Abstract
Objective
Metabolic syndrome (MetS) is a metabolic condition with high prevalence worldwide. This study aims to examine the relationship between serum concentrations of gastrointestinal hormones such as cholecystokinin (CCK), ghrelin, peptide YY (PYY), and high sensitive C-reactive protein (hs-CRP) and the ingredients of MetS in obese population.
Subjects and Methods
This case-control study included 40 obese subjects (20 with MetS and 20 BMI and age-matched control individuals). The age range of the participants was 20-50 years and the participants’ anthropometric characteristics were measured. Serum lipids and the concentrations of oxidized low density lipoprotein (Ox-LDL), insulin, hs-CRP, CCK, PYY, and ghrelin were assessed with commercial ELISA kits.
Results
Serum levels of hs-CRP, total cholesterol (TC) and triglycerides (TG) in patients with MetS were significantly higher while CCK and insulin concentrations were higher in obese non- MetS group (P <0.05). PYY had a negative association with waist circumference (WC) and high density lipoprotein cholesterol (HDL-C) and ghrelin had a positive association with systolic blood pressure (SBP) and TC in obese control group (P < 0.05). In obese patients with MetS, hs-CRP had a strong positive association with TG.
Conclusion
The current study revealed the possible role of hs-CRP and several GI- hormones in the pathogenesis of obesity-associated diseases and MetS. Additional works are needed to elucidate the possible underlying mechanisms and clarify several controversies in this issue.
Keywords: Metabolic Syndrome, CCK, PYY, hs-CRP, Ghrelin
Introduction
Metabolic syndrome (MetS) is a complex disease characterized by raised blood pressure, central obesity, dyslipidemia and hyperglycemia and it is related to increased risk of cardiovascular mortality, stroke, diabetes mellitus type 2, and even death (1). The prevalence of MetS is increasing with body mass index (BMI) and age (2). In a cross-sectional study by Ervin RB et al. overweight females and males were 5-6 times and obese females and males were 17-32 times more likely to have metabolic syndrome (2). In Iran also a high prevalence of MetS has been identified; in a relatively large sample study, the unadjusted prevalence of MetS in the study participants was 30.1% and age-standardized prevalence was 33.7%. The prevalence of MetS increased with age in both genders and was more prevalent in female than in male (3-5). Several prior studies showed that MetS is associated with a 3 to 4.3 fold increase in mortality from cardiovascular disease (CVD) (6) and subjects with MetS were 3.5 to 5 times more likely to develop type 2 diabetes (7-9). Within recent years, measuring serum high sensitivity C-reactive protein (hs-CRP) concentrations as an indicator of chronic inflammation was considered as a potent prognostic marker for diabetes and CVD. Moreover, the level of hs-CRP is related to the MetS and its components. Early identification of the MetS is desirable as lifestyle interventions and adequate treatment of its risk factors can prevent CVD and improve quality of life (10-12). Numerous studies have revealed that hs-CRP is increased in obese patients concurrently to the number of available existing components of the MetS (13). Engelsen and colleagues reported that hs-CRP could be a prognostic marker to detect MetS in patients with central obesity (11).
There are limited studies evaluating the role of gastrointestinal hormones in metabolic syndrome or obesity; cholecystokinin (CCK) is the most extensively studied gastrointestinal satiety hormone and is secreted from endocrine cells (I-cells) within the proximal intestinal tract, the jejunum and duodenum and also is largely distributed through the central nervous system. CCK is secreted primarily by the ingestion of fatty acids and protein in chime (14). In normal lean individuals, its secretion reaches at its peak concentrations 15 minutes after meal ingestion and its concentrations are higher in women than in men because of the effects of estrogen (15). CCK sends its satiety signals into the brain through CCK receptor 1 and chronic CCK administration reduces meal size and might increase meal frequency and is a strong stimulator of ghrelin secretion through mucosal cells of gastric fundus (16-18).
