Abstract
Smart drug delivery nano-systems show significant changes in their physical or chemical properties in response to slight change in environmental physical and/or chemical signals, and further releasing drugs adjusted to the progression of the disease at the right target and rate intelligently. Two-dimensional materials possess dramatic status extend all over various scientific and technological disciplines by reason of their exceptional unique properties in application of smart drug delivery nano-systems. In this review, we summarized current progress to highlight various kinds of two-dimensional materials drug carriers which are widely explored in smart drug delivery systems as well as classification of stimuli responsive two-dimensional materials and the advantages and disadvantages of their applications. Consequently, we anticipate that this review might inspire the development of new two-dimensional materials with smart drug delivery systems, and deepen researchers’ understanding of smart nano-carries based on two-dimensional materials.
Keywords: Two-dimensional materials, Smart drug delivery system, Nano-carriers, Disease
Graphical abstract
The fabrication, encapsulation, various stimuli response and applications of 2D materials smart drug delivery systems were reviewed. With unique 2D structure and sensitive response to external stimuli, 2D materials are expected to conquer any obstacle in maximizing the therapeutic effect of drugs.
Highlights
-
•
Introduction to the exfoliation method of two-dimensional materials for biomedical applications.
-
•
Summary of the encapsulation of two-dimensional materials smart drug delivery systems.
-
•
Discussion of the classification of stimuli responsive two-dimensional materials smart drug delivery systems.
-
•
Overview of the application of two-dimensional materials smart drug delivery systems.
1. Introduction
Smart drug delivery systems (SDDSs) is a kind of drug delivery system which could transfer the signal, respond, deliver the drug, and stop delivering automatically [1]. All kinds of the smart drug delivery achieve a particular aim of delivering the drug by expected quantity at expected time in a proper point. The control signals of SDDSs are not only limited to internal in nature such as redox, pH, concentration of specific biomolecules, and enzymatic activity [[2], [3], [4], [5]], but also including external in nature such as light of various wavelengths, magnetic fields, electric fields, and ultrasound [[6], [7], [8], [9]]. Carriers are the base of SDDSs. These smart carriers include polymers [[10], [11], [12], [13], [14]], hydrogels [[15], [16], [17]], liposomes [[18], [19], [20], [21]], nanoparticles [[22], [23], [24]], nanosheet [[25], [26], [27]], micelles [[28], [29], [30]] etc. Two-dimensional (2D) materials refer to the material size is reduced to the limit of atomic layer thickness in one dimension, while in the other two dimensions the material size is relatively large [31]. In 2004, Novoselov et al. [32] reported that graphene was obtained from the exfoliation of graphite by using Scotch tape. It's proved that it has unique and excellent electrical properties. Since then, 2D nanomaterials represented by graphene have gained rapid development [[33], [34], [35], [36], [37]], and new two-dimensional materials have sprung up [[38], [39], [40]]. In addition to graphene, the family of 2D materials generally also include transition metal dichalcogenides (MoS2 [[41], [42], [43]], WSe2 [44], TiS2 [45,46], SnS [[47], [48], [49]], etc. [[50], [51], [52]]), single element (graphene [[53], [54], [55]], selenium [38,56], boron [57], tellurium [58], bismuth [[59], [60], [61]] and black phosphorus [[62], [63], [64], [65]], etc [60,66,67].), main group of metallic sulfide compounds (GaS、InSe、SnS、SnS2) [48,[68], [69], [70]] and other two-dimensional materials [[71], [72], [73], [74], [75], [76]]. These 2D materials have completely different energy band structures and electrical properties, including photo-electronics, and water splitting and energy storage [[77], [78], [79], [80]]. In addition, they reveal interesting characteristics in medicine field, which may provide new opportunities for SDDSs.
SDDSs show broad prospect in the application field in pharmacy, and have rich research content [[81], [82], [83]]. Various intelligent drug delivery systems can be released at fixed point, time quantitatively [[84], [85], [86]]. However, up to now, most of the studies on such drug delivery systems are still in the experimental stage, and there are still many problems applying them in clinical practice. For example, with the deepening understanding of cancer, researchers have found that the traditional nano-smart drug delivery system has obvious deficiencies [87]. The low drug load of carrier severely limits the application of multi-mode therapy. And that single treatment (such as chemotherapy and immunotherapy) is limited for the treatment of illness [[88], [89], [90]]. It is difficult to effectively inhibit tumor development, metastasis, and to achieve the precision treatment and visualization for cancer [91].
2D materials have great light photodynamic properties and heat conversion efficiency, so they maintain diverse advantages in medical applications. Which also endow them with great potentials in medicine fields, such as sensing [[92], [93], [94]], imaging [[95], [96], [97]], and therapy [[98], [99], [100], [101], [102]]. Meanwhile, 2D materials show a formidable capability in drug delivery with a lot of advantages. 2D nano materials have one of the lamella structures of the unique essential characteristics, which ensure the huge surface area for high drug loading. One of the unique essential characteristics of 2D nano materials is the lamella structure, which provides the vast surface area for high-efficiency of drug loading [[103], [104], [105]]. In addition, due to graphene and their derivatives have special sp [2]-bond of carbon atoms and a large number of surface contacts, they can strongly interact with drugs by hydrophobic interactions and supra-molecular stacking [[106], [107], [108], [109], [110], [111]]. These characteristics of the 2D materials enable them to have good prospects for the application of SDDSs [86] (Fig. 1). In this review, we emphasize the current development of SDDSs for a great deal of smart 2D materials based nanocarriers, including polymers, hydrogels, liposomes, nanoparticles, nanosheets, micelles, etc. This review also correlates classification of stimuli responsive 2D materials and the application fields of 2D material SDDSs. Finally, merits and demerits of 2D materials for SDDSs are also presented.
Fig. 1.
Schematic illustration of 2D material as a smart drug delivery with multimodal imaging guidance.
2. Two-dimensional material preparation
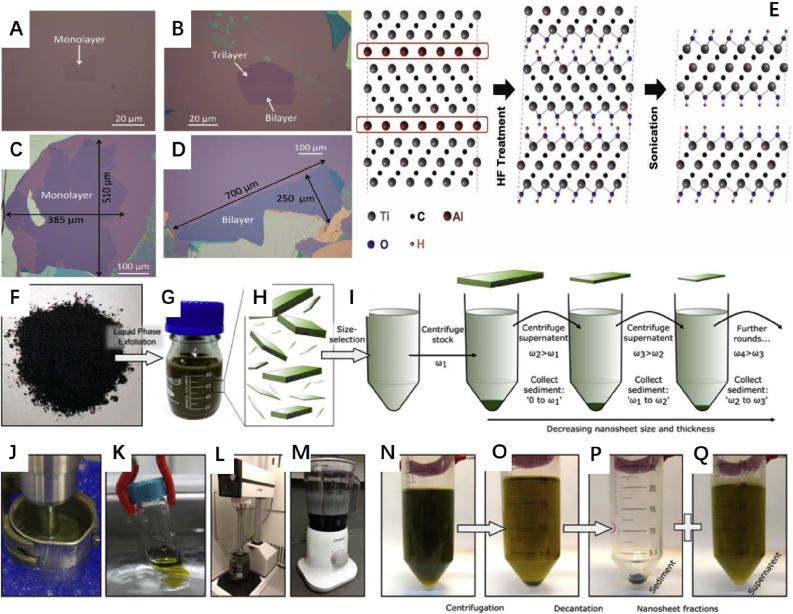
Since Novoselo et al. obtained monolayer graphene by micromechanical exfoliation in 2004, a variety of methods have been developed for the preparation of 2D materials. However, not all preparation methods are suitable for the application of drug loading platforms. Micromechanical exfoliation is a simple method widely used to obtain 2D material. Micromechanical exfoliated 2D materials, which exhibit considerable lateral size, few defects, high purity, Ideal surface as well as great crystallinity, are suitable for high-precision application such as photodetector and field effect transistor. In 2004, Novoselov et al. [112] cleavage high quality graphene with single-atomic thickness from the highly-oriented pyrolytic graphite by micromechanical exfoliation and opened the era of 2D materials. After the starting material (graphite) was attached to the photo-resist, the scotch tape was used to repeatedly process the surface of graphite. The mechanical force interrupted the fragile van der Waals force between the layers, while the stronger covalent bonds in the layer were retained. Then, the few-layer graphene attached to the tape is released in acetone with ultrasound assistance and captured by the silicon wafer in the solution. Fortunately, perhaps owing to van der Waals as well as capillary forces, graphene with a thickness of less than 10 nm can be better attached to the silicon wafer, so that most of the thick sheets can be removed by ultrasonic-assisted acetone washing. Thanks to optical interference, researcher can easily distinguish graphene with thicknesses higher and less than 1.5 nm, the former is able to be observed under an optical microscope and the latter not. The high-quality 2D materials obtained by micromechanical exfoliation showed a lot of novel physical properties and realized a great number of amazing applications. However, the low yield and the small radial size of as-prepared single-layer nanosheets limit the large-scale industrialization. In order to solve this problem, Huang et al. [113] designed an improved micromechanical exfoliation method, the yield of which is increased by 20–60 times compared to the conventional method (Fig. 2A–D). Unlike the initial use of silicon wafers to passively collect the few-layer 2D material attached on tape, the silicon wafers are utilized to exfoliate the 2D material from the thick bulk material held by the tape in the improved method. To enhance the van der Waals force between silicon wafers and outermost layer of the graphite sheet on the surface of tape, which is the key factor for obtaining ideal graphene, oxygen plasma is used to clean the surface of substrate and sufficient reaction time and temperature (100 °C, 2–5 min) between the substrate and the tape with the material is guaranteed. Notably, the tape is only treated 3 to 4 times after removing the thick graphite sheet from the highly-oriented pyrolytic graphite to ensure the diameter of graphene. Despite some progress, the manual operation during the micromechanical stripping method without the aid of equipment has inevitably led to the product randomness and limited yield as well when brought convenience. As a result, in the study of the 2D material-based drug carrier, the most commonly used method is the liquid phase exfoliation described below.
Fig. 2.
A~D [113]: Optical images of graphene obtained by micromechanical exfoliation with standard methods (A and B) and modified methods (C and D) improved micromechanical exfoliation method (C and D). Reprinted with permission from Ref. [113] E [120]: Compared with the traditional methods, graphene exfoliated by modified method exhibit a much larger size. After Al is etched by HF and replaced with OH, Ti3C2 layers are connected by weak hydrogen bonds, which can be interrupted by sonication. Reprinted with permission from Ref. [120] F ~ Q [121]: Illustration of the liquid phase exfoliation method. F: Typical starting material (here WS2 powder). G:2D material disperse colloidally in organic solvent after liquid phase exfoliation. H:nanosheets with various size and thicknesses disperse in the suspension. I: Schematic of cascaded centrifugation. The increasing speed ω enables efficient purification of 2D materials with specific size. J: Probe sonication. K: bath sonication. L: rotor stator mixer. M: The household kitchen blender, which can also be used to exfoliate 2D material as long as there is sufficient speed. N-Q Typical collection process for 2D materials with specific size. After the nanosheets mixture (N) is centrifuged at a suitable speed and time, nanosheets with a specific size is deposited to the bottom of the centrifuge tube (O). The supernatant was removed as completely as possible to ensure uniformity of nanosheets in the sediment (P). Finally, the nanosheets are redispersed to get suspension with any desired concentration (Q). Reprinted with permission from Ref. [121].
The liquid phase exfoliation is one of the most widely used method to achieve large-scale 2D material fabrication. Unlike the micromechanical exfoliation, the 2D material obtained by the liquid phase exfoliation tends to be small, which limits its electrical application but is very suitable in the field of biomedicine. What's more, the size of the 2D materials obtained by this method is controllable and reproducible with the liquid cascade centrifugation, indicating a great potential in the clinical application [114]. The process and equipment of the liquid phase exfoliation method are shown in Fig. 2F–Q. There are three key steps in the process of liquid phase exfoliation: First, minimize the energy required for exfoliation, and there are many strategies developed for this aim. The most common path to achieve this goal is to disperse the bulk material in a suitable solvent where the energy required to break the van der Waals force between the layers of the material matches the surface energy of the solvent itself. It is worth noting that the solvent provides not only surface energy required for exfoliation but also benefit protection, which is very useful for some 2D materials such as black phosphorus. The commonly used solvent, such as isopropyl alcohol and N-methyl-2-pyrrolidone, have toxicity and require multiple wash with deionized water before they can be used as drug delivery platform. Fortunately, in recent years, some biocompatible solvents have also demonstrated the ability to fabricate 2D materials. For example, Tao et al. [115] obtained a black phosphorus nanosheet with an average size of 120 nm and thickness of 1–2 nm using deoxygenated water, which achieved significant tumor treatment effects as a drug carrier, and a variety of characterizations confirm the higher quality of the resulting nanosheets. Xue et al. [116] utilize ethanol as solvent to exfoliate antimonene (radial size about 400 nm, thickness about 3 nm) with excellent miRNA detection ability. In addition to pure solvent, mixed solvent or added solvents are also popular to prepare 2D materials [117]. Surfactants are a common additive to enhance the stripping efficiency of 2 materials in aqueous solutions. The difficulty of completely removing the surfactant from the surface of obtained 2D material limits the application of this technology in a lot of conventional fields. However, a suitable surfactant that remains on the surface of 2D material will further enhance the biological effects rather than bring problem. Wang et al. [118] used human serum albumin as a surfactant to obtain 2D black phosphorus successfully, and the attached human serum albumin improves the dispersibility and stability of the black phosphorus nanosheets as well as load the drug. In addition to the common method of reducing the exfoliation energy by the surface energy of the solvent, a specific oxidation-assisted strategy has been developed for special 2D material, mainly graphene. This method uses a strong oxidizing agent to form many oxygen-containing groups on the surface of each layer of graphite to increase the interlayer spacing [119]. The van der Waals force between the obtained graphite oxide layers is obviously weakened, and a large amount of graphene oxide nanosheets can be obtained after sonication. Graphene oxide (GO) and the related reduced GO (rGO) possess abundant oxygen-containing groups on their surface, which ensure the strong drug-loading ability, modification, and hydrophilicity. However, the oxygen-containing groups also bring a lot of uncertainties to the toxicity of GO, which should be solved before clinical application. It is worth noting that a recent report pointed out that borophene, the neighbor of graphene on the periodic table, can also be prepared by oxidation-assisted liquid phase exfoliation. As the lightest 2D material, borophene has received extensive attention. However, with non-layered structure, it is difficult to fabricate ultrathin borophene by the conventional top-down exfoliation method. Ji et al. [57] oxidized the boron sheet (thicker than 15 nm) at a high temperature (650 °C) to transform the surface of the boron sheet to B2O3. After that, the B2O3-coated boron sheet was dispersed in water, where B2O3 dissolved away and expose the ultrathin borophene with an average size of 100 nm and thickness less than 5 nm. In addition to oxidation, selective etching can also assist liquid phase exfoliation to fabricate 2D materials (for example, Mxene),as shown in Fig. 2E. Second, enough tensile stress is necessary to interrupt the interlayer force of material. The sonication and shear forces are the two most common approach to provide the energy needed for liquid phase exfoliation, while the former requires less time and the latter have a greater production scale as well as larger radial size. During the exfoliation, the bubble induced by liquid cavitation is the most important contribution. Along with the bubble rupture, a strong shock wave can effectively break the interlayer force of the material to achieve the exfoliation of the 2D materials. Unlike traditional concepts, thanks to their anisotropic chain structure and fragile van der Waals forces between the chains, non-layered materials, such as tellurium and selenium, can also be exfoliated into 2D nanosheets with sonication-assisted liquid phase exfoliation [38,56,58].
