Abstract
Objective
To observe the impact of quercetin and isoquercitrin on gluconeogenesis in hepatocytes.
Methods
Mouse primary hepatocytes were cultured with lactic acid and pyruvic acid. After treatment with quercetin and isoquercitrin for 24 hours, the glucose concentration in the culture supernatant was determined. RT-PCR was used to detect the mRNAs of PEPCK, G6Pase, LKB1, and AMPKα. Protein levels of LKB1, AMPKα, and Thr172 phosphorylation were evaluated by Western blot.
Results
The glucose concentration in the gluconeogenesis group (GN) was significantly higher than in the control group (C), but the glucose concentrations in the high level quercetin(group 80Q) and high level isoquercitrin (group 80I) were significantly lower than in the group GN, P<0.01. In the group 80Q, and group 80I, the mRNA levels of PEPCK and LKB1were significantly lower than in the group GN (P<0.01), and the G6Pase mRNA were significantly lower than in the group GN (P<0.05). The protein levels of LKB1 and the phosphorylation of AMPKα Thr172 in the group 80Q, group 40I, and group 80I were higher than in the group GN. The effects of quercetin and isoquercitrin on LKB1 and AMPKα were similar to those of metformin.
Conclusions
Quercetin and isoquercitrin inhibit gluconeogenesis in hepatocytes, which may be related to the LKB1 upregulation and phosphorylation of AMPKα.
Keywords: quercetin, isoquercitrin, gluconeogenesis, liver kinase B1 (LKB1), AMP-activated protein kinase α (AMPKα)
Introduction
According to the 2013 China Diabetes Epidemiological Survey, 10.9% of Chinese adults have diabetes, and the pre-diabetes prevalence rate is 35.7% (1). Gluconeogenesis refers to the process by which non-sugar precursors, such as lactic acid, pyruvic acid, amino acids, or glycerol are converted to glucose. Excessive gluconeogenesis in the liver leads to an increase in hepatic glucose output, which is an important mechanism of diabetes and early glucose metabolism disorders. Reducing abnormally active hepatic gluconeogenesis is an important target for the prevention and treatment of diabetes. Recent studies have found that Okra can improve blood glucose in diabetic patients and prevent early glucose metabolism disorders (2). Possible mechanisms include reducing intestinal glucose absorption, inhibiting DPP-4, or reducing islet β cell damage (3-5). Our previous studies have found that Okra extract can reduce blood glucose and inhibit hepatic gluconeogenesis in pre-diabetes mice. However, the extract of Okra is complex and rich in flavonoids and polysaccharides, which have been confirmed to be able to regulate glucose metabolism (6). Among them, flavonoids isoquercitrin and quercetin are found to be able to regulate glucose and lipid metabolism disorders in pre-diabetes rats and improve hepatocyte morphology and functional damage (7-9), suggesting that these two monomer components may reduce blood glucose by regulating the hepatic glucose metabolism. Therefore, it is necessary to further confirm whether isoquercitrin and quercetin can regulate blood sugar by affecting hepatic gluconeogenesis, thereby providing more basis for studying the hypoglycemic effect of Okra. This study investigated the impact of quercetin and isoquercitrin on glucose production in the hepatocytes cultured in vitro and explored the effects and mechanisms of quercetin and isoquercitrin on hepatic gluconeogenesis.
MATERIALS AND METHODS
Isolation of primary liver cells from mice
Male C57/BL mice, weighing 18-20 g, were firstly intraperitoneally injected 1% pentobarbital for anesthesia, as well as intramuscular injection of 0.01-0.02 mL heparin sodium for anticoagulation; then, each mouse had the skin disinfected using 75% alcohol, inserted one vein indwelling needle into the hepatic portal vein, and fixed the needle using silk thread. The liver was then isolated after in vivo infusion of 4°C pre-cooled Ca-free Hanks buffer, in vitro infused 0.05% type IV collagenase prepared by 37°C Ca-free Hanks, and separated the liver cells to prepare the liver cell suspension. One 100 μm sieve was then used to filtrate the suspension, followed by 5-min centrifugation at 4°C and 500 rpm. After discarding the supernatant, the cells were washed with PBS, centrifuged again at low temperature, transferred to the hepatocyte culture medium, and seeded in 6-well plates with the density as 5 × 105 ~ 1 × 106 cells/mL. After 24 h, the cells grew wall-adhered and then cultured in sugar-free DMEM medium containing a mixture of 10% FBS and 1% mycillin. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai University of Traditional Chinese Medicine.
