Abstract
Background:
Electroconvulsive therapy (ECT) is usually reserved for treatment of severe major depressive disorder (MDD), but may be equally effective in the treatment of moderate-severity MDD. This possibility, however, has only been studied to a very limited extent. We therefore investigated the efficacy of ECT after stratifying patients into severe MDD and moderate-severity MDD.
Methods:
We used data from the Prolonging Remission in Depressed Elderly (PRIDE) study, in which 240 patients (≥60 years) with MDD were treated with right unilateral ultrabrief pulse ECT, combined with venlafaxine. We used the six-item core depression subscale (HAM-D6) of the Hamilton Depression Rating Scale to define depression severity. Participants with baseline total scores ≥12 on the HAM-D6 were considered to have severe MDD, while those with HAM-D6 total scores ≤11 were considered to have moderate-severity MDD.
Results:
Among the participants with severe MDD and moderate-severity MDD, the mean change in the HAM-D6 total score from baseline to endpoint was −8.2 (95% confidence interval (95%CI) = −7.5; −9.0, paired t-test: p<0.001) and −5.9 (95%CI = −5.1; −6.6, paired t-test: p<0.001), respectively. A total of 63% of those with severe MDD and 75% of those with moderate-severity MDD achieved remission (HAM-D6 total score ≤4) (Pearson’s 2-sample chi-squared test of difference between groups: p=0.27).
Limitations:
The PRIDE study was not designed to address this research question.
Conclusions:
ECT combined with venlafaxine appears to be an effective treatment for moderate-severity MDD. It may be appropriate to expand the indications for ECT to include patients with moderate-severity MDD.
Introduction
Electroconvulsive therapy (ECT) has remained a mainstay in the treatment for severe major depressive disorder (MDD), largely due to the efficacy and the speed with which it induces response and remission (Kellner, et al, 2010). ECT has been reserved, almost exclusively, for the most seriously ill psychiatric patients, including those who have not adequately responded to multiple prior pharmacotherapy trials (Kellner, et al, 2012) or psychotic MDD (Leadholm, et al, 2013). Ongoing refinement of the procedure, most notably resulting in the decrease in cognitive side-effects (Kolshus, et al, 2017), has led to the question of whether ECT could be considered as a treatment for patients with less severe MDD (Beale and Kellner, 2000).
The question of the appropriateness of ECT for less severe depressive illness comes from both practitioners and patients (Beale and Kellner, 2000), some of whom might opt for ECT over sequential antidepressant trials, were it available to them (Ross, et al, 2018). The transition of ECT from an exclusively inpatient procedure to include treatment of outpatients, also facilitates this possibility (Fink and Kellner, 1996). Given the recent FDA order in which the only two indications for ECT, that was re-classified from Class III (high risk) to Class II (moderate risk), are severe major depression and catatonia (Food and Drug Administration, HHS, 2018) addressing the question regarding ECT for less severe depression seems particularly pertinent.
Severity of depressive illness has been investigated as a predictor for ECT response and remission. The data on this issue are mixed, as some studies found that increased baseline severity of depressive symptoms predicts better response (van Diermen, et al, 2018a; van Diermen, et al, 2018b), while others found no association or inconclusive results (Haq, 2015). We are, however, aware of only few studies studies that have specifically investigated whether patients with moderate levels of baseline depression severity also do well with ECT (Brus, et al, 2017; Nordenskjold, et al, 2012). Here, we sought to provide information on this issue by using data from phase 1 of the Prolonging Remission in Depressed Elderly (PRIDE) study (Kellner, et al, 2016) in which all patients received ECT. We divided this cohort of patients into those with severe depression and those with moderate-severity depression and compared their response to ECT. Our hypothesis was that the patients with moderate levels of severity would benefit as much from ECT as those with severe MDD.
Method
The data for this analysis stems from phase 1 of the PRIDE study, which evaluated the efficacy of right unilateral ultrabrief pulse ECT combined with venlafaxine for the treatment of MDD among the elderly (Kellner, et al, 2016). The PRIDE study has been described in detail elsewhere (Kellner, et al, 2016) – and is briefly summarized below.
Sample
In order to be eligible for the PRIDE study, patients had to i) be referred for inpatient or outpatient ECT, ii) be at least 60 years old, iii) have a diagnosis of MDD according to the DSM-IV (American Psychiatric Association, 1994), and iv) have a score of at least 21 on the 24-item Hamilton Depression Rating Scale (HAM-D24). Exclusion criteria included diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, dementia, intellectual disability, substance abuse/dependence (past six months); an active neurological or medical condition that could affect treatment response or cognition; contraindications to venlafaxine or lithium; and failure to respond (within the current episode) to an adequate trial of venlafaxine plus lithium or to ECT (Kellner, et al, 2016).
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the institutional review boards of the participating study sites (Kellner, et al, 2016). All patients provided written informed consent.
Washout
Psychotropic medications were withheld for one week prior to ECT, with the exception of lorazepam, up to 3 mg, to treat anxiety, if needed (Kellner, et al, 2016).
Venlafaxine
Venlafaxine was initiated (open label) 1–5 days prior to ECT or up to 2 days after the first ECT treatment. The initial dosage was 37.5 mg/day, which was increased by 37.5 mg every 3 days (or as tolerated), with an eventual target dose of 225 mg (Kellner, et al, 2016).
ECT procedure
ECT procedures were standardized across study sites, using right unilateral electrode placement and ultrabrief pulse width stimuli (either 0.25 or 0.3 ms), empirical dose titration at the first session, and 6X seizure threshold at subsequent treatments. Anesthesia was with standard doses of methohexital and succinylcholine. ECT treatments were given three times per week (Kellner, et al, 2016).