Notably, sonication may induce some defects, which is harmful in electrical applications but is beneficial for the application of nanomedicine carriers due to the enhanced photothermal effect. Compared to sonication, the shear forces has less application in the field of drug carriers, probably due to its relatively short history. However, as an attractive fabrication method with the largest yield, the shear-assisted liquid phase exfoliation has unique advantages over other methods. This method utilizes the rotor stator mixer to induce shear forces on the surface of material, which can trigger the large scale 2D materials exfoliation. At present, this method has been able to obtain several hundred liters of 2D material dispersion at one time. Finally, centrifugation is also an essential step to obtain 2D materials with a relatively uniform size. Liquid cascade centrifugation is especially suitable for biological applications with the consideration of the close relationship between the toxicity and size of 2D materials.
3. Encapsulation of 2D materials
Nanoscience derived from drug delivery vehicles is the center of researchers’ main interest. Using nanotechnology, nano drug can overcome many defects of traditional drugs with poor solubility and low bioavailability, which provide a new way for the research of new drug delivery systems. For example, nanoparticles (NPs) may enhancing drug biocompatibility through increasing the drug stability during transport in the blood and improving cell absorption in the process of entering the cell [122]. Smart NPs, which exhibit dramatic conformational changes in their physical/chemical properties in response to mild change in environmental physical and/or chemical signals, further play an important role in synergism and attenuation through increasing the drug concentration in the target tissues or cells and reducing the drug release in the normal tissues or cells. Due to the ultrahigh specific surface area, 2D materials exhibit great potential in the drug delivery application. Unfortunately, 2D materials may agglomerate into larger particles in physiological conditions, which limit its clinical application. To solve this problem, researchers used a variety of methods to encapsulate the 2D material, which significantly improved the biocompatibility and dispersion of 2D material. In this section, we discussed various techniques for encapsulating 2D materials (Fig. 3).
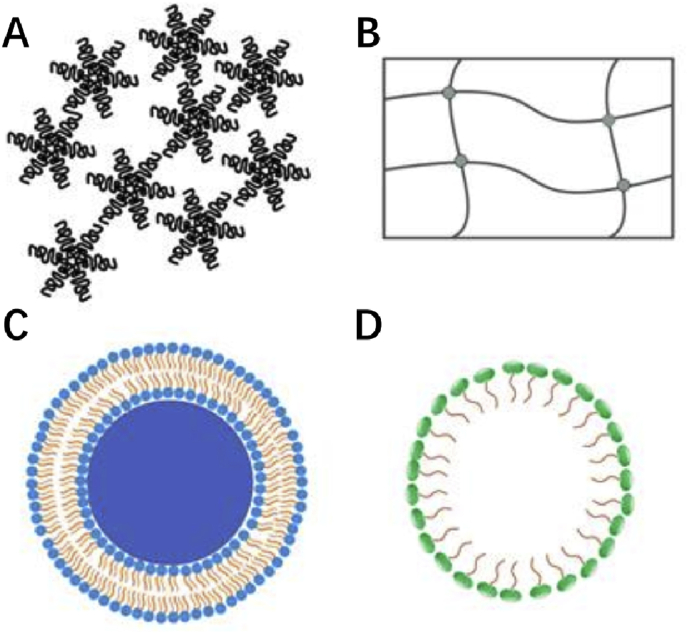
Fig. 3.
Schematic representation of the nanocarriers used in smart drug delivery. polymers(A), hydrogels(B), liposomes(C), micelles (D).
3.1. Encapsulation of 2D materials by polymers
Innovations in SDDSs field allow the introduction of 2D polymers since they could deliver drug at the appropriate time and site of function and establish a link between therapeutic need and drug delivery [123,124]. To this end, researchers have made use of characteristics of 2D materials polymers, because they can regulate large reversible, physical, or chemical fluctuations as responses to small changes in environmental conditions, such as temperature, light and pH.
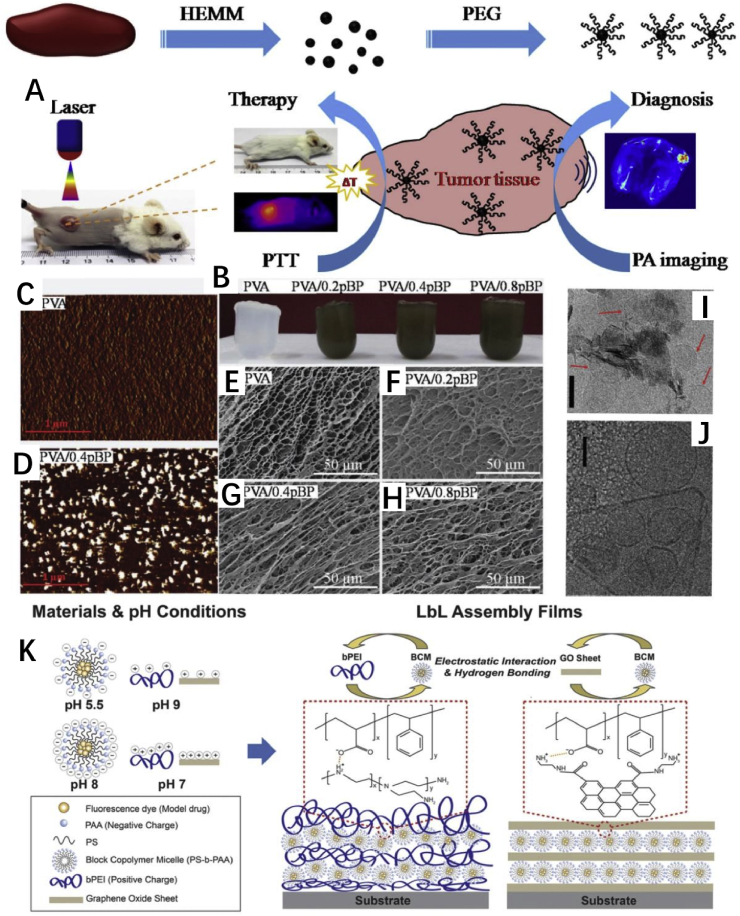
GO was prepared from purified natural graphite on the basis of an improved Hummers' method. Afterwards, to introduce amine groups, GO was functionalized by adipic acid dihydrazide. To obtain GO-SA conjugate, sodium alginate (SA) was covalently conjugated to GO via the formation of amide bonds. The polymers maintained a low drug release under physiological conditions, but achieved a higher drug release under tumor cell microenvironment. In fact, the release rate can increase with the decrease of pH [125]. Furthermore, in the study of Li, Tan et al. [126], PEGylated nanographene oxide and polyethylenimine (PEI, 25 kDa) solution were mixed at 80° °C for 2 h. In this way, they successfully synthesized nanographene oxide (nrGO) conjugate (PEG-nrGO-PEI) with high gene delivery efficiency. The 25 min irradiation (2 W/cm2) of 808 nm laser does not enhance the gene transfection efficiency of PEG-nGO/PEI, but augments gene transfection efficiency of PEG-nrGO-PEI. By one-pot solventless high energy mechanical milling technique, biocompatible PEGylated and water-soluble black phosphorus (BP) nanoparticles were prepared. Because the new BP nanoparticles exhibit excellent photostability, and can efficiently convert near-infrared (NIR) light into heat, they can be applied to photothermal therapy of cancer and photoacoustic imaging (Fig. 4A) [127]. Yang et al. [128] reported MoS2 nanoparticles coated by polyvinyl pyrrolidone with high drug loading capacity having good synergistic effect on tumor photothermal and chemotherapy. MoS2 NPs release drug via a controlled NIR- and pH- dual responsive manner, and the porous structure of MoS2 NPs is conducive to the improvement of the drug loading rate (adjustable drug loading percentage is 7–72%).
Fig. 4.
(A) Schematic illustration of PEGylated BP for cancer photothermal therapy and PA imaging [127] Reprinted with permission from Ref. [127]. (B) Digital pictures of hydrogels and hydrogels encapsulated BP. (C and D) Are the AFM phase images of hydrogels and hydrogels encapsulated BP, respectively. (E–H) Are the cross-section SEM images of hydrogels encapsulated BP with various pBP contents. Cryo-TEM image of liposomes modified (I) WS2 and (J) GO (scale bars: 100 nm). The arrowheads in (I) point at liposome features [135]. Reprinted with permission from Ref. [135]. (K) Schematic illustration of micelle encapsulated GO, the layer by layer (lbl) assembly building block (left), and bPEI/BCM and GO/BCM multilayer films assembled by the lbl assembly [147]. Reprinted with permission from Ref. [147].
Now research shows that 2D materials polymers as carriers of SDDSs can effectively inhibit tumor growth, and significantly reduce the toxic side effects of drugs. At the same time, 2D materials polymers have the ability to enhance uptake and to target cancer tissues by cancer cell, in addition to increasing fast release of payloads in response to a stimulus [[129], [130], [131], [132]].
3.2. Encapsulation of 2D materials by hydrogels
Hydrogels are three-dimensional hydrophilic polymeric networks that can swell in an aqueous environment [133]. They are rich in water molecules but insoluble in water. They are immensely biocompatible because of similarity in extracellular matrix. Physical, chemical, and biological properties can be controlled by adding specific 2D materials to hydrogels, usually introduce physical or chemical methods [134,135], because of their efficiency, ease and simplicity (Fig. 4B–J). These modifications can enable materials reacting to external stimuli, where they trigger or perform chemical and physical change or a specific function upon interaction with pH, temperature and light or other stimuli.
Nanosheets acting as excellent drug nanocarriers can combine with a hydrogel system to increase the performances of drug loading and release. It is also possible to utilize a thermo-sensitive deformable hydrogel to control the rate of release effectively, or to deploy the lamellar surface modification function of the polymer to develop a targeted precision medical therapy [136]. Through the in situ reduction of adsorbed Ag+ by hydroquinone in a citrate buffer solution, GO nanosheets impregnated with Ag NPs were prepared. The as-prepared composite material has stronger antibacterial activity [137]. Furthermore, by the action of concentrated sulfuric acid, using dextrin as material and cetyltrimethyl ammonium bromide as stabiliserdextrin, graphene carbon nanosheets were synthesized. Oxidation of such sheets produces GO like materials. The results showed that carbon nano-sheets have no effect on normal cells, but induced apoptosis on cancer cells [138]. In the investigation of Li and Xiang et al., soybean phospholipid-encapsulated MoS2 (SP–MoS2) nanosheets were successfully synthesized in a rotary evaporator. The results displayed the SP-MoS2 nanosheet was a promising platform for breast tumor photothermal therapyin vitro and in vivo [139]. NIR photothermal-responsive 2D smart materials display supernormal advantages in biomedical applications bacause of their prominent tissue-penetration ability. Herein, exploiting the high photothermal conversion efficiency of polydopamine modified black phosphorus, these and composite hydrogels are in good cellular interaction and biocompatibility, and demonstrated an on-demand NIR-responsive drug release behavior [135]. Recently, 2D materials have been shown to be promising for applications in anticancer therapy. Zhu, Xiali et al. [140] prepared a novel biodegradable and thermo-sensitive hydrogel. The hydrogel is made of chitosan and beta-glycerophosphate salt. Super paramagnetic GO modified with PEI was used as a minimally invasive treatment of cancer lesions through magnetically inducing local thermotherapy. A drug delivery system was prepared by adding adriamycin (DOX) into hydrogel which was preloaded on GO. The GO-based SDDSs showed higher antitumor efficacy than free DOX on MCF-7 cells in vitro. Moreover, Zhang and Qian et al. [141] prepared a new GO-based supramolecular hybrid nanohydrogel, in which GO as the cross-links was put in the hydrogel through non-covalent bind. They loaded doxorubicin hydrochloride into the mixed hydrogel as a model drug. According to the research in vitro and in vivo experiments, hydrogel showed the inhibition of tumor cell proliferation and tumor growth.
3.3. Encapsulation of 2D materials by liposomes
Liposomes are naturally occurring drug carriers based on amphipathic phospholipid [142]. Phospholipids act as an important part of the cell membrane. It consists of a hydrophilic head based on phospholipid and a hydrophobic tail based on fatty acid. When the phospholipids are added into an aqueous medium, they can self-assemble into a bilayer vesicle, with the polar ends facing the water and the non-polar ends forming a bilayer. The cores formed by the bilayer can be water-soluble or entrap water drugs. During the past years, liposomes have attracted extensive attention as pharmaceutical carriers with great potential, on account of their extraordinary properties with low toxicity, biocompatibility, biodegradability and immunogenicity. Many types of 2D materials have already been interacted with liposomes, including MoS2, WS2 and graphene-related materials. Comprehensive studies have been performed on 2D materials.
Recently, Sahu, Abhishek et al. [143] have prepared nanographene oxide integrated liposomes. The embedded nrGOs could act as a molecular switcher for NIR light controlled drug release from the liposomes. Through irradiation of pulsed NIR lasers into the nrGO-liposome suspensions, the drug delivery can be intelligently controlled. Furthermore, through the thin film hydration method, nanoliposomes were prepared. Graphene nanosheets and Dox were added to the liposomes during the hydration of the lipid film [144]. In addition, Liu, Jue et al. [145] have prepared hybrid materials made of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and transition metal dichalcogenides liposomes. WS2 synergistically adsorbed DOPC and DOX liposomes. On bare WS2, the adsorption capacity of DOPC was about one fourth of the weight of WS2. After DOX was adsorbed on WS2, the adsorption capacity of liposome increased approximately to 110%. Thus hydrogen bonding also promoted the adsorption of liposome on DOX-loaded WS2.
3.4. Encapsulation of 2D materials by micelle
Polymer micelle is a kind of drug carriers. As amphiphilic molecules, polymer micelle have both hydrophilic and hydrophobic portions, and exhibit particular self-assembly characteristics of self-assembly in solvent [146]. It consists of two individual parts. One is hydrophobic core and the other is hydrophilic shell. Polymer micelle form amphiphilic “core-shell” structure. Hydrophilic shell can improve the stability of drug delivery system, and hydrophobic core has the function of drug delivery. Han, Uiyoung et al. [147], reported the basic properties of layer by layer films (Fig. 4K). These films composed of different substances, such as 2D-shaped GO, and branched polyethylenimine (bPEI), and polystyrene-b-poly (acrylic acid) amphiphilic block copolymer micelles (BCM). Because each material has different pH dependence, the results displayed significant differences. For instance, in pH 2 and pH 7.4 PBS buffer, the drug release rates of the GO/BCM film are faster than the (bPEI/BCM) film. Furthermore, rapid drug release can be induced by inserting GO layers into the bPEI/BCM multilayer.
4. Classification of stimuli responsive two-dimensional materials SDDSs
Due to the unique quantum confinement effect and abundant chemical active sites on the surface, 2D materials can respond sensitively to a variety of external stimulus. As a result, the 2D material-based drug delivery system can realize image-guide intelligent drug release. In this section, we introduce the response of 2D materials to light, magnetic field and PH.