Induction and detection of gluconeogenesis in hepatocytes
After the primary hepatocytes grew wall-adhered for 24 h, the cells were cultured in sugar-free DMEM for 24 h. The mixture of 10 mmol/L lactic acid +1 mmol/L pyruvate was used as the gluconeogenesis substrate, and 100 nmol dexamethasone + 100 μmol/L cAMP were added as the gluconeogenesis inducer. According to the concentrations of quercetin, isoquercitrin, and metformin in the culture medium, the grouping was as follows: the blank control group (group C), the gluconeogenesis induction group (group GN), 40 μmol/L quercetin group (group 40Q), 80 μmol/L quercetin group (group 80Q), 40 μmol/L isoquercitrin group (group 40I), 80 μmol/L isoquercitrin group (group 80I), and 500 μmol/L metformin group (group Met). After 24-hr culture, 200 μL of cell culture supernatant was sampled from each group for 10-min centrifugation at 4°C and 2000 rpm. The supernatant was then added to the reaction solution according to the glucose content test kit, water-bathed at 37°C for 20 min, and it was detected the absorbance at 550 nm wavelength (A) for calculating the production of cellular glucose based on the absorbance values of the standards and the measured samples.
RT-PCR
The mouse liver primary cells were firstly seeded in 6-well plates, treated according to the above experimental conditions, cultured for 24 hours, discarded the supernatant, washed twice with PBS, extracted the total RNA by Trizol method, and measured the level of total RNA using one 752 ultraviolet spectrophotometer (Shanghai Third Analytical Instrument Factory, Shanghai, China). The absorbance of A260/A280 within 1.8 to 2.0 was considered to be high purity of RNA extraction. The reverse transcription reaction was carried out according to the reagent instructions with GADPH being used as the internal reference for correction. Primer sequences: phosphoenolpyruvate carboxykinase (PEPCK) upstream 5'-AGCATT CAACGCCAGGTTC-3', downstream 5'-CGAGTCTGTCAGTTCAATACCAA-3'; glucose-6-phosphatedehydrogenase (glucose-6-phosphatase, G6Pase),upstream 5'-TACAGCAACACTTCCGTGCC-3', downstream 5'-CGTAGTATACACCTGCTGTGCC-3'; AMPKα, upstream 5'-TCTGAGGGGCACCAAGAAAC-3', downstream 5-GTGGGTGTTGACGGAGAAGAG-3'; liver kinase B1(LKB1), upstream 5’-TCAAGGCAGCACACCATCATCATC-3’, downstream 5’-GGTCATCGAGCAGCAGTTCATCC-3’; and GADPH, upstream 5'-AGGTCGGTGTGAACGGATTTG-3'. PCR amplification conditions: pre-denaturation at 94°C for 2 min, 94°C for 30 s, 57°C for 30 s, and 72°C for 45 s, 30 cycles, 7-min 72°C for extension. After the amplification, the Ct (amplification cycle) value was read, and 2-ΔΔCT was calculated based on the Ct values of the target gene and the internal reference to indicate the relative expression level of the target gene mRNA.
Western blot
The mouse liver primary cells were firstly seeded in 6-well plates, treated according to the above experimental conditions, cultured for 24 hours, discarded the supernatant, washed twice with PBS, and added 200 μL of RIPA lysate as well as 100 μg of PMSF protease inhibitor / PhosSTOP phosphatase inhibitor per well. The plates were then kept on ice for 5 min and scraped the cells, which were transferred into 1.5 ml centrifuge tubes for 15-min centrifugation at 4°C and 12000 rpm. The supernatant was then taken and tested the protein concentration inside using the Coomassie Brilliant Blue G250 Protein Quantitative Assay. 6% concentrated gel and 12% separating gel were then prepared for later use. The proteins were then wet-transferred onto polyvinylidene fluoride (PVDF) membranes, incubated with 5% skim milk powder in TBST solution for 30 min at 37°C, and then cultured overnight with mouse anti-LKB1 antibody, mouse anti-T-AMPK/Thr172p-AMPKα antibody as well as mouse anti-β-actin antibody (1:6000, Abcam, Cambridge, UK) at 4°C. Finally, alkaline phosphatase-labeled goat anti-rabbit IgG and horse anti-mouse IgG were added (1:2000) for 1-hr incubation at 37°C, followed by 3-time TBST washing, β-naphthol phosphate coloration, and image analysis using one SH-EHE-368 automatic gel imager (Shanghai Ehe Instrument Co., Ltd. Shanghai, China).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. The measurement data were expressed as ±S, the inter-group mean comparison was analyzed by ANOVA, and the LSD test was used for comparison between groups, with P < 0.05 being considered as statistical significance.
