Abstract
Objective
To evaluate the neuroprotective effect of therapeutic hypothermia (TH) induced by phase changing material (PCM) on MRI biomarkers in infants with hypoxic-ischaemic encephalopathy (HIE) in a low-resource setting.
Design
Open-label randomised controlled trial.
Setting
One neonatal intensive care unit in a tertiary care centre in India.
Patients
50 term/near-term infants admitted within 5 hours after birth with predefined physiological criteria and signs of moderate/severe HIE.
Interventions
Standard care (n=25) or standard care plus 72 hours of hypothermia (33.5°C±0.5°C, n=25) induced by PCM.
Main outcome measures
Primary outcome was fractional anisotropy (FA) in the posterior limb of the internal capsule (PLIC) on neonatal diffusion tensor imaging analysed according to intention to treat.
Results
Primary outcome was available for 22 infants (44%, 11 in each group). Diffusion tensor imaging showed significantly higher FA in the cooled than the non-cooled infants in left PLIC and several white matter tracts. After adjusting for sex, birth weight and gestational age, the mean difference in PLIC FA between groups was 0.026 (95% CI 0.004 to 0.048, p=0.023). Conventional MRI was available for 46 infants and demonstrated significantly less moderate/severe abnormalities in the cooled (n=2, 9%) than in the non-cooled (n=10, 43%) infants. There was no difference in adverse events between groups.
Conclusions
This study confirmed that TH induced by PCM reduced brain injury detected on MRI in infants with moderate HIE in a neonatal intensive care unit in India. Future research should focus on optimal supportive treatment during hypothermia rather than looking at efficacy of TH in low-resource settings.
Trial registration number
CTRI/2013/05/003693.
Keywords: neonatology, imaging, neuropathology
What is already known on this topic?
Therapeutic hypothermia is the only treatment shown to reduce death or disability for infants with moderate to severe hypoxic-ischaemic encephalopathy (HIE).
Treatment effect is based on studies from high-income settings, although the majority of these infants are born in low-resource settings.
Small trials in low and middle-income countries using different cooling devices have not demonstrated the benefit of cooling.
What this study adds?
This study confirms a neuroprotective effect of therapeutic hypothermia induced by phase changing material in newborns with moderate HIE in a neonatal unit in India.
The beneficial effect on MRI biomarkers was found even though the use of sedatives/analgesics and mechanical ventilation was limited.
This supports the implementation of therapeutic hypothermia in tertiary care neonatal units in resource-limited settings.
Introduction
More than 95% of deaths from perinatal asphyxia and hypoxic-ischaemic encephalopathy (HIE) occur in low and middle-income countries (LMICs)1; globally, 1.2 million infants survive with disability annually.2 Therapeutic hypothermia (TH) is the only treatment found to reduce mortality and major disability based on randomised controlled trials (RCT) from high-income countries (HICs).3 Trials from LMICs, however, have not consistently demonstrated benefits of cooling,4–6 and two recent reviews concluded that evidence for the safety or efficacy of TH as a neuroprotectant for HIE in LMICs is lacking and that TH in this setting is experimental.4 7 There are only three RCTs from LMICs (China and India) reporting neurodevelopmental outcomes beyond 12 months of age, and all concluded with a beneficial effect of TH.8–10 Population characteristics with higher incidence of fetal growth restriction and different comorbidities (eg, higher incidences of sepsis and meconium aspiration), facility characteristics with limited staffing with limited training and unavailability of expensive servo-controlled cooling equipment have been suggested as reasons for why TH can still not be recommended as standard of care for moderate to severe HIE in low-resource settings.
India carries the largest burden of neonatal deaths worldwide, and perinatal asphyxia and HIE account for a quarter of these deaths and an unknown number of survivors with lifelong disability.11 A survey from 2015 showed that many of the neonatal units in India which do not offer TH are unable to do so due to lack of cooling device and trained staff.12 Phase changing material (PCM) has been shown to provide very stable temperatures,13 14 but its efficacy in inducing a rapid fall in temperature or in maintaining stable temperatures during the cooling phase irrespective of the ambient temperature has been questioned.15 PCMs consist of salt hydride, fatty acid and esters or paraffin, melting at a set point, and with the ability to store and release heat at a nearly constant temperature.14 The very low cost of PCM, even when compared with so-called low-cost, servo-controlled devices,15 makes it an excellent alternative to servo-controlled devices if efficacy is comparable.