Peptide YY (PYY), another satiety GI hormone, exists in two forms of PYY3-36 and PYY1-36 (17). The circulating PYY3-36 as the predominant form is a major endocrine mediator of satiation and reduces body weight and its deficiency is a potent inducer of obesity (19, 20). Its effects on inhibition of food intake mostly occur through its high affinity to presynaptic inhibitory neurons (17).
Ghrelin, a peptide of 28 amino acid polypeptide, was first identified by Kojima et al. in rat and human stomachs as an endogenous ligand of growth hormone secretagogue receptor (21). In addition to the growth hormone secretion, ghrelin has been reported to be implicated in other processes such as food intake, insulin release, gastric acid secretion and body weight gain (22). In contrast to previously mentioned satiation GI peptides (PYY and CCK), ghrelin increases GI motility and decreases insulin secretion. Ghrelin concentration reduces in response to high doses of PYY3-36 in the pre-meal period (23). There are limited studies reporting the possible role of GI hormones in the MetS. In the study by Zwirska-Korczala (17), in comparison of basal concentrations of CCK, PYY, ghrelin and gastrin in a relatively low sample size of healthy lean (n=8), obese (n=12) and morbid obese (n=18) patients with MetS, reported higher CCK and PYY concentrations in lean controls compared with obese patients with MetS while ghrelin concentrations were higher in morbid obese patients with MetS than in obese patients with MetS (P <0.05). No association of these hormones with MetS ingredients was reported. In several other studies the possible role of different gene variants of PYY or ghrelin as potent predictor of MetS and its components in different ethnic populations had also been reported (24, 25). The above introduction elucidates the possible role of GI hormones in association with MetS and its ingredients.
However, according to our review of literature, no study was available to assess the possible relationship of these GI hormones with MetS components in obese subjects. Therefore, the present study was aimed to assess the possible relationship between GI hormones and hs-CRP with serum lipids, insulin, blood pressure, appetite and central obesity in obese individuals with and without MetS.
Materials and methods
Subjects and sample collection
The present case-control study was carried out between August and October of 2016, in Tabriz, Iran. The MetS was diagnosed according to guidelines from the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III) (26). MetS can be diagnosed when patients have ≥ 3 of 5 following criteria: increased level of triglycerides (TG) (≥ 150 mg/dL), waist circumference (WC) ≥ 88 cm in women and ≥ 102 cm in men, low serum high density lipoprotein cholesterol (HDL-C) (≤ 40 mg/dL for men and≤ 50 mg/dL for women), diastolic blood pressure (DBP ≥85 mmHg) or systolic blood pressure (SBP) ≥130 mmHg and fasting blood glucose (FBG) ≥ 100 mg/dL.
Therefore, groups included 20 obese patients with MetS and 20 obese subjects without MetS age and BMI- matched apparently healthy control subjects. Inclusion criteria were having BMI ≥ 30 kg/m2 and being aged between 20-50 years. We excluded the patients with the history of kidney or cardiovascular complications, cancer, atherosclerosis, recent surgery and those treated by anti-depressive, diuretics, glucocorticoids, anti-hypertensive, hypoglycemic and/or hypolipidemic drugs in the past three months. Other exclusion criteria were diet for losing weight, being pregnant or lactating and menopause in the previous 3 months. Participants were informed about the protocol and gave their written consent before initiation of the study. The project was approved by the ethical committee of Tabriz University of Medical Science. The study was conducted in accordance with the Declaration of Helsinki.
Figure 1.
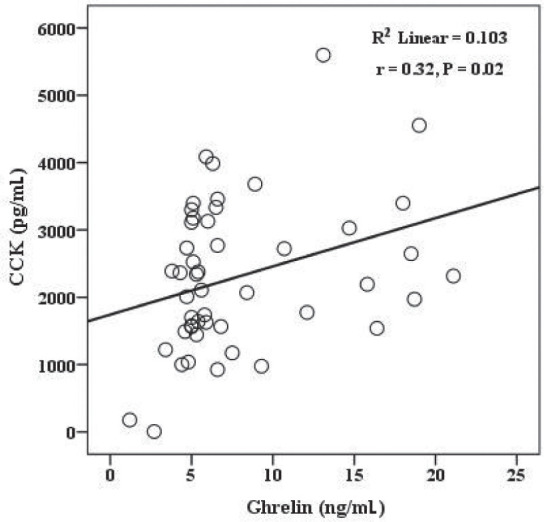
The association between serum ghrelin and CCK concentrations in obese individuals (r=0.32, P = 0.02).