4.1. Light-sensitive SDDSs
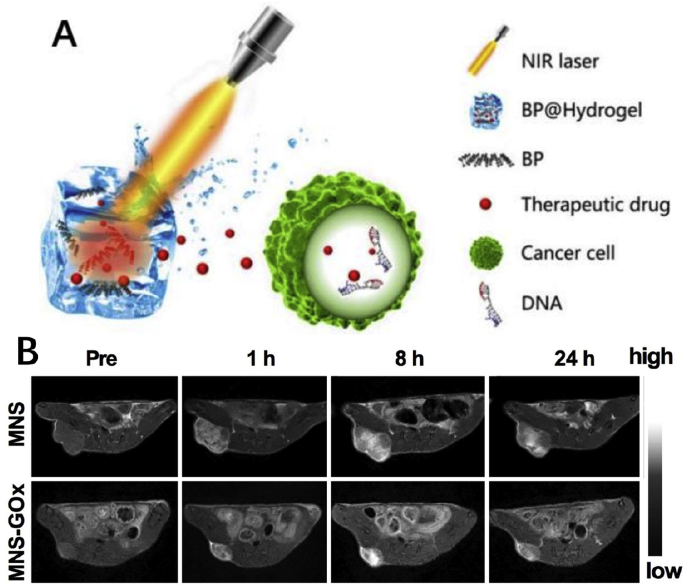
One of the most widespread used stimuli for stimuli-responsive 2D materials is light, since it has practical advantage and it is easily controlled in vivo and in vitro. Light, either visible or NIR, is ideal for applications of SDDSs as a source of energy [78,148,149]. But NIR part of the spectrum is more useful than visible light due to less harmful nature and deeper penetration ability in tissue. In general, the NIR light at the range of 700–1000 nm wavelength is widely used in biomedicine [150]. NIR-responsive nano systems can release chemotherapeutic drugs by NIR-absorbing plasmonic materials to transform the energy of the absorbed photons into heat (Fig. 5A). The main advantages of NIR radiation therapy are less toxic to normal cells, and deeper penetrating, with less biological tissue damage [62].
Fig. 5.
A: Schematic diagram of the working principle of BP@Hydrogel. BP@Hydrogel released the encapsulated chemotherapeutics under NIR-light irradiation to broken the DNA chains, leading to the apoptosis induction [158]. Reprinted with permission from Ref. [158] B: T1-weigthed MR images of mice treated with MNS or MNS-GOx at 0, 1, 8, 24 h post-injection [157]. Reprinted with permission from Ref. [157].
On the base of GO for NIR light controlling chemotherapeutic/photodynamic (PDT)/photothermal (PTT) trimodal synergistic therapy, a multifunctional antitumor drug delivery system was synthesized. Indocyanine green-wedelolactone-GO could effectively absorb and transform optical energy to heat under NIR laser irradiation, generate damage and ablate tumor cells by reactive oxygen species [151]. Fan, Jing et al. [152] reported the novel sandwich nanomedicine with NIR light responsive release of nitric oxide (NO) has been constructed by a NO donor BNN6 (N,N′-di-sec-butylN,N′-dinitroso-1,4-phenylenediamine) through self-assembly of GO nanosheets. The GO-BNN6 nanomedicine has high NIR responsiveness, and good thermal stability, and a high drug loading capacity. In addition, a novel shape memory polymer composite with excellent shape memory performance and biodegradability was prepared by using polyurethane as thermal response material and BP as near infrared photothermal nano-filler. The material can be controlled by remote NIR and degraded gradually after it has completed its function in vivo [153]. Under NIR light irradiation, BP nanosheets decorated with Ag nanoparticles can rapidly destroy the bacterial membrane. In vivo, Ag@BP can effectively reduce the bacterial burden in mice and the tissue damage related to infection. In addition, Ag@BP has excellent biocompatibility, which guarantees their biosafety in future clinical applications [154]. BP nanosheets are combined with [poly (d,l-lactide)-poly (ethylene glycol)-poly (d,l-lactide) (PDLLA‐PEG‐PDLLA: PLEL)]. The BP@PLEL hydrogel exhibits a rapid NIR-induced sol-gel transition and excellent NIR photothermal performance [155].
4.2. Magnetic field-responsive SDDSs
Due to the high tissue penetration and spatial resolution, magnetic resonance imaging (MRI) has been widely used in clinic. To achieve a good imaging effect, a suitable MRI contrast agents is essential. The signals of traditional MRI contrast agents are not tumor-specific, which makes it difficult to distinguish the target from the background signal. To solve this problem, researchers design a novel MRI strategy based on two-dimensional MnO2 nanosheets. In 2014, Zhao et al. [156] fabricated MnO2 nanosheets with a size of ~141 nm and a thickness of ~1.5 nm as MRI contrast agents, which exhibited tumor-specific MRI signals. Due to the modification of aptamers, MnO2 nanosheets can effectively target cancer cells. After the endocytosis of MnO2 nanosheets into cancer cells, MnO2 was reduced into Mn2+ ions by intracellular GSH and shows obvious MRI signals, while the MnO2 shows a negligible MRI background signal in normal tissues. Compared with MnO2 nanosheets, the longitudinal relaxation rate (1/T1) and lateral relaxation rate (1/T2) of the reduced products are enhanced by 48 times and 120 times respectively, indicating the great potential of MnO2 nanosheets as targeting MRI contrast agents. After that, another researcher used MnO2 to realize the MRI-guided cancer starvation therapy (Fig. 5B). He et al. [157] used melanin as a biotemplate to prepare MnO2 nanosheets with a size of ~70 nm and a thickness of ~2 nm under neutral conditions. Due to abundant catechol groups, melanin can be adsorbed on the surface of MnO2 nanosheets effectively and achieve enhanced stability, water solubility and photothermal effect. In addition, the surface of melanin-modified MnO2 has abundant carboxyls, which can graft glucose oxidase (GOx) through EDC/NHS reaction. MnO2 can catalyze the decomposition reaction of H2O2 to produce oxygen and improve the GOx-based cancer starvation therapy. Then, under the combination of the acidic condition in the tumor and the degradation products of glucose, MnO2 decomposes into Mn2+ ions, which can produce a significant MRI signal. In summary, MnO2 nanosheets can be used as a drug delivery platform to achieve effective MRI imaging-guided tumor treatment.
4.3. pH-responsive SDDSs
The pH-responsive SDDSs have attracted more and more attention because they are able to deliver drugs in a controllable manner at a specific time and site. The pH changes can occur at different parts of the digestive tracts (stomach, intestine) and genital duct (vagina) and at different organelles (lysosome and endosome) and at the extracellular sites (such as cancer cells). For example, compared with more slightly basic intracellular pH, the extracellular pH in tumors is an acidic environment. As a result, pH has been considered to be an effective physiological feature for SDDSs to the targeted tumor sites. Normally, the pH-sensitive carriers stabilize and store drug at physiological pH, but only rapidly release in the acidic environment of cancer cells, which ensure that the concentration of drug reaches a peak in cell. There have been many reports of 2D materials pH-responsive SDDSs in the literature.
In the study of Yang, huihui et al. [159], the functionalized graphene-based material were synthesized by GO modified with carboxymethyl chitosan (CMC), fluorescein isothiocyanate (FI) and hyaluronic acid (HA). Then, DOX was loaded on the conjugate (GO-CMC–FI–HA). The drug release rate under pH 5.8 was dramatically higher than that under pH 7.4, and the drug loads were as high as 95%. Studies of cellular uptake maintain that GO-CMC–FI–HA/DOX can effectively inhibit cancer cell growth and particularly target cancer cells over-expressing CD44 receptors. In the work of Xie, bei and others [160], the conjugate of doxorubicin/graphene oxidepolyethyleneimine/p53 plasmid were synthesized. The results contend that the complexes were able to release DOX significantly higher under tumor cell microenvironment than physiological conditions. A novel multifunctional GO drug carrier was developed by surface modified GO with PEI sequentially derivatised with PEG-linked lactobionic acid and fluorescein isothiocyanate, and acetylation of remaining terminal amines of the PEI. The studies showed that the release rate of DOX from complexes was significantly faster at pH 5.8 than at pH 7.4 in vitro [161]. A new type of thermo/pH sensitive Nanogels was prepared by acrylic acid (AA) and salep modified GO with branched N-isopropylacrylamide. Compound loaded DOX showed slow drug release at lower temperature and neutral pH, but increased significantly in higher temperature and acidic pH without any burst release [162].
Different response exhibits different advantages and disadvantages. The energy of the laser is concentrated, so the phototherapy performance is obvious at low material concentration. However, the penetration of the laser limits the clinical application of phototherapy based on 2D materials. Unlike lasers, magnetic field can easily penetrate deep tissue. MRI imaging based on magnetic field response has been extensively studied and has mature applications in the clinic. However, due to the large concentration of materials required, there are not as many researches related to magnetic thermotherapy as photothermal therapy. As an important feature of tumor microenvironment, acidic PH value has received extensive attention in the field of drug delivery. The acidic environment at the tumor site can significantly affect the rate of drug release and material degradation, and enhance the targeting of drugs. However, some studies have shown that the PH value in tumor cells is neutral or even weakly alkaline. As a result, PH-responsive drug delivery vehicles may have difficulty functioning inside the cells, which affects the final therapeutic effect.
5. The field of 2D materials SDDSs therapy
5.1. Cancer
At present, cancer has become one of the major diseases that seriously affect human health and threaten human life all over the world. Conventional treatments for cancer, such as surgery, chemotherapy and radiotherapy, have some major defects, such as severe side effects, drug resistance, limited efficacy, and so on. In order to solve these issues, people have developed many new cancer treatment methods including PTT, PDT, etc [85,163,164]. Nanotechnology and nanoscience have made amazing progress, and offer unprecedented opportunities for cancer treatment over the past decades. So far, nano-materials have been widely explored for biomedical fields, such as graphene, carbon nanotubes, magnetic iron oxides, quantum dots, up-conversion nanoparticles, Au nanostructures [165,166]. Due to their unique chemical/physical properties, 2D materials, as novel nanoplatforms for SDDSs, have become a hot topic in recent years.
One of the most effective anti-tumor strategies against certain cancer-cells-specific is nano-sized active targeting drug delivery systems in vivo [167]. Multidrug resistance of cancers towards chemotherapeutic drugs is the main cause for the failure of chemotherapy [168]. Therefore, it is critical to improve biocompatibility of target drugs to cancer cells, improve drug delivery in cells, and overcome multiple drug resistance. The results revealed that the targeting nanohybrids not only had good antitumor activity, but also little systemic toxicity to B16 tumor-bearing mice [169]. Yu, Jiantao et al. [170] synthesized reduced GO nanocomposite modified with a specific peptide conjugated polydopamine (PDA). They loaded antiarrhythmic peptide 10 (AAP10) onto the surface of dopamine-modified rGO. Through NIR radiation, AAP10-pDA/rGO can effectively ablate breast tumor in tumor-bearing mice, and inhibit tumor growth nearly 100%. Through immobilizing gold nanoparticles on folate modified dendritic mesoporous silica-coated reduced GO nanosheets, a novel sandwich-like nanocomposite was prepared as cell imaging and a multifunctional intelligent nanocarrier for curcumin (Cur) targeted delivery. This new drug delivery system exhibits a lot of interesting properties, such as suitable surface area, biodegradability, good biocompatibility, and high drug loading capacity. In addition, the nanocarrier showed pH-responsive and sustained-release properties. The obtained results displayed that new drug delivery system can be used as an effective anticancer drug in targeted cancer therapy of breast cancer [171]. Xing, Chenyang et al. reported that the intrinsically characteristic of naked BP is easy to be oxidized (or degrade naturally) and deposited in the tumor microenvironment, which can result in uneven photothermal effect and short-term treatment. The injectable hydrogel based on BP nanometer sheet and cellulose has good anticancer effect on PTT. Importantly, the hydrogel nanometer platform is completely biocompatible and harmless in vivo and in vitro [172].
5.2. Antibacterial
Since the discovery of bacteria in the 19th century, the battles between these tiny pathogens and human beings have continued for hundreds of years. With the gradual expansion of antibiotic indication, the abuse of antibiotics is very common, and the emergence of antibiotic-resistant pathogens in the treatment of infectious diseases has become a greatly threat to human beings [173]. At the same time, the public health is still seriously threatened by the infections bacause of some pathogenic microorganisms. Therefore, it is very imperative to find new antibacterial drugs. In recent years, the appearance of 2D materials in the field of biomedicine has attracted extensive attention. It is noteworthy that some 2D materials have been found to be effective in antifungal and antibacterial.
Zeng, Guangjian et al. [174] prepared the MoS2-PDA-Ag nanocomposites by combining microwave irradiation with mussel inspired chemistry. In alkaline aqueous solution, PDA films were formed by self-polymerization of dopamine. Then Ag nanoparticles were easily modified to the surface of MoS2-PDA by using microwave irradiation via in situ reduction. The results believed that the complex had higher antibacterial activity than pure MoS2-PDA and MoS2. Through introducing arginine (Arg) into the surface of GO/Ag nanostructure electrospun polycaprolactone (PCL) nanocomposite were fabricated. (PCL)-GO/Ag/Arg nanocomposites not only have great antibacterial activity on Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), but also have suitable biocompatibility to L929 fibroblastic cell [175]. Athinarayanan, Jegan et al. [176] developed an environmental and simple chemistry method to synthesize Cu2O/graphene nanocomposites. Compared with GO and Cu2O nano particles, the Cu2O/G nanocomposites exhibited outstanding bactericidal activity, that inhibited the gram-positive and gram-negative bacterial growth at 300 mu/g. Zhang, Lingling et al. [177] reported a photon-controlled antibacterial platform, through encapsulating drug and BP quantum dots inside a thermal-sensitive liposome, which can kill drug-resistant bacteria, and efficiently avoid the generation of new bacterial resistance. Since the BP quantum dots can generate heat to disrupt the liposome under NIR stimulation, the antibacterial platform can release drug in a temporal, spatial, and dosage controlled manner. This novel SDDSs can significantly reduce the antibiotic dosage, thus preventing the generation of superbugs and avoiding the indiscriminate use of antibiotics.
5.3. Central nervous system
Brain disease is one of the biggest threats to public health. Brain disease include the treatments of inflammatory brain disorders, stroke, psychiatric diseases, neurodegenerative disease, and neurodevelopmental disease [178]. An intact blood-brain barrier (BBB) impedes access by therapeutic agents with high hydrophilic indices or large molecular masses. BBB has become an urgent problem that drugs enter the brain to treat brain diseases.
GO quantum dots (GQDs) were good carriers with their high hydrophobicity and low toxicity. Studies have shown that the aggregation of A beta peptides is a key factor leading to Alzheimer's disease (AD). Inhibition of the A beta peptide aggregation has become one of the most primary strategies to treatment of AD. GQDs may be small enough to cross the BBB and inhibit the aggregation of A beta peptides. Therefore, it is believed that GQDs may be therapeutic agents against AD [179]. In addition, Go (120) showed better inhibition than Go (60) by providing a larger effective contact surface area when the total number of atoms was the same [180]. GQDs can rescue synaptic loss and neuronal death, and reduce Lewy neurite and Lewy body formation. Furthermore, GQDs can ameliorate mitochondrial dysfunctions. GQDs could penetrate the BBB and breast tumor inhibit the formation of alpha-synuclein (alpha-syn) aggregation in Parkinson's disease. In addition, GQDs have no significant toxicity in vitro and vivo, which is expected to be a clinical drug for the treatment of Parkinson's disease [181]. In the treatment of central nervous system diseases, direct conversion into nerve cells without inducing pluripotency has a better therapeutic effect. In a mouse model, Parkinson's disease symptoms were alleviated by facilitating the production of induced neurons by transmission of reprogramming factors into the brain by oxide-polyethylenimine complexes [182].
