Abstract
Background
Elevated HIV viral load (HIV-VL) in pregnancy has been linked to increased risk of mortality, immunological abnormalities, infectious morbidity and restricted growth among HIV-exposed uninfected (HEU) children, but little is known about effects on child development.
Methods
HIV-infected women initiating lifelong ART (tenofovir+emtricitabine+efavirenz) antenatally were followed from first antenatal visit through delivery and with their breastfed infants postpartum. Cognitive, motor and expressive language development (Bayley Scales of Infant and Toddler Development, BSID-III; delay defined as score <85) were assessed on a subset of HEU infants. HIV-VL was measured at ART initiation, in third trimester and around delivery. Cumulative viraemia in pregnancy was expressed as log10 VL copies x year/mL (viraemia copy-years, VCY). Relationships between VCY and development were examined after adjusting for socio-economic, behavioural and psychosocial confounders.
Results
Women (median pre-ART log10 VL 4.1, CD4 349 cells/mm3) commonly reported adverse social circumstances (44% informal housing, 63% unemployed, 29% risky drinking). Among 214 infants (median age 13 months; 53% male; 13% born <37 weeks’ gestation), viraemia predicted lower motor and expressive language, but not cognitive, scores in crude and adjusted analysis [per log10 VCY increase, aβ (95%CI): motor, −2.94 (−5.77; −0.11); language, −3.71 (−6.73; −0.69) and cognitive-2.19 (−5.02; 0.65)]. Increasing VCY also predicted higher relative odds of motor delay [adjusted odds ratio, aOR 3.32 (95% CI 1.36; 8.14)] and expressive language delay [aOR 2.79 (95% CI 1.57; 4.94), but not cognitive delay [aOR 1.68 (0.84; 3.34)].
Conclusion
Cumulative maternal HIV viraemia in pregnancy may have adverse implications for HEU child development.
Keywords: Africa, HIV exposed uninfected (HEU) children, Neurodevelopment, Prevention of mother-to-child transmission (PMTCT), Viremia
Elevated HIV viral load in pregnancy has been linked to increased risk of mortality, immunologic abnormalities, infectious morbidity and restricted growth among HIV-exposed uninfected (HEU) children globally.1,2 However, few data are available on the relationship between antepartum maternal HIV viremia and neurodevelopment of HEU children3,4, and little is known about this putative relationship in the context of breastfeeding with universal triple-drug antiretroviral therapy (ART).5 In a study of HIV-infected women and their children living in Cape Town, South Africa, we investigated the relationship between antepartum HIV viremia and neurodevelopmental outcomes of HEU children in the context of universal maternal ART initiated in pregnancy.6
METHODS
We conducted developmental assessments in HEU children enrolled into the Maternal and Child Health Antiretroviral Therapy study (MCH-ART), a large multi-phase implementation science study evaluating strategies to optimize postpartum ART services for women and children. Study methodology for the main study has been published previously.5,7 Briefly, pregnant HIV-infected women initiating universal ART (tenofovir+emtricitabine+efavirenz) were followed prospectively through delivery and with breastfeeding children until approximately 1 year of age. According to local guidelines at the time, all infants received daily nevirapine for a minimum of 6 weeks; those considered at high risk of vertical transmission also received zidovudine for 4 weeks.8 Trained interviewers administered standardized instruments assessing physical health, socio-economic and psychosocial factors throughout antenatal (1–3 visits) and postnatal (up to 5 visits) study follow-up.6 Maternal blood was collected for batched HIV viral load testing at each visit (Abbot Realtime HIV-1; Abbott Laboratories, Waltham, MA), including at ART initiation, at least once during the third trimester and within 2 weeks before or after delivery. Birth anthropometry and other clinical information were abstracted from medical records. Repeated HIV-PCR (CAP/CTM v 2.0, Roche Molecular Systems, Inc., Branchberg, NJ) testing excluded infection among children.
Mothers of eligible children (HIV-uninfected, ≥28 weeks’ gestation at birth without known congenital abnormalities or cerebral palsy) were approached for enrolment into the neurodevelopmental sub-study when the children were approximately 13 months (window, 11–18 months) old. Following informed consent, cognitive, expressive language, fine and gross motor development were tested using the Bayley Scales of Infant and Toddler Development, 3rd edition (BSID-III, PsychCorp, 2006). The developmental assessment team, comprising three pediatric occupational therapists and one child health physician, received systematic supervised training in the use of the BSID-III by a developmental pediatrician and were assisted by a trained, isi-Xhosa-speaking counsellor. Ten assessments were video-graphed for independent blinded scoring as a quality assurance exercise. Interrater agreement was high, with correlation coefficients above 0.9 for cognitive and motor scores and ranging from 0.7 to 0.98 for expressive language scores. There was perfect agreement for “any” vs. “no delay” classifications across domains. Composite cognitive and motor scores were generated from cognitive, fine and gross motor subtest scaled scores using BSID-III normative and conversion tables, which account for gestation at delivery (adjusting for preterm birth) and child age at time of assessment.9 Receptive language testing proved contextually challenging, necessitating a focus on expressive language only. Expressive language subtest scaled scores were transformed to have a similar distribution to composite scores (expected mean 100, standard deviation 15). Untransformed expressive language subtest scaled scores were analyzed separately in sensitivity analysis.