MRI of the brain is considered the best single predictor of outcome in infants with HIE, and conventional MRI (cMRI) in the neonatal period is highly predictive of later neurodevelopment.16 17 Diffusion tensor imaging (DTI) is another MR method which has the ability to assess the microstructural organisation of white matter (WM),18 and may be used as a surrogate outcome for neurodevelopment.19 20 Fractional anisotropy (FA), a DTI metric, is a measure of the overall directionality of water, and decreased FA is a common feature of cerebral WM abnormalities.21 Mean diffusivity (MD), another DTI metric, can be compared with the apparent diffusion coefficient (ADC) derived from diffusion-weighted imaging (DWI) and both are commonly used markers of hypoxic-ischaemic brain injury.22
The aim of this study was to evaluate the neuroprotective effect of TH achieved by a PCM-based cooling device in infants with moderate to severe HIE in a neonatal intensive care unit (NICU) in India. Outcome measures were neonatal MRI biomarkers, indicating severity of brain injury.
Methods
Patients
This open-label RCT was conducted between September 2013 and October 2015 in the NICU at the Christian Medical College (CMC) Vellore, a large tertiary care teaching hospital in south India. The hospital has around 15 000 deliveries annually and 75 neonatal beds with a nurse-patient ratio of 1:3–4 for the sickest infants. This was a collaborative study between CMC and the Norwegian University of Science and Technology (NTNU) and St Olavs Hospital, Trondheim University Hospital, both located in Trondheim, Norway, and involved specialists from all centres.
Infants born at gestational age (GA) >35 weeks and birth weight >1800 g admitted within 5 hours after birth with a history of perinatal asphyxia were considered for inclusion. Physiological inclusion criteria were arterial blood gas (umbilical cord or first postnatal hour) pH <7.0 or base deficit ≥12, 5 min Apgar score ≤5, or need of positive pressure ventilation for at least 10 min as part of resuscitation at birth. For outborn infants, no cry at birth was a sufficient criterion. Eligible infants were further screened for neurological criteria, including seizures or evidence of moderate or severe HIE identical to the NICHD trial.23 Infants with major congenital anomalies or with imminent death anticipated at the time of assessment were excluded. Written parental consent was obtained after giving the parents an information leaflet and oral explanation.
Randomisation
Infants were stratified according to the severity of encephalopathy and randomly assigned to treatment group in a 1:1 ratio. The allocation sequences were concealed in sequentially numbered, sealed and opaque envelopes with block sizes of 4 and 6, which were created by the Department of Biostatistics at CMC. A research officer ensured adequate recruitment and collection of clinical data.
Procedures
Infants assigned to hypothermia (TH group) were placed on a PCM-based cooling device (MiraCradle Neonate Cooler, Pluss Advanced Technologies, India).14 The target core temperature was 33.5°C±0.5°C for 72 hours followed by controlled rewarming at 0.2°C–0.5°C per hour until the temperature was above 36.5°C. Infants assigned to standard care (SC group) were placed under radiant warmer, and target core temperature was 37.0°C±0.5°C. The environmental temperature in the NICU is typically 27°C–30°C year-round.
Rectal temperature was monitored continuously in all infants and recorded every 15 min for the first 4 hours, thereafter hourly during the intervention and another 24 hours. Oxygen saturation, heart rate, blood pressure and respiratory rate were monitored continuously while urine output was monitored every 6 hours. Neurological examination using the Thompson score and modified Sarnat was done at recruitment and repeated daily until 4 days of life. Blood gas, electrolytes, glucose, kidney function, liver enzymes, coagulation parameters and full blood counts were monitored, and investigation for infection was done as clinically indicated. All treatments including medications were as per existing treatment protocols. Sedatives/analgesics were given to babies who had excessive shivering or a pain score of >4 on Neonatal Infant Pain Scale as per treating physician’s discretion. Mechanical ventilation was not routinely used but was provided for infants with respiratory failure. Seizures were managed with a loading dose of phenobarbitone. Second-line anticonvulsant was phenytoin and third was levetiracetam.
MRI acquisition and analysis
All infants underwent cerebral MRI at 5±1 days of life with the same 3.0T Philips Achieva scanner (Philips Healthcare, Best, Netherlands; software V.3.2.3.1) using an 8-channel head coil. MRI scans were performed under pulse oximetry surveillance, and chloral hydrate (50 mg/kg) or midazolam (0.10–0.15 mg/kg) sedation was used if needed. Infants were wrapped, foam pads placed around the head and ear protection was applied. Conventional T1 and T2-weighted images, DWI, MR spectroscopy (MRS) and DTI were obtained (online supplementary eTable1). The duration of the MRI acquisition was 25 min. All MR images were stored deidentified on a password-protected hard drive which was brought from CMC to NTNU in person by one of the investigators.
fetalneonatal-2019-317311supp001.pdf (254.6KB, pdf)
The DTI data were analysed using Tract-Based Spatial Statistics (TBSS) and a region-of-interest (ROI) approach. The DTI analysis plan is given in the online supplementary material with ROI locations (online supplementary eFigure1). The clinical images were reviewed by one neonatologist (MMB) with experience in neonatal neuroimaging and scored in accordance with Rutherford et al.16 The MR spectra were automatically processed on the Philips workstation, and one voxel of interest in the basal ganglia from each infant was chosen. Peak area of major metabolites was calculated, including N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), myoinositol, and glutamate and glutamine. The peak area values were expressed relative to Cho and Cr.
Outcomes
The primary outcome was FA in the posterior limb of the internal capsule (PLIC) analysed by the TBSS and ROI approach. A priori defined secondary outcomes were FA and MD in thalami, lentiform nuclei, genu and splenium of the corpus callosum, and midbrain, peak area of major metabolites on MRS and abnormalities on cMRI. All assessors of MRI were blinded to the intervention and clinical characteristics except sex, birth weight and GA.
Severe adverse events were major cardiac arrhythmia (except sinus bradycardia), persistent hypotension (defined as mean blood pressure <GA+postnatal age in days, despite two inotropes), prolonged coagulation time (prothrombin time >20 s, activated partial thromboplastin time >60 s), thrombocytopenia (platelet count <100x109/L), severe haemorrhage, persistent pulmonary hypertension, culture-positive sepsis and death or withdrawal of care. The Data Safety Monitoring Board at the CMC oversaw the study and adverse events were reported.
Statistical analysis
A sample size of 20 in each arm was calculated to detect a 10% difference in mean PLIC FA values, with a power of 90% for a two-sided t-test at a significance level of 5%.19 To account for the assumption that up to 20% of included infants may die before MRI, 25 infants were included in each arm. The primary outcome was analysed using TBSS based on intention to treat. In addition, the FA and MD values obtained from the ROI approach were analysed using multivariable linear regression including sex, birth weight and GA as covariates in addition to treatment group. A linear mixed model was used for PLIC, thalami, lentiform nuclei and midbrain due to dependent measurements from the left and right sides, accounting for within-subject correlation by subject-specific random intercept. Group differences in adverse events, postnatal complications, cMRI and metabolite levels from MRS were analysed by χ2 tests or Mann-Whitney U tests, as appropriate. P values <0.05 were considered significant.
Demographic factors and clinical characteristics were summarised with counts (percentages) for categorical variables and means (SD) or medians (IQR) for continuous variables. All analyses were performed using SPSS V.24 and V.25 (IBM).
Results
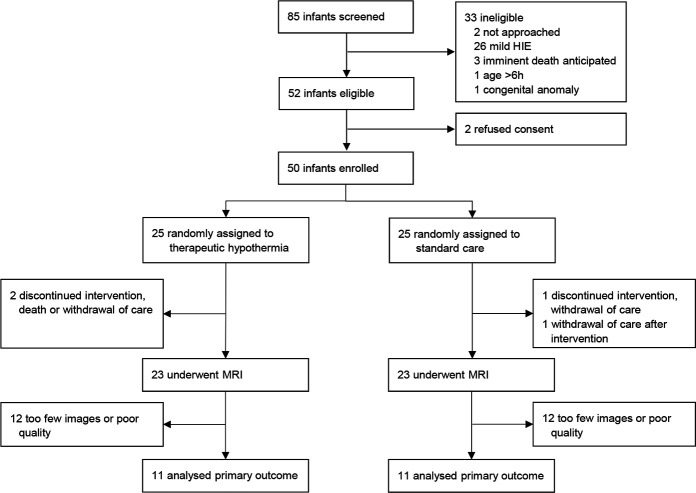
A total of 85 infants were assessed for eligibility, of whom 50 infants were randomly allocated hypothermia (n=25) or SC (n=25) (figure 1). TH was discontinued in two infants due to death or discharge against medical advice (DAMA). In the SC group, two infants were DAMA before MRI was acquired. All infants who were DAMA were noted as having poor prognosis, and withdrawal of care had been recommended. There were no baseline differences in delivery and neonatal demographics between groups (table 1). Mean rectal temperature during the intervention was 33.5°C (SD 0.27) and 36.9°C (SD 0.35) in the TH and SC groups, respectively (online supplementary eFigure 2).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. HIE, hypoxic-ischaemic encephalopathy.
Table 1.
Neonatal baseline characteristics*
| Characteristic | Therapeutic hypothermia (n=25) |
Standard care (n=25) |
| Inborn, n/N (%) | 13/25 (52) | 17/25 (68) |
| Mode of delivery, n/N (%) | ||
| Vaginal | 9/25 (36) | 10/25 (40) |
| Vacuum/forceps | 5/25 (20) | 5/25 (20) |
| Emergency caesarean section | 11/25 (44) | 10/25 (40) |
| Male sex, n/N (%) | 17/25 (68) | 16/25 (64) |
| Gestational age (weeks), mean (SD)† | 39.1 (1.3) | 39.2 (1.4) |
| Birth weight (g), mean (SD) | 2911 (483) | 2960 (553) |
| Length (cm), mean (SD)‡ | 48.5 (2.5) | 48.6 (3.1) |
| Head circumference (cm), mean (SD)§ | 34.0 (1.1) | 34.4 (1.9) |
| Small for gestational age, n/N (%)¶ | 7/25 (28) | 7/23 (30) |
| 1 min Apgar score, n/N (%) | ||
| 0–3 | 17/21 (81) | 13/21 (62) |
| 4–6 | 3/21 (14) | 7/21 (33) |
| 7–10 | 1/21 (5) | 1/21 (5) |
| 5 min Apgar score, n/N (%) | ||
| 0–3 | 5/22 (23) | 1/21 (5) |
| 4–6 | 13/22 (59) | 12/21 (57) |
| 7–10 | 4/22 (18) | 8/21 (38) |
| Cord/blood <60 min pH, mean (SD)** | 6.81 (0.12) | 6.93 (0.18) |
| Cord/blood <60 min base excess, mean (SD)†† | −19.6 (3.2) | −16.5 (4.6) |
| Resuscitation at birth, n/N (%) | ||
| Bag mask ventilation | 19/19 (100) | 22/23 (96) |
| Intubation | 12/19 (63) | 16/23 (70) |
| Chest compressions | 0/19 | 3/23 (13) |
| Epinephrine | 0/19 | 0/23 |
| Temperature on admission (°C), mean (SD)‡‡ | 35.8 (0.8) | 36.2 (0.6) |
| Age at randomisation (hour), mean (SD) | 3.14 (1.48) | 3.27 (1.50) |
| Seizures before randomisation, n/N (%) | 13/25 (52) | 13/25 (52) |
| Thompson score at randomisation, median (IQR) | 9 (6–13) | 9 (6–16) |
*Percentages are based on the number of infants for whom data were available.
†Data were unavailable for two infants in standard care group.
‡Data were unavailable for one infant in therapeutic hypothermia group.
§Data were unavailable for one infant in each group.
¶Small for gestational age defined as birth weight less than the 10th percentile according to the Intergrowth 21st chart.
**Data were unavailable for 13 infants in therapeutic hypothermia group and seven in standard care group. Cord pH was not available in outborn infants.
††Data were unavailable for 14 infants in therapeutic hypothermia group and eight in standard care group. Cord base excess was not available in outborn infants.
‡‡Data were unavailable for three infants in therapeutic hypothermia group and five in standard care group.
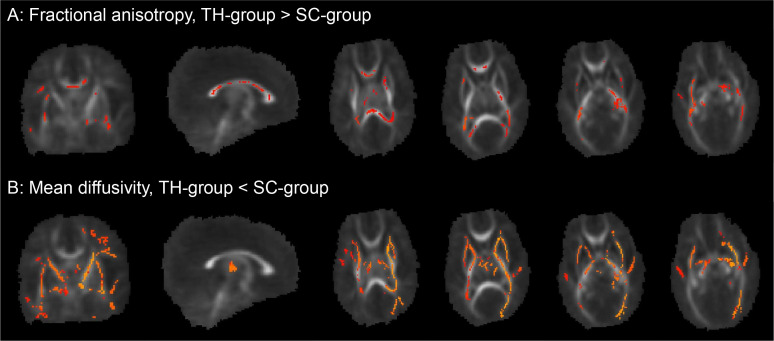
Primary outcome data with DTI were available for 22 infants (44%): 4 died or were DAMA, 1 did not have a DTI sequence, 9 had too few DTI images to be analysed and 14 had poor quality images due to artefacts (figure 1). The TBSS analysis showed higher FA values in the TH group compared with the SC group in the left PLIC (figure 2). Mean FA value in left PLIC was 0.407 (SD 0.032) and 0.390 (SD 0.029) in the TH and SC groups, respectively; in right PLIC 0.409 (SD 0.038) and 0.390 (SD 0.036), respectively. Higher FA and lower MD values were also found in several WM tracts (figure 2). Using the ROI approach, the unadjusted mean FA value in PLIC was 0.414 in the TH group compared with 0.397 in the SC group, and after adjusting for sex, birth weight and GA there was a mean difference between groups of 0.026 (95% CI 0.004 to 0.048, p=0.023). DTI results from the ROI analysis are shown in table 2.
Figure 2.
Tract-based spatial statistical analysis of whole-brain white matter. (A) There was significantly higher fractional anisotropy in the therapeutic hypothermia group (TH group) compared with the standard care group (SC group) (p<0.05, non-parametric permutation test, corrected for multiple comparisons, sex, birth weight and gestational age). Areas include the posterior and anterior limb of internal capsule in the left hemisphere, bilaterally in the external capsule, the genu, splenium and body of corpus callosum, forceps major, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, uncinate fasciculus; superior corona radiata on the left side, and cerebral peduncle and superior longitudinal fasciculus in the right hemisphere. (B) There was significantly lower mean diffusivity in the TH group compared with the SC group. Areas include the posterior and anterior limb of the internal capsule, external capsule, superior and inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, cingulum, uncinate fasciculus, splenium of corpus callosum, corticospinal tract, cerebral peduncle and thalami bilaterally; and centrum semiovale, anterior, superior and posterior corona radiata in the left hemisphere.
Table 2.
Between-group comparisons of selected regions of interest*
| Region | Mean difference FA (95% CI) | P value | Mean difference MD (95% CI)† | P value |
| PLIC | 0.026 (0.004 to 0.048) | 0.023 | −0.051 (−0.087 to −0.014) | 0.007 |
| Thalami | 0.008 (−0.009 to 0.024) | 0.354 | −0.061 (−0.104 to −0.017) | 0.007 |
| Lentiform nuclei | 0.006 (−0.012 to 0.023) | 0.484 | −0.059 (−0.093 to −0.024) | 0.001 |
| Midbrain | 0.012 (−0.005 to 0.029) | 0.147 | −0.037 (−0.070 to −0.005) | 0.026 |
| Genu of corpus callosum | 0.033 (−0.002 to 0.068) | 0.062 | −0.020 (−0.092 to 0.053) | 0.575 |
| Splenium of corpus callosum | 0.054 (0.021 to 0.087) | 0.003 | −0.048 (−0.104 to 0.008) | 0.087 |
*Adjusted for sex, birth weight and gestational age.
†Values reported as 10−3.
FA, fractional anisotropy; MD, mean diffusivity; PLIC, posterior limb of the internal capsule.
cMRI (n=46) demonstrated moderate/severe abnormalities in 2 (9%) and 10 (43%) infants in the TH and SC groups, respectively (p=0.007). Four (all in the SC group) had a global injury pattern (severe basal ganglia and thalami (BGT), WM and cortical injury). Six infants had moderate BGT injury, four of them with moderate/severe WM injury (all in SC group) and two with equivocal PLIC (one in each group). One infant in each group had normal/mild BGT and severe WM injury; the infant in SC group also had severe cortical injury.
MRS was done in all 46 infants with MRI, but 18 failed processing (7 in the TH group and 11 in the SC group) and 2 were excluded due to poor quality of images and voxels chosen mainly within WM (TH group). Median NAA/Cho ratio was 0.628 (IQR 0.556–0.684) and 0.559 (IQR 0.472–0.625) in TH and SC groups, respectively (p=0.013). The other MRS measures were non-significant.
Adverse events and postnatal complications are shown in table 3. Fentanyl was given as infusion (maximum 2 µg/kg/hour) to one infant in each group, and as a single bolus to one infant (SC group). Bolus doses of midazolam (0.10–0.15 mg/kg) and/or chloral hydrate (50 mg/kg) were given to 10 and 3 infants in TH and SC groups, respectively. Data on sedatives/analgesics are missing for nine infants. Forty-one infants (82%) with clinical seizures after admission received phenobarbitone, and 21 (42%) received two or more anticonvulsants.
Table 3.
Major adverse events and postnatal complications
| Therapeutic hypothermia (n=25) |
Standard care (n=25) |
P value | |
| Major adverse events | |||
| Death or withdrawal of care during intervention | 2 (8) | 1 (4) | 0.55 |
| Major cardiac arrhythmia | 0 | 0 | – |
| Persistent hypotension | 0 | 0 | – |
| Prolonged coagulation time | 23 (92) | 19 (76) | 0.12 |
| Thrombocytopenia | 4 (16) | 2 (8) | 0.38 |
| Severe haemorrhage | 0 | 2 (8)* | 0.15 |
| PPHN | 0 | 0 | – |
| Culture-positive sepsis | 0 | 0 | – |
| Postnatal complications | |||
| Death or withdrawal of care after intervention | 0 | 4 (17)† | 0.04 |
| Clinical seizures | 20 (80) | 22 (88) | 0.44 |
| ≥2 anticonvulsants | 9 (36) | 12 (48) | 0.39 |
| Mechanical ventilation >24 hours | 2 (8) | 6 (24) | 0.12 |
| Inotropic support | 14 (56) | 17 (68) | 0.38 |
| Treated coagulopathy | 10 (40) | 8 (32) | 0.56 |
| Hypoglycaemia | 2 (8) | 1 (4) | 0.55 |
| Hyperglycaemia requiring insulin | 1 (4) | 1 (4) | – |
Data are n (%).
*Both were subgaleal bleed requiring packed cell transfusion.
†Denominator 24 due to one withdrawal of care during the intervention.
PPHN, persistent pulmonary hypertension of the newborn.
Discussion
This RCT of TH using a PCM-based cooling device for neonatal HIE confirms a neuroprotective effect in a low-resource setting. There was higher FA in the left PLIC and several WM tracts in cooled compared with non-cooled infants. Infants in the TH group had less abnormalities on cMRI and higher NAA/Cho ratio in the basal ganglia compared with SC group. The beneficial effect of cooling demonstrated on several MRI biomarkers occurred even though the use of sedatives/analgesics was very limited, and the majority of infants were not mechanically ventilated.
The favourable effect of TH demonstrated by higher FA in the left PLIC, corpus callosum and other WM tracts is in accordance with the findings of Porter et al.19 Treatment effect on WM integrity found by the voxel-based analysis (TBSS) was confirmed by the ROI analysis. TBSS has the advantage of being faster, more time effective and reproducible, and can investigate the entire WM. Decreased FA values reflect tissue damage and are strongly associated with neurodevelopmental outcomes,20 24 and our findings support the use of FA as a sensitive marker for brain injury after HIE.
The finding of lower MD values in the TH group than the SC group is of more uncertain significance. Although lower MD in cooled versus non-cooled infants has been shown also by Artzi et al,25 most studies demonstrate lower ADC values in injured brain tissue on early MRI.26–29 ADC and MD may, in contrast to FA, pseudonormalise after an acute hypoxic-ischaemic insult, and this process is affected by TH.26 The interpretation of MD will, therefore, depend strongly on time of imaging and injury severity.
The difference between treatment groups shown on DTI was supported by findings on cMRI and MRS. The injury pattern on cMRI was predominantly BGT with or without WM and/or cortical injury, which is the injury pattern most commonly seen in infants with HIE in HICs and comparable to the TH trials.16 30 In a study of infants with HIE admitted to a NICU in Kerala, India, more than 90% had WM injury and only 27% had any BGT injury.31 The predominance of WM injury could be partly explained by a large proportion of infants with mild HIE (56%) in that study, but question remains if there are differences in injury pattern between populations. It has been speculated that a high incidence of fetal growth restriction in LMICs may be related to prenatally established brain injury and, thus, reduced efficacy of TH.5 15 32 Our study, where almost a third of infants were small for gestational age, did not support this.
There were no significant differences in major adverse events or postnatal complications between the groups and no life-threatening adverse events in the TH group. In accordance with our results, the PCM-based cooling device has recently been shown feasible and safe when used in 11 tertiary NICUs in India.13 Our study confirms very stable temperatures in cooled infants with fluctuation of temperature (SD) of 0.27°C during intervention. The PCM mattress is produced locally and costs one-tenth of a servo-controlled, automated cooling device commonly used in HICs. Compared with other low-cost, non-servo-controlled cooling devices PCM provides excellent temperature stability and requires less nursing input.13 14 33
Very few infants received continuous infusion of sedatives/analgesics and respiratory support in the present study. This suggests that routine sedation requiring mechanical ventilation is not necessary to achieve a neuroprotective effect of TH. However, our study was not designed to detect the effect of sedatives/analgesics, and results should be interpreted with caution. A sedative effect of the anticonvulsants used must also be anticipated. There is still little knowledge about the optimal level of sedation during TH.34 Many centres in HICs electively intubate and sedate infants during cooling, while others use clinical indications to assess the need for sedatives/analgesics and ventilatory support. Although the issue of deep sedation necessitating mechanical ventilation is of particular importance in resource-limited settings, a better understanding of the neuroprotective and/or toxic effects of sedatives/analgesics is important in all settings where TH is provided.
Limitations
The very low proportion of infants with severe HIE (2 of 50) is a limitation of the present study and is most likely due to selection bias. Infants with imminent death anticipated at the time of enrolment, and infants without established respiration at 30 min of age are not offered intensive care in the study hospital due to the poor prognosis and were not included. The sickest outborn infants may not have been referred or arrived beyond 5 hours of age to the tertiary care hospital. This is supported by a very low proportion of severe HIE also among screened infants. Thus, the results of the present trial cannot be extrapolated to infants with severe HIE.
DTI data were available for only 44% of included infants due to artefacts and incomplete data sets. The sample size was lower than expected, and the results are therefore vulnerable to the variability in the data. Despite this, we found significant differences between the treatment groups. The fact that four infants (all in the SC group) with global injury on cMRI did not contribute to the group differences found on DTI, suggests that the beneficial effect of cooling is underestimated in the present study.
Despite proper randomisation procedures, there is a risk of imbalance between groups in small trials. The randomisation procedure in the present study included allocation concealment and stratification by HIE stage. Unfortunately, umbilical blood gas analysis was not available in any of the referral hospitals and could, therefore, not be used to assess severity of the hypoxic-ischaemic insult.35 36
Conclusion
TH reduced brain injury detected on all MRI biomarkers in infants with moderate HIE admitted to an NICU in India and supports that TH is feasible and neuroprotective in this setting. Future research should focus on finding the optimal supportive treatment during TH. This is of particular importance in LMICs where the burden of disease is highest.
Acknowledgments
The authors thank all the staff of the Department of Neonatology, CMC, Vellore, who took care of the study babies and the research officers for adequate recruitment and collection of clinical data. We thank Associate Professor in Statistics Turid Follestad (Unit of Applied Clinical Research, NTNU) for valuable support on statistical analyses. We thank members of the Data Safety Monitoring Board at the CMC for monitoring the study. We also thank Associate Professor Emerita Nancy Eik-Nes (Department of Language and Literature, NTNU) for language editing a draft of the manuscript. The language editing was done as part of a PhD course in scientific writing.
Footnotes
Contributors: NT was the PI of the study. KA did the literature search, conceptualised and designed the study, drafted the initial manuscript, analysed and interpreted the data, and reviewed and revised the manuscript. RS did the literature search, conceptualised and designed the study, analysed and interpreted the data, and reviewed and revised the manuscript. LE made the MRI protocol, did the DTI analysis, interpreted the data, and reviewed and revised the manuscript. MMB did the conventional MRI analysis, and reviewed and revised the manuscript. IN made the MRI protocol, implemented and adapted the protocol on the local scanner, interpreted the data, and reviewed and revised the manuscript. AKH made the MRI protocol, interpreted the data, and reviewed and revised the manuscript. SG was responsible for the MRI acquisition, and reviewed and revised the manuscript. NT did the literature search, conceptualised and designed the study, enrolled the patients, collected clinical data, analysed and interpreted the data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. KA and NT contributed equally to this work.
Funding: The trial was funded by the Liaison Committee for Education, Research and Innovation in Central Norway, and the Joint Research Committee between St Olavs Hospital, Trondheim University Hospital, and the Faculty of Medicine, NTNU, Trondheim, Norway.
Disclaimer: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Institutional Review Board at the CMC (number 2013/8223) and the Regional Committee for Medical and Health Research Ethics in central Norway (number 2013/2167).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Lehtonen L, Gimeno A, Parra-Llorca A, et al. Early neonatal death: a challenge worldwide. Semin Fetal Neonatal Med 2017;22:153–60. 10.1016/j.siny.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 2. Azra Haider B, Bhutta ZA. Birth asphyxia in developing countries: current status and public health implications. Curr Probl Pediatr Adolesc Health Care 2006;36:178–88. 10.1016/j.cppeds.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 3. Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;69 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montaldo P, Pauliah SS, Lally PJ, et al. Cooling in a low-resource environment: lost in translation. Semin Fetal Neonatal Med 2015;20:72–9. 10.1016/j.siny.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 5. Pauliah SS, Shankaran S, Wade A, et al. Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: a systematic review and meta-analysis. PLoS One 2013;8:e58834 10.1371/journal.pone.0058834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tagin M, Abdel-Hady H, ur Rahman S, et al. Neuroprotection for perinatal hypoxic ischemic encephalopathy in low- and middle-income countries. J Pediatr 2015;167:25–8. 10.1016/j.jpeds.2015.02.056 [DOI] [PubMed] [Google Scholar]
- 7. Montaldo P, Lally PJ, Oliveira V, et al. International perspectives: hypothermic neuroprotection for neonatal encephalopathy in low- and middle-income countries: a new approach to an old problem. Neoreviews 2018;19:e735–41. 10.1542/neo.19-12-e735 [DOI] [Google Scholar]
- 8. Li T, Xu F, Cheng X, et al. Systemic hypothermia induced within 10 hours after birth improved neurological outcome in newborns with hypoxic-ischemic encephalopathy. Hosp Pract 2009;37:147–52. 10.3810/hp.2009.12.269 [DOI] [PubMed] [Google Scholar]
- 9. Zhou W-hao, Cheng G-qiang, Shao X-mei, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157:367–72. 10.1016/j.jpeds.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 10. Gane BD, Bhat V, Rao R, et al. Effect of therapeutic hypothermia on DNA damage and neurodevelopmental outcome among term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr 2014;60:134–40. 10.1093/tropej/fmt098 [DOI] [PubMed] [Google Scholar]
- 11. Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. The Lancet 2014;384:189–205. 10.1016/S0140-6736(14)60496-7 [DOI] [PubMed] [Google Scholar]
- 12. Chandrasekaran M, Swamy R, Ramji S, et al. Therapeutic hypothermia for neonatal encephalopathy in Indian neonatal units: a survey of national practices. Indian Pediatr 2017;54:969–70. 10.1007/s13312-017-1194-z [DOI] [PubMed] [Google Scholar]
- 13. Thomas N, Abiramalatha T, Bhat V, et al. Phase Changing Material for Therapeutic Hypothermia in Neonates with Hypoxic Ischemic Encephalopathy - A Multi-centric Study. Indian Pediatr 2018;55:201–5. 10.1007/s13312-018-1317-1 [DOI] [PubMed] [Google Scholar]
- 14. Thomas N, Chakrapani Y, Rebekah G, et al. Phase changing material: an alternative method for cooling babies with hypoxic ischaemic encephalopathy. Neonatology 2015;107:266–70. 10.1159/000375286 [DOI] [PubMed] [Google Scholar]
- 15. Oliveira V, Kumutha JR, E N, et al. Hypothermia for encephalopathy in low-income and middle-income countries: feasibility of whole-body cooling using a low-cost servo-controlled device. Bmjpo 2018;2:e000245 10.1136/bmjpo-2017-000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45. 10.1016/S1474-4422(09)70295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liauw L, van der Grond J, van den Berg-Huysmans AA, et al. Hypoxic-Ischemic encephalopathy: diagnostic value of conventional MR imaging pulse sequences in term-born neonates. Radiology 2008;247:204–12. 10.1148/radiol.2471070812 [DOI] [PubMed] [Google Scholar]
- 18. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–19. 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- 19. Porter EJ, Counsell SJ, Edwards AD, et al. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr Res 2010;68:205–9. 10.1203/PDR.0b013e3181e9f1ba [DOI] [PubMed] [Google Scholar]
- 20. Massaro AN, Evangelou I, Fatemi A, et al. White matter tract integrity and developmental outcome in newborn infants with hypoxic-ischemic encephalopathy treated with hypothermia. Dev Med Child Neurol 2015;57:441–8. 10.1111/dmcn.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ward P, et al. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics 2006;117:e619–30. 10.1542/peds.2005-0545 [DOI] [PubMed] [Google Scholar]
- 22. Muir KW, Buchan A, von Kummer R, et al. Imaging of acute stroke. Lancet Neurol 2006;5:755–68. 10.1016/S1474-4422(06)70545-2 [DOI] [PubMed] [Google Scholar]
- 23. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-Body hypothermia for neonates with Hypoxic–Ischemic encephalopathy. N Engl J Med 2005;353:1574–84. 10.1056/NEJMcps050929 [DOI] [PubMed] [Google Scholar]
- 24. Tusor N, Wusthoff C, Smee N, et al. Prediction of neurodevelopmental outcome after hypoxic–ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr Res 2012;72:63–9. 10.1038/pr.2012.40 [DOI] [PubMed] [Google Scholar]
- 25. Artzi M, Sira LB, Bassan H, et al. Brain diffusivity in infants with Hypoxic–Ischemic encephalopathy following whole body hypothermia. J Child Neurol 2011;26:1230–6. 10.1177/0883073811402346 [DOI] [PubMed] [Google Scholar]
- 26. Bednarek N, Mathur A, Inder T, et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 2012;78:1420–7. 10.1212/WNL.0b013e318253d589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alderliesten T, de Vries LS, Khalil Y, et al. Therapeutic hypothermia modifies perinatal asphyxia-induced changes of the corpus callosum and outcome in neonates. PLoS One 2015;10:e0123230 10.1371/journal.pone.0123230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunt RW, et al. Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 2004;114:999–1003. 10.1542/peds.2003-0935-L [DOI] [PubMed] [Google Scholar]
- 29. Liauw L, van Wezel-Meijler G, Veen S, et al. Do apparent diffusion coefficient measurements predict outcome in children with neonatal hypoxic-ischemic encephalopathy? AJNR Am J Neuroradiol 2009;30:264–70. 10.3174/ajnr.A1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rutherford M, Biarge MM, Allsop J, et al. Mri of perinatal brain injury. Pediatr Radiol 2010;40:819–33. 10.1007/s00247-010-1620-z [DOI] [PubMed] [Google Scholar]
- 31. Lally PJ, Price DL, Pauliah SS, et al. Neonatal encephalopathic cerebral injury in South India assessed by perinatal magnetic resonance biomarkers and early childhood neurodevelopmental outcome. PLoS One 2014;9:e87874 10.1371/journal.pone.0087874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilkinson DJ, Thayyil S, Robertson NJ. Ethical and practical issues relating to the global use of therapeutic hypothermia for perinatal asphyxial encephalopathy. Arch Dis Child Fetal Neonatal Ed 2011;96:F75–8. 10.1136/adc.2010.184689 [DOI] [PubMed] [Google Scholar]
- 33. Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011;159:851–8. 10.1016/j.jpeds.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Natarajan G, Shankaran S, Laptook AR, et al. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol 2018;38:1060–7. 10.1038/s41372-018-0126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelly R, Ramaiah SM, Sheridan H, et al. Dose-Dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2018;103:F567–72. 10.1136/archdischild-2017-314034 [DOI] [PubMed] [Google Scholar]
- 36. Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG 2012;119:824–31. 10.1111/j.1471-0528.2012.03335.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fetalneonatal-2019-317311supp001.pdf (254.6KB, pdf)