5.4. Orthopedic
2D materials have superb physical, chemical properties and biological properties, and they have bone repairing ability and drug release property. According to reports, graphene can promote stem cell proliferation and osteogenic differentiation. Therefore, it has broad application prospects in the field of orthopedic biomaterials. The study focuses on the aspects of bone tissue engineering scaffold, bone repair, bone graft materials, etc.
Saravanan, Sekaran et al. [183] developed a thermosensitive and injectable Chitosan hydrogel containing GO and its properties were studied. Through up-regulation of Runt-related transcription factor 2, Type-1 collagen, Alkaline phosphatase, and osteocalcin under osteogenic conditions, the hydrogel facilitated osteogenic differentiation of mouse mesenchymal stem cells. In addition, a new graphene nanosheet (GNS) composite has been fabricated. It is doped with micro-arc oxidized-AZ91D based calcium phosphate (CaP)-CS. In the early stage, extracts from GNS-CaP-CS/AZ91D could prominently extend calcium mineral deposition, alkaline phosphatase activity and osteoblast-related genes expression of human bone marrow mesenchymal stem cells [184]. Furthermore, Zhao, Bin et al. synthesized a GO/silk fibroin (GO/SF) barrier film loaded with simvastatin (SIM) by a freeze-drying method. The experimental results of against defective skulls of Sprague Dawley rats showed that the GO/SF/SIM exhibited a better biocompatibility in vitro among the four membranes (SF membrane, GO/SF membrane, GO/SF/SIM membrane and SF/SIM membrane) and the best bone repairing ability [185]. Zhang, Xiaodi et al. [186] reported that few-layer MoS2 nanoflakes were assembled on substrate by a facile hydrothermal method and then nanostructured MoS2 biointerface was establish. The results revealed that MoS2 interface the with nanoporous structured can not only accelerate the mesenchymal stem cells (MSCs) osteogenesis, but also promote the MSCs attachment and spreading.
5.5. Diabetes
Human islet amyloid polypeptide (hIAPP) aggregation has been found to be directly related with insulin deficiency and pancreatic p-cell death in type 2 diabetes. Therefore, destroying the pre-formed hIAPP fibrils is expected to be an effective way to treat patients with type 2 diabetes [187]. Recently, engineered nanoparticles have been exploited as anti-aggregation nanodrugs. In the presence of GO, GO can reduce aggregation of hIAPP and inhibit effect on hIAPP aggregation [188]. Furthermore, stable GO-PEI complex was prepared by modification of PEI on the surface of GO by using covalent binding method. The result showed that GO-PEI is more efficient than GO in inhibiting hIAPP fibril formation [189]. In the study of Zhou, Xianbo et al. [190], nGO nanocomposite modified with PEG and an insulin-derived peptide (EALYLV) was synthesized, which can inhibit the aggregation of hIAPP, protect INS-1 cells from the toxicity of hIAPP, stabilize mitochondrial membrane potential and decrease intracellular reactive oxygen species.
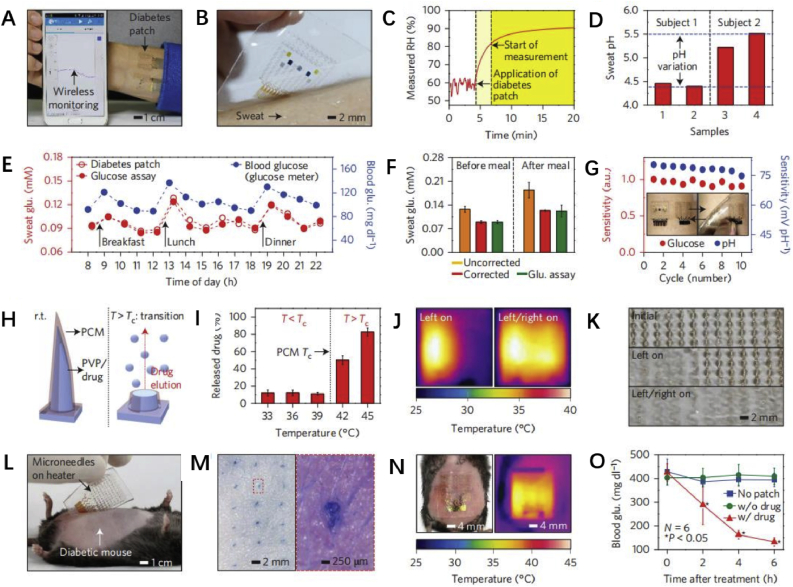
Lee, H et al. [191] reported that a flexible translucent patch was fabricated by adding gold particles to graphene and combining them with a gold mesh Fig. 6). The patch contains a series of sensors that can detect humidity, glucose, pH and temperature. The results show that the patch can deliver metformin via thermally activated process and reduce blood glucose levels when the patch monitor high glucose levels in sweat. Several different types of smart drug delivery systems and their application were summarized in Table 1.
Fig. 6.
Demonstration of the wearable diabetes monitoring and therapy system in vivo. A, Optical image of the integrated wearable diabetes monitoring and therapy system connected to a portable electrochemical analyser. The electrochemical analyser wirelessly communicates with external devices via Bluetooth. B, Optical image of the GP - hybrid electrochemical device array on the human skin with perspiration. C, RH measurement by the diabetes patch. D, Measurement of the pH variation in two human sweat samples from two subjects. E, One-day monitoring of glucose concentrations in the sweat and blood of a human (subject 2 in d). F, Comparison of the average glucose concentrations with the commercial glucose assay data in e before and after correction using the measured pH (error bars show the standard deviation). G, Plots showing the stable sensitivity of the glucose and pH sensors after multiple reuses of the patch. H, Schematic illustrations of bioresorbable microneedles. I, Drug release from the microneedles at different temperatures (N = 3, error bars show the standard deviation). J, Infrared camera images of multichannel heaters showing the stepwise drug release. K, Optical images of the stepwise dissolution of the microneedles. L, Optical image of the heater integrated with the microneedles, which is laminated on the skin near the abdomen of the db/db mouse. The hair on the skin was shaved off before treatment with the microneedles. M, Optical image (left) and its magnified view (right) of the db/db mouse skin stained with trypan blue to visualize the micro-sized holes made by the penetration of the microneedles. N, Optical (left) and infrared (right) camera images of the patch with the thermal actuation. O, Blood glucose concentrations of db/db mice for the treated group (with the drug) and control groups (without the patch and without the drug). The error bars show the standard deviation in each group and small P values show that the results are statistically reliable. The asterisks indicate significant difference (P < 0.05) between the treated (red) and the non-treated group (blue and green) on each time point [191]. Reprinted with permission from Ref. [191].
Table 1.
Summary of SDDSs based on 2D nanomaterials.
| 2D materials | modification of 2D materials | Response | clinical application | Biological model | Ref |
|---|---|---|---|---|---|
| BP | Polymer | Light | anticancer | 4T1 tumor | [127] |
| MoS2 | Polymer | Light &pH | antitumor | HT29 tumor | [128] |
| GO | Ag | N.A. | antibacterial | antibacterial | [137] |
| GO | N.A. | N.A. | anticancer | anticancer | [138] |
| MoS2 | Liposomes | Light | antitumor | 4T1 | [139] |
| GO | Polymer | pH | antitumor | Hela cells | [125] |
| BP | Polymer | Light | anticancer | MCF7 breast tumor | [165] |
| MnO2 | Aptamer | Magnetic field | antitumor | N.A. | |
| MnO2 | glucose oxidase | Light &Magnetic field | antitumor | A375 tumor | |
| GO | Hydrogel | Magnetic field | antitumor | S180 tumor | [140] |
| GO | Hydrogels | N.A. | antitumor | antitumor | [141] |
| GO | Liposomes | Light | N.A. | N.A. | [143] |
| graphene | Liposomes | Light | anticancer | MCF-7 cells | [144] |
| WS2/MoS2 | Liposomes | N.A. | anticancer | HeLa cells | [145] |
| GO | Films | pH | the control of coating techniques | N.A. | [147] |
| GO | BNN6 | Light | anticancer | 143B cells | [152] |
| BP | Polymer | Light | intelligent implantable devices | N.A. | [153] |
| BP | Pt | Light | antitumor | 4T1 | [154] |
| BP | Hydrogels | Light | postoperative treatment of cancer | HeLa | [155] |
| GO | Polymer | pH | anticancer | Hela and L929 cells | [159] |
| GO | Polymer | pH | antitumor | HeLa cells | [160] |
| GO | Polymer | pH | anticancer | SMMC-7721 cells | [161] |
| GO | Hydrogels | Light &pH | anticancer | HeLa cells | [162] |
| GO | Polymer | redox | antitumor | B16 tumor | [169] |
| rGO | Polymer | Light | anticancer | 4T1 tumor | [170] |
| rGO | gold and folic acid | Light &pH | anticancer | MCF-7 cells | [171] |
| BP | Hydrogels | Light | anticancer | SMMC-7721 cells | [172] |
| MoS2 | Polymer | N.A. | antibacterial | S. aureus | [174] |
| GO | Polymer | N.A. | antibacterial | S. aureus and E. coli | [175] |
| graphene | Cu2O | N.A. | antibacterial | Vibrio cholerae and Salmonella typhimurium | [176] |
| BP | Liposomes | Light | antibacterial | Methicillin-resistant Staphylococcus aureus |
[177] |
| GO | N.A. | N.A. | anti-Alzheimer's disease | N.A. | [180] |
| graphene | N.A. | N.A. | antiparkinsonian | N.A. | [181] |
| GO | Polymer | N.A. | antiparkinsonian | N.A. | [182] |
| GO | Hydrogels | thermosensitive | bone tissue repair | Human MG-63 cells | [183] |
| graphene | Polymer | N.A. | bone tissue repair | SaOS-2 cell | [184] |
| GO | Films | N.A. | bone tissue repair | Defective skulls of Sprague Dawley rats | [185] |
| MoS2 | Films | N.A. | bone tissue repair | rat bone marrow mesenchymal stem cells | [186] |
| GO | N.A. | N.A. | diabetes | NIT-1 cells | [188] |
| GO | Polymer | N.A. | diabetes | N.A. | [189] |
| GO | Polymer | N.A. | diabetes | INS-1 cells | [190] |
| graphene | Gold | temperature | diabetes | N.A. | [191] |
| BP | Polymer | Light | anticancer | Hela tumor | [200] |
| graphene | Antibody | pH | anticancer | MDA-MB-231 cell | [201] |
| GO | Hydrogels | Light/redox | anticancer | HeLa cells | [202] |
| graphene | N.A. | N.A. | respiratory diseases | BEAS-2B cells | [205] |
| GO | Polymer | pH | anticancer | Hela and L929 cells | [206] |
6. Advantages and disadvantages of two-dimensional materials in intelligent drug delivery system
6.1. Advantages
Due to sensitive response to external stimuli, 2D materials are more controllable compared to traditional drug delivery platform (such as polymers). 2D materials have a huge specific surface area, resulting in an effective load of fluorescent dyes, targeting molecules and therapeutic drugs [[192], [193], [194], [195]]. In addition, many 2D materials exhibit great phototherapy performance, which makes combined therapy possible [[196], [197], [198], [199]]. Gao, Nansha et al. [200] prepared a nanocapsule by coating the BP nanosheets with PDA. Then a novel nanoparticle was prepared by introducing the targeting polymer mercapto group-PEG-folic acid into the nanocapsule and loaded with adriamycin. This BP-based nanoparticle exhibited better photothermal conversion performance, biocompatibility and targeting ability for cancer cells.
The surface of targeted GQDs could be loaded with cisplatin up to 50% at neutral conditions and, and reduce the common systemic toxicity of cisplatin. Its release behavior showed slow release property and pH dependence. Cell experiments displayed that it has low cytotoxicity, high selective uptake rate of tumor cells and good tumor targeting. The toxicity of targeted cisplatin-loaded nanocarriers on MDA-MB-231 cells was significantly higher than non-targeted ones [201]. By combining 2D GO nanopletlets with thermosensitive matrix, a hybrid nanogels with photothermal effect they and good stability can be acquired. GO nanopletlets with well-dispersed in the nanogels not only increased the encapsulation efficiency of an anticancer drug, but also exhibited an enhanced photothermal effect [202].
6.2. Disadvantage
2D nanomaterials may interact with biological systems including tissues, organs, cells and biomolecules, which can lead to acute and chronic toxicity. The ultra-high surface area of GNS is composed of purely of carbon. Compared with other nanocarriers, GNS has the advantages of high drug-loading rate. However, there is a downside to such a large surface area. Due to GNS can improve interactions with blood components, and formation of precipitates with serum proteins or red blood cells after in vivo administration [203]. On the other study, GNS can cause side effects abnormal cells. For example, graphene causes the transforming growthfactor-β-related signaling pathways, and induces apoptosis of mitogen-activated protein kinases; graphene increases intracellular ROS and destroys the mitochondrial Membrane potential to cause cytotoxicity; graphene derivatives showed dose-dependent hemolytic activity [204]. It has been reported that unmodified graphene can induce apoptosis by epidermal growth factor receptors in lung epithelial cells. By activating IP3 pathway, graphene induced an increase in Ca2+ transportation. In fact, cystolic Ca2+ increase stimulates Ca2+ mediated apoptosis with increased Ca2+ transportation [205].
Few layer thick graphene, high dose and long exposure of GO can impart mitochondrial dysfunction. Lammel et al. [206] found that exposuring to GO and carboxyl graphene can disrupt mitochondrial structure and function. Inhalation of graphene can lead to accumulation in lungs, and result in severe health issues. When mice were exposed to 10mg/body weight dosage of GO for 14days, the results showed severe accumulation of graphene in lungs leading to granulomatous lesions, pulmonary edema, inflammatory cell infiltration and fibrosis [207].
7. Conclusions and perspectives
Nano-scale drug-delivery systems can increase drug loading rate, improve drug biological distribution, and endue drug slow-release performance, thus reducing drug toxicity and enhancing drug efficacy. Therefore, nano-scale drug-delivery systems has attracted more and more attention in the field of medicine and generated a strong impetus. In addition to natural drug carriers, biomedical enterprises and pharmaceutical have invested massive money, energy and time to research potent nanocarrier systems.
2D materials have attracted much attention due to their diverse physical and chemical properties and their broad application prospects in the fields of devices, sensors, catalysis, medicine and energy. 2D materials also show strong ability in drug delivery. Compared with traditional drug delivery systems, the unique essential characteristics of 2D materials is the ultrathin nanosheet structure, which is closely related to the huge specific surface area, thickness-dependent band gap and sensitive responses to various external stimuli. 2D materials can be used for highly efficient photodynamic and photothermal therapy of diseases. In addition, 2D nano materials are the lamella structures that ensure the huge surface area for high-efficiency drug loading. Meanwhile, with surface modification of 2D material, 2D materials can have active targeting. Drugs can be accurately delivered to the focus, and drug side effects are reduced. At the same time, with the NIR response and pH response of 2D materials, 2D SDDSs can release drugs accurately, and effectively control the blood concentration, so as to achieve the best therapeutic effect. These characteristics will provide an opportunity for multimodality treatment of disease. These unique advantages make research of 2D materials in SDDSs more and more popular. However, there is still a long way to go before they can be applied in clinical practice, and there are still some urgent problems to be solved, such as the long-term degradability and long-term toxicity of 2D materials; Besides, the research of 2D materials is only limited to in animal land in vitro, and further in vitro experiments are an indispensable step in its clinical applications. It is anticipated that in the future, with the in-depth research and more in vivo experiments, the applications of 2D layered materials and their derivatives in SDDSs eventually will be applied in clinical practice. In this review we highlight the recent development of SDDSs for a lot of smart 2D materials carriers, including polymers, hydrogels, liposomes, nanoparticles, nanosheet, micelles, etc. It is one of the main biological applications of 2D materials to act modified 2D materials as drug delivery carriers. Using 2D materials as drug carrier can improve the therapeutic effect of drugs on diseases by controlled release, sustained release and improving the targeting of drugs. Consequently, 2D materials have a bright future in the development of the next generation of SDDSs.