MCH-ART was approved by Columbia University Medical Centre Institutional Review Board and University of Cape Town’s Faculty of Health Sciences Research Ethics Committee (Clinicaltrials.gov NCT01933477).
Statistical methods
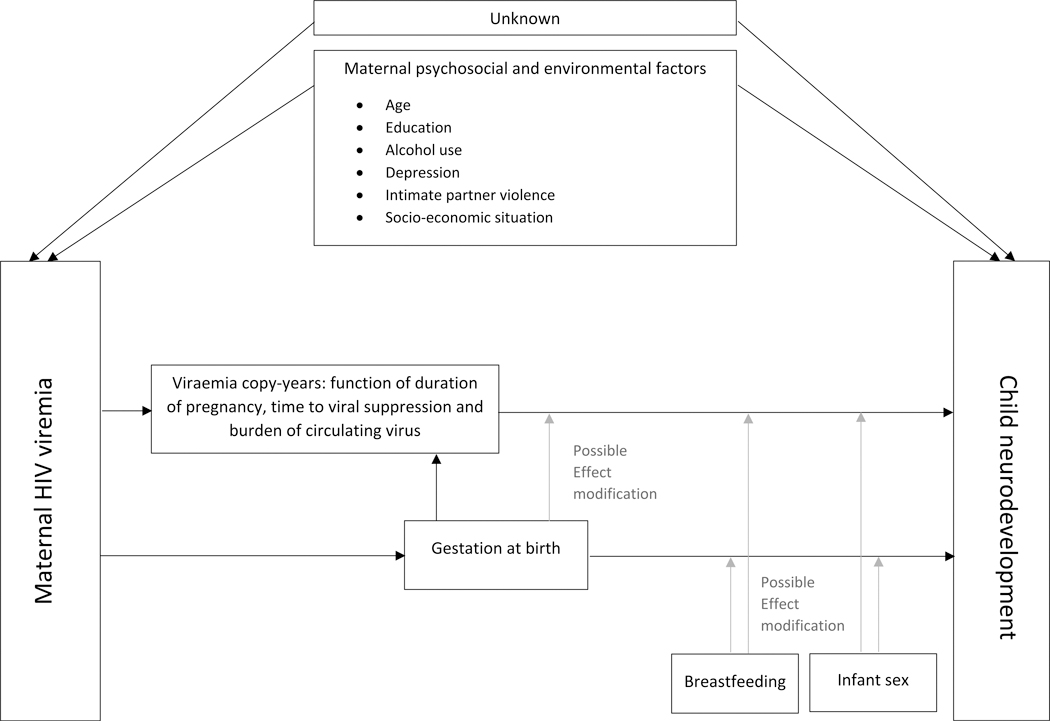
Cognitive, motor and expressive language scores [expected mean (standard deviation, SD) of 100 (15)] were modeled separately in continuous and binary form. Based on international recommendations, “any delay” was defined a priori as composite score <85 (vs. ≥85, “no delay”).10 Viremia during pregnancy was expressed as log10 viral copies X year/mL (viremia copy-years, VCY), a cumulative measure accounting for both burden and duration of viraemia.11 For calculation of VCY, a minimum of two viral load measures was required; missing data were assumed to be missing at random. A review of childhood neurodevelopmental literature informed the choice of potential third variables, guided by a directed acyclic graph developed a priori (Figure 1).12–14 Socio-economic, psychosocial and behavioral measures were evaluated during model building; those that improved model fit (based on Akaike’s Information Criterion, AIC) were retained in the final model. Maternal history of current/prior tuberculosis was chosen as clinical indicator for symptomatic HIV disease, as this is the most common stage III condition in our context.15 Preterm birth (gestation <37 completed weeks) was considered as both potential mediator (potentially on causal pathway between viremia and development) and confounder (earlier delivery resulting in shorter duration of fetal exposure to viremia) in separate models. Analyses (Stata 14.0, StataCorp, College Station Tx) used linear and logistic regression; point estimates are presented with 95% confidence intervals (CI).