Assessments of anthropometric, blood pressure and appetite
Anthropometric variables such as BMI, WC, height and weight were measured by trained interviewers.
Weight was assessed by using a digital scale with 0.1-kg precision and the height was assessed by a stadiometer with a precision of 0.5 cm. WC was assessed in the standing position at the level midway between the anterior iliac crest and the lower border of the rib. BMI was assessed as weight (kg) /height (m2). Blood pressure was recorded by a standard mercury sphygmomanometer twice after 10 minutes of rest. The mean of the two readings was assigned as DBP and SBP measurement.
In this study, appetite profile was assessed by anchored 100-mm visual analogue scales (VAS) (27). Participants were asked to respond to 10 questions relating to hunger, appetite, fullness, thirst, satiety, desire to eat and prospective food intake by VAS for each question.
Biochemical assessments
After 12-14 hours fasting, venous blood sample was collected from each individual. The plasma and serum samples were separated and stored at −70°C till further use. Serum PYY, CCK and ghrelin levels were measured by commercial active ELISA kits (Hangzhou East Biopharm Co, LTD, USA). The inter-assay and intra-assay coefficients of variation (CV) for PYY, CCK and ghrelin were <12 % and <10 % respectively. Serum CRP concentrations were determined by commercial ELISA kits (Monobind Inc, Lake Forest, CA, USA) with the inter-assay and intra-assay CV of <10 %. Serum insulin was assessed by Diametra assay ELISA kit with the inter-assay and intra-assay CV of ≤10 % and ≤ 5 %, respectively. Serum Ox-LDL was assessed by Bioassay Technology Laboratory ELISA kit with the inter-assay and intra-assay CV of < 10% and < 8%, respectively. Concentrations of serum lipids (TC, TG and HDL-C) were assessed by enzymatic methods and serum LDL-C levels were estimated by Friedewald formula (28). All of the biochemical analyses were carried out blind by a trained lab assistant.
Statistical analysis
All statistical analysis was carried out using Statistical Package for Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). Categorical and continuous variables were presented as frequency (%) and mean ± standard deviation, respectively. Also, chi-square test and independent sample t-test were used for comparison of categorical and continuous variables, respectively. Partial correlation analysis with adjustment for the confounders of BMI and age were performed for identifying the correlation between CCK, PYY, Ghrelin and hs-CRP and components of MetS. Sample size calculation was carried out based on 80% power and a type I error of 5% as explained before (29).
RESULTS
The general demographic characteristics of the 40 subjects are reported in Table 1. Baseline values of WC and DBP were significantly higher in patients with MetS compared with control group (P < 0.05). However, no significant difference was seen between the groups regarding appetite, BMI age, gender distribution, and SBP. The comparison between biochemical variables including serum lipids, CCK, PYY, hs-CRP, ghrelin and insulin concentrations are shown in Table 2. As can be observed in this table, TG, TC and hs-CRP concentrations in patients with MetS were significantly higher while CCK and insulin concentrations were higher in obese control group (P <0.05). In evaluating the relations between CCK, PYY, ghrelin and hs-CRP concentrations in study groups (Table 3), PYY was in negative association with HDL-C and WC and ghrelin had a positive association with SBP and TC in obese control group (P < 0.05). In obese patients with MetS, hs-CRP had a strong positive association with TG (r = 0.59, P = 0.04).
Table 1.