Declaration of competing interest
None.
Acknowledgements
Thanks to Prof. Han Zhang for his helpful suggestions on the article. The research was partially supported by the National Natural Science Foundation of China (NSFC) (U1803128, 81960334 81960648); Science and Technology Innovation Commission of Shenzhen (KQTD2015032416270385); Science and Technology Development Fund (STDF) (007/2017/A1) of Macao SAR; China and the Postgraduate Innovation Development Fund Project of Shenzhen University (PIDFPZR2018004).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Wen Chen, Email: chen-wen2000@126.com.
Bing Wang, Email: wangbing@szu.edu.cn.
Abbreviations
- Smart drug delivery systems
SDDSs (SDDSs)
- Two-dimensional
2D
- Graphene oxide
GO
- reduced Graphene oxide
rGO
- nanoparticles
NPs
- sodium alginate
SA
- Polyethylene glycol
PEG
- polyethylenimine
PEI
- black phosphorus
BP
- near-infrared
NIR
- soybean phospholipid-encapsulated MoS2
SP-MoS2
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
DOPC
- branched polyethylenimine
bPEI
- block copolymer micelles
BCM
- photodynamic
PDT
- photothermal
PTT
- nitric oxide
NO
- (N,N′-di-sec-butylN,N′-dinitroso-1,4-phenylenediamine)
BNN6
- poly(d,l‐lactide)‐poly(ethylene glycol)‐poly(d,l‐lactide)
PLEL
- magnetic resonance imaging
MRI
- carboxymethyl chitosan
CMC
- fluorescein isothiocyanate
FI
- hyaluronic acid
HA
- adriamycin
DOX
- acrylic acid
AA
- polydopamine
PDA
- antiarrhythmic peptide 10
AAP10
- arginine
Arg
- polycaprolactone
PCL
- Staphylococcus aureus
S. aureus
- Escherichia coli
E. coli
- blood-brain barrier
BBB
- GO quantum dots
GQDs
- Alzheimer's disease
AD
- calcium phosphate
CaP
- simvastatin
SIM
- mesenchymal stem cells
MSCs
- Human islet amyloid polypeptide
hIAPP
References
- 1.Fang Z., Wan L.-Y., Chu L.-Y., Zhang Y.-Q., Wu J.-F. Smart' nanoparticles as drug delivery systems for applications in tumor therapy. Expet Opin. Drug Deliv. 2015;12(12):1943–1953. doi: 10.1517/17425247.2015.1071352. [DOI] [PubMed] [Google Scholar]
- 2.Balamuralidhara V., Kumar T.M.P., Gupta N.V., Gangadharappa H.V. pH-dependent gradient release microspheres of lercanidipine hydrochloride: in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2012;22(6):493–499. [Google Scholar]
- 3.Karimi M., Basri S.M.M., Vossoughi M., Pakchin P.S., Mirshekari H., Hamblin M.R. Redox-sensitive smart nanosystems for drug and gene delivery. Curr. Org. Chem. 2016;20(28):2949–2959. [Google Scholar]
- 4.de la Rica R., Aili D., Stevens M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012;64(11):967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Rashmi R., Nedungadi D., Podder A., Mishra N., Bhuniya S. Monitoring of topoisomerase (I) inhibitor camptothecin release from endogenous redox-stimulated GO-polymer hybrid carrier. J. Photochem. Photobiol. B Biol. 2018;189:14–20. doi: 10.1016/j.jphotobiol.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Xiong F., Huang S., Gu N. Magnetic nanoparticles: recent developments in drug delivery system. Drug Dev. Ind. Pharm. 2018;44(5):697–706. doi: 10.1080/03639045.2017.1421961. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Zhang H., Zhu X., Zhang X., Chen Q., Chen J., Hou L., Zhang Z. Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2+ liberating and artemisinin delivery. Oncotarget. 2017;8(35):58738–58753. doi: 10.18632/oncotarget.17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolosnjaj-Tabi J., Gibot L., Fourquaux I., Golzio M., Rols M.-P. Electric field-responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2018;138:56–67. doi: 10.1016/j.addr.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Mullick Chowdhury S., Lee T., Willmann J.K. Ultrasound-guided drug delivery in cancer. Ultrasonography (Seoul, Korea) 2017;36(3):171–184. doi: 10.14366/usg.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C., Zheng P., Cao Z., Ma Y., Li J., Qian H., Tao W., Yang X. PEGylated hyperbranched polyphosphoester based nanocarriers for redox-responsive delivery of doxorubicin. Biomater. Sci. 2016;4(3):412–417. doi: 10.1039/c5bm00440c. [DOI] [PubMed] [Google Scholar]
- 11.Hamidi M., Shahbazi M.-A., Rostamizadeh K. Copolymers: efficient carriers for intelligent nanoparticulate drug targeting and gene therapy. Macromol. Biosci. 2012;12(2):144–164. doi: 10.1002/mabi.201100193. [DOI] [PubMed] [Google Scholar]
- 12.Hunter A.C., Moghimi S.M. Smart polymers in drug delivery: a biological perspective. Polym. Chem. 2017;8(1):41–51. [Google Scholar]
- 13.James H.P., John R., Alex A., Anoop K.R. Smart polymers for the controlled delivery of drugs - a concise overview. Acta Pharm. Sin. B. 2014;4(2):120–127. doi: 10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P.-C., Chen H.-H., Chen S.-Y., Wang W.-L., Yang K.-L., Huang C.-H., Kao H.-F., Chang J.-C., Hsu C.-L.L., Wang J.-Y., Chou T.-M., Kuo W.-S. Graphene oxide conjugated with polymers: a study of culture condition to determine whether a bacterial growth stimulant or an antimicrobial agent? J. Nanobiotechnol. 2018;16 doi: 10.1186/s12951-017-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.C., Kwon I.K., Park K. Hydrogels for delivery of bioactive agents: a historical perspective. Adv. Drug Deliv. Rev. 2013;65(1):17–20. doi: 10.1016/j.addr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deen G.R., Loh X.J. Stimuli-responsive cationic hydrogels in drug delivery applications. Gels (Basel, Switzerland) 2018;4(1):13. doi: 10.3390/gels4010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira N.N., Ferreira L.M.B., Cardoso V.M.O., Boni F.I., Souza A.L.R., Gremiao M.P.D. Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur. Polym. J. 2018;99:117–133. [Google Scholar]
- 18.Fernandes L.F., Bruch G.E., Massensini A.R., Frezard F. Recent advances in the therapeutic and diagnostic use of liposomes and carbon nanomaterials in ischemic stroke. Front. Neurosci. 2018;12:453. doi: 10.3389/fnins.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif. Cell Nanomed. Biotechnol. 2016;44(1):381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- 20.Sawant R.R., Torchilin V.P. Liposomes as 'smart' pharmaceutical nanocarriers. Soft Matter. 2010;6(17):4026–4044. [Google Scholar]
- 21.Zangabad P.S., Mirkiani S., Shahsavari S., Masoudi B., Masroor M., Hamed H., Jafari Z., Taghipour Y.D., Hashemi H., Karimi M., Hamblin M.R. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018;7(1):95–122. doi: 10.1515/ntrev-2017-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suhrland C., Truman J.-P., Obeid L.M., Sitharaman B. Oxidized graphene nanoparticles as a delivery system for the pro-apoptotic sphingolipid C-6 ceramide. J. Biomed. Mater. Res. 2019;107(1):25–37. doi: 10.1002/jbm.a.36474. [DOI] [PubMed] [Google Scholar]
- 23.Kong X., Liu Y., Huang X., Huang S., Gao F., Rong P., Zhang S., Zhang K., Zeng W. Cancer therapy based on smart drug delivery with advanced nanoparticles. Anti Canc. Agents Med. Chem. 2019;19(6):720–730. doi: 10.2174/1871520619666190212124944. [DOI] [PubMed] [Google Scholar]
- 24.Ye G., Jiang Y., Yang X., Hu H., Wang B., Sun L., Yang V.C., Sun D., Gao W. Smart nanoparticles undergo phase transition for enhanced cellular uptake and subsequent intracellular drug release in a tumor microenvironment. ACS Appl. Mater. Interfaces. 2018;10(1):278–289. doi: 10.1021/acsami.7b15978. [DOI] [PubMed] [Google Scholar]
- 25.Cao R., Chen S., Liu H., Liu H., Zhang X. Fabrication and characterization of thermo-responsive GO nanosheets with controllable grafting of poly(hexadecyl acrylate) chains. J. Mater. Sci. 2018;53(6):4103–4117. [Google Scholar]
- 26.Cai R., Yang D., Wu J., Zhang L., Wu C., Chen X., Wang Y., Wan S., Hou F., Yan Q., Tan W. Fabrication of ultrathin Zn(OH)(2) nanosheets as drug carriers. Nano Res. 2016;9(8):2520–2530. doi: 10.1007/s12274-016-1138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao P., Zhu Y., Yang X., Shen J., Jiang X., Zong J., Li C. Multifunctional MnO2 nanosheet-modified Fe3O4@SiO2/NaYF4:Yb, Er nanocomposites as novel drug carriers. Dalton Trans. 2014;43(2):451–457. doi: 10.1039/c3dt52066h. [DOI] [PubMed] [Google Scholar]
- 28.He L., Sun M., Cheng X., Xu Y., Lv X., Wang X., Tang R. pH/redox dual-sensitive platinum (IV)-based micelles with greatly enhanced antitumor effect for combination chemotherapy. J. Colloid Interface Sci. 2019;541:30–41. doi: 10.1016/j.jcis.2019.01.076. [DOI] [PubMed] [Google Scholar]
- 29.Cajot S., Schol D., Danhier F., Preat V., De Pauw M.-C.G., Jerome C. In vitro investigations of smart drug delivery systems based on redox-sensitive cross-linked micelles. Macromol. Biosci. 2013;13(12):1661–1670. doi: 10.1002/mabi.201300250. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z., Li S., Xu Y., Ren H., Zhang X., Hu G., Huang F., Yue T. Extracting pulmonary surfactants to form inverse micelles on suspended graphene nanosheets. Environ. Sci. Nano. 2018;5(1):130–140. [Google Scholar]
- 31.Duan F., Zhang S., Yang L., Zhang Z., He L., Wang M. Bifunctional aptasensor based on novel two-dimensional nanocomposite of MoS2 quantum dots and g-C3N4 nanosheets decorated with chitosan-stabilized Au nanoparticles for selectively detecting prostate specific antigen. Anal. Chim. Acta. 2018;1036:121–132. doi: 10.1016/j.aca.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 32.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 33.Bao Q., Zhang H., Wang Y., Ni Z., Yan Y., Shen Z.X., Loh K.P., Tang D.Y. Atomic-layer graphene as a saturable absorber for ultrafast pulsed lasers. Adv. Funct. Mater. 2009;19(19):3077–3083. [Google Scholar]
- 34.Miao L., Jiang Y., Lu S., Shi B., Zhao C., Zhang H., Wen S. Broadband ultrafast nonlinear optical response of few-layers graphene: toward the mid-infrared regime. Photon. Res. 2015;3(5):214. [Google Scholar]
- 35.Song Y., Liang Z., Zhang H., Zhang Q., Zhao L., Shen D., Tang D. Period-doubling and quadrupling bifurcation of vector soliton bunches in a graphene mode locked fiber laser. IEEE Photonics J. 2017;9(5):1–8. [Google Scholar]
- 36.Wang Z.T., Chen Y., Zhao C.J., Zhang H., Wen S.C. Switchable dual-wavelength synchronously Q-switched erbium-doped fiber laser based on graphene saturable absorber. IEEE Photonics J. 2012;4(3):869–876. [Google Scholar]
- 37.Zhang H., Bao Q., Tang D., Zhao L., Loh K. Large energy soliton erbium-doped fiber laser with a graphene-polymer composite mode locker. Appl. Phys. Lett. 2009;95(14):141103. [Google Scholar]
- 38.Fan T., Xie Z., Huang W., Li Z., Zhang H. Two-dimensional non-layered selenium nanoflakes: facile fabrications and applications for self-powered photo-detector. Nanotechnology. 2019;30(11):114002. doi: 10.1088/1361-6528/aafc0f. [DOI] [PubMed] [Google Scholar]
- 39.Xing C., Xie Z., Liang Z., Liang W., Fan T., Ponraj J.S., Dhanabalan S.C., Fan D., Zhang H. Selenium nanosheets: 2D nonlayered selenium nanosheets: facile synthesis, photoluminescence, and ultrafast photonics. Adv. Mater. Optic. 2017;5(24):1700884. [Google Scholar]
- 40.Zhang H., Liu J., Liu J., Wang J., Su L., Ma W., Guo Z. Dual-wavelength Q-switched Er:SrF2 laser with a black phosphorus absorber in the mid-infrared region. Optic Express. 2016;24(26):30289. doi: 10.1364/OE.24.030289. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z., Han W., Tang H., Ren L., Chander D.S., Qi X., Zhang H. Photoelectrochemical-type sunlight photodetector based on MoS2/graphene heterostructure. 2D Mater. 2015;2(3) 035011. [Google Scholar]
- 42.Jiang Y., Miao L., Jiang G., Chen Y., Qi X., Jiang X.F., Zhang H., Wen S. Broadband and enhanced nonlinear optical response of MoS2/graphene nanocomposites for ultrafast photonics applications. Sci. Rep. 2015;5:16372. doi: 10.1038/srep16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Xue Y.X., Zhenda Xie Z.X., Zhilin Ye Z.Y., Xiaopeng Hu X.H., Jinlong Xu J.X., Han Zhang H.Z. Enhanced saturable absorption of MoS2 black phosphorus composite in 2 μm passively Q-switched Tm: YAP laser. Chin. Optic Lett. 2018;16(2) [Google Scholar]
- 44.Shi J., Li Z., Sang D.K., Xiang Y., Li J., Zhang S., Zhang H. THz photonics in two dimensional materials and metamaterials: properties, devices and prospects. J. Mater. Chem. C. 2018;6(6):1291–1306. [Google Scholar]
- 45.Ge Y., Zhu Z., Xu Y., Chen Y., Chen S., Liang Z., Song Y., Zou Y., Zeng H., Xu S., Zhang H., Fan D. Broadband nonlinear photoresponse of 2D TiS2 for ultrashort pulse generation and all-optical thresholding devices. Adv. Mater. Optic. 2018;6(4):1701166. [Google Scholar]
- 46.Zhu X., Chen S., Zhang M., Chen L., Wu Q., Zhao J., Jiang Q., Zheng Z., Zhang H. TiS2-based saturable absorber for ultrafast fiber lasers. Photon. Res. 2018;6(10):C44. [Google Scholar]
- 47.Huang W., Xie Z., Fan T., Li J., Wang Y., Wu L., Ma D., Li Z., Ge Y., Huang Z.N., Dai X., Xiang Y., Li J., Zhu X., Zhang H. Black-phosphorus-analogue tin monosulfide: an emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C. 2018;6(36):9582–9593. [Google Scholar]
- 48.Xie Z., Wang D., Fan T., Xing C., Li Z., Tao W., Liu L., Bao S., Fan D., Zhang H. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B. 2018;6(29):4747–4755. doi: 10.1039/c8tb00729b. [DOI] [PubMed] [Google Scholar]
- 49.Xie Z., Zhang F., Liang Z., Fan T., Li Z., Jiang X., Chen H., Li J., Zhang H. Revealing of the ultrafast third-order nonlinear optical response and enabled photonic application in two-dimensional tin sulfide. Photon. Res. 2019;7(5):494–502. [Google Scholar]
- 50.Ge Y., Zhu Z., Xu Y., Chen Y., Si C., Liang Z., Song Y., Zou Y., Zeng H., Xu S. Ultrafast photonics: broadband nonlinear photoresponse of 2D TiS 2 for ultrashort pulse generation and all-optical thresholding devices advanced. Opt. Mater. 2018;6(4):1870014. [Google Scholar]
- 51.Huang W., Xing C., Wang Y., Li Z., Wu L., Ma D., Dai X., Xiang Y., Li J., Fan D., Zhang H. Facile fabrication and characterization of two-dimensional bismuth(iii) sulfide nanosheets for high-performance photodetector applications under ambient conditions. Nanoscale. 2018;10(5):2404–2412. doi: 10.1039/c7nr09046c. [DOI] [PubMed] [Google Scholar]
- 52.Qiyuan H., Zhiyuan Z., Zongyou Y., Hai L., Shixin W., Xiao H., Hua Z. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small. 2012;8(19):2994–2999. doi: 10.1002/smll.201201224. [DOI] [PubMed] [Google Scholar]
- 53.Bao Q., Zhang H., Ni Z., Wang Y., Polavarapu L., Shen Z., Xu Q.-H., Tang D., Loh K.P. Monolayer graphene as a saturable absorber in a mode-locked laser. Nano Res. 2010;4(3):297–307. [Google Scholar]
- 54.Bao Q., Zhang H., Wang B., Ni Z., Lim C.H.Y.X., Wang Y., Tang D.Y., Loh K.P. Broadband graphene polarizer. Nat. Photon. 2011;5(7):411–415. [Google Scholar]
- 55.Chen Y., Jiang G., Chen S., Guo Z., Yu X., Zhao C., Zhang H., Bao Q., Wen S., Tang D., Fan D. Mechanically exfoliated black phosphorus as a new saturable absorber for both Q-switching and Mode-locking laser operation. Optic Express. 2015;23(10):12823–12833. doi: 10.1364/OE.23.012823. [DOI] [PubMed] [Google Scholar]
- 56.Xing C., Xie Z., Liang Z., Liang W., Fan T., Ponraj J.S., Dhanabalan S.C., Fan D., Zhang H. 2D nonlayered selenium nanosheets: facile synthesis, photoluminescence, and ultrafast photonics. Adv. Mater. Optic. 2017;5(24):1700884. [Google Scholar]
- 57.Ji X., Kong N., Wang J., Li W., Xiao Y., Gan S.T., Zhang Y., Li Y., Song X., Xiong Q., Shi S., Li Z., Tao W., Zhang H., Mei L., Shi J. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 2018 doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Z., Xing C., Huang W., Fan T., Li Z., Zhao J., Xiang Y., Guo Z., Li J., Yang Z. Ultrathin 2D nonlayered tellurium nanosheets: facile liquid‐phase exfoliation, characterization, and photoresponse with high performance and enhanced stability. Adv. Funct. Mater. 2018;28(16):1705833. [Google Scholar]
- 59.Guo B., Wang S.H., Wu Z.X., Wang Z.X., Wang D.H., Huang H., Zhang F., Ge Y.Q., Zhang H. Sub-200 fs soliton mode-locked fiber laser based on bismuthene saturable absorber. Optic Express. 2018;26(18):22750–22760. doi: 10.1364/OE.26.022750. [DOI] [PubMed] [Google Scholar]
- 60.Huang H., Ren X., Li Z., Wang H., Huang Z., Qiao H., Tang P., Zhao J., Liang W., Ge Y., Liu J., Li J., Qi X., Zhang H. Two-dimensional bismuth nanosheets as prospective photo-detector with tunable optoelectronic performance. Nanotechnology. 2018;29(23):235201. doi: 10.1088/1361-6528/aab6ee. [DOI] [PubMed] [Google Scholar]
- 61.Xing C., Huang W., Xie Z., Zhao J., Ma D., Fan T., Liang W., Ge Y., Dong B., Li J., Zhang H. Ultrasmall bismuth quantum dots: facile liquid-phase exfoliation, characterization, and application in high-performance UV–vis photodetector. ACS Photonics. 2017;5(2):621–629. [Google Scholar]
- 62.Fan T., Zhou Y., Qiu M., Zhang H. Black phosphorus: a novel nanoplatform with potential in the field of bio-photonic nanomedicine. J. Innovat. Optic. Health Sci. 2018;11(6):1830003. [Google Scholar]
- 63.Luo M., Fan T., Zhou Y., Zhang H., Mei L. 2D black phosphorus–based biomedical applications. Adv. Funct. Mater. 2019;29(13):1808306. [Google Scholar]
- 64.Tang X., Liang W., Zhao J., Li Z., Qiu M., Fan T., Luo C.S., Zhou Y., Li Y., Guo Z. Fluorinated phosphorene: electrochemical synthesis, atomistic fluorination, and enhanced stability. Small. 2017;13(47):1702739. doi: 10.1002/smll.201702739. [DOI] [PubMed] [Google Scholar]
- 65.Wang H.-D., Sang D.K., Guo Z.-N., Cao R., Zhao J.-L., Ullah Shah M.N., Fan T.-J., Fan D.-Y., Zhang H. Black phosphorus-based field effect transistor devices for Ag ions detection. Chin. Phys. B. 2018;27(8) [Google Scholar]
- 66.Liu J., Huang H., Zhang F., Zhang Z., Liu J., Zhang H., Su L. Bismuth nanosheets as a Q-switcher for a mid-infrared erbium-doped SrF2 laser. Photon. Res. 2018;6(8):762. [Google Scholar]
- 67.Mu H., Wang Z., Yuan J., Xiao S., Chen C., Chen Y., Chen Y., Song J., Wang Y., Xue Y., Zhang H., Bao Q. Graphene–Bi2Te3 heterostructure as saturable absorber for short pulse generation. ACS Photonics. 2015;2(7):832–841. [Google Scholar]
- 68.Feng X.-Y., Ding B.-Y., Liang W.-Y., Zhang F., Ning T.-Y., Liu J., Zhang H. MXene Ti3C2T x absorber for a 1.06 μm passively Q-switched ceramic laser. Laser Phys. Lett. 2018;15(8) 085805. [Google Scholar]
- 69.Jiang X., Liu S., Liang W., Luo S., He Z., Ge Y., Wang H., Rui C., Feng Z., Qiao W. Broadband nonlinear photonics in few‐layer MXene Ti3C2Tx (T = F, O, or OH) Laser Photon. Rev. 2017;12(2):1700229. [Google Scholar]
- 70.Zu Y., Zhang C., Guo X., Liang W., Liu J., Su L., Zhang H. A solid-state passively Q-switched Tm,Gd:CaF2 laser with a Ti3C2T x MXene absorber near 2 μm. Laser Phys. Lett. 2018;16(1) 015803. [Google Scholar]
- 71.Chen J., Fan T., Xie Z., Zeng Q., Xue P., Zheng T., Chen Y., Luo X., Zhang H. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials. 2020;237:119827. doi: 10.1016/j.biomaterials.2020.119827. [DOI] [PubMed] [Google Scholar]
- 72.Ding H., Shu X., Jin Y., Fan T., Zhang H. Recent advances in nanomaterial-enabled acoustic devices for audible sound generation and detection. Nanoscale. 2019;11(13):5839–5860. doi: 10.1039/c8nr09736d. [DOI] [PubMed] [Google Scholar]
- 73.Wang R., Jiang X., Gao S., Zhao J., Zhang F., Huang W., Fan T., Liang W., Li Z., Huang H., Guo Z., Wang H., Zhang Y., Zhang X., Luo Z., Zhang H. Unveiling the stimulated robust carrier lifetime of surface‐bound excitons and their photoresponse in InSe. Adv. Mater. Interfaces. 2019;6(13):1900171. [Google Scholar]
- 74.Wang Y., Qiu M., Won M., Jung E., Fan T., Xie N., Chi S.-G., Zhang H., Kim J.S. Emerging 2D material-based nanocarrier for cancer therapy beyond graphene. Coord. Chem. Rev. 2019;400 UNSP 213041. [Google Scholar]
- 75.Xie Z., Chen S., Duo Y., Zhu Y., Fan T., Zou Q., Qu M., Lin Z., Zhao J., Li Y., Liu L., Bao S., Chen H., Fan D., Zhang H. Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl. Mater. Interfaces. 2019;11(25):22129–22140. doi: 10.1021/acsami.9b04628. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B., Fan T., Xie N., Nie G., Zhang H. Versatile applications of metal single-atom @ 2D material nanoplatforms. Adv. Sci. 2019;6(21):1901787. doi: 10.1002/advs.201901787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie Z., Xing C., Huang W., Fan T., Li Z., Zhao J., Xiang Y., Guo Z., Li J., Yang Z., Dong B., Qu J., Fan D., Zhang H. Ultrathin 2D nonlayered tellurium nanosheets: facile liquid-phase exfoliation, characterization, and photoresponse with high performance and enhanced stability. Adv. Funct. Mater. 2018;28(16):1705833. [Google Scholar]
- 78.Ren X., Zhou J., Qi X., Liu Y., Huang Z., Li Z., Ge Y., Dhanabalan S.C., Ponraj J.S., Wang S., Zhong J., Zhang H. Few-layer black phosphorus nanosheets as electrocatalysts for highly efficient oxygen evolution reaction. Adv. Energy Mater. 2017;7(19):1700396. [Google Scholar]
- 79.Ren X., Li Z., Huang Z., Sang D., Qiao H., Qi X., Li J., Zhong J., Zhang H. Environmentally robust black phosphorus nanosheets in solution: application for self-powered photodetector. Adv. Funct. Mater. 2017;27(18):1606834. [Google Scholar]
- 80.Li Z., Qiao H., Guo Z., Ren X., Huang Z., Qi X., Dhanabalan S.C., Ponraj J.S., Zhang D., Li J., Zhao J., Zhong J., Zhang H. High-performance photo-electrochemical photodetector based on liquid-exfoliated few-layered InSe nanosheets with enhanced stability. Adv. Funct. Mater. 2018;28(16):1705237. [Google Scholar]
- 81.Huang H., Li Y., Sa Z., Sun Y., Wang Y., Wang J. A smart drug delivery system from charge-conversion polymer-drug conjugate for enhancing tumor therapy and tunable drug release. Macromol. Biosci. 2014;14(4):485–490. doi: 10.1002/mabi.201300337. [DOI] [PubMed] [Google Scholar]
- 82.Prasanna A., Pooja R., Suchithra V., Ravikumar A., Gupta P.K., Niranjan V. Smart drug delivery systems for cancer treatment using nanomaterials. Mater. Today Proc. 2018;5(10):21047–21054. [Google Scholar]
- 83.Liu M., Song X., Wen Y., Zhu J., Li J. Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl. Mater. Interfaces. 2017;9(41):35673–35682. doi: 10.1021/acsami.7b12849. [DOI] [PubMed] [Google Scholar]
- 84.Unsoy G., Gunduz U. Smart drug delivery systems in cancer therapy. Curr. Drug Targets. 2018;19(3):202–212. doi: 10.2174/1389450117666160401124624. [DOI] [PubMed] [Google Scholar]
- 85.Wei Y., Zhou F., Zhang D., Chen Q., Xing D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale. 2016;8(6):3530–3538. doi: 10.1039/c5nr07785k. [DOI] [PubMed] [Google Scholar]
- 86.Fan H., Yan G., Zhao Z., Hu X., Zhang W., Liu H., Fu X., Fu T., Zhang X.-B., Tan W. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem. Int. Ed. 2016;55(18):5477–5482. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen S., Wu Y., Liu Y., Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int. J. Nanomed. 2017;12:4085–4109. doi: 10.2147/IJN.S132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goffredo R., Accoto D., Guglielmelli E. Swallowable smart pills for local drug delivery: present status and future perspectives. Expet Rev. Med. Dev. 2015;12(5):585–599. doi: 10.1586/17434440.2015.1061933. [DOI] [PubMed] [Google Scholar]
- 89.Hossen S., Hossain M.K., Basher M.K., Mia M.N.H., Rahman M.T., Uddin M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J. Adv. Res. 2019;15:1–18. doi: 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Y., Li B., Chen X., Zhu M. Preparation and characterization of a prolonged and sustained drug delivery system: linear polyacrylamide in poly(N-isopropylacrylamide)/clay hydrogels. J. Appl. Polym. Sci. 2012;125 E148-E156. [Google Scholar]
- 91.Kalaydina R.-V., Bajwa K., Qorri B., Decarlo A., Szewczuk M.R. Recent advances in "smart" delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018;13:4727–4745. doi: 10.2147/IJN.S168053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang X., Du A., Kou L. Gas sensing and capturing based on two-dimensional layered materials: overview from theoretical perspective. Wiley Interdiscipl. Rev. Comput. Mol. Sci. 2018;8(4) [Google Scholar]
- 93.Yadav V., Roy S., Singh P., Khan Z., Jaiswal A. 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications. Small. 2019;15(1):1803706. doi: 10.1002/smll.201803706. [DOI] [PubMed] [Google Scholar]
- 94.Yang T., Cui Y., Chen H., Li W. Controllable preparation of two dimensional metal- or covalent organic frameworks for chemical sensing and biosensing. Hua Hsueh Hsueh Pao. 2017;75(4):339–350. [Google Scholar]
- 95.Chen H., Liu T., Su Z., Shang L., Wei G. 2D transition metal dichalcogenide nanosheets for photo/thermo-based tumor imaging and therapy. Nanoscale Horiz. 2018;3(2):74–89. doi: 10.1039/c7nh00158d. [DOI] [PubMed] [Google Scholar]
- 96.Khadir S., Bon P., Vignaud D., Galopin E., McEvoy N., McCloskey D., Monneret S., Baffou G. Optical imaging and characterization of graphene and other 2D materials using quantitative phase microscopy. ACS Photonics. 2017;4(12):3130–3139. [Google Scholar]
- 97.Wu M.T., Chu F.L. Penetrative imaging of 2D conductors embedded in planar multilayer media. Electron. Lett. 1996;32(11):976–977. [Google Scholar]
- 98.Chen Y., Wang L., Shi J. Two-dimensional non-carbonaceous materials-enabled efficient photothermal cancer therapy. Nano Today. 2016;11(3):292–308. [Google Scholar]
- 99.Ji X., Kong N., Wang J., Li W., Xiao Y., Gan S.T., Zhang Y., Li Y., Song X., Xiong Q., Shi S., Li Z., Tao W., Zhang H., Mei L., Shi J. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 2018;30(36) doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin J., Guo M., Liu J., Liu J., Zhou H., Li J., Wang L., Liu H., Li Y., Zhao Y., Chen C. Graphdiyne nanosheet-based drug delivery platform for photothermal/chemotherapy combination treatment of cancer. ACS Appl. Mater. Interfaces. 2018;10(10):8436–8442. doi: 10.1021/acsami.7b17219. [DOI] [PubMed] [Google Scholar]
- 101.Lin L.-S., Cong Z.-X., Li J., Ke K.-M., Guo S.-S., Yang H.-H., Chen G.-N. Graphitic-phase C3N4 nanosheets as efficient photosensitizers and pH-responsive drug nanocarriers for cancer imaging and therapy. J. Mater. Chem. B. 2014;2(8):1031–1037. doi: 10.1039/c3tb21479f. [DOI] [PubMed] [Google Scholar]
- 102.Liu Z., Chen H., Jia Y., Zhang W., Zhao H., Fan W., Zhang W., Zhong H., Ni Y., Guo Z. A two-dimensional fingerprint nanoprobe based on black phosphorus for bio-SERS analysis and chemo-photothermal therapy. Nanoscale. 2018;10(39):18795–18804. doi: 10.1039/c8nr05300f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J., Liu Z., Han Y., Wang L., Li J., Deng L., Liu Y.-N., Guo S. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017;29(5) doi: 10.1002/adma.201603864. UNSP 1603864. [DOI] [PubMed] [Google Scholar]
- 104.Peng L., Mei X., He J., Xu J., Zhang W., Liang R., Wei M., Evans D.G., Duan X. Monolayer nanosheets with an extremely high drug loading toward controlled delivery and cancer theranostics. Adv. Mater. 2018;30(16):1707389. doi: 10.1002/adma.201707389. [DOI] [PubMed] [Google Scholar]
- 105.Xie Z., Wang D., Fan T., Xing C., Li Z., Tao W., Liu L., Bao S., Fan D., Zhang H. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B. 2018;6(29):4747–4755. doi: 10.1039/c8tb00729b. [DOI] [PubMed] [Google Scholar]
- 106.Cheng C.-C., Muhabie A.A., Huang S.-Y., Wu C.-Y., Gebeyehu B.T., Lee A.-W., Lai J.-Y., Lee D.-J. Dual stimuli-responsive supramolecular boron nitride with tunable physical properties for controlled drug delivery. Nanoscale. 2019;11(21):10393–10401. doi: 10.1039/c8nr09537j. [DOI] [PubMed] [Google Scholar]
- 107.Chimene D., Alge D.L., Gaharwar A.K. Two-dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv. Mater. 2015;27(45):7261–7284. doi: 10.1002/adma.201502422. [DOI] [PubMed] [Google Scholar]
- 108.Choi S.-J., Kim I.-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018;14(3):221–260. [Google Scholar]
- 109.Tao W., Ji X., Zhu X., Li L., Wang J., Zhang Y., Saw P.E., Li W., Kong N., Islam M.A., Gan T., Zeng X., Zhang H., Mahmoudi M., Tearney G.J., Farokhzad O.C. Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv. Mater. 2018;30(38):11896–11900. doi: 10.1002/adma.201802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tu Z., Wycisk V., Cheng C., Chen W., Adeli M., Haag R. Functionalized graphene sheets for intracellular controlled release of therapeutic agents. Nanoscale. 2017;9(47):18931–18939. doi: 10.1039/c7nr06588d. [DOI] [PubMed] [Google Scholar]
- 111.Xing C., Chen S., Liang X., Liu Q., Qu M., Zou Q., Li J., Tan H., Liu L., Fan D., Zhang H. Two-dimensional MXene (Ti3C2)-integrated cellulose hydrogels: toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces. 2018;10(33):27631–27643. doi: 10.1021/acsami.8b08314. [DOI] [PubMed] [Google Scholar]
- 112.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric field effect in atomically thin carbon films. Science. 2004;306:666. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 113.Huang Y., Sutter E., Shi N.N., Zheng J., Yang T., Englund D., Gao H.-J., Sutter P. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano. 2015;9(11):10612–10620. doi: 10.1021/acsnano.5b04258. [DOI] [PubMed] [Google Scholar]
- 114.Coleman J.N., Lotya M., O'Neill A., Bergin S.D., King P.J., Khan U., Young K., Gaucher A., De S., Smith R.J., Shvets I.V., Arora S.K., Stanton G., Kim H.Y., Lee K., Kim G.T., Duesberg G.S., Hallam T., Boland J.J., Wang J.J., Donegan J.F., Grunlan J.C., Moriarty G., Shmeliov A., Nicholls R.J., Perkins J.M., Grieveson E.M., Theuwissen K., McComb D.W., Nellist P.D., Nicolosi V. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science. 2011;331(6017):568–571. doi: 10.1126/science.1194975. [DOI] [PubMed] [Google Scholar]
- 115.Tao W., Zhu X., Yu X., Zeng X., Xiao Q., Zhang X., Ji X., Wang X., Shi J., Zhang H., Mei L. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv. Mater. 2017;29(1) doi: 10.1002/adma.201603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xue T., Liang W., Li Y., Sun Y., Xiang Y., Zhang Y., Dai Z., Duo Y., Wu L., Qi K., Shivananju B.N., Zhang L., Cui X., Zhang H., Bao Q. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019;10(1):28. doi: 10.1038/s41467-018-07947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo Z., Chen S., Wang Z., Yang Z., Liu F., Xu Y., Wang J., Yi Y., Zhang H., Liao L., Chu P.K., Yu X.F. Metal-ion-modified black phosphorus with enhanced stability and transistor performance. Adv. Mater. 2017;29(42) doi: 10.1002/adma.201703811. [DOI] [PubMed] [Google Scholar]
- 118.Wang S., Weng J., Fu X., Lin J., Fan W., Lu N., Qu J., Chen S., Wang T., Huang P. Black phosphorus nanosheets for mild hyperthermia-enhanced chemotherapy and chemo-photothermal combination therapy. Nanotheranostics. 2017;1(2):208–216. doi: 10.7150/ntno.18767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Y., Murali S., Cai W., Li X., Suk J.W., Potts J.R., Ruoff R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010;22(35):3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 120.Naguib M., Kurtoglu M., Presser V., Lu J., Niu J., Heon M., Hultman L., Gogotsi Y., Barsoum M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv. Mater. 2011;23(37):4248–4253. doi: 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- 121.Backes C., Higgins T.M., Kelly A., Boland C., Harvey A., Hanlon D., Coleman J.N. Guidelines for exfoliation, characterization and processing of layered materials produced by liquid exfoliation. Chem. Mater. 2016;29(1):243–255. [Google Scholar]
- 122.Zeng X., Luo M., Liu G., Wang X., Tao W., Lin Y., Ji X., Nie L., Mei L. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv. Sci. 2018;5(10) doi: 10.1002/advs.201800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu Y., He L., Ma W., Chen L. Reduced graphene oxide-based bortezomib delivery system for photothermal chemotherapy with enhanced therapeutic efficacy. Polym. Int. 2018;67(12):1648–1654. [Google Scholar]
- 124.Siriviriyanun A., Tsai Y.-J., Voon S.H., Kiew S.F., Imae T., Kiew L.V., Looi C.Y., Wong W.F., Lee H.B., Chung L.Y. Cyclodextrin- and dendrimer-conjugated graphene oxide as a nanocarrier for the delivery of selected chemotherapeutic and photosensitizing agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;89:307–315. doi: 10.1016/j.msec.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 125.Fan L., Ge H., Zou S., Xiao Y., Wen H., Li Y., Feng H., Nie M. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int. J. Biol. Macromol. 2016;93:582–590. doi: 10.1016/j.ijbiomac.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 126.Li T., Wu L., Zhang J., Xi G., Pang Y., Wang X., Chen T. Hydrothermal reduction of polyethylenimine and polyethylene glycol dual-functionalized nanographene oxide for high-efficiency gene delivery. ACS Appl. Mater. Interfaces. 2016;8(45):31311–31320. doi: 10.1021/acsami.6b09915. [DOI] [PubMed] [Google Scholar]
- 127.Sun C., Wen L., Zeng J., Wang Y., Sun Q., Deng L., Zhao C., Li Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 128.Yang H., Zhao J., Wu C., Ye C., Zou D., Wang S. Facile synthesis of colloidal stable MoS2 nanoparticles for combined tumor therapy. Chem. Eng. J. 2018;351:548–558. [Google Scholar]
- 129.Liu J., Zhang D., Lian S., Zheng J., Li B., Li T., Jia L. Redox-responsive hyaluronic acid-functionalized graphene oxide nanosheets for targeted delivery of water-insoluble cancer drugs. Int. J. Nanomed. 2018;13:7457–7472. doi: 10.2147/IJN.S173889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu J., Bremner D.H., Niu S., Wu H., Wu J., Wang H., Li H., Zhu L.-M. Functionalized MoS2 nanosheet-capped periodic mesoporous organosilicas as a multifunctional platform for synergistic targeted chemo-photothermal therapy. Chem. Eng. J. 2018;342:90–102. [Google Scholar]
- 131.Fong Y.T., Chen C.-H., Chen J.-P. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials. 2017;7(11) doi: 10.3390/nano7110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han S., Su L., Zhai M., Ma L., Liu S., Teng Y. A molecularly imprinted composite based on graphene oxide for targeted drug delivery to tumor cells. J. Mater. Sci. 2019;54(4):3331–3341. [Google Scholar]
- 133.Chen Y., Cheng W., Teng L., Jin M., Lu B., Ren L., Wang Y. Graphene oxide hybrid supramolecular hydrogels with self-healable, bioadhesive and stimuli-responsive properties and drug delivery application. Macromol. Mater. Eng. 2018;303(8) [Google Scholar]
- 134.Huang K., Wu H., Jiang F., Shen G., Wang L. On the near-infrared light-responsive and mechanical properties of PNIPAM-based nanocomposite hydrogels. Polym. Degrad. Stabil. 2018;156:228–233. [Google Scholar]
- 135.Yang G., Wan X., Gu Z., Zeng X., Tang J. Near infrared photothermal-responsive poly(vinyl alcohol)/black phosphorus composite hydrogels with excellent on-demand drug release capacity. J. Mater. Chem. B. 2018;6(11):1622–1632. doi: 10.1039/c7tb03090h. [DOI] [PubMed] [Google Scholar]
- 136.Li M., Guan Y., Chen Z., Gao N., Ren J., Dong K., Qu X. Platinum-coordinated graphitic carbon nitride nanosheet used for targeted inhibition of amyloid beta-peptide aggregation. Nano Res. 2016;9(8):2411–2423. [Google Scholar]
- 137.Bao Q., Zhang D., Qi P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011;360(2):463–470. doi: 10.1016/j.jcis.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 138.Chattopadhyay A.P., Mandal P., Sarkar R., Samadder A., Khuda-Bukhsh A.R., Yadav P., Sarkar K. Synthesis and characterization of graphene like carbon nanosheet: interaction with some drug molecules and anticancer activity. Chemistryselect. 2017;2(12):3516–3526. [Google Scholar]
- 139.Li X., Gong Y., Zhou X., Jin H., Yan H., Wang S., Liu J. Facile synthesis of soybean phospholipid-encapsulated MoS2 nanosheets for efficient in vitro and in vivo photothermal regression of breast tumor. Int. J. Nanomed. 2016;11:1819–1833. doi: 10.2147/IJN.S104198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu X., Zhang H., Huang H., Zhang Y., Hou L., Zhang Z. Functionalized graphene oxide-based thermosensitive hydrogel for magnetic hyperthermia therapy on tumors. Nanotechnology. 2015;26(36) doi: 10.1088/0957-4484/26/36/365103. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Q., Deng H., Li H., Song K., Zeng C., Rong L. Preparation of graphene oxide-based supramolecular hybrid nanohydrogel through host-guest interaction and its application in drug delivery. J. Biomed. Nanotechnol. 2018;14(12):2056–2065. doi: 10.1166/jbn.2018.2648. [DOI] [PubMed] [Google Scholar]
- 142.Hashemi M., Omidi M., Muralidharan B., Tayebi L., Herpin M.J., Mohagheghi M.A., Mohammadi J., Smyth H.D.C., Milner T.E. Layer-by-layer assembly of graphene oxide on thermosensitive liposomes for photo-chemotherapy. Acta Biomater. 2018;65:376–392. doi: 10.1016/j.actbio.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 143.Sahu A., Kim M., Ryu J., Son J.-G., Lee E., Noh D.Y., Tae G. Nanographene oxide as a switch for CW/pulsed NIR laser triggered drug release from liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;82:19–24. doi: 10.1016/j.msec.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 144.Tajvar S., Mohammadi S., Askari A., Janfaza S., Nikkhah M., Tamjid E., Hosseinkhani S. Preparation of liposomal doxorubicin-graphene nanosheet and evaluation of its in vitro anti-cancer effects. J. Liposome Res. 2018:1–28. doi: 10.1080/08982104.2018.1524481. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y., Liu J. Hybrid nanomaterials of WS2 or MoS2 nanosheets with liposomes: biointerfaces and multiplexed drug delivery. Nanoscale. 2017;9(35):13187–13194. doi: 10.1039/c7nr04199c. [DOI] [PubMed] [Google Scholar]
- 146.Xie M., Yu L., Li Z., Zheng Z., Wang X. Synthesis and character of novel polycarbonate for constructing biodegradable multi-stimuli responsive delivery system. J. Polym. Sci. Polym. Chem. 2016;54(22):3583–3592. [Google Scholar]
- 147.Han U., Seo Y., Hong J. Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 2016;6 doi: 10.1038/srep24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liang X., Ye X., Wang C., Xing C., Miao Q., Xie Z., Chen X., Zhang X., Zhang H., Mei L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Contr. Release. 2019;296:150–161. doi: 10.1016/j.jconrel.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 149.Han Z., Jie L., Chu Z., Guo Z. 2 μm passively Q-switched laser based on black phosphorus. Opt. Mater. Express. 2016;6(7):2374. [Google Scholar]
- 150.Zhao Y., Tong L., Li Z., Yang N., Fu H., Wu L., Cui H., Zhou W., Wang J., Wang H., Chu P.K., Yu X.-F. Stable and multifunctional dye-modified black phosphorus nanosheets for near-infrared imaging-guided photothermal therapy. Chem. Mater. 2017;29(17):7131–7139. [Google Scholar]
- 151.Zhang X., Luo L., Li L., He Y., Cao W., Liu H., Niu K., Gao D. Trimodal synergistic antitumor drug delivery system based on graphene oxide. Nanomed. Nanotechnol. Biol. Med. 2019;15(1):142–152. doi: 10.1016/j.nano.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 152.Fan J., He N., He Q., Liu Y., Ma Y., Fu X., Liu Y., Huang P., Chen X. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale. 2015;7(47):20055–20062. doi: 10.1039/c5nr06630a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie H., Shao J., Ma Y., Wang J., Huang H., Yang N., Wang H., Ruan C., Luo Y., Wang Q.-Q., Chu P.K., Yu X.-F. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials. 2018;164:11–21. doi: 10.1016/j.biomaterials.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 154.Ouyang J., Deng Y., Chen W., Xu Q., Wang L., Liu Z., Tang F., Deng L., Liu Y.-N. Marriage of artificial catalase and black phosphorus nanosheets for photodynamic antitumor therapy. J. Mater. Chem. B. 2018;6(14):2057–2064. doi: 10.1039/c8tb00371h. [DOI] [PubMed] [Google Scholar]
- 155.Shao J., Ruan C., Xie H., Li Z., Wang H., Chu P.K., Yu X.-F. Black-phosphorus-incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer. Adv. Sci. 2018;5(5) doi: 10.1002/advs.201700848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao Z., Fan H., Zhou G., Bai H., Liang H., Wang R., Zhang X., Tan W. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet–aptamer nanoprobe. J. Am. Chem. Soc. 2014;136(32):11220–11223. doi: 10.1021/ja5029364. [DOI] [PubMed] [Google Scholar]
- 157.He T., Xu H., Zhang Y., Yi S., Cui R., Xing S., Wei C., Lin J., Huang P. Glucose oxidase-instructed traceable self-oxygenation/hyperthermia dually enhanced cancer starvation therapy. Theranostics. 2020;10(4):1544–1554. doi: 10.7150/thno.40439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qiu M., Wang D., Liang W., Liu L., Zhang Y., Chen X., Sang D.K., Xing C., Li Z., Dong B., Xing F., Fan D., Bao S., Zhang H., Cao Y. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 2018;115(3):501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang H., Bremner D.H., Tao L., Li H., Hu J., Zhu L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016;135:72–78. doi: 10.1016/j.carbpol.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 160.Xie B., Yi J., Peng J., Zhang X., Lei L., Zhao D., Lei Z., Nie H. Characterization of synergistic anti-tumor effects of doxorubicin and p53 via graphene oxide-polyethyleneimine nanocarriers. J. Mater. Sci. Technol. 2017;33(8):807–814. [Google Scholar]
- 161.Lv Y., Tao L., Bligh S.W.A., Yang H., Pan Q., Zhu L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;59:652–660. doi: 10.1016/j.msec.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 162.Bardajee G.R., Hooshyar Z., Farsi M., Mobini A., Sang G. Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;72:558–565. doi: 10.1016/j.msec.2016.11.109. [DOI] [PubMed] [Google Scholar]
- 163.Wang H., Zhong L., Liu Y., Xu X., Xing C., Wang M., Bai S.-M., Lu C.-H., Yang H.-H. A black phosphorus nanosheet-based siRNA delivery system for synergistic photothermal and gene therapy. Chem. Commun. 2018;54(25):3142–3145. doi: 10.1039/c8cc00931g. [DOI] [PubMed] [Google Scholar]
- 164.Zhao J., Xie P., Ye C., Wu C., Han W., Huang M., Wang S., Chen H. Outside-in synthesis of mesoporous silica/molybdenum disulfide nanoparticles for antitumor application. Chem. Eng. J. 2018;351:157–168. [Google Scholar]
- 165.Shao J., Xie H., Huang H., Li Z., Sun Z., Xu Y., Xiao Q., Yu X.F., Zhao Y., Zhang H., Wang H., Chu P.K. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016;7:12967. doi: 10.1038/ncomms12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tao W., Ji X., Xu X., Ariful I.M., Li Z., Chen S., Saw P.E., Zhang H., Bharwani Z., Guo Z. Antimonene quantum dots: synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew Chem. Int. Ed. Engl. 2017;56(39):11896. doi: 10.1002/anie.201703657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X., Yang C., Zhou J., Huo M. Somatostatin receptor-mediated tumor-targeting nanocarriers based on octreotide-PEG conjugated nanographene oxide for combined chemo and photothermal therapy. Small. 2016;12(26):3578–3590. doi: 10.1002/smll.201600618. [DOI] [PubMed] [Google Scholar]
- 168.Yao H.J., Sun L., Liu Y., Jiang S., Pu Y., Li J., Zhang Y. Monodistearoylphosphatidylethanolamine-hyaluronic acid functionalization of single-walled carbon nanotubes for targeting intracellular drug delivery to overcome multidrug resistance of cancer cells. Carbon. 2016;96:362–376. [Google Scholar]
- 169.Huang C., Wu J., Jiang W., Liu R., Li Z., Luan Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;89:15–24. doi: 10.1016/j.msec.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 170.Yu J., Lin Y.-H., Yang L., Huang C.-C., Chen L., Wang W.-C., Chen G.-W., Yan J., Sawettanun S., Lin C.-H. Improved anticancer photothermal therapy using the bystander effect enhanced by antiarrhythmic peptide conjugated dopamine-modified reduced graphene oxide nanocomposite. Adv. Healthc. Mater. 2017;6(2) doi: 10.1002/adhm.201600804. [DOI] [PubMed] [Google Scholar]
- 171.Malekmohammadi S., Hadadzadeh H., Farrokhpour H., Amirghofran Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: a new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter. 2018;14(12):2400–2410. doi: 10.1039/c7sm02248d. [DOI] [PubMed] [Google Scholar]
- 172.Xing C., Chen S., Qiu M., Liang X., Liu Q., Zou Q., Li Z., Xie Z., Wang D., Dong B., Liu L., Fan D., Zhang H. Conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthc. Mater. 2018;7(7) doi: 10.1002/adhm.201701510. [DOI] [PubMed] [Google Scholar]
- 173.Yang F., Feng Y., Fan X., Zhang M., Wang C., Zhao W., Zhao C. Biocompatible graphene-based nanoagent with NIR and magnetism dual-responses for effective bacterial killing and removal. Colloids Surf. B Biointerfaces. 2019;173:266–275. doi: 10.1016/j.colsurfb.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 174.Zeng G., Huang L., Huang Q., Liu M., Xu D., Huang H., Yang Z., Deng F., Zhang X., Wei Y. Rapid synthesis of MoS2-PDA-Ag nanocomposites as heterogeneous catalysts and antimicrobial agents via microwave irradiation. Appl. Surf. Sci. 2018;459:588–595. [Google Scholar]
- 175.Shahmoradi S., Golzar H., Hashemi M., Mansouri V., Omidi M., Yazdian F., Yadegari A., Tayebi L. Optimizing the nanostructure of graphene oxide/silver/arginine for effective wound healing. Nanotechnology. 2018;29(47) doi: 10.1088/1361-6528/aadedc. [DOI] [PubMed] [Google Scholar]
- 176.Athinarayanan J., Periasamy V.S., Krishnamoorthy R., Alshatwi A.A. Evaluation of antibacterial and cytotoxic properties of green synthesized Cu2O/Graphene nanosheets. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:242–253. doi: 10.1016/j.msec.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 177.Zhang L., Wang Y., Wang J., Wang Y., Chen A., Wang C., Mo W., Li Y., Yuan Q., Zhang Y. A photon-responsive antibacterial nanoplatform for synergistic photothermal-/pharmaco- therapy of skin infection. ACS Appl. Mater. Interfaces. 2018 doi: 10.1021/acsami.8b18146. [DOI] [PubMed] [Google Scholar]
- 178.Suh M., Lee D.S. Brain theranostics and radiotheranostics: exosomes and graphenes in vivo as novel brain theranostics. Nucl. Med. Mol. Imag. 2018;52(6):407–419. doi: 10.1007/s13139-018-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Y., Xu L.-P., Dai W., Dong H., Wen Y., Zhang X. Graphene quantum dots for the inhibition of beta amyloid aggregation. Nanoscale. 2015;7(45):19060–19065. doi: 10.1039/c5nr06282a. [DOI] [PubMed] [Google Scholar]
- 180.Chen Y., Chen Z., Sun Y., Lei J., Wei G. Mechanistic insights into the inhibition and size effects of graphene oxide nanosheets on the aggregation of an amyloid-beta peptide fragment. Nanoscale. 2018;10(19):8989–8997. doi: 10.1039/c8nr01041b. [DOI] [PubMed] [Google Scholar]
- 181.Kim D., Yoo J.M., Hwang H., Lee J., Lee S.H., Yun S.P., Park M.J., Lee M., Choi S., Kwon S.H., Lee S., Kwon S.-H., Kim S., Park Y.J., Kinoshita M., Lee Y.-H., Shin S., Paik S.R., Lee S.J., Lee S., Hong B.H., Ko H.S. Graphene quantum dots prevent alpha-synucleinopathy in Parkinson's disease. Nat. Nanotechnol. 2018;13(9):812–+. doi: 10.1038/s41565-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baek S., Oh J., Song J., Choi H., Yoo J., Park G.-Y., Han J., Chang Y., Park H., Kim H., Cho S.-G., Kim B.-S., Kim J. Generation of integration-free induced neurons using graphene oxide-polyethylenimine. Small. 2017;13(5) doi: 10.1002/smll.201601993. [DOI] [PubMed] [Google Scholar]
- 183.Saravanan S., Vimalraj S., Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018;107:908–917. doi: 10.1016/j.biopha.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 184.Zhao M., Dai Y., Li X., Li Y., Zhang Y., Wu H., Wen Z., Dai C. Evaluation of long-term biocompatibility and osteogenic differentiation of graphene nanosheet doped calcium phosphate-chitosan AZ91D composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;90:365–378. doi: 10.1016/j.msec.2018.04.082. [DOI] [PubMed] [Google Scholar]
- 185.Zhao B., Wu F., Bai Y.-y., Fang M., Wang L. Preparation and biological properties of a graphene oxide/silk fibroin barrier membrane loaded with simvastatin. N. Carbon Mater. 2018;33(5):460–468. [Google Scholar]
- 186.Zhang X., Nie J., Yang X., Liu Z., Guo W., Qiu J., Wang S., Yu X., Guan Y., Liu H., Li L. Nanostructured molybdenum disulfide biointerface for adhesion and osteogenic differentiation of mesenchymal stem cells. Appl. Mater. Today. 2018;10:164–172. [Google Scholar]
- 187.Li S., Lin D., Hu X., Yang X. Directly probing the dissociation effects of graphene oxide nanosheets on hIAPP fibrils. Nanotechnology. 2018;29(49) doi: 10.1088/1361-6528/aae143. [DOI] [PubMed] [Google Scholar]
- 188.Nedumpully-Govindan P., Gurzov E.N., Chen P., Pilkington E.H., Stanley W.J., Litwak S.A., Davis T.P., Ke P.C., Ding F. Graphene oxide inhibits hIAPP amyloid fibrillation and toxicity in insulin-producing NIT-1 cells. Phys. Chem. Chem. Phys. 2016;18(1):94–100. doi: 10.1039/c5cp05924k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu T., Wang C., Zhu Z., Jiang W., Huo M., Li F. Polyethyleneimine functionalized graphene oxide against hIAPP amyloid aggregation. Chem. J. Chin. Univ. Chin. 2018;39(6):1274–1280. [Google Scholar]
- 190.Zhou X., Cao C., Chen Q., Yu Q., Liu Y., Yin T., Liu J. PEG modified graphene oxide loaded with EALYLV peptides for inhibiting the aggregation of hIAPP associated with type-2 diabetes. J. Mater. Chem. B. 2015;3(35):7055–7067. doi: 10.1039/c5tb00487j. [DOI] [PubMed] [Google Scholar]
- 191.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L., Choi H.J., Chung T.D., Lu N., Hyeon T., Choi S.H., Kim D.-H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016;11(6):566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 192.Tao W., Kong N., Ji X., Zhang Y., Sharma A., Ouyang J., Qi B., Wang J., Xie N., Kang C., Zhang H., Farokhzad O.C., Kim J.S. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019;48(11):2891–2912. doi: 10.1039/c8cs00823j. [DOI] [PubMed] [Google Scholar]
- 193.Tang Z., Kong N., Ouyang J., Feng C., Kim N.Y., Ji X., Wang C., Farokhzad O.C., Zhang H., Tao W. Phosphorus science-oriented design and synthesis of multifunctional nanomaterials for biomedical applications. Matter. 2020;2(2):297–322. [Google Scholar]
- 194.Kong N., Ji X., Wang J., Sun X., Chen G., Fan T., Liang W., Zhang H., Xie A., Farokhzad O.C., Tao W. ROS-mediated selective killing effect of black phosphorus: mechanistic understanding and its guidance for safe biomedical applications. Nano Lett. 2020;20(5):3943–3955. doi: 10.1021/acs.nanolett.0c01098. [DOI] [PubMed] [Google Scholar]
- 195.Kong N., Ding L., Zeng X., Wang J., Li W., Shi S., Gan S.T., Zhu X., Tao W., Ji X. Comprehensive insights into intracellular fate of WS2 nanosheets for enhanced photothermal therapeutic outcomes via exocytosis inhibition. Nanophotonics. 2019;8(12):2331–2346. [Google Scholar]
- 196.Tao W., Wang J., Parak W.J., Farokhzad O.C., Shi J. Nanobuffering of pH-responsive polymers: a known but sometimes overlooked phenomenon and its biological applications. ACS Nano. 2019;13(5):4876–4882. doi: 10.1021/acsnano.9b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhu X., Ji X., Kong N., Chen Y., Mahmoudi M., Xu X., Ding L., Tao W., Cai T., Li Y., Gan T., Barrett A., Bharwani Z., Chen H., Farokhzad O.C. Intracellular mechanistic understanding of 2D MoS2 nanosheets for anti-exocytosis-enhanced synergistic cancer therapy. ACS Nano. 2018;12(3):2922–2938. doi: 10.1021/acsnano.8b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ji X., Kang Y., Ouyang J., Chen Y., Artzi D., Zeng X., Xiao Y., Feng C., Qi B., Kim N.Y., Saw P.E., Kong N., Farokhzad O.C., Tao W. Synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy. Adv. Sci. 2019;6(19):1901211. doi: 10.1002/advs.201901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ouyang J., Feng C., Ji X., Li L., Gutti H.K., Kim N.Y., Artzi D., Xie A., Kong N., Liu Y.-N., Tearney G.J., Sui X., Tao W., Farokhzad O.C. 2D monoelemental germanene quantum dots: synthesis as robust photothermal agents for photonic cancer nanomedicine. Angew Chem. Int. Ed. Engl. 2019;58(38):13405–13410. doi: 10.1002/anie.201908377. [DOI] [PubMed] [Google Scholar]
- 200.Gao N., Nie J., Wang H., Xing C., Mei L., Xiong W., Zeng X., Peng Z. A versatile platform based on black phosphorus nanosheets with enhanced stability for cancer synergistic therapy. J. Biomed. Nanotechnol. 2018;14(11):1883–1897. doi: 10.1166/jbn.2018.2632. [DOI] [PubMed] [Google Scholar]
- 201.Nasrollahi F., Koh Y.R., Chen P., Varshosaz J., Khodadadi A.A., Lim S. Targeting graphene quantum dots to epidermal growth factor receptor for delivery of cisplatin and cellular imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;94:247–257. doi: 10.1016/j.msec.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 202.Ji P., Zhang W., Ai S., Zhang Y., Liu J., Liu J., He P., Li Y. Hybridization of graphene oxide into nanogels to acquire higher photothermal effects for therapeutic delivery. Nanotechnology. 2018 doi: 10.1088/1361-6528/aaf8e4. [DOI] [PubMed] [Google Scholar]
- 203.Shim G., Kim M.-G., Park J.Y., Oh Y.-K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016;105:205–227. doi: 10.1016/j.addr.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Q., Wu Z., Li N., Pu Y., Wang B., Zhang T., Tao J. Advanced review of graphene-based nanomaterials in drug delivery systems: synthesis, modification, toxicity and application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;77:1363–1375. doi: 10.1016/j.msec.2017.03.196. [DOI] [PubMed] [Google Scholar]
- 205.Tsai S.-M., Bangalore P., Chen E.Y., Lu D., Chiu M.-H., Suh A., Gehring M., Cangco J.P., Garcia S.G., Chin W.-C. Graphene-induced apoptosis in lung epithelial cells through EGFR. J. Nanoparticle Res. 2017;19(7) [Google Scholar]
- 206.Yang H., Bremner D.H., Tao L., Li H., Hu J., Zhu L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016;135:72–78. doi: 10.1016/j.carbpol.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 207.Zhang X., Yin J., Peng C., Hu W., Zhu Z., Li W., Fan C., Huang Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon. 2011;49(3):986–995. [Google Scholar]