Figure 1.
Directed acyclic graph: Maternal HIV viremia and HIV-exposed uninfected child development
RESULTS
At ART initiation, most mothers (n=214; median age 29 years) reported poor socio-economic status (SES) with multiple psychosocial stressors (see Figure, Supplemental Digital Content 1, and Table, Supplemental Digital Content 2): 63% were unemployed; 44% living in informal housing; 72% did not complete high school, with high prevalence of risky drinking (29%) and intimate partner violence (IPV, 20%). Median (interquartile range, IQR) pre-ART CD4 count was 349 (239–522) cells/mm3 and log10 HIV viral load, 4.1 (3.6–4.7). Overall, 999 viral load measures were available for calculation of antepartum VCY, with the majority of women (82%) having five viral load measures. Six women only had 2 values. At time of delivery, the median accumulated log10 VCY was 2.2 (IQR 1.7–2.8); most women (75%) had achieved viral suppression <50 copies/mL (Table 1). Of 214 HEU children [median age 13 (IQR 13–14) months; 53% male], 13% were born preterm with median gestation at birth 39 (IQR 38–40) weeks. The median duration of breastfeeding was 6 (IQR 1–12) months (see Table, Supplemental Digital Content 2).
Table 1.
Linear regression results for association between maternal HIV viremia in pregnancy and mean BSID-III scores for cognitive, motor and expressive language development of HIV-exposed uninfected infants
| Variable | Composite cognitive score10, 11 | Composite motor score10, 11 | Expressive language* score10, 11 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | aβ (95% CI) | β (95% CI) | aβ (95% CI) | β (95% CI) | aβ (95% CI) | |
| Maternal variables | ||||||
| Log10 viral copies x year/mL (VCY) | −1.87 (−4.59; 0.84) | −2.19 (−5.02; 0.65) | −2.82 (−5.60; −0.04) | −2.94 (−5.77; −0.11) | −3.71 (−6.60; −0.83) | −3.71 (−6.73; −0.69) |
| Log10 HIV viral load, ART initiation | −1.96 (−4.30;0.38) | - | −2.91 (−5.30; −0.52) | - | −3.22 (−5.70; −0.73) | - |
| HIV VL ≥50 copies/mL vs. <50, delivery | −1.10 (−5.84; 3.64) | - | −5.14 (−9.98; −0.30) | - | −2.90 (−8.08; 2.28) | - |
| HIV VL ≥1000 copies/mL vs. <1000, delivery | −2.90 (−11.78; 5.98) | - | −5.89 (−14.99; 3.21) | - | 0.10 (−9.64; 9.84) | - |
| Nadir CD4 cell count (cells/mm3) | 0.01 (0.00; 0.02) | - | 0.01 (−0.004; 0.02) | - | 0.01 (0.002; 0.02) | - |
| Nadir CD4 cell count categories: | ||||||
| <200 | Ref | - | Ref | - | Ref | - |
| 200 – 349 | 0.92 (−5.28; 7.11) | - | 1.44 (−5.10; 7.98) | - | 4.85 (−1.94; 11.65) | - |
| 350 – 499 | −1.15 (−7.82; 5.53) | - | 2.23 (−4.85; 9.32) | - | 3.55 (−3.76; 10.87) | - |
| ≥ 500 | 3.90 (−2.52; 10.33) | - | 4.12 (−2.67; 10.91) | - | 8.98 (1.98; 15.98) | - |
| Current/prior tuberculosis vs. none | −3.20 (−9.28; 2.88) | - | −5.49 (−11.81; 0.82) | - | −3.01 (−9.66; 3.64) | - |
| Hemoglobin (g/dL) | 0.65 (−1.09; 2.39) | - | 1.47 (−0.33; 3.27) | - | 1.38 (−0.53; 3.30) | - |
| Anemic (<11 g/dL vs ≥11 g/dL) | −0.97 (−5.18; 3.24) | - | −2.19 (−6.55; 2.17) | - | −1.85 (−6.48; 2.77) | - |
| Maternal age (years) | 0.30 (−0.05; 0.66) | - | 0.24 (−0.13; 0.61) | - | 0.24 (−0.15; 0.63) | - |
| Age ≥ 30 years (vs. <30) | 2.01 (−2.11; 6.14) | 1.33 (−2.94;5.61) | 2.29 (−2.00; 6.57) | 1.15 (−3.18; 5.48) | 1.28 (−3.22; 5.79) | 0.04 (−4.62; 4.69) |
| Poverty score 1 | 1.25 (−0.63; 3.13) | 1.23 (−0.76; 3.23) | 1.81 (−0.14; 3.75) | 2.11 (0.09; 4.13) | 0.27 (−1.80; 2.33) | −0.21 (−2.38; 1.97) |
| Education 2 | 0.57 (−4.04; 5.17) | 0.02 (−4.80; 4.83) | −0.43 (−5.21; 4.35) | −1.58 (−6.46; 3.30) | 3.31 (−1.69; 8.31) | 3.98 (−1.23; 9.20) |