RESULTS
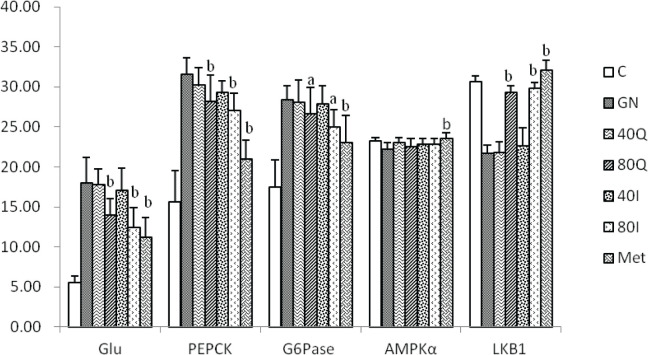
Impact of quercetin and isoquercitrin on glucose production and mRNA expressions of PEPCK, G6Pase, and LKB1.
This study detected the concentration of glucose in the supernatant of culture medium to reflect the gluconeogenesis level in the primary liver cells of mice. After 24-hr culture, the concentration of glucose in group C was 55.17±8.03 μmol/L, and that in group GN was significantly higher than in group C (P<0.01). After quercetin and isoquercitrin intervention, the glucose concentrations in the supernatant of group 80Q, group 80I, and group Met were significantly lower than in group GN (P<0.01). After 24-hr culture, the expressions of PEPCK and G6Pase in group GN were significantly higher than in group C (P<0.01). The expressions of PEPCK in group 80Q and group 80I were significantly lower than in group GN (P<0.01). The expressions of G6Pase in group 80Q and group 80I were significantly lower than in group GN (P<0.05). The expressions of PEPCK and G6Pase in group Met were significantly lower than in group GN (P<0.01). The expressions of LKB1 in group 80Q, group 80I, and group Met were significantly higher than in group GN (P<0.01). There was no significant difference in the mRNA expression of AMPKα among each quercetin and isoquercitrin groups (Table 1).
Table 1.
Comparison of glucose production and expressions of PEPCK and G6Pase (x̄ , n = 8)
| Glucose production (μmol/L) | PEPCK (/GADPH) | G6Pase (/GADPH) | AMPKα (/GADPH) | LKB1 (/GADPH) | |
|---|---|---|---|---|---|
| C | 55.17±8.03 | 15.66±3.91 | 17.44±3.40 | 23.25±0.40 | 30.63±0.73 |
| GN | 179.50±31.85 | 31.53±2.13 | 28.41±1.68 | 22.20±0.86 | 21.71±1.05 |
| 40Q | 177.33±20.07 | 30.27±2.17 | 28.09±2.79 | 23.06±0.59 | 21.75±1.38 |
| 80Q | 139.50±21.29b | 28.21±3.21 b | 26.58±3.31 a | 22.52±0.99 | 29.29±0.82b |
| 40I | 170.75±27.29 | 29.34±1.37 | 27.87±2.24 | 22.85±0.65 | 22.58±2.33 |
| 80I | 124.62±24.33b | 27.07±2.13b | 24.94±2.23a | 22.83±0.75 | 29.81±0.75b |
| Met | 111.50±25.42b | 21.00±2.30b | 23.05±3.40b | 23.58±0.68b | 32.04±1.23b |
Note: Compared with group GN, a, P <0.05; b, P <0.01.
Impact of quercetin and isoquercitrin on LKB1 and phosphorylation of AMPKα and AMPKαThr172
The results of Western blot showed that LKB1, AMPKα protein and pT172-AMPK were significantly down-regulated in group GN. LKB1 levels in group 80Q, group 40I, group 80I, and group Met were significantly higher than in GN. The phosphorylation levels of AMPKαThr172 in group 80Q, group 40I, group 80I, and group Met were significantly higher than in group GN. AMPKα levels were significantly higher in group 80I and group Met (Fig. 1).
Figure 1.
