Abstract
Context
Craniopharyngiomas (CPH) are benign tumors, rarely encountered in children, representing 5-6% of all intracranial tumors.
Objective
This study aimed to analyze the surgical management and quality of life in a series of CPH pediatric cases.
Design
This was a multicenter study performed over a 25-year period (1994 - 2019) in Bucharest.
Subjects and Methods
152 children (0-17 years old) were treated for CPH. Preoperative manifestations were intracranial hypertension, endocrine dysfunction, visual impairment, ataxia, intellectual performance decrease.
Results
Considering all surgical approaches used, we advocate for pterional approach to best fit in CPH. We achieved gross-total removal (GTR) in 83 cases (54.4%), near-total resection (NTR) in 13 cases (9%), partial resection (PTR) in 51 cases (33.3%). 5 cases were biopsies (3.2%). Gamma Knife Surgery was performed in 10 cases (6.5%), all recurrences. At 6 months GOS revealed: Good Recovery 70 cases (46.2%), Moderate Disability 62 cases (40.7%), Severe Disability 13 (8.5%), Vegetative State 2 cases (1.3%), Deceased 5 cases (3.2%). Complications were: diabetes insipidus (89.3%); hypopituitarism (66.4%); hypothalamic damage (17.7%); visual deterioration (18.4%).
Conclusions
Surgery remains the main option, but GTR complications prove the necessity for a multidisciplinary approach. Outcome predicting factors are: age, tumor size, hydrocephalus degree, hypothalamic dysfunction.
Keywords: Craniopharyngiomas, MRI, Children, CPH Scale, Gamma Knife Surgery, Glasgow Outcome Scale, Quality of life
Introduction
Craniopharyngiomas are the most common non-glial tumours in children. In contrast to their benign character, craniopharyngiomas show to be clinically very aggressive exhibiting a strong adhesive behaviour on the vital structures that usually surround them, such as the hypothalamus, infundibular stalk, basal vessels and important nerves (1). In terms of appearance and epidemiology, they indicate a bimodal arising behaviour with 2 peaks: one in children between 5-14 years and the other one in adults between 65-74 years and an overall epidemiology of 0.13-2 to 100 000 persons per year (2). Among the primary brain tumours, craniopharyngiomas are encountered in 2-5% patients and their frequency in terms of the intracranial tumours in children is 5.6-15% (3). Clinical manifestations depend on the proximity with the surrounding structures varying as authors encountered in their study endocrinological dysfunction, diabetes insipidus to visual disturbances and others (Fig 1A and B).
Figure 1.
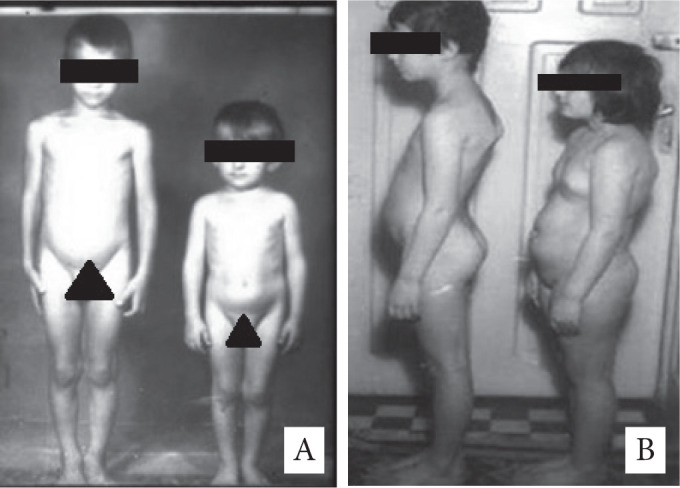
A – Two boys at the same age (11 years old) – the left one is endocrinologically normal and the right one is hypostatural, hypoponderal with no gonadal development. B – Two girls at the same age (10 years old) – the left one is endocrinologically normal and the right one presents early puberty and obesity.