| Depression 3 | −2.85 (−10.07; 4.36) | - | −2.30 (−9.72; 5.13) | - | −6.64 (−14.50; 1.22) | - |
| Intimate partner violence 4 | −0.16 (−5.35; 5.04) | 0.06 (−5.33; 5.45) | −4.21 (−9.52; 1.10) | −3.37 (−8.76; 2.02) | 1.66 (−3.96; 7.27) | 3.06 (−2.75; 8.89) |
| Risky drinking 5 | 0.13 (−4.44; 4.69) | 0.79 (−4.00; 5.58) | −3.63 (−8.38; 1.13) | −1.65 (−6.52; 3.23) | −2.07 (−7.06; 2.91) | −2.27 (−7.49; 2.94) |
| Infant variables | ||||||
| Gestation at delivery (weeks) 6 | 0.95 (0.05; 1.85) | 1.11 (0.17; 2.04) | 1.27 (0.31; 2.23) | 1.46 (0.48; 2.44) | −0.08 (−1.07; 0.91) | 0.10 (−0.91; 1.11) |
| Preterm (<37 weeks) vs. term | −2.76 (−0.92; 3.39) | - | −4.83 (−11.34; 1.68) | - | 1.92 (−4.82; 8.67) | - |
| Infant sex: male vs. female | 1.30 (−2.81; 5.41) | - | 1.45 (−2.82; 5.72) | - | −0.71 (−5.20; 3.79) | - |
| Small-for-gestational age 7 | −2.72 (−8.69; 3.25) | - | −0.69 (−7.03; 5.64) | - | −0.28 (−6.83; 6.27) | - |
| Duration of any breastfeeding (months) 8 | 0.16 (−0.17; 0.49) | - | 0.27 (−0.08; 0.62) | - | 0.17 (−0.19; 0.54) | - |
Language score based on expressive language sub-scale only; Abbreviations – BSID-III, Bayley Scales of Infant and Toddler Development Third Edition; ART, antiretroviral therapy; Hb, hemoglobin; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; VCY, viral copy-years (cumulative measure of duration and severity of HIV viremia during pregnancy)
Except where otherwise indicated, maternal variables represent measures taken at first antenatal clinic booking, prior to ART initiation
Calculated using standardized asset score (access to flush toilet, running water, electricity in home, refrigerator, telephone, television, formal housing) and maternal employment; higher score indicates less relative disadvantage
Education: comparing women who completed secondary schooling vs. those who did not
Depression: EPDS (Edinburgh postnatal depression scale) score of ≥ 13, vs. score <13
Any physical, sexual or psychological violence as measured with World Health Organization violence against women questionnaire (compared to no reported violence)
Risky drinking, defined as Alcohol use disorders identification test (AUDIT-C) score ≥3 (compared to scores <3)
Gestation at delivery: based on early ultrasound and/or last menstrual period as indicated
SGA, birthweight below 10th centile for gestational age (Intergrowth-21st reference standards); compared to appropriate for gestational age
Breastfeeding durations determined by maternal self-report: last study visit at which breastfeeding reported taken as last day of breastfeeding
Interaction terms (models adjusted for maternal age, education, intimate partner violence, risky drinking, poverty score and gestation): VCY-gestational age for cognitive, p=0.67; motor, p=0.67; expressive language, p=0.93; VCY-sex: cognitive, p=0.16; motor, p=0.40; expressive language, p=0.75; VCY-breastfeeding duration: cognitive, p=0.73; motor, p=0.13; expressive language, p=0.65
Sensitivity analysis results:
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score (not including gestational age): cognitive aβ −1.63 (95% CI −4.46; 1.19); motor aβ −2.25 (95% CI −5.10; 0.60); expressive language aβ −3.66 (95% CI −6.65; −0.68).
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score; maternal history of tuberculosis and CD4 cell count (not including gestational age): cognitive aβ −0.90 (95% CI −4.17; 2.36); motor aβ −1.60 (95% CI −5.00; 1.81); expressive language aβ −2.54 (95% CI −6.09; 1.00).
