facilitating the overcoming of cellular stress during cancer progression (Wang et al. 2016a, b; Gugnoni et al. 2016; Mathew et al. 2007). For the first time, Jo et al. (2012) reported that the increased expression of ATG10 was correlated with lymphatic vascular invasion and lymph node metastasis in CRC. ATG10 may be a new prognostic biomarker for gastric cancer (Cao et al. 2016). Xie et al. (2016) found that ATG10 rs10514231 may affect lung adenocarcinoma. Recently, miR-221 has been reported to induce autophagy by inhibiting HDAC6 expression and to promote apoptosis of pancreatic cancer cells (Yang et al. 2018). MiR-221 targets TP53INP11 to inhibit autophagy of CRC cells (Liao et al. 2018). The above data indicated that autophagy has a complex impact on the development of cancer. However, the regulation of miR-221/222 on autophagy is still unclear, especially regarding their role in autophagy regulation in PTC. Wang et al. (2016a) evaluated the ability of autophagy by detecting the expression level of LC3-I, LC3-II and P62. In the present study, we demonstrated that miR-221/222 inhibit autophagy activity and apoptosis of PTC cells by targeting tumor protein ATG10.
Our study suggested that miR-221/222 are significantly associated with aggressive clinicopathological phenotypes of PTC. We also demonstrated that miR-221/222 may significantly promote migration and invasion, suppress autophagy and apoptosis of PTC cells by down-regulation of ATG10 expression. Thus, ATG10 is a new target of miR-221/222 in PTC cells and miR-221/222 might be a promising therapeutic target for APTC.
Author contributions
Conceptualization and funding acquisition: XY. Data curation: HS and ZL. Formal analysis: HY and LW. Investigation: HS, ZL, BM and HL. Methodology:BY, SH and ZL. Writing—original draft: HS and ZL. Writing—review and editing: HS, WL, JT and HY. All authors read and approved the final manuscript.
Funding
This work was sponsored by the third batch of ten hundred thousand project of health and family planning system in Jing’An district, Shanghai (JWRC2014Q13).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee (number: 2016-39) of Jing’An District Central Hospital (Shanghai, China). Written informed consent forms were obtained from all the subjects and their patients in this study.
Patient consent for publication
All patients provided informed consent and agreed to publish.
Footnotes
Hao Shen and Zaikai Lin equal contributors.
References
- Cao QH, Liu F, Yang ZL, et al. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res. 2016;8:3831–3847. [PMC free article] [PubMed] [Google Scholar]
- Costa AM, Herrero A, Fresno MF, et al. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrinol. 2008;68(4):618–634. doi: 10.1111/j.1365-2265.2007.03077.x. [DOI] [PubMed] [Google Scholar]
- Dai L, Wang Y, Chen L, Zheng J, Li J, Wu X. MiR-221, a potential prognostic biomarker for recurrence in papillary thyroid cancer. World J Surg Oncol. 2017;15:11. doi: 10.1186/s12957-016-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysh BP, Rieger EY, Sinha DK, Evers CV, Cote GJ, Cabanillas ME, Hofmann M-C. Long-term vemurafenib treatment drives inhibitor resistance through a spontaneous KRAS G12D mutation in a BRAF V600E papillary thyroid carcinoma model. Oncotarget. 2016;7:30907–30923. doi: 10.18632/oncotarget.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Lei Q, Wang Y, et al. Downregulation of miR-221-3p and upregulation of its target gene PARP1 are prognostic biomarkers for triple negative breast cancer patients and associated with poor prognosis. Oncotarget. 2017;8:108712–108725. doi: 10.18632/oncotarget.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino MT, Rossi M, Caracciolo D, Gulla A, Tagliaferri P, Tassone P. Mir-221/222 are promising targets for innovative anticancer therapy. Expert Opin Ther Targets. 2016;20:1099–1108. doi: 10.1517/14728222.2016.1164693. [DOI] [PubMed] [Google Scholar]
- Diao Y, Fu H, Wang Q. MiR-221 exacerbate cell proliferation and invasion by targeting TIMP3 in papillary thyroid carcinoma. Am J Ther. 2017;24:e317–e328. doi: 10.1097/MJT.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eszlinger M, Lau L, Ghaznavi S, Symonds C, Chandarana SP, Khalil M, Paschke R. Molecular profiling of thyroid nodule fine-needle aspiration cytology. Nat Rev Endocrinol. 2017;13:415–424. doi: 10.1038/nrendo.2017.24. [DOI] [PubMed] [Google Scholar]