There is a controversy in the literature regarding the treatment of choice for this type of tumours, considering the tumour unpredictable behaviour and our increasing awareness about the risks that some treatments may involve (4, 5). The currently available options are gross-total resection GTR, partial surgery PTR with or without radiotherapy, PTR with Gamma-Knife Surgery (GKS), cysts decompression with insertion of an Ommaya reservoir, or other adjuvant therapies (endocrine substitution). Nowadays, microsurgical techniques and advancements in MRI investigation improve the quality of surgical approach making GTR more accessible. GTR has some limitations especially when important neurovascular structures are involved. Thus, even with high survival rates, QoL is an important aspect that should be taken in consideration (6). Regarding the conservative treatment, adjuvant therapy seems to provide good results, worth mentioning being the reduction of pituitary hormone deficits without significant tumour recurrence (7). Thus, some authors may choose PTR to improve the neurological symptoms, afterwards opting for radiotherapy to decrease the tumour volume. However, close proximity with the optic nerve may lead to visual deterioration, so radiotherapy benefits should be weighted carefully. In this study authors analysed the general aspects of craniopharyngiomas management in terms of diagnosis, treatment, QoL and global outcome.
Materials and Methods
Our multicentre study included 152 of consecutive operated paediatric cases, treated at “Bagdasar – Arseni” Teaching Hospital, Bucharest and at “Sanador Medical Center” Hospital, Bucharest on a period of 25 years between 1995 – 2019. Following the approval of the hospitals’ boards, data were collected from medical reports, data history, pre-/postoperative Imagistics and later follow-up CTs and MRIs. Significant data such as clinical manifestation, histological type of the tumour, level of resection, surgical approach, postoperative outcome and complications were accumulated in this retrospective study. Full endocrinological examination, hormonal measures and indices were assessed separately from their centre and authors did not include these methods of investigation here. Postoperatively, majority of patients were recommended to the “C.I. Parhon” National Institute of Endocrinology. The goal in our series of cases was to remove the tumour as much as it was permitted (Table 1).
Table 1.
Demographic features and clinical manifestations
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| male | 80 (52.2) |
| female | 72 (47.7) |
| Age at surgery (years) | |
| mean | 10.7 |
| 0-3 | 5 (3.2) |
| 3-6 | 34 (22.3) |
| 7-10 | 66 (43.4) |
| 11-16 | 47 (30.9) |
| Clinical symptoms | |
| Headache | 114 (75) |
| Vomiting | 78 (51.3) |
| Raised ICP syndrome | 88 (57.8) |
| Endocrine dysfunction | 118 (77.6) |
| Visual impairment | 104 (68.4) |
| Gait disturbances | 17 (11.1) |
| Psychological disturbances | 22 (14.4) |
| Other signs: pallor, aphasia | 14 (9.2) |
ICP - Intracranial Pressure.
Thus, authors adapted each case to a suitable surgical approach from one of the 5 wide-used interventions in such situations: pterional, bilateral subfrontal, unilateral frontal, midline interhemispheric and transcallosal. Authors advocate for a pterional since it permits a good access to almost every tumour. Dissections of the prechiasmatic, retrochiasmatic space and opticocarotid triangle were extended as much as possible, but with a careful approach since the tumour could be found in tangent with the hypothalamus (8). Lesion of the optic chiasm followed by visual impairment and of the hypothalamus followed by obesity are well-documented complications in such procedures (9). A translaminar approach was used to remove parts of the tumour from the third ventricle. Same procedure is used for tumours arising from tuber cinereum, that develop entirely into the ventricle, however these cases are rare (8, 10). More complicated cases are those which develop in the 3rd ventricle but also spread into the interpeduncular and chiasmatic cisterns. In this case, Konovalov (2014), recommended a combined approach of transcallosal with a subfrontal or pterional route (11).
Results
According to Table 1, authors reported no significant difference in terms of males: females’ ratio, with 80 males and 72 females with the highest frequency of age between 7-10 years (43.4%), followed by 11-16 years’ interval (30.9%) and 3-6 years’ interval (22.3%), with mean age 10.7 years. Regarding the clinical features, the most frequent and specific characteristics were endocrine dysfunctions (118 cases – 77.6%) and visual impairment (104 cases – 68.4%), followed by headaches (114 cases – 75%), vomiting (78 cases – 51.3%), raised ICP (Intracranial Pressure) syndrome (88 cases – 57.8%), gait disturbances (17 – 11.1%), psychological disturbances (22 cases – 14.4%), and other signs – pallor, aphasia, etc. (14 cases – 9.2%).
Case presentation
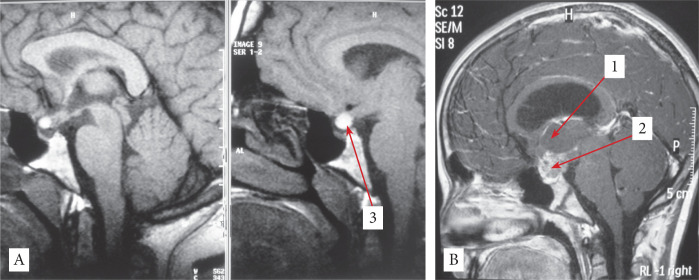
Seventeen years old girl diagnosed with giant CPH presenting with endocrine dysfunction (obesity), raised ICP, visual impairment. The surgical approach used was unilateral subfrontal and GTR was obtained. After surgery no recurrences at 8 years’ follow-up and good recovery (Fig 2A-B).
Figure 2.
A - Sagittal View Postoperative MRI; B - Sagittal View Preoperative MRI; 1- thalamus; 2 - tumor; 3-pituitary stalk at the postoperative control.
Craniopharyngiomas usually are found in the sella and suprasellar area, around the pituitary gland and hypothalamo-hypophyseal axis. Regarding the location of the tumour, M. Samii et al. in 1997 proposed the most renowned classification of them, taking the optic chiasm as the reference point, he approximately noted that one-third are retrochiasmatic, one-third are subchiasmatic, 20% are prechiasmatic, and 10-15% are intrasellar (12). Authors already introduced a personal CPH grading scale and a personal classification according to which CPH are divided into 5 categories: intrasellar, suprasellar and retrochiasmatic – the most frequent, suprasellar retrochiasmatic with anterior extension, suprasellar retrochiasmatic with posterior extension and suprasellar retrochiasmatic with 3rd ventricle extension (13). In our study authors reported the suprasellar retrochiasmatic area (97 cases – 63.8%) to be the most frequent location for this type of tumours (Table 2).
Table 2.
Histopathological aspects of the tumour
| Histological Type | Cases (%) |
|---|---|
| Adamantinomatous CPH | 138 (90.7) |
| Papillary CPH | 14 (9.2) |
| Cystic | 81 (53) |
| Solid | 21 (14) |
| Mixed | 40 (33) |
| Total | 152 |
According to Table 2, regarding in terms of pathological aspects of the tumour, authors accounted about 138 cases (90.7%) of craniopharyngiomas in our study to be the adamantinomatous type and 14 cases (9.3%) of the papillary type, which is in accordance with the frequency reported in the literature (14). The impact that this difference between these types of tumour has on the prognostic and future clinical complications is still debatable. Generally, adamantinomatous type is more aggressive and adhesive to the 3rd ventricle, infundibular stalk, hypothalamus and the important vessels while papillary type seems to be less aggressive and better demarcated (15,16). Also, Adamantinomatous CPH type is often composed of a cystic structure (authors reported 53% of the tumours as cystic) which may contain an oil-like fluid which, in case of rupture or during surgery, could lead to chemical meningitis (13). On the other hand, papillary craniopharyngiomas, which represent 9.2% of total in our study, appear exclusively in adults and are better demarcated from the other structures (17).
Our surgical approaches were: bilateral subfrontal (46 cases – 30.2%), pterional (39 – 25.6% cases), unilateral frontal (29 cases – 19%), midline interhemispheric (24 cases), transcallosal (5 cases – 3.2%) and combined approaches (9 cases – 5.9%). Authors obtained gross-total resection (GTR) in 83 cases (55%), near-total resection (NTR) in 13 cases (9%), partially total resection (PTR) in 51 cases (33%) and biopsy (B) of cystic tumour in 5 cases (3%) (Table 3).
Table 3.
Surgical approaches and shunt placement in children < 16 years
| Type of Surgery | Cases (%) |
|---|---|
| Unilateral lateral frontal | 29 (19) |
| Bilateral subfrontal | 46 (30.2) |
| Midline interhemispheric | 24 (15.7) |
| Pterional | 39 (25.6) |
| Transcallosal | 5 (3.2) |
| Combined approaches | 9 (5.9) |
| Hydrocephalus | 26 (17.1) |
| VP shunt | 16 (10.5) |
| Extension of Surgery | |
| Gross-Total Resection | 83 (55%) |
| Near-Total Resection | 13 (9%) |
| Partially Total Resection | 51 (33%) |
| Biopsy | 5 (3%) |
Case presentation
A twelve years old girl diagnosed with giant CPH presenting with endocrine dysfunction, raised ICP, visual impairment. The surgical approach used was bilateral subfrontal and GTR was obtained. After surgery no recurrences at 5 years’ follow-up and good recovery (Fig 3A-B).
Figure 3.
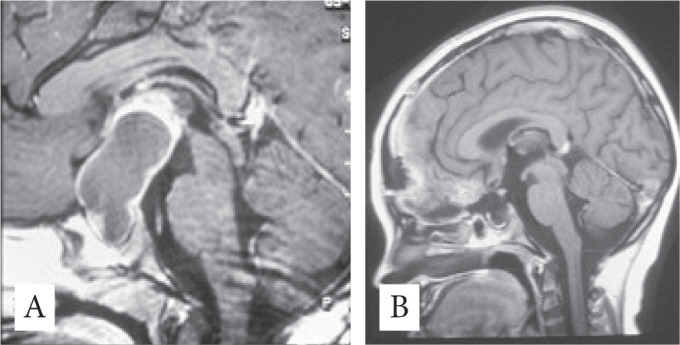
A - Sagittal View Preoperative MRI; B - Sagittal View Postoperative MRI.
In accordance to other literature data, authors recommend GTR including the capsule as the golden standard for the treatment of this pathology (13). Authors defined PTR in patients with > 5mm residual tumour. Patients who underwent a partial resection were usually in an advanced stage of tumour growth, with tumour invading fine elements, or intense level of calcification, thus authors opted for preservation of the neurovascular structures. Biopsies were performed in diagnosis or in similar cases as mentioned above. According to the National Centre for Excellence in Neurosurgery Bucharest strategy, authors recommended endocrine therapy to the patients, but authors do not advocate for local radiation or chemotherapy with intracystic bleomycin. Our policy for patient management includes follow-up at every 6 months with neurological, ophthalmological and endocrinological examination. From the 152 patients that were operated and examined at our centres, authors reported 7 cases of recurrences (9.7% of those who were gross-total removed) at 6 months post operatory. Authors also reported 25 cases of regrowth (36.6% of those who are NTR, PTR, or biopsied). Among the 32 cases of recurrent craniopharyngioma, 10 were treated by Gamma-Knife Surgery (GKS). Considering the side-effects of radiotherapy especially in paediatric cases, authors did not use radiotherapy in this series. In addition, 26 patients (17.1%) have been presented with hydrocephalus that was treated with VP shunt in 16 cases and 10 cases by surgical decompression. Global Outcome (GOS) at 6 months’ follow-up after the surgery: 70 patients (46%) reported good recovery (GR). Authors reported 62 patients (41%) with moderate disability (MD), 13 patients (9%) with severe disability (SD), 2 patients (1%) in vegetative state (VS) and 5 deaths (3%) (D) (Table 4).
Table 4.
General clinical status at 6 months after surgery (GOS)
| Outcome | No. of patients (%) |
|---|---|
| Recovery | |
| Good recovery | 70 (46) |
| Moderate disability | 62 (41) |
| Severe disability | 13 (9) |
| Vegetative state | 2 (1) |
| Death | 5 (3) |
| Recurrence GTR | 7/83 (9.7) |
| Regrowth NTR/PTR/B | 25/69 (36.6) |
| GKS after recurrence | 10 (6.5) |
At 6 months postoperatory: authors reported 135 cases (88.8%) of transient DI status, from which DI permanent 88 cases (57%). Authors also reported anosmia in 70 cases (46%), visual disturbance in 28 cases (18.4%), hypothalamic dysfunction 27 cases (17.7%), 3rd nerve palsy 14 cases (19.2%), seizures and other complications (Table 5). Further, hormonal evaluation was performed at “C.I. Parhon” National Institute of Endocrinology.
Table 5.
Complications of surgical treatment
| Complication | No. of patients (%) |
|---|---|
| DI transient | 135 (88.8%) |
| DI permanent | 88 (57%) |
| Anosmia | 70 (46%) |
| Visual disturbance | 28 (18.4%) |
| Hypopituitarism | 101 (66.4%) |
| Hypothalamic Dysfunction | 27 (17.7%) |
| 3rd Nerve palsy | 14 (9.2%) |
| ACA injuries | 3 (1.9%) |
| CSF leak | 2 (1.3%) |
| Seizures | 13 (8.5%) |
| Subdural effusion | 10 (6.5%) |
Discussion
Craniopharyngioma treatment was for long-time discussed. Survival rate of this tumour type has been improved with 7% in recent years, with an overall survival rate at 5 years of 93% (18). With this low-mortalities and high-survival rates, the focus on tumour management has been changed, QoL being now one of the most important aspects to be considered. Personally, authors reported some important prognostic factors affecting QoL after CPH treatment: age (< 5 years), size of the tumour and degree of the intracranial compartments involved, hydrocephalus status and preoperative signs of the hypothalamic dysfunction (13). Surgery leads to modifications of hormonal status, however it is still the best approach to an effective treatment.
Studies have reported that craniopharyngioma treatment is very often associated with visual disturbances, permanent DI, hypopituitarism (1,19). As mentioned in Table 5 regarding postoperative complications, our data is consistent with the literature (Hypothalamic dysfunction 27 – 17%, hypopituitarism 101 – 66.4%, visual disturbances 28 - 18.4%). Moreover, when compared with adults, children seem to be more affected after surgery in terms of hormonal dysfunction (20). Hypothalamus dysfunction with obesity postoperative complication is an important issue and a predictor factor for the outcome of the primary surgery (21). In case of hypothalamic disturbance authors advocate for conservative treatment. Choosing between GTR, PTR with radiosurgery or just radiosurgery is one of the important topics in the literature. From a personal experience authors advocate for GTR, or PTR coupled with GKS, trying to avoid as much as possible the side effects of radiotherapy in children.
The ability to obtain GTR varies a lot in the recent literature between 40-87% (21, 22). Authors reported 83 cases (54%) of GTR with no remnant tumour on postoperative scan. Most authors optate for GTR because of its low-recurrence rate between 18.2% and 37% and avoidance of the radiation in children due to side-effects (19, 23). In our 83 patients treated with GTR authors reported 7 cases (9.7%) recurrences. Following GTR, PTR with adjuvant therapy report a higher or similar recurrence rate and PTR alone with the highest rate (22). Besides the extent of the surgery, the recurrence rate depends also on the type of the tumour and size tumours > 3 cm being more predisposed to recur.
From a personal experience, authors could report that reasons for incomplete removal were: severe bradycardia, adherences to the hypothalamus, adherences to the major vessels, thin capsule and major calcifications (13).
Partial tumor removal surgery followed by radiosurgery may lead to good outcomes (24). The moment of time for radiotherapy after primary surgery is still discussed. In adults, radiosurgery should follow immediately the PTR, in order to prevent recurrences, however, in children the potential harmful effects of radiations on the endocrine system and brain myelination postpone the usage of radiotherapy till is strictly necessary in case of a possible recurrence (25).
From a personal experience, authors would rather recommend GKS than radiotherapy. GKS has been associated with good long-term outcomes, patient prognostic depending on the tumour size and the effective dose administered (26). Comparing to the radiotherapy the advantage of GKS is the precision of irradiation and less normal tissue radiated. Tumour growth control rate varies from 80-94% and is associated with few complications (27). Another important topic on craniopharyngiomas is regarding the transcortical (TC) and transsphenoidal approaches (TS). As mentioned above, craniopharyngiomas are tumours with high-recurrence rate, thus regrowth should be expected in a remnant tumour. Some authors have experienced low mortality even in recurrent open surgeries while others reported higher-mortality or lower-survival rate when operation was performed in a recurrent case (8, 28-30). It should be mentioned that the small number of cases operated so far with TS was due to the high-risk of LCR fistula and consequently other complications. Despite these risks, TS approach seems to be a good choice since it involves less brain retraction, direct access to the tumour lesion, less hypothalamus lesion and lower morbidity rate (31). Advantages of TS were also noted during tumour resection when used both in prior TC or TS patients (32). Even though, so far TS was used to treat only small tumours arising from sella, currently it was shown that also suprasellar tumour or tumours with hypothalamic involvement are suitable (7,33).
The new endoscopic CPH classification emphasizes the origin of the tumour independently of the recognition pituitary stalk and helps determine the extent of hypothalamus injury prior to surgery. CPH were classified as central or peripheral, the latter one being further classified as hypothalamic stalk CPH, suprasellar stalk CPH or intrasellar stalk CPH (34). Regarding the extent of the surgery, TS shows a higher rate of complete removal as a primary treatment and also in the recurrent cases (85% and 53%) when compared to TC approach (45% and 21%) (35). Recurrence rate for TS is also low - 9% (33).
In addition to those mentioned above, cyst decompression via insertion of a drain connected to an Ommaya reservoir or adjuvant therapy with intracavitary administration of bleomycin were used in the past as treatments (36-38). However, due to bleomycin toxicity and side-effects, clinics nowadays have shifted their therapy to interferon-α administration, which is a non-neurotoxic agent (7). Interferon-α, intracavity, treatment shows a good-control rate, no mortality and low morbidity (39). Finally, authors advocate for an interdisciplinary perspective, including medical, behavioural and physical support, when considering CPH management (40).
In conclusion, craniopharyngioma are benign tumors with high-recurrence rate. Surgical treatment outcome depends on the type of the tumor, tumor invasion, extent of the tumor, patient age, preclinical manifestations. This pathology has been associated with many complications, thus a multidisciplinary perspective should be considered. Authors in this study advocates for a maximum safe removal when possible considering the low-recurrence rate, however quality of life may be the one which should be a priority in choosing the treatment.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Caldarelli M, Massimi L, Tamburrini G, Cappa M, Di Rocco C. Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome. Child’s Nervous System. 2005;21(8-9):747–757. doi: 10.1007/s00381-005-1186-5. [DOI] [PubMed] [Google Scholar]
- 2.Garnett MR, Puget S, Grill J, Sainte-Rose C. Craniopharyngioma. Orphanet J Rare Dis. 2007;2(1):1–7. doi: 10.1186/1750-1172-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karavitaki N, Cudlip S, Adams CBT, Wass JAH. Craniopharyngiomas. Endocr. Rev. 2006;27(4):371–397. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 4.Bidur K, Prasad D. Outcome following surgical resection of craniopharyngiomas: A case series. Asian J. Neurosurg. 2017;12(3):514–518. doi: 10.4103/1793-5482.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graffeo CS, Perry A, Link MJ, Daniels DJ. Pediatric Craniopharyngiomas: A Primer for the Skull Base Surgeon. J. Neurol. Surgery, Part B Skull Base. 2018;79(1):65–80. doi: 10.1055/s-0037-1621738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Pohl F, Sörensen N, Calaminus G. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: Results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur. J. Endocrinol. 2011;165(1):17–24. doi: 10.1530/EJE-11-0158. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M, Bartels U, Branson H, Kulkarni AV, Hamilton J. Trends in treatment and outcomes of pediatric craniopharyngioma, (1975-2011) Neuro. Oncol. 2013;15(6):767–774. doi: 10.1093/neuonc/not026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. Journal of Neurosurgery. 1990;73(1):3–11. doi: 10.3171/jns.1990.73.1.0003. [DOI] [PubMed] [Google Scholar]
- 9.Karavitaki N, Brufani C, Warner JT, Adams CBT, Richards P, Ansorge O, Shine B, Turner HE, Wass JAH. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. (Oxf). 2005;62(4):397–409. doi: 10.1111/j.1365-2265.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 10.Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: Trans-lamina terminalis approach. Neurosurgery. 2000;47(4):857–865. doi: 10.1097/00006123-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Konovalov AN. Third Ventricle Craniopharyngiomas. World Neurosurgery. 2014;82(6):1023–1025. doi: 10.1016/j.wneu.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Samii M., Tatagiba M. Surgical Management of Craniopharyngiomas: A Review. Neurologia Medico-Chirurgica. 1997;37(2):141–149. doi: 10.2176/nmc.37.141. [DOI] [PubMed] [Google Scholar]
- 13.Ciurea AV, Mircea D, Coman Teodora, Vionescu D, Tascu A, Lisievici M. Our policy in craniopharyngiomas – comparative studies in children and adults – a personal craniopharyngiomas grading scale, In: Brain Tumor Surgery ; Management Strategies and Navigator / Neuroendoscope Osaka, Medica Co. Noboru Sakai. 2003;1:145–157. [Google Scholar]
- 14.Yachnis T. Craniopharyngioma: Embryology, Pathology, and Molecular Aspects. In: Kenning TJ, Evans JJ, editors. Craniopharyngiomas: A Comprehensive Guide to Diagnosis, Treatment and Outcome. Elsevier Science; 2015. pp. 95–105. [Google Scholar]
- 15.Stache C, Hölsken A, Fahlbusch R, Flitsch J, Schlaffer S-M, Buchfelder M, Buslei R. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro. Oncol. 2014;16(2):256–264. doi: 10.1093/neuonc/not195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamson T. E., Wiestler O. D., Kleihues P., Yasargil G. M. Correlation of clinical and pathological features in surgically treated craniopharyngiomas. Journal of Neurosurgery. 1990;73(1):12–17. doi: 10.3171/jns.1990.73.1.0012. [DOI] [PubMed] [Google Scholar]
- 17.Hölsken A, Sill M, Merkle J, Schweizer L, Buchfelder M, Flitsch J, Fahlbusch R, Metzler M, Kool M, Pfister SM, von Deimling A, Capper D, Jones DTW, Buslei R. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol. Commun. 2016;4(1):20. doi: 10.1186/s40478-016-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbok P. Craniopharyngioma in Children: Long-term outcomes. Neurol. Med. Chir. (Tokyo). 2015;55(9):722–726. doi: 10.2176/nmc.ra.2015-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, Lustig RH, Kun LE. Craniopharyngioma: The St. Jude Children’s Research Hospital experience 1984-2001. Int. J. Radiat. Oncol. Biol. Phys. 2002;53(3):533–542. doi: 10.1016/s0360-3016(02)02799-2. [DOI] [PubMed] [Google Scholar]
- 20.Capatina C, Vintila M, Gherlan I, Dumitrascu A, Caragheorgheopol A, Procopiuc C, Ciubotaru V, Poiana C. Craniopharyngioma - Clinical and Therapeutic Outcome Data in A Mixed Cohort of Adult and Paediatric Cases. Acta Endocrinologica-Bucharest. 2018;14(4):549–555. doi: 10.4183/aeb.2018.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott RE, Hsieh K, Hochman T, Belitskaya-Levy I, Wisoff J, Wisoff JH. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children: Clinical article. J. Neurosurg. Pediatr. 2010;5(1):30–48. doi: 10.3171/2009.7.PEDS09215. [DOI] [PubMed] [Google Scholar]
- 22.Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, Gupta N. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst. 2013;29(2):231–238. doi: 10.1007/s00381-012-1926-2. [DOI] [PubMed] [Google Scholar]
- 23.Duff JM, Meyer FB, Ilstrup DM, Laws ER, Schleck CD, Scheithauer BW. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000;46(2):291–305. doi: 10.1097/00006123-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CI, Wang KC, Cho BK. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas - A study in single institute. Child’s Nerv. Syst. 2005;21(8-9):799–807. doi: 10.1007/s00381-005-1189-2. [DOI] [PubMed] [Google Scholar]
- 25.Varlotto J, DiMaio C, Grassberger C, Tangel M, Mackley H, Pavelic M, Specht C, Sogge S, Nguyen D, Glantz M, Saw C, Upadhyay U, Moser R, Yunus S, Rava P, Fitzgerald T, Glanzman J, Sheehan J. Multi-modality management of craniopharyngioma: a review of various treatments and their outcomes. Neuro-Oncology Practice. 2015;3(3):173–187. doi: 10.1093/nop/npv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi T, Tsugawa T, Hatano M, Hashizume C, Mori Y, Shibamoto Y. Gamma knife radiosurgery of craniopharyngioma: results of 30 cases treated at Nagoya Radiosurgery Center. Nagoya J Med Sci. 2015;77(3):447–454. [PMC free article] [PubMed] [Google Scholar]