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score; maternal history of tuberculosis, CD4 cell count AND gestational age: cognitive aβ −1.32 (95% CI −4.65; 2.01); motor aβ −2.36 (95% CI −5.75; 1.03); expressive language aβ −2.85 (95% CI −6.17; 1.01).
Differences between children with and without developmental assessments
There were limited differences by completion of developmental assessment. Overall, mother-child pairs who attended study follow-up through 12 months (vs. those who discontinued follow-up prior to 12 months) were older (mean age 29 vs. 27 years), with somewhat higher log10 HIV viral load at ART initiation (median, 4.0 vs. 3.8); similar proportions achieved viral suppression by delivery (77% vs. 72%). Among all mother-child pairs who completed 12 months of follow-up, those who also completed developmental assessments (vs. those who did not) had similar age and psychosocial characteristics but lived in better socio-economic circumstances. Median log10 HIV viral load results were similar at ART initiation (4.1 vs. 4.0); similar proportions achieved viral suppression by delivery (75% vs. 78%). There were no differences in history of tuberculosis, CD4 cell count at ART initiation or duration of breastfeeding.
Developmental outcomes
Overall, mean (SD) scores of HEU children approximated the BSID-III reference standards: cognitive, 101 (15); motor, 99 (15); and expressive language, 100 (17).9 In crude linear regression, VCY [median 2.2 (IQR 1.7; 2.8)] predicted lower expressive language and motor scores [β (95%CI): −2.82 (−5.60; −0.04) and −3.71 (−6.60; −0.83), respectively], with limited effect on cognitive score [β −1.87 (95%CI: −4.59; 0.84); Table 1]. Estimates did not change appreciably after adjusting for multiple confounders; similar associations were seen using other indicators of maternal disease severity in pregnancy (Table 1). In sensitivity analysis, addition of nadir CD4 cell count and history of tuberculosis decreased precision without changing associations (Table 1, footnotes). Similar results were seen in analysis restricted to full-term children: per log10 increase in VCY, aβ (95%CI) for motor, −1.75 (−4.63; 1.13); expressive language, −3.38 (−6.59; −0.17) and cognitive scores, −1.19 (−4.14; 1.76).
Increasing VCY was persistently associated with higher relative odds of motor and expressive language delay [aOR (95% CI): 3.32 (1.36; 8.14) and 2.79 (1.57; 4.94), respectively], but not with cognitive delay (Table 2). In sensitivity analysis, estimates and precision did not change significantly following further adjustment for CD4 cell count and history of tuberculosis (Table 2, footnotes). Increasing gestational age was protective against cognitive and motor, but not expressive language, delay (Table 2). Results from analysis restricted to term-born children (n=183) approximated those for the full cohort albeit with somewhat lower precision. Adjusting for maternal age, socio-economic status, IPV and maternal alcohol use, increasing VCY was association with increased relative odds of motor delay [aOR 2.55 (95% CI 0.99; 6.61)] and expressive language delay [aOR 2.62 (95% CI1.45; 4.75)], but not cognitive delay [aOR 1.46 (95% CI 0.70; 3.06)] in HEU children born at term. Inferences were unchanged in analyses using subtest scaled scores.
Table 2.
Logistic regression analysis of odds of delay (BSID-III score<85), for cognitive, motor and expressive language domains by maternal HIV viremia in pregnancy
| Variable | Cognitive delay10, 11 | Motor delay10, 11 | Expressive language* delay10, 11 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Maternal variables | ||||||
| Log10 viral copies x year/mL (VCY) | 1.24 (0.67; 2.30) | 1.68 (0.84; 3.34) | 1.72 (0.89; 3.32) | 3.32 (1.36; 8.14) | 2.42 (1.45; 4.04) | 2.79 (1.57; 4.94) |
| Log10 HIV viral load, ART initiation | 1.41 (0.79; 2.51) | - | 1.83 (0.96; 3.49) | - | 2.65 (1.58; 4.45) | - |
| HIV VL ≥50 copies/mL vs. <50, delivery | 1.00 (0.34; 2.90) | - | 2.56 (0.95; 6.88) | - | 2.05 (0.97; 4.33) | - |
| HIV VL ≥1000 copies/mL vs. <1000, delivery | 2.02 (0.41; 9.95) | - | 4.00 (0.98; 16.36) | - | 2.47 (0.70; 8.67) | - |
| Nadir CD4 cell count (cells/mm3) | 0.999 (0.996; 1.001) | - | 0.999 (0.997; 1.002) | - | 0.996 (0.994; 0.999) | - |
| <200 | Ref | - | Ref | - | Ref | - |