The baseline characteristics of the study subjects
| Variables | The study groups | P-value | |
|---|---|---|---|
| Obese control subjects (n=20) | Obese subjects with MetS (n=20) | ||
| Age (Year) | 35.46± 8.01 | 32.15± 9.97 | 0.339* |
| Female gender | 15 (75) | 17 (85) | 0.211** |
| Appetite | 190.00± 34.01 | 204.23± 46.43 | 0.360* |
| WC (cm) | 95.13± 7.01 | 102.07± 8.55 | 0.026* |
| BMI (kg/m2) | 32.60± 2.49 | 33.70± 3.51 | 0.343* |
| SBP (mmHg) | 122.00± 6.76 | 126.10± 9.60 | 0.193* |
| DBP (mmHg) | 79.33± 4.57 | 83.84± 5.06 | 0.020* |
*P-value was reported based on Independent Sample T test. ** P-value was reported based on Chi-square test. BMI: Body Mass Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MetS: Metabolic Syndrome. Data are presented as mean± SD.
Table 2.
The comparison of biochemical parameters among study groups
| Variables | The study groups | P-value* | |
|---|---|---|---|
| Obese Control subjects (n=20) | Obese Subjects with MetS (n=20) | ||
| TG (mg/dL) | 128.46 ± 57.67 | 186.08 ± 73.65 | 0.028 |
| HDL (mg/dL) | 34.00 ± 11.26 | 31.61± 8.62 | 0.540 |
| LDL (mg/dL) | 115.82± 24.65 | 126.33± 27.28 | 0.134 |
| Total cholesterol (mg/dL) | 177.13± 31.81 | 195.69± 31.40 | 0.02 |
| CCK (pg/mL) | 11.96±9.40 | 5.77±2.42 | 0.017 |
| PYY (pg/mL) | 267.29 ±84.08 | 270.32 ± 90.18 | 0.92 |
| Ghrelin (ng/mL) | 8.82 ±5.74 | 8.34 ± 5.35 | 0.78 |
| OX-LDL (ng/L) | 2776.69 ± 593.36 | 1963.98± 632.15 | 0.36 |
| Insulin (μIU/L) | 11.96 ± 9.40 | 5.77± 2.42 | 0.017 |
| hs-CRP (μg/mL) | 2.92 ± 0.56 | 4.89 ± 1.69 | 0.02 |
*P-value was reported based on Independent Sample T test. TG: triglyceride; HDL: High Density Lipoprotein; WC: Waist Circumference; LDL: Low density Lipoprotein.
Table 3.
The correlation between CCK, PYY, Ghrelin and hs-CRP with appetite and components of metabolic syndrome in study groups
| Study groups | Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Obese control group | CCK | PYY | Ghrelin | hs-CRP | |||||
| Variables | r | P | r | P | r | P | r | P | |
| Appetite | 0.032 | 0.84 | -0.012 | 0.94 | -0.05 | 0.73 | -0.02 | 0.88 | |
| SBP | 0.12 | 0.09 | 0.23 | 0.65 | 0.45 | 0.02 | 0.34 | 0.97 | |
| DBP | 0.23 | 0.90 | 0.14 | 0.48 | 0.11 | 0.07 | 0.01 | 0.98 | |
| TC | 0.26 | 0.10 | -0.14 | 0.39 | 0.28 | 0.049 | -0.12 | 0.49 | |
| TG | 0.12 | 0.44 | 0.21 | 0.19 | -0.18 | 0.28 | 0.08 | 0.62 | |
| HDL | 0.09 | 0.54 | -0.34 | 0.039 | 0.13 | 0.42 | -0.08 | 0.64 | |
| WC | 0.06 | 0.71 | -0.27 | 0.05 | 0.06 | 0.71 | -0.07 | 0.66 | |
| LDL | 0.05 | 0.72 | -0.08 | 0.62 | 0.02 | 0.90 | -0.19 | 0.27 | |
| OX-LDL | -0.04 | 0.98 | -0.28 | 0.22 | 0.17 | 0.48 | -0.37 | 0.11 | |
| Obese MetS group | Appetite | -0.03 | 0.92 | -0.24 | 0.45 | -0.16 | 0.61 | 0.09 | 0.77 |
| SBP | 0.56 | 0.87 | 0.45 | 0.34 | 0.23 | 0.54 | 0.12 | 0.09 | |
| DBP | 0.98 | 0.65 | 0.09 | 0.09 | 0.65 | 0.65 | 0.34 | 0.34 | |
| TC | 0.26 | 0.43 | 0.012 | 0.97 | 0.01 | 0.96 | 0.10 | 0.75 | |
| TG | 0.35 | 0.27 | 0.015 | 0.97 | -0.23 | 0.44 | 0.59 | 0.04 | |
| HDL | -0.33 | 0.32 | 0.07 | 0.81 | -0.16 | 0.6 | -0.3 | 0.34 | |
| WC | -0.29 | 0.38 | -0.37 | 0.29 | -0.32 | 0.28 | 0.29 | 0.35 | |
| LDL | 0.26 | 0.43 | 0.04 | 0.89 | 0.21 | 0.47 | -0.09 | 0.78 | |
| OX-LDL | -0.27 | 0.51 | -0.47 | 0.2 | 0.4 | 0.28 | -0.42 | 0.25 | |
Data analysis was done by partial correlation adjusted for age and BMI. MetS: Metabolic Syndrome; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; TG: triglyceride; HDL: High Density Lipoprotein; WC: Waist Circumference; LDL: Low density Lipoprotein.