- 27.Yomo S, Hayashi M, Chernov M, Tamura N, Izawa M, Okada Y, Hori T, Iseki H. Stereotactic Radiosurgery of Residual or Recurrent Craniopharyngioma: New Treatment Concept Using Leksell Gamma Knife Model C with Automatic Positioning System. Stereotactic and Functional Neurosurgery. 2009;87(6):360–367. doi: 10.1159/000236370. [DOI] [PubMed] [Google Scholar]
- 28.Minamida Y, Mikami T, Hashi K, Houkin K. Surgical management of the recurrence and regrowth of craniopharyngiomas. Journal of Neurosurgery. 2005;103(2):224–232. doi: 10.3171/jns.2005.103.2.0224. [DOI] [PubMed] [Google Scholar]
- 29.Vinchon M, Dhellemmes P. Craniopharyngiomas in children: Recurrence, reoperation and outcome. Child’s Nerv. Syst. 2008;24(2):211–217. doi: 10.1007/s00381-007-0456-9. [DOI] [PubMed] [Google Scholar]
- 30.Matson DD, Crigler JF. Management of craniopharyngioma in childhood. J. Neurosurg. 1969;30(4):377–390. doi: 10.3171/jns.1969.30.4.0377. [DOI] [PubMed] [Google Scholar]
- 31.Elliott RE, Jane JA, Wisoff JH. Surgical management of craniopharyngiomas in children: Meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69(3):630–643. doi: 10.1227/NEU.0b013e31821a872d. [DOI] [PubMed] [Google Scholar]
- 32.Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, Carrau RL, Kassam AB, Cappabianca P. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. Journal of Neurosurgery. 2009;111(3):578–589. doi: 10.3171/2009.2.JNS081026. [DOI] [PubMed] [Google Scholar]
- 33.Alalade AF, Ogando-Rivas E, Boatey J, Souweidane MM, Anand VK, Greenfield JP, Schwartz TH. Suprasellar and recurrent pediatric craniopharyngiomas: expanding indications for the extended endoscopic transsphenoidal approach. Journal of Neurosurgery: Pediatrics PED. 2018;21(1):72–80. doi: 10.3171/2017.7.PEDS17295. [DOI] [PubMed] [Google Scholar]
- 34.Tang B, Xie SH, Xiao LM, Huang G L, Wang ZG, Yang L, Yang XY, Xu S, Chen YY, Ji YQ, Zeng EM, Hong T. A novel endoscopic classification for craniopharyngioma based on its origin. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-28282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J. Neurosurg. 1999;90(2):237–250. doi: 10.3171/jns.1999.90.2.0237. [DOI] [PubMed] [Google Scholar]
- 36.Mottolese C, Stan H, Hermier M. P., Berlier Convert J, Frappaz D, Lapras C. Intracystic chemotherapy with bleomycin in the treatment of craniopharyngiomas. Child’s Nerv. Syst. 2001;17(12):724–730. doi: 10.1007/s00381-001-0524-5. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi H, Yamaguchi F, Teramoto A. Long-term outcome and reconsideration of intracystic chemotherapy with bleomycin for craniopharyngioma in children. Child’s Nerv. Syst. 2005;21(8-9):701–704. doi: 10.1007/s00381-005-1208-3. [DOI] [PubMed] [Google Scholar]
- 38.Moussa AH, Kerasha AA, Mahmoud ME. Surprising outcome of ommaya reservoir in treating cystic craniopharyngioma: A retrospective study. Br. J. Neurosurg. 2013;27(3):370–373. doi: 10.3109/02688697.2012.741732. [DOI] [PubMed] [Google Scholar]
- 39.Cavalheiro S, Di Rocco C, Valenzuela S, Dastoli PA, Tamburrini G, Massimi L, Nicacio JM, Faquini IV, Ierardi DF, Silva NS, Pettorini BL, Toledo SRC. Craniopharyngiomas: intratumoral chemotherapy with interferon-α: a multicenter preliminary study with 60 cases. Neurosurgical Focus. 2010;28(4):E12. doi: 10.3171/2010.1.FOCUS09310. [DOI] [PubMed] [Google Scholar]
- 40.Rakhshani N, Jeffery AS, Schulte F, Barrera M, Atenafu EG, Hamilton JK. Evaluation of a comprehensive care clinic model for children with brain tumor and risk for hypothalamic obesity. Obesity. 2010;18(9):1768–1774. doi: 10.1038/oby.2009.491. [DOI] [PubMed] [Google Scholar]