Depression rating and trial completion
Participants were rated on the 24-item version of the Hamilton Depression Rating Scale (HAM-D24) at baseline and subsequently three times per week – prior to the administration of ECT. The participating patients exited phase 1 of the study if they were remitters, non-remitters or dropouts. Here, remission (not to be mistaken with the HAM-D6 defined remission used as outcome measure in the present reanalysis of the PRIDE data) was based on two consecutive HAM-D24 ratings. Specifically, participants were considered remitters if their HAM-D24 total scores were ≤10 on two consecutive ratings, and the HAM-D24 total score did not decrease >3 points on the second consecutive HAM-D24 rating, or remained ≤6. Participants were considered non-remitters if they did not reach the remission criteria after at least 10 treatments, and reached a plateau defined as no clinical improvement (<3-point decrease in HRSD24 total score after the last two consecutive treatments). Participants were considered dropouts if neither remission nor non-remission criteria were met, or if the consent for ECT or study participation was withdrawn, or if ECT was discontinued for clinical or other reasons. The minimum number of ECT treatments required for remission status was two, while there was no maximum. Non-remitters were required to have at least 12 ECT treatments to be study completers, but could continue ECT treatment beyond the 12 treatments if no plateau of improvement had been reached (<3-point decrease in HAM-D24 score after last two consecutive treatments) (Kellner, et al, 2016)
Neurocognitive measures
As described in detail elsewhere (Lisanby, et al, 2020), a neuropsychological test battery including the following instruments was employed at baseline and within 72 hours following the last ECT session: The Autobiographical Memory Interview-Short Form (AMI-SF) (McElhiney, et al, 2001) California Verbal Learning Test-ll (CVLT-II) (Delis, et al, 2000), Delis-Kaplan Executive Function System (D-KEFS) Verbal Fluency (Condition 1: Letter Fluency) Test (Delis and Kaplan, 2001), Dementia Rating Scale-2nd Edition Initiation Perseveration Index (DRS-2) (Jurica, et al, 2001), Stroop Color and Word Test, and Trail Making Test Parts A and B (TMT A and B) (Golden and Freshwater, 2002). Global cognitive function was assessed with the Mini Mental State Examination (Folstein, et al, 1975).
Definition of severe and non-severe depression
As the objective of this study was to compare the treatment outcome between participants with severe depression and moderate levels of depression, respectively, we grouped the participants according to their baseline score on the six-item core depression subscale (HAM-D6) of the Hamilton Depression Rating Scale (Bech, et al, 1975; Timmerby, et al, 2017). In accordance with Bech (Bech, 2012), those with baseline total scores ≥12 on the HAM-D6 were defined as having severe MDD. Those with HAM-D6 total scores ≤11 were defined as having moderate-severity MDD. The reason for using HAM-D6 for this purpose is that this subscale, unlike the 17-, 21, and 24-item versions of the Hamilton Depression Rating Scale that are multidimensional (Kyle, et al, 2016), is unidimensional (Bech, et al, 1981; Kyle, et al, 2016; Ostergaard, et al, 2014a; Ostergaard, et al, 2014b; Ostergaard, et al, 2016). Unidimensionality is present when all items in a rating scale add unique information regarding the severity of the underlying latent syndrome - and is a prerequisite for using the total score of a rating scale as a meaningful measure of depression symptom severity (Bech, 2012). Another reason for preferring HAM-D6 (which consists of item 1 – depressed mood, item 2 – guilt feelings, item 7 – work and interests, item 8 – psychomotor retardation, item 10 – psychic anxiety, and item 13 – general somatic symptoms) is that it is less sensitive to the side effects of common antidepressant medications (Bech, 2010; Hieronymus, et al, 2016; Ostergaard, 2017). Such side effects are likely to interfere with the ratings on certain Hamilton items, in particular item 12 – gastrointestinal symptoms (antidepressants can cause nausea, reflux, diarrhea and constipation), item 14 – sexual dysfunction (antidepressants can cause erectile dysfunction, delayed/premature ejaculation and anorgasmia), item 16 – loss of weight (antidepressants can cause unintended weight gain), and items 4-6 on sleep difficulties (antidepressants can cause unintended sedation), which are all included in the 17-, 21-, and 24-item versions of the Hamilton Depression Rating Scale (Bech, 2010; Hieronymus, et al, 2016; Ostergaard, 2017) and complicate the interpretation of results obtained with these longer scales. Therefore, since the PRIDE study also involved treatment with an antidepressant (venlafaxine) the HAM-D6 is a particularly appropriate outcome measure for this analysis.
Imputation of missing neurocognitive data and statistical analysis of change from baseline
There were no missing data on HAM-D24 and hence no missing data for HAM-D6 either. There were however missing data for the neurocognitive outcomes. Therefore, as reported elsewhere (Lisanby, et al, 2020), imputation was carried out as follows: “Ninety-five percent CI and paired t-tests obtained using multiple imputation procedure for missing data were used, respectively, to estimate the change from baseline to end of Phase 1 and determine if the change was statistically significant for each neurocognitive outcome. First, as part of the multiple imputation procedure, logistic regression, with the dichotomous-dependent variable missing/not missing, was used to identify the variables predictive of missing values for the neurocognitive variables. The multiple imputation process was then carried out using the Markov Chain Monte Carlo (MCMC) method to obtain 100 completed data subsets for the neurocognitive variables. Variables included in the imputation model were outcome status (remitter, non-remitter, dropout), age, baseline and last observed” HAM-D24 “total score, sex, education, baseline and last observed MMSE total raw score, and number of ECT sessions in Phase 1. Next, in the multiple imputation procedure, results were combined across the 100 imputed subsets for final estimation of change from baseline (raw effect size) using 95% Cl and inference using paired t-tests” (Lisanby, et al, 2020).