DISCUSSION
In the present work, we found higher hs-CRP concentrations in patients with MetS and its positive association with serum TG in these patients. In obese apparently healthy individuals, CCK and insulin concentrations were higher compared with other group; moreover, in this group, PYY was in negative association with HDL and WC and ghrelin in a positive association with SBP and TC.
Similar to our findings, in a population based study by Engelsen et al. (11), hs-CRP concentrations in centrally obese patients with MetS were higher compared with patients with central obesity but without MetS. Moreover, increased components of metabolic syndrome were in parallel of increased hs-CRP concentrations in these patients. Accordingly the authors suggested that hs-CRP levels can be used as a diagnostic indicator of MetS. High hs-CRP concentration in patients with MetS is in accordance with higher TG, LDL, TC and lower HDL concentrations as also confirmed in our study.
This positive association arises from several key mechanisms including a) the role of hs-CRP in impairing insulin signaling through suppressing insulin-induced nitric oxide (NO) production, inhibiting the phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS), and stimulating the phosphorylation of Insulin receptor substrate 1 (IRS-1) in a dose-dependent manner (30-32); b) the role of hs-CRP in endothelial dysfunction and suppressing endothelial reactivity and reducing NO synthesis from endothelial cells (33) and c) the pro-inflammatory role of CRP in monocyte–macrophages include induction of tissue factor, pro-inflammatory cytokines, reactive oxygen species (ROS), matrix metalloproteinases release and oxidized LDL-C uptake (34).
The higher CCK and insulin levels in obese subjects without MetS compared with obese patients with Mets were another result of the present study. In an animal model by Chun-Min Lo (35), CCK-deficient mice developed lower insulin secretion and higher insulin sensitivity. In their study, CCK was introduced as a gut peptide involved in glucose intolerance, insulin resistance and suggested the chronic effects of CCK on β-cell adaptation to diet. Moreover, obesity is a CCK resistant state that makes them less sensitive to the satiety effect of CCK than lean individuals (36).
In the present study, serum PYY was in negative relationship with central obesity and HDL-C concentrations in obese individuals. Although PYY is an appetite suppressing hormone and several studies revealed that its deficiency is associated with greater BMI, however, the data in this issue is controversial. Similar to our findings, Cahill et al. (37) found that fasting PYY was positively related to adiposity measures only among women. The female dominant population of the current study also makes similar findings which can be explained by gender-specific difference in the PYY concentrations and its association with the components of MetS. The positive relationship between ghrelin and TC or SBP in the current study is in agreement with the previous findings revealing the positive association of ghrelin with MetS and its positive association with the components of MetS (24, 38, 39) further confirming the possible role of this GI hormone in metabolic syndrome, obesity and T2DM.
In conclusion, the current study revealed the possible role of hs-CRP and several GI- hormones in the pathogenesis of obesity-related metabolic disorders and MetS. There are several controversial findings in the previous literatures about the possible role of GI hormones in the pathogenesis of metabolic disorders. Elucidating the possible underlying mechanisms and confirming the results of our findings warrants further researches.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
We thank all of the project participants. The study protocol has been approved by the ethics committee of the Tabriz University of Medical Sciences.
References
- 1.Mirinazhad MM, Farhangi MA, Jahangiri L, Yaghoubi A. Serum adiponectin concentrations in relation to lipid profile, anthropometric variables and insulin resistance in patients with metabolic syndrome. Mal J Nutr. 2014;20(3):283–289. [Google Scholar]
- 2.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. National health statistics reports. 2009;13:1–8. [PubMed] [Google Scholar]
- 3.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diab Res Clin Pract. 2003;61(1):29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 4.Farhangi MA, Jahangiry L, Mirinazhad MM, Shojaeezade D, Montazeri A, Yaghoubi A. A web-based interactive lifestyle modification program improves lipid profile and serum adiponectin concentrations in patients with metabolic syndrome: the “Red Ruby” study. Int J Diab Develop Ctrs. 2017;37(1):21–30. [Google Scholar]
- 5.Farhangi MA, Jahangiry L, Asghari-Jafarabadi M, Najafi M. Association between dietary patterns and metabolic syndrome in a sample of Tehranian adults. Obes Res Clin Pract. 2016;10(1):S64–S73. doi: 10.1016/j.orcp.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Lakka H-M, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 7.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J Am Coll Cardiol. 2012;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Sokhanvar S, Khoshi A, Hajiaghaei S, Mousavinasab SN, Golmohammadi Z. Association between Apo-lipoprotein-B levels at admission of patients and short-term morbidity and mortality after myocardial infarction. J Cardiovasc Thorac Res. 2012;4(3):61–64. doi: 10.5681/jcvtr.2012.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal A, Aggarwal S, Sharma V. Cardiovascular risk factors in young patients of coronary artery disease: Differences over a decade. J Cardiovasc Thorac Res. 2014;6(3):169–173. doi: 10.15171/jcvtr.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. Vitamin A supplementation, serum lipids, liver enzymes and C-reactive protein concentrations in obese women of reproductive age. Annals Clin Biochem. 2013;50(1):25–30. doi: 10.1258/acb.2012.012096. [DOI] [PubMed] [Google Scholar]
- 11.Engelsen C, Rutten GE, Gorter KJ, van den Donk M, Koekkoek PS, Salomé PL. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: a cross-sectional analysis. Cardiovasc Diabetol. 2012;11(1):25–32. doi: 10.1186/1475-2840-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. Vitamin A supplementation, serum lipids, liver enzymes and C-reactive protein concentrations in obese women of reproductive age. Ann Clin Biochem. 2013;50(Pt 1):25–30. doi: 10.1258/acb.2012.012096. [DOI] [PubMed] [Google Scholar]
- 13.Ford E. The metabolic syndrome and C-reactive protein, fibrinogen, and leucocyte count: finding from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003;168:351–358. doi: 10.1016/s0021-9150(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 14.Strader AD. Gastrointestinal hormones and satiety. Agro FOOD industry hi-tech. 2005;15(5):6–9. [Google Scholar]
- 15.Xia Y, Ran L, Yan D. Regulative effects of ovarian steroids on rat gastric motility and sensitivity. Acta Physiol Sin. 2006;58:275–280. [PubMed] [Google Scholar]
- 16.Marchal-Victorion S, Vionnet N, Escrieut C. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics. 2002;12:23–30. doi: 10.1097/00008571-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and Postprandial Plasma Levels of Pyy, Ghrelin. J Physiol Pharmacol. 2007;58(1):13–35. [PubMed] [Google Scholar]
- 18.Heshmat R, Shafiee G, Qorbani M, Azizi-Soleiman F, Djalalinia S, Esmaeil Motlagh M, Ardalan G, Ahadi Z, Safari O, Safiri S, Kelishadi R. Association of ghrelin with cardiometabolic risk factors in Iranian adolescents: the CASPIAN-III study. J Cardiovasc Thorac Res. 2016;8(3):107–112. doi: 10.15171/jcvtr.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batterham RL, Cohen MA, Ellis SM. Inhibition of food intake in obese subjects by peptide YY3-36. NEJM. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 20.Batterham RL, Cowley MA, Small CJ. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 22.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 23.Cummings DE. Ghrelin and the short- and long-regulation of appetite and body weight. Physiol Behavior. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Mora M, Adam V, Palomera E, Blesa S, Díaz G, Buquet X, Serra-Prat M, Martín-Escudero JC, Palanca A, Chaves JF, Puig-Domingo M Mataró Aging Study Group. Ghrelin gene variants influence on metabolic syndrome components in aged Spanish population. PloS one. 2015;10(9):e0136931. doi: 10.1371/journal.pone.0136931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih PB, Wang L, Chiron S, Wen G, Nievergelt C, Mahata M, Khandrika S, Rao F, Fung MM, Mahata SK, Hamilton BA, O’Connor DT. Peptide YY (PYY) gene polymorphisms in the 3′-untranslated and proximal promoter regions regulate cellular gene expression and PYY secretion and metabolic syndrome traits in vivo. J Clin Endocrinol Metab. 2009;94(11):4557–4566. doi: 10.1210/jc.2009-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy SM, Hansen B, Smith SC. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific Issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 27.Smeets AJ, Westerterp-Plantenga MS. Acute effects on metabolism and appetite profile of one meal difference in the lower range of meal frequency. Br J Nutr. 2008;99(6):1316–1321. doi: 10.1017/S0007114507877646. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Zeinalian R, Farhangi MA, Shariat A, Saghafi-Asl M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial. BMC Complement Altern Med. 2017;17(1):225–233. doi: 10.1186/s12906-017-1670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu JW, Morita I, Ikeda K. C-reactive protein suppresses insulin signaling in endothelial cells: role of spleen tyrosine kinase. Mol Endocrinol. 2007;21:564–573. doi: 10.1210/me.2006-0354. [DOI] [PubMed] [Google Scholar]
- 31.Shafayi FS, Akef M, Sadegi H, Niknazhad AS. A comparison of physical activity and nutritional practices in hypertensive and non- hypertensive pregnant women. J Cardiovasc Thorac Res. 2012;4(2):53–56. doi: 10.5681/jcvtr.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi N, Mahdavi R, Ebrahimi-Mameghani M. Perceived barriers to weight loss programs for overweight or obese women. Health Prom Perspect. 2013;3(1):11–22. doi: 10.5681/hpp.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S, Wang CH, Li SH. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 34.Cermak J, Key NS, Bach RR. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 35.Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D’Alessio D, Woods SC, Tso P. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diab Res Clin Pract. 2011;60(7):2000–2007. doi: 10.2337/db10-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niederau C, Meereis-Schwanke K, Klonowski-Stumpe H, Herberg L. CCK-resistance in Zucker obese versus lean rats. Regulat Peptid. 1997;70(2):97–104. doi: 10.1016/s0167-0115(97)00014-1. [DOI] [PubMed] [Google Scholar]
- 37.Cahill F, Ji Y, Wadden D, Amini P, Randell E, Vasdev S, Gulliver W, Sun G. The association of serum total peptide YY (PYY) with obesity and body fat measures in the CODING study. PLoS One. 2014;9(4):e95235. doi: 10.1371/journal.pone.0095235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulkkinen L, Ukkola O, Kolehmainen M, Uusitupa M. Ghrelin in diabetes and metabolic syndrome. Int J Peptid. 2010;2010(248948):1–11. doi: 10.1155/2010/248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E. Ghrelin and the metabolic syndrome in older adults. J Clin Endocrinol Metab. 2005;90(12):6448–6453. doi: 10.1210/jc.2005-1358. [DOI] [PubMed] [Google Scholar]