| 200 – 349 | 1.49 (0.37; 6.01) | - | 0.92 (0.25; 3.38) | - | 0.71 (0.29; 1.76) | - |
| 350 – 499 | 1.65 (0.38; 7.11) | - | 0.37 (0.06; 2.14) | - | 0.34 (0.11; 1.04) | - |
| ≥ 500 | 0.63 (0.12; 3.33) | - | 0.79 (0.20; 3.17) | - | 0.17 (0.05; 0.59) | - |
| Current/prior tuberculosis vs. none | 2.42 (0.80; 7.29) | - | 3.95 (1.34; 11.64) | - | 2.53 (1.04; 6.14) | - |
| Hemoglobin (g/dL) | 0.68 (0.46; 0.99) | - | 0.60 (0.39; 0.91) | - | 0.82 (0.61; 1.10) | - |
| Anemic (<11 g/dL vs ≥11 g/dL) | 1.64 (0.64; 4.19) | - | 2.00 (0.71; 5.64) | - | 1.15 (0.56; 2.34) | - |
| Maternal age (years) | 1.06 (0.98; 1.14) | - | 1.02 (0.93; 1.11) | - | 0.99 (0.93; 1.05) | - |
| Age ≥ 30 years (vs. <30) | 1.61 (0.64; 4.05) | 0.89 (0.71; 5.03) | 1.02 (0.39; 2.70) | 1.26 (0.38; 4.19) | 0.89 (0.44; 1.81) | 1.08 (0.50; 2.32) |
| Poverty score 1 | 0.85 (0.55; 1.29) | 0.84 (0.52; 1.34) | 0.98 (0.63; 1.53) | 0.82 (0.47; 1.43) | 1.07 (0.77; 1.48) | 1.26 (0.88; 1.80) |
| Education 2 | 0.44 (0.12; 1.56) | 0.37 (0.10; 1.48) | 1.01 (0.34; 2.98) | 0.70 (0.17; 2.96) | 0.43 (0.17; 1.10) | 0.26 (0.09; 0.76) |
| Depression 3 | 2.92 (0.86; 9.84) | - | 2.14 (0.56; 8.17) | - | 2.32 (0.82; 6.56) | - |
| Intimate partner violence 4 | 0.99 (0.32; 3.14) | 1.34 (0.33; 3.92) | 3.58 (1.31; 9.73) | 4.72 (1.36; 16.32) | 0.86 (0.35; 2.11) | 0.62 (0.23; 1.66) |
| Risky drinking 5 | 0.58 (0.19; 1.82) | 0.51 (0.15; 1.74) | 1.30 (0.46; 3.64) | 0.58 (0.16; 2.17) | 1.58 (0.75; 3.30) | 1.65 (0.72; 3.76) |
| Infant characteristics | ||||||
| Gestation at delivery (weeks) 6 | 0.82 (0.70; 0.96) | 0.77 (0.64; 0.92) | 0.65 (0.53; 0.78) | 0.54 (0.42; 0.70) | 1.14 (0.95; 1.38) | 1.11 (0.90; 1.37) |
| Preterm (<37 weeks) vs. term | 2.58 (0.85; 7.78) | - | 8.09 (2.82; 23.24) | - | 0.54 (0.15; 1.90) | - |
| Infant sex: male vs. female | 0.88 (0.35;2.22) | - | 1.16 (0.44; 3.07) | - | 0.97 (0.48; 1.96) | - |
| Small-for-gestational age 7 | 1.13 (0.31; 4.11) | - | 2.06 (0.62; 6.80) | - | 1.97 (0.80; 4.86) | - |
| Duration of any breastfeeding (months) 8 | 1.03 (0.95; 1.11) | - | 0.99 (0.91;1.07) | - | 0.98 (0.92; 1.04) | - |
| Duration of exclusive breastfeeding (weeks) 9 | 1.02 (0.97; 1.06) | - | 1.00 (0.96; 1.05) | - | 1.00 (0.97; 1.03) | - |
Language score based on expressive language subscale only; Abbreviations – BSID-III, Bayley Scales of Infant and Toddler Development Third Edition; ART, antiretroviral therapy; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Except where otherwise indicated, maternal variables represent measures taken at first antenatal clinic booking, prior to ART initiation
Calculated using standardized asset score (access to flush toilet, running water, electricity in home, refrigerator, telephone, television, formal housing) and maternal employment; higher score indicates less relative disadvantage
Education: comparing women who completed secondary schooling vs. those who did not
Depression: EPDS (Edinburgh postnatal depression scale) score of ≥ 13, compared to score <13
Any physical, sexual or psychological violence as measured with World Health Organization violence against women questionnaire (compared to no IPV)
Risky drinking, defined as Alcohol use disorders identification test (AUDIT-C) score ≥3 (compared to scores <3)
Gestation at delivery: based on early ultrasound and/or last menstrual period as indicated
SGA, birthweight below 10th centile for gestational age (Intergrowth-21st reference standards); compared to appropriate for gestational age
Breastfeeding durations determined by maternal self-report: last study visit at which breastfeeding reported taken as last day of breastfeeding
Exclusivity defined as receiving only breastmilk and medication; non-time-varying; duration of exclusive breastfeeding among those who never exclusively fed set as zero
Interaction terms: VCY-gestational age for cognitive, p=0.84; motor, p=0.46; expressive language, p=0.40; VCY-sex: cognitive, p=0.13; motor, p=0.30; expressive language, p=0.16; VCY-breastfeeding duration: cognitive, p=0.10; motor, p=0.74; expressive language, p=0.62
Sensitivity analysis results:
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score (not including gestational age): cognitive aOR 1.39 (95% CI 0.73; 2.63); motor aOR 1.70 (95% CI 0.87; 3.33); expressive language aOR 2.89 (95% CI 1.63; 5.11).
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score; maternal history of tuberculosis and CD4 cell count (not including gestational age): cognitive aOR 1.21 (95% CI 0.57; 2.58); motor aOR 1.76 (95% CI 0.80; 3.90); expressive language aOR 2.04 (95% CI 1.05; 3.94).
Adjusting for maternal age, education, intimate partner violence, risky drinking and poverty score; maternal history of tuberculosis, CD4 cell count AND gestational age: cognitive aOR 1.55 (95% CI 0.70; 3.42); motor aOR 3.72 (95% CI 1.35; 10.26); expressive language aOR 1.89 (95% CI 0.96; 3.71).
DISCUSSION
In this unique cohort of young, breastfed, HEU children born under conditions of universal ART, antepartum burden of HIV viremia was associated with higher relative odds of developmental delay in motor and language domains. These associations, largely independent of preterm birth, were seen despite reasonably high median pre-ART CD4 counts and successful viral suppression at delivery among most women.
Few data are available on the relationship between antepartum HIV viremia and neurodevelopment of breastfed HEU children in the context of universal maternal ART. Similar associations have however been described in other contexts. In a large US-based cohort of young (median age 20 months) HEU children, increasing maternal viral load in pregnancy predicted incrementally lower BSID-II mental (MDI) and psychomotor developmental indices (PDI).3 Although not all mothers had received ART, longer duration of any antiretroviral use was associated with higher MDI scores.3 In Tanzania, antepartum clinical HIV disease severity in the absence of antiretroviral drugs predicted lower PDI scores among 311 HEU children.4
There is growing evidence to support the biological plausibility of this association. Emerging evidence from animal, human and epidemiological studies point to significant alterations in brain development during in utero exposure to maternal immune activation.16 In adults with chronic HIV infection, the degree and persistence of systemic immune activation and inflammation, even during suppressive ART, is directly proportional to disease severity at ART initiation.17 Higher levels and duration of maternal HIV viremia in pregnancy may reflect magnitude and duration of maternal immune activation. Furthermore, direct exposure to circulating HIV antigens may result in fetal immune activation; chronic activation of fetal microglia may alter synaptic plasticity. Additionally, maternal co-infections, such as with cytomegalovirus (CMV), are often associated with higher levels of HIV viremia, and may further contribute to peripartum mother-infant immune activation, as well as having possible direct adverse effects on infant neurodevelopment.18–20
These data come from a well-characterized mother-infant cohort wherein all mothers received the same perinatal care and universal ART regimens, and all HEU children were breastfed.6 For these reasons, our cohort may not be fully representative of the larger HIV-affected mother-infant population in Southern Africa. The average developmental scores of the children in our cohort were within age expectations on measures of cognition, expressive language, and motor development, while absolute differences associated with maternal viremia were small. These findings align with other recent publications on developmental outcomes of HEU children in Africa.7,21,22
Study strengths include prospective, repeated viral load testing and extensive measures of maternal psycho-social and economic factors. Nevertheless, our findings should be interpreted with caution given the cross-sectional nature of our developmental measure, low precision for sub-group analyses and lack of receptive language measurement. Inability to screen for maternal co-infections, such as CMV, and lack of inflammatory markers preclude in-depth evaluation of possible underlying causal mechanisms. Nonetheless, our cohort is adequately powered to detect differences in developmental outcomes using several measures of HIV disease severity in pregnancy. Included in these measures we use a novel application of VCY.11 While VCY is increasingly used in the adult HIV literature, to our knowledge this is the first application thereof in perinatal epidemiology of HEU child health.
Supplementary Material
SDC 1. Study flow diagram
SDC 2. Characteristics of HIV-infected women and HIV-exposed uninfected children
ACKNOWLEDGEMENTS
The authors thank all participating mothers and children, the dedicated study staff (including Andrea Strandvik, Liza Esterhuyse and Robyn Meissner) and the health services at Gugulethu Midwife Obstetric Unit. This research was supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research
SLR assisted with collection of data, conducted the analysis and wrote the first draft of the manuscript. KD provided training and supervision of developmental assessments; KD and MK provided supervision for all child health aspects of the study. LM and EJA conceived the MCH-ART study, and were responsible for study design, funding, implementation and overall leadership. TKP was the study coordinator. TKP and KB were responsible for data management and oversight. LE conducted developmental assessments and assisted with data management. ML contributed to the data analysis. AZ was the senior study manager and provided oversight of all study administration processes. All authors contributed to and approved the final manuscript.
Footnotes
Conflicts of interest
The authors have no conflict of interest to declare.
REFERENCES
- 1.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. The Lancet infectious diseases. 2016;16(6):e92–e107. [DOI] [PubMed] [Google Scholar]
- 2.le Roux SM, Abrams EJ, Nguyen K, et al. Clinical outcomes of HIV-exposed, HIV-uninfected children in sub-Saharan Africa. Tropical medicine & international health : TM & IH. 2016;21(7):829–845. [DOI] [PubMed] [Google Scholar]
- 3.Williams PL, Marino M, Malee K, et al. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics. 2010;125(2):e250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald CM, Manji KP, Kupka R, et al. Stunting and wasting are associated with poorer psychomotor and mental development in HIV-exposed tanzanian infants. Journal of Nutrition. 2013;143(2):204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McHenry MS, McAteer CI, Oyungu E, et al. Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics. 2018;141 (2):e20172888 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myer L, Phillips TK, Zerbe A, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH): Rationale and Design of the MCH-ART Study. Journal of acquired immune deficiency syndromes (1999). 2016;72 Suppl 2:S189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.le Roux SM, Donald KA, Brittain K, et al. Neurodevelopment of breastfed HIV-exposed uninfected and HIV-unexposed children in South Africa. AIDS (London, England). 2018;32(13):1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Western Cape Government (Health), Cape Town South Africa. PMTCT Clinical Guidelines Update, June 2014. [Google Scholar]
- 9.Bayley N Bayley Scales of Infant and Toddler Development - third edition Pearson clinical assessments, San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- 10.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatric research. 2014;75(5):670–674. [DOI] [PubMed] [Google Scholar]
- 11.Cole SR, Napravnik S, Mugavero MJ, et al. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. American journal of epidemiology. 2010;171(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black MM, Walker SP, Fernald LC, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udo IE, Sharps P, Bronner Y, et al. Maternal Intimate Partner Violence: Relationships with Language and Neurological Development of Infants and Toddlers. Maternal and child health journal. 2016;20(7):1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen SKG, Berens AE, Nelson CA. Effects of poverty on interacting biological systems underlying child development. The Lancet Child & Adolescent Health. 2017;1(3):225–239. [DOI] [PubMed] [Google Scholar]
- 15.Teck R, Ascurra O, Gomani P, et al. WHO clinical staging of HIV infection and disease, tuberculosis and eligibility for antiretroviral treatment: relationship to CD4 lymphocyte counts. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2005;9(3):258–262. [PubMed] [Google Scholar]
- 16.Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nature reviews. Neurology. 2014;10(11):643–660. [DOI] [PubMed] [Google Scholar]
- 17.Younas M, Psomas C, Reynes J, et al. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV medicine. 2016;17(2):89–105. [DOI] [PubMed] [Google Scholar]
- 18.Deayton JR, Sabin CA, Johnson MA, et al. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. The Lancet. 2004;363(9427):2116–2121. [DOI] [PubMed] [Google Scholar]
- 19.Filteau S, Rowland-Jones S. Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children. Frontiers in immunology. 2016;7:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianella S, Letendre S. Cytomegalovirus and HIV: A Dangerous Pas de Deux. The Journal of infectious diseases. 2016;214 Suppl 2:S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhury S, Williams PL, Mayondi GK, et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics. 2017;140(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer PE, Slogrove AL, Laughton B, et al. Neurodevelopmental outcome of HIV-exposed but uninfected infants in the Mother and Infants Health Study, Cape Town, South Africa. Tropical medicine & international health : TM & IH. 2018;23(1):69–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1. Study flow diagram
SDC 2. Characteristics of HIV-infected women and HIV-exposed uninfected children