Statistical analysis of HAM-D6 outcomes
The mean change in HAM-D6 total scores from baseline to endpoint was calculated and subsequently tested for significance by means of the paired t-test within the severe MDD and moderate-severity MDD groups, respectively. The proportion of participants from the two groups that obtained remission (HAM-D6 total score ≤4) (Timmerby, et al, 2017) was compared between groups (severe vs. moderate-severity MDD) using Pearson’s 2-sample chi-square test. A significance level of .05 was employed in these tests.
Statistical software
The analyses were carried out using the statistical package “R” and SAS statistical software, Version 9.4. Cary, NC: SAS Institute Inc: 2014.
Results
The patients who participated in the PRIDE study are described in full elsewhere (Kellner, et al, 2016). In brief, the study recruited 240 participants of whom 58% were female. The mean age was 70 years (standard deviation (SD) = 8). Eighty-eight percent of the participants had recurrent depression, 59% had melancholic depression, and 12% had psychotic depression. The mean total score on the HAM-D24 at baseline was 31 (SD = 7) (Kellner, et al, 2016). Of the 240 participants, 156 (65%) had severe MDD and 84 (35%) had moderate-severity MDD at study baseline according to the HAM-D6 total score. The baseline demographic and clinical characteristics of the participants with severe and moderate-severity MDD, respectively, are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics of the 240 study participants by severity of depression per HAM-D6 total score cut-off (<12 vs. ≥12)
| Characteristic | Total Sample (N=240) | HAM-D6 total score | Comparison: moderate-severity versus severe MDD | |
|---|---|---|---|---|
| <12 Moderate-severity MDD (n=84) | ≥12 Severe MDD (n=156) |
|||
| Mean ± SD | Mean ± SD | Mean ± SD | p-value* | |
| Age (years) | 69.9 ± 7.6 | 71.0 ± 7.5 | 69.3 ± 7.6 | 0.091 |
| Education (years)a | 14.5 ± 3.3 | 14.6 ± 3.5 | 14.5 ± 3.1 | 0.778 |
| Hamilton Depression Rating Scale (24-item) score | 31.2 ± 7.3 | 25.9 ± 3.6 | 34.1 ± 7.1 | <0.0001 |
| Hamilton Depression Rating Scale (6-item) score | 12.5 ± 2.4 | 10.0 ± 1.2 | 13.9 ± 1.8 | <0.0001 |
| Mini-Mental State Examination raw scorea | 27.5 ± 2.4 | 27.7 ± 2.3 | 27.4 ± 2.4 | 0.453 |
| Clinical Global /Impression severity scorea | 5.3 ± 0.9 | 4.9 ± 0.8 | 5.4 ± 0.9 | <0.0001 |
| Lifetime psychiatric hospitalizationsb | 2.4 ± 3.4 | 2.0 ± 2.2 | 2.6 ± 3.8 | 0.213 |
| Cumulative Illness Rating Scale for Geriatrics scorec | 8.6 ± 4.2 | 8.1 ± 4.1 | 8.9 ± 4.3 | 0.214 |
| Number of antidepressants trials in current episode d | 2.4 ± 1.6 | 2.0 ± 1.7 | 2.4 ± 1.6 | 0.943 |
| N (%) | N (%) | N (%) | p-value | |
| Female | 138 (57.5) | 47 (56.0) | 91 (58.3) | 0.722 |
| White | 228 (95.0) | 81 (96.4) | 147 (94.2) | 0.549 |
| Hispanice | 9 (3.8) | 6 (7.3) | 3 (1.9) | 0.067 |
| Recurrent depression | 210 (87.5) | 72 (85.7) | 138 (88.5) | 0.539 |
| Psychotic depression | 28 (11.7) | 3 (3.6) | 25 (16.0) | 0.004 |
| Melancholic depressiona | 141 (59.0) | 48 (57.1) | 93 (60.0) | 0.668 |
| Atypical depressiona | 5 (2.1) | 4 (4.8) | 1 (0.7) | 0.053 |
| Beck Scale for Suicide Ideation, % with score of 0e | 105 (52.0) | 45 (60.0) | 60 (47.2) | 0.080 |
| Family history of psychiatric illnessf | 160 (68.7) | 52 (63.4) | 108 (71.5) | 0.203 |
| Family history of mood disorderg | 143 (61.9) | 46 (56.8) | 97 (64.7) | 0.240 |
| Family history of MDD h | 136 (59.1) | 43 (53.8) | 93 (62.0) | 0.225 |
| Family history of bipolar disordersg | 33 (14.3) | 9 (11.3) | 24 (15.9) | 0.337 |
Data missing for 1 participant
Data missing for 11 participants
Data missing for 3 participants
Data missing for 34 participants
Data missing for 38 participants
Data missing for 7 participants
Data missing for 9 participants
Data missing for 10 participants
SD = Standard deviation
For comparisons between severe and moderate-severity groups (t-test or chi-square test as appropriate)
The two groups were comparable with regard to the vast majority of the characteristics – with expected exceptions, namely that those with severe MDD (according to the HAM-D6 total score) had significantly higher scores on the HAM-D24, HAM-D6 and on the Clinical Global Impression Severity score, and were significantly more likely to meet the criteria for psychotic depression, compared to those with moderate-severity depression.
On average, the patients with severe MDD underwent 7.2 (SD = 3.6) ECT sessions, while those with moderate-severity MDD underwent 7.0 (SD = 3.7) – p-value of t-test for difference between groups = 0.704. With regard to trial completion, using the original HAM-D24-based definitions (see the method section), there were 59.6% remitters, 9.6% non-remitters and 30.8% dropouts among those with severe MDD, and 65.5% remitters, 10.7% non-remitters and 23.8% drop-outs among those with moderate-severity depression (chi-square test for difference between groups = 0.521).
The HAM-D6 total score trajectory of the participants - including stratification on depression severity at baseline - is shown in Figure 1.
Figure 1. Mean HAM-D6 total scores for study participants – stratified by depression severity.
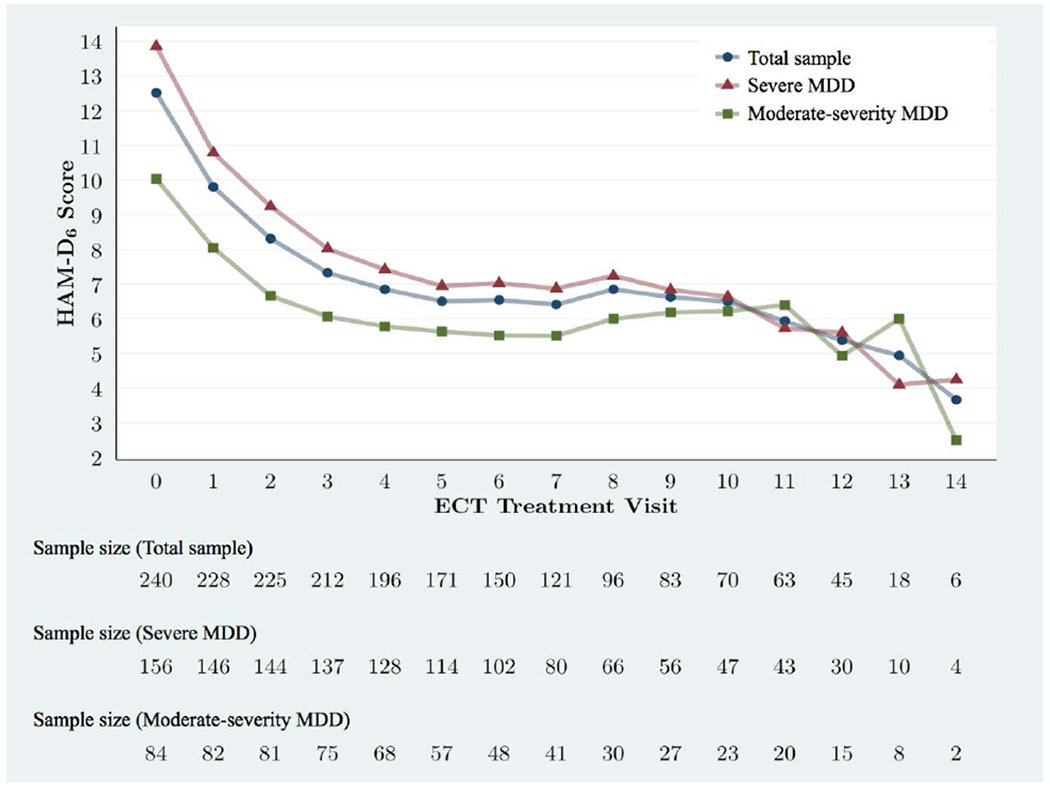
HAM-D6 mean total score trajectories from baseline to visit 14 for the entire sample and stratified into severe MDD (HAM-D6 total score ≥12) or moderate-severity MDD (HAM-D6 total score ≤11). Visits 15-17 are omitted due to the small sample size late in the trial (n=1-3) resulting in unstable means.
Among the participants with severe MDD, the mean change in the HAM-D6 total score from baseline to endpoint was −8.2 (95% confidence interval (95%-CI) = −7.5; −9.0). According to the paired t-test, this decrease was statistically significant (p<0.001). Similarly, among the participants with moderate-severity MDD, the mean change in the HAM-D6 total score from baseline to endpoint was −5.9 (95%-CI = −5.1; −6.6). According to the paired t-test, this decrease was also statistically significant (p<0.001).
Among the participants with severe MDD, using the HAM-D6-based definition, 63% reached remission during the study. Among the participants with moderate-severity MDD, 75% reached remission during the study. Pearson’s 2-sample chi-square test showed that these proportions were not significantly different (p=0.27). The rate at which remission was achieved is illustrated in Figure 2. Of the participants with moderate-severity MDD reaching remission during the study, more than 90% had done so after five treatments. It took 10 treatments before 90% of the patients with severe MDD who reached remission had done so.
Figure 2. Speed of remission – stratified by depression severity.
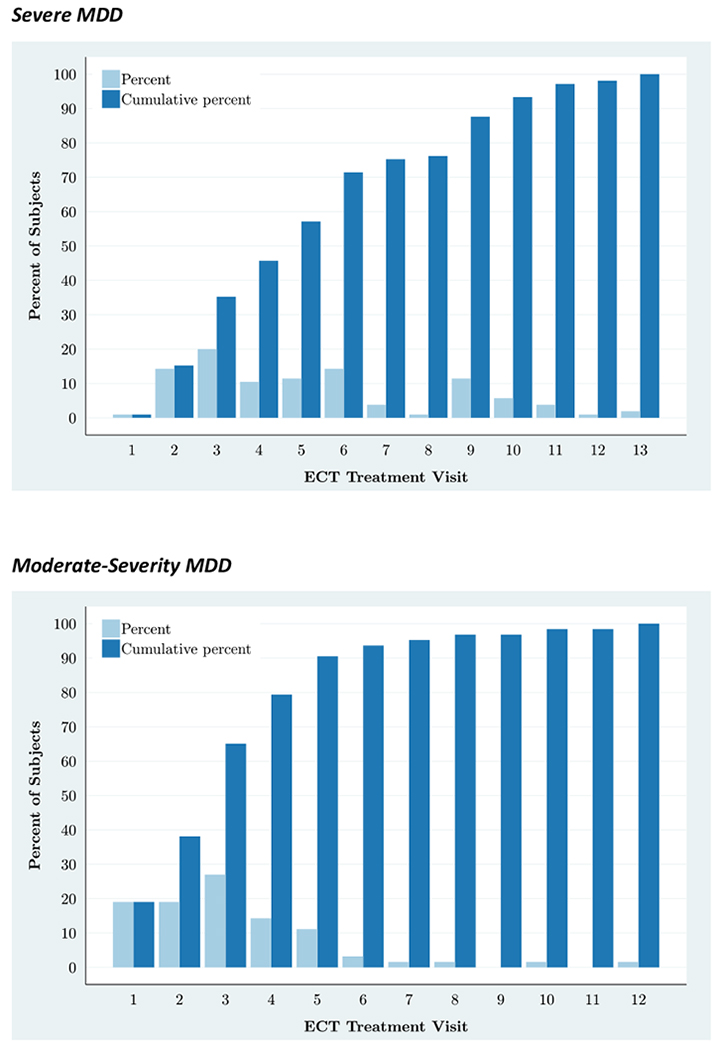
Speed of remission (HAM-D6 total score ≤4) among participants with severe MDD (top) and moderate-severity MDD (bottom), who reached remission during the study.
Regarding neurocognitive function, both depression severity groups showed similar neurocognitive changes from baseline to endpoint (see Table 2). Out of 12 neurocognitive variables, only the difference between the severity groups in change in processing speed as measured by the Stroop Color and Word Test Color Naming variable was statistically significant (p=0.044).
Table 2.
Baseline, last and change from baseline assessment for cognitive outcomes of the 240 study participants by severity of depression per HAM-D6 total cut-off (<12 vs. ≥12) after multiple imputation*.
| Neuropsychological Assessment | HAM-D6 total score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <12 Moderate-severity MDD (n=84) | ≥12 Severe MDD (n=156) | Comparison: moderate-severity versus severe MDD | |||||||
| Baseline mean (SD) | Last mean (SD) | Change mean (95%CI) | Baseline mean (SD) | Last mean (SD) | Change mean (95%CI) | Difference (95%CI) | p-value** | DF | |
| Autobiographical Memory Interview-Short Form (AMI-SF) | |||||||||
| Total score | 51.3 (0.7) | 38.9 (1.2) | −12.4 (−14.3,10.5) | 49.7 (0.6) | 36.3 (0.9) | −13.4 (−14.9,−1.9 | 1.0 (−1.4, 3.3) | 0.408 | 67.8 |
| California Verbal Learning Test – II (CVLT-II) | |||||||||
| Trial 1 -5 total recall T-score | 43.1 (1.4) | 41.9 (1.4) | −1.2 (−3.9, 1.6) | 40.9 (1.0) | 39.7 (1.1) | −1.1 (−3.4, 1.3) | −0.1 (−3.5, .5) | 0.979 | 69.3 |
| Short delay free recall z-score | −0.8 (0.1) | −1.1 (0.1) | −0.3 (−0.6, 0.0) | −1.0 (0.1) | −1.3 (0.1) | −0.3 (−0.5, −0.1) | −0.0 (−0.4, 3) | 0.921 | 67.5 |
| Long delay free recall z-score | −1 (0.2) | −1.2 (0.1) | −0.3 (−0.6, 0.0) | −1.2 (0.1) | −1.4 (0.1) | −0.1 (−0.4, 0.2) | −0.2 (−0.6, 3) | 0.447 | 70.3 |
| Recognition discrimination z-score | −0.8 (0.1) | −1.1 (0.2) | −0.3 (−0.7, 0.0) | −0.7 (0.1) | −1.2 (0.1) | −0.4 (−0.7, −0.1) | 0.1 (−0.4, 0.5) | 0.676 | 69.4 |
| Delis-Kaplan Executive Function System (D-KEFS) | |||||||||
| Letter fluency scaled score | 9.2 (0.5) | 7.2 (0.4) | −2.0 (−2.7, 1.2) | 8.3 (0.3) | 7.0 (0.3) | −1.3 (−1.9, −0.7) | −0.7 (−1.6, 3) | 0.184 | 65.9 |
| Dementia Rating Scale-2nd Edition (DRS-2) | |||||||||
| Initiation/Perseveration Index scaled score | 8.2 (0.4) | 7.6 (0.4) | −0.6 (−1.4, 0.2) | 7.5 (0.3) | 7.1 (0.3) | −0.4 (−1.0, 0.3) | −0.2 (−1.3, 8) | 0.661 | 65.1 |
| Stroop Color and Word Test (Stroop) | |||||||||
| Word T-score | 35.5 (1.5) | 31.8 (1.4) | −3.8 (−6.0, 1.6) | 32.7 (0.9) | 30.3 (0.9) | −2.4 (−4.4, −0.5) | −1.4 (−4.3, 5) | 0.345 | 69.4 |
| Color T-score | 32.1 (1.5) | 30.7 (1.4) | −1.3 (−3.9, 1.3) | 28.2 (1.0) | 30 (1.0) | 1.9 (−0.1, 3.) | −3.2 (−6.3, −1) | 0.044 | 64.8 |
| Color-Word T-score | 40.1 (1.1) | 38.8 (1.2) | −1.3 (−37, 1.1) | 39.2 (0.9) | 37.8 (1.0) | −1.4 (−3.1, 0.3) | 0.1 (−2.7, 2.9) | 0.940 | 65.1 |
| Trail Making Test | |||||||||
| Part A scaled score | 8.6 (0.3) | 7.6 (0.4) | −1.0 (−1.6, −0.3) | 7.8 (0.2) | 6.9 (0.3) | −0.9 (−1.5, −0.4) | −0.0 (−0.8, 8) | 0.987 | 63.5 |
| Part B scaled score | 8.1 (0.4) | 7.1 (0.5) | −1.0 (−1.9, 0.2) | 7.3 (0.3) | 6.1 (0.4) | −1.2 (−1.9, −0.4) | 0.1 (−0.9, 2) | 0.798 | 54.9 |
Means and standard deviations for baseline and last and means and 95% confidence intervals for change from baseline.
P-values obtained from pooled t-test
Discussion
In this analysis of data from 240 patients with geriatric depression treated with ECT and venlafaxine we found that the response to treatment among the participants with moderate-severity MDD was equivalent to that seen among those with severe MDD. These results are consistent with those obtained by (Brus, et al, 2017; Nordenskjold, et al, 2012) using naturalistic data from the Swedish Quality register for ECT.
The numerically larger decrease in the HAM-D6 total score from baseline to endpoint observed among patients with severe MDD compared to those with moderate-severity MDD likely represents a floor effect of the relatively lower baseline scores of the latter group. The same is the case for the numerically greater rate of remission among patients with moderate-severity MDD, as compared with those with severe MDD.
While it may seem intuitively obvious that this should be so, there are few data in the literature to date, to document that ECT is useful for patients with less severe depression. Consequently, it is likely that there is a large cohort of depressed patients who have not previously been considered candidates for ECT because of the perception that it should be reserved exclusively for the most severely ill, and because of misperceptions about its tolerability profile (Aki, et al, 2013).
ECT has traditionally been reserved for patients with highly severe, persistent and/or psychotic MDD (Kellner, et al, 2012; Leadholm, et al, 2013). Conversely, it has generally not been considered a mainstream treatment for patients with less severe depression, because of the more involved logistics of treatment delivery (general anesthesia in a procedure room, often hospital-based) and concerns about severe adverse effects (Torring, et al, 2017), particularly transient cognitive impairment (Aki, et al, 2013). There is no clinical or theoretical reason to believe that ECT would not be effective for less severe depression. If response/remission rates were found to be lower in patients with less severe depression (as opposed to our current findings), one possible explanation would be lower rates of psychotic and melancholic subtypes, both of which predict particularly good ECT response and are often associated with greater severity (Petrides, et al, 2001; van Diermen, et al, 2017).
The results of this secondary analysis of the data from the PRIDE study suggest that ECT may be no less effective in elderly patients with less severe levels of MDD. Further research should evaluate whether this is also the case in younger cohorts. Right unilateral electrode placement and ultrabrief stimuli are technical advances that allow effective antidepressant treatment with far fewer adverse cognitive effects than previous forms of ECT (Kolshus, et al, 2017). Indeed, we found no clinically meaningful differences in neurocognitive changes from baseline to endpoint between the severity groups. Specifically, out of 12 neurocognitive variables only the difference between the severity groups in change in processing speed was statistically significant. However, this difference was not clinically significant/meaningful as the performance in both groups was similar. Specifically, both groups at baseline and after ECT showed moderately impaired processing speed. Hence, we extend the main neurocognitive findings of the PRIDE study (Lisanby, et al, 2020), which showed that elderly adults showed relative cognitive tolerability to ECT provided with the combination of right unilateral electrode placement and ultrabrief stimuli, by documenting that this tolerability appears to be independent of baseline depression severity. This is in agreement with prior research that has found no significant relationship between depression symptom severity and neurocognitive performance in patients with MDD (Keilp, et al, 2018; McClintock, et al, 2010b) including those referred for treatment with ECT (McClintock, et al, 2010a). Fortunately across the globe, the shift in many countries to predominantly outpatient practice facilitates access to ECT for a greater number of patients (Fink and Kellner, 1996) to receive antidepressant treatment with ECT parameters that can confer relative cognitive tolerability.
The four main limitations of phase 1 of the PRIDE study, and therefore also of this reanalysis, are that: i) it involved single-arm (non-randomized) open-label treatment with ECT (no sham), ii), it included concomitant treatment with venlafaxine, iii) it only involved elderly patients (≥ 60 years), and iv) The PRIDE study was not designed to address the research question proposed here. A consequence of these limitations is that the observed decrease in depression severity scores was potentially driven by multiple mechanisms: effect of ECT, effect of venlafaxine, placebo effect and the spontaneous course of illness. Also, it cannot be excluded that the study raters consciously/subconsciously underestimated the severity of depression during the study (after baseline) – motivated by a wish to see a strong treatment effect in this uncontrolled study. While these limitations are indeed important for the interpretation of the absolute reductions in depressive severity in the entire PRIDE sample, and have been discussed elsewhere (Kellner, et al, 2016), they are of less importance for the interpretation of the comparative reductions between the study participants with severe- and moderate-severity MDD, respectively. In other words, assuming that the majority of the reduction in the severity of depression is due to ECT, this effect does not seem to differ between those with severe- and moderate-severity MDD. Furthermore, due to the age restriction in the inclusion criteria, the findings of this study may not generalize to younger patients with depression (older age is a well known predictor of response to ECT (van Diermen, et al, 2018b)). Finally, due to the fact that this study represents a secondary analysis of the data from PRIDE, we consider it exploratory in nature and in need of replication in future studies.
In conclusion, our data suggest high remission rates in a cohort of elderly patients with moderate-severity MDD treated with ECT, combined with venlafaxine. Hence, patients with moderate-severity, depressive illness might well be helped by ECT, which may reduce the risk of protracted depressive episodes (Patten, 2006) and sequential unsuccessful antidepressant medication trials (Gaynes, et al, 2009) – thereby improving their prognosis and reducing their risk for suicide. This possibility would benefit from further study.
Highlights.
Electroconvulsive therapy (ECT) has been reserved, almost exclusively, for the most seriously ill psychiatric patients, including those with treatment-resistant or psychotic depression.
The question has been raised as to whether ECT could also be considered as a treatment for patients with less severe depression.
Here we show that patients with severe depression and moderate-severity depression respond equally well to the combination of ECT and venlafaxine.
Acknowledgements
We acknowledge the contributions of the extended CORE/PRIDE work group to the conduct of the PRIDE study and the collection of these data, including Drs. Joan Prudic, Peter Rosenquist, Matt Rudorfer, Richard Weiner and Robert Young. We are grateful to Dr. Rene B.K. Brund and Ms. Mary Dooley for statistical assistance.
Role of the funding source:
The PRIDE study, which provided data for this analysis, was supported by the following grants from the National Institute of Mental Health: U01MH055495, U01MH081362, U01MH086127, U01MH086127, U01MH086130, U01MH08612005, U01MH084241, and U01MH086122. The National Institute of Mental Health had no influence on the design of this secondary data analysis, the interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflicts of interest
Dr. Østergaard and Dr. Speed report no conflicts of interest. Dr. Kellner has previously received grant support from the National Institute of Mental Health; he receives fees from UpToDate, Psychiatric Times and Northwell Health; he receives royalties from Cambridge University Press. Dr. McClintock has received research support from the National Institutes of Health and he is a consultant to Pearson. Dr. Husain reports research grant support from: the National Institute of Health, the National Institute of Mental Health, the National Institute of Drug Abuse, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Alliance for Research on Schizophrenia, Stanley Medical Foundation, Cyberonics, Neuronetics (past), and St. Jude Medical/Abbott. Dr. Petrides reports grants from the National Institute of Mental Health and support for clinical trials from St. Jude Medical, Alkermes, Lundbeck, Proteus, and the Stanley Foundation. Dr. McCall receives research support from MECTA Corp, Vistagen, Merck and PCORI, is a paid scientific advisor for Sage, Jazz, and Janssen pharmaceutical companies and receives royalties from Wolters Kluwer. Dr. Lisanby has received grant support from the Brain and Behavior Research Foundation, the Stanley Medical Research Foundation, Neosync, Nexstim, NIH, and Brainsway. The opinions expressed in this article are the author’s own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aki OE, Ak S, Sonmez YE, Demir B, 2013. Knowledge of and attitudes toward electroconvulsive therapy among medical students, psychology students, and the general public. J. ECT 29, 45–50. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Washington, DC. [Google Scholar]
- Beale MD and Kellner CH, 2000. ECT in treatment algorithms: no need to save the best for last. J. ECT 16, 1–2. [DOI] [PubMed] [Google Scholar]
- Bech P, Gram LF, Dein E, Jacobsen O, Vitger J, Bolwig TG, 1975. Quantitative rating of depressive states. Acta Psychiatr. Scand 51, 161–170. [DOI] [PubMed] [Google Scholar]
- Bech P, Allerup P, Gram LF, Reisby N, Rosenberg R, Jacobsen O, Nagy A, 1981. The Flamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatr. Scand 63, 290–299. [DOI] [PubMed] [Google Scholar]
- Bech P, 2010. Is the antidepressive effect of second-generation antidepressants a myth? Psychol. Med 40, 181–186. [DOI] [PubMed] [Google Scholar]
- Bech P, 2012. Clinical Psychometrics. Wiley-Blackwell, Oxford, UK. [Google Scholar]
- Brus O, Cao Y, Gustafsson E, Flulten M, Landen M, Lundberg J, Nordanskog P, Nordenskjold A, 2017. Self-assessed remission rates after electroconvulsive therapy of depressive disorders. Eur. Psychiatry 45, 154–160. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. California Verbal Learning Test-Second Edition Adult Version. Manual, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Delis DC, Kaplan E, 2001. Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Fink M and Kellner CH, 1996. A second quiet revolution: ambulatory ECT. Convuls. Ther 12, 1–2. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, HHS, 2018. Neurological Devices; Reclassification of Electroconvulsive Therapy Devices; Effective Date of Requirement for Premarket Approval for Electroconvulsive Therapy Devices for Certain Specified Intended Uses. Final order. Fed. Regist 83, 66103–66124. [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ, 2009. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr. Serv 60, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM, 2002. Stroop Color and Word Test: A Manual for Clinical and Experimental Use. Wood Dale, IL: Stoelting Co. [Google Scholar]
- Haq AU, 2015. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J. Clin. Psychiatry 76, 1374–1384. [DOI] [PubMed] [Google Scholar]
- Hieronymus F, Emilsson JF, Nilsson S, Eriksson E, 2016. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol. Psychiatry 21, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S, 2001. DRS-2: Dementia Rating Scale-2 Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Keilp JG, Madden SP, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, 2018. The lack of meaningful association between depression severity measures and neurocognitive performance. Journal of affective disorders. 241, 164–172. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, McClintock SM, Tobias KG, Martino C, Mueller M, Bailine SH, Fink M, Petrides G, 2010. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br. J. Psychiatry 196, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM, 2012. ECT in treatment-resistant depression. Am. J. Psychiatry 169, 1238–1244. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM, Mueller M, Prudic J, Greenberg RM, Weiner RD, Bailine SH, Rosenquist PB, Raza A, Kaliora S, Latoussakis V, Tobias KG, Briggs MC, Liebman LS, Geduldig ET, Teklehaimanot AA, Lisanby SH, CORE/PRIDE Work Group, 2016. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am. J. Psychiatry 173, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolshus E, Jelovac A, McLoughlin DM, 2017. Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials. Psychol. Med 47, 518–530. [DOI] [PubMed] [Google Scholar]
- Kyle PR, Lemming OM, Timmerby N, Sondergaard S, Andreasson K, Bech P, 2016. The Validity of the Different Versions of the Hamilton Depression Scale in Separating Remission Rates of Placebo and Antidepressants in Clinical Trials of Major Depression. J. Clin. Psychopharmacol 36, 453–456. [DOI] [PubMed] [Google Scholar]
- Leadholm AK, Rothschild AJ, Nolen WA, Bech P, Munk-Jorgensen P, Ostergaard SD, 2013. The treatment of psychotic depression: is there consensus among guidelines and psychiatrists? J. Affect. Disord 145, 214–220. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, McClintock SM, Alexopoulos G, Bailine SH, Bernhardt E, Briggs MC, Cullum CM, Deng Z, Dooley M, Geduldig ET, Greenberg RM, Husain MM, Kaliora S, Knapp RG, Latoussakis V, Liebman LS, McCall WV, Mueller M, Petrides G, Prudic J, Rosenquist PB, 2020. Neurocognitive Effects of Combined Electroconvulsive Therapy (ECT) and Venlafaxine in Geriatric Depression: Phase 1 of the PRIDE Study. American journal of geriatric psychiatry. 28, 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Cullum M, Husain MM, Rush AJ, Knapp RG, Mueller M, Petrides G, Sampson S, Kellner CH, 2010a. Evaluation of the Effects of Severe Depression on Global Cognitive Function and Memory. CNS spectrums. 15, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM, 2010b. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 24, 9–34. [DOI] [PubMed] [Google Scholar]
- McElhiney MC, Moody BJ, Sackeim HA, 2001. The Autobiographical Memory Interview Short Form: Manual for Administration and Scoring, New York, Department of Psychiatry, New York State Psychiatric Institute. [Google Scholar]
- Nordenskjold A, von Knorring L, Engstrom I, 2012. Predictors of the short-term responder rate of Electroconvulsive therapy in depressive disorders--a population based study. BMC Psychiatry. 12, 115-244X-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard SD, Bech P, Trivedi MH, Wisniewski SR, Rush AJ, Fava M, 2014a. Brief, unidimensional melancholia rating scales are highly sensitive to the effect of citalopram and may have biological validity: Implications for the Research Domain Criteria (RDoC). J. Affect. Disord 163, 18–24. [DOI] [PubMed] [Google Scholar]
- Ostergaard SD, Meyers BS, Flint AJ, Mulsant BH, Whyte EM, Ulbricht CM, Bech P, Rothschild AJ, on behalf of the STOP-PD Study Group, 2014b. Measuring psychotic depression. Acta Psychiatr. Scand 129, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard SD, Bech P, Miskowiak KW, 2016. Fewer study participants needed to demonstrate superior antidepressant efficacy when using the Hamilton melancholia subscale (HAM-D(6)) as outcome measure. J. Affect. Disord 190, 842–845. [DOI] [PubMed] [Google Scholar]
- Ostergaard SD, 2017. Do not blame the SSRIs: blame the Hamilton Depression Rating Scale. Acta Neuropsychiatr, 1–3. [DOI] [PubMed] [Google Scholar]
- Patten SB, 2006. A major depression prognosis calculator based on episode duration. Clin. Pract. Epidemiol. Ment. Health. 2, 13-0179-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, Rummans TA, O’Connor KM, Rasmussen KG Jr, Bernstein HJ, Biggs M, Bailine SH, Kellner CH, 2001. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J. ECT 17, 244–253. [DOI] [PubMed] [Google Scholar]
- Ross EL, Zivin K, Maixner DF, 2018. Cost-effectiveness of Electroconvulsive Therapy vs Pharmacotherapy/Psychotherapy for Treatment-Resistant Depression in the United States. JAMA Psychiatry. 75, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerby N, Andersen JH, Sondergaard S, Ostergaard SD, Bech P, 2017. A Systematic Review of the Clinimetric Properties of the 6-Item Version of the Hamilton Depression Rating Scale (HAM-D6). Psychother. Psychosom 86, 141–149. [DOI] [PubMed] [Google Scholar]
- Torring N, Sanghani SN, Petrides G, Kellner CH, Ostergaard SD, 2017. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr. Scand 135, 388–397. [DOI] [PubMed] [Google Scholar]
- van Diermen L, Schrijvers D, Cools O, Birkenhager TK, Fransen E, Sabbe BGC, 2017. Distinguishing Subgroups Based on Psychomotor Functioning among Patients with Major Depressive Disorder. Neuropsychobiology. 76, 199–208. [DOI] [PubMed] [Google Scholar]
- van Diermen L, Hebbrecht K, Schrijvers D, Sabbe BCG, Fransen E, Birkenhager TK, 2018a. The Maudsley Staging Method as predictor of electroconvulsive therapy effectiveness in depression. Acta Psychiatr. Scand 138, 605–614. [DOI] [PubMed] [Google Scholar]
- van Diermen L, van den Ameele S, Kamperman AM, Sabbe BCG, Vermeulen T, Schrijvers D, Birkenhager TK, 2018b. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br. J. Psychiatry 212, 71–80. [DOI] [PubMed] [Google Scholar]