- Galardi S, Mercatelli N, Farace MG, Ciafre SA. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39:3892–3902. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell. 2014;54(2):224–233. doi: 10.1016/j.molcel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Gugnoni M, Sancisi V, Manzotti G, Gandolfi G, Ciarrocchi A. Autophagy and epithelial-mesenchymal transition: an intricate interplay in cancer. Cell Death Dis. 2016;7:e2520. doi: 10.1038/cddis.2016.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HM, Lim BJ, Chang HS, Hong S. The definition of minimal extrathyroid extension in thyroid pathology by analyzing sizable intra- and extrathyroid blood vessels. Korean J Pathol. 2012;46:548–553. doi: 10.4132/KoreanJPathol.2012.46.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong G, Lim YH, Kim NJ, Wee G, Kim YK. Knockout of miR-221 and miR-222 reveals common and specific targets for paralogous miRNAs. RNA Biol. 2017;14:197–205. doi: 10.1080/15476286.2016.1269994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YK, Kim SC, Park IJ, et al. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PLoS ONE. 2012;7:e52705. doi: 10.1371/journal.pone.0052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaş HE, Gözüaçik D. Autophagy and cancer. Turkish J Biol. 2014;38(6):720–739. doi: 10.3906/biy-1408-16. [DOI] [Google Scholar]
- Kim KM, Park JB, Bae KS, Kang SJ. Analysis of prognostic factors in patients with multiple recurrences of papillary thyroid carcinoma. Surg Oncol. 2012;21:185–190. doi: 10.1016/j.suronc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Zhao JT, Clifton-Bligh RJ, et al. MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer. 2013;119:4358–4365. doi: 10.1002/cncr.28254. [DOI] [PubMed] [Google Scholar]
- Lemcke H, David R. Potential mechanisms of miRNA mobility. Traffic. 2018;19:910–917. doi: 10.1111/tra.12606. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Li T, Ye C, et al. miR-221 inhibits autophagy and targets TP53INP1 in colorectal cancer cells. Exp Ther Med. 2018;15:1712–1717. doi: 10.3892/etm.2017.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Ning T, Zhang H, Wang X, et al. miR-221 and miR-222 synergistically regulate hepatocyte growth factor activator inhibitor type 1 to promote cell proliferation and migration in gastric cancer. Tumour Biol. 2017;39:1010428317701636. doi: 10.1177/1010428317701636. [DOI] [PubMed] [Google Scholar]
- Shah MY, Calin GA. MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome Med. 2011;3:56. doi: 10.1186/gm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- Song J, Ouyang Y, Che J, et al. Potential value of miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers for diseases. Front Immunol. 2017;8:56. doi: 10.3389/fimmu.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredni ST, Bonaldo Mde F, Costa FF, et al. Upregulation of mir-221 and mir-222 in atypical teratoid/rhabdoid tumors: potential therapeutic targets. Childs Nerv Syst. 2010;26:279–283. doi: 10.1007/s00381-009-1028-y. [DOI] [PubMed] [Google Scholar]
- Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer—recent advances and future directions. Nat Rev Endocrinol. 2018;14:670–683. doi: 10.1038/s41574-018-0080-7. [DOI] [PubMed] [Google Scholar]
- Wang S, Xia P, Huang G, et al. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat Commun. 2016;7:11023. doi: 10.1038/ncomms11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SF, Wu MY, Cai CZ, Li M, Lu JH. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J Ethnopharmacol. 2016;194:861–876. doi: 10.1016/j.jep.2016.10.069. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Xie K, Liang C, Li Q, et al. Role of ATG10 expression quantitative trait loci in non-small cell lung cancer survival. Int J Cancer. 2016;139:1564–1573. doi: 10.1002/ijc.30205. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao X, Li HX. MiR-221 and miR-222 simultaneously target ARID1A and enhance proliferation and invasion of cervical cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:1509–1515. [PubMed] [Google Scholar]
- Yang Y, Sun Y, Wang H, et al. MicroRNA-221 induces autophagy through suppressing HDAC6 expression and promoting apoptosis in pancreatic cancer. Oncol Lett. 2018;16:7295–7301. doi: 10.3892/ol.2018.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Gorantla SP, Muller-Rudorf A, et al. Phosphorylation of Beclin-1 by BCR-ABL suppresses autophagy in chronic myeloid leukemia. Haematologica. 2019 doi: 10.3324/haematol.2018.212027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Pang B, Xin T, et al. Plasma miR-221/222 family as novel descriptive and prognostic biomarkers for glioma. Mol Neurobiol. 2016;53:1452–1460. doi: 10.1007/s12035-014-9079-9. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Hu ZY, Zhang JP, et al. Dual roles of two isoforms of autophagy-related gene ATG10 in HCV-subgenomic replicon mediated autophagy flux and innate immunity. Sci Rep. 2017;7(1):11250. doi: 10.1038/s41598-017-11105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BAKT, Holford TR, Han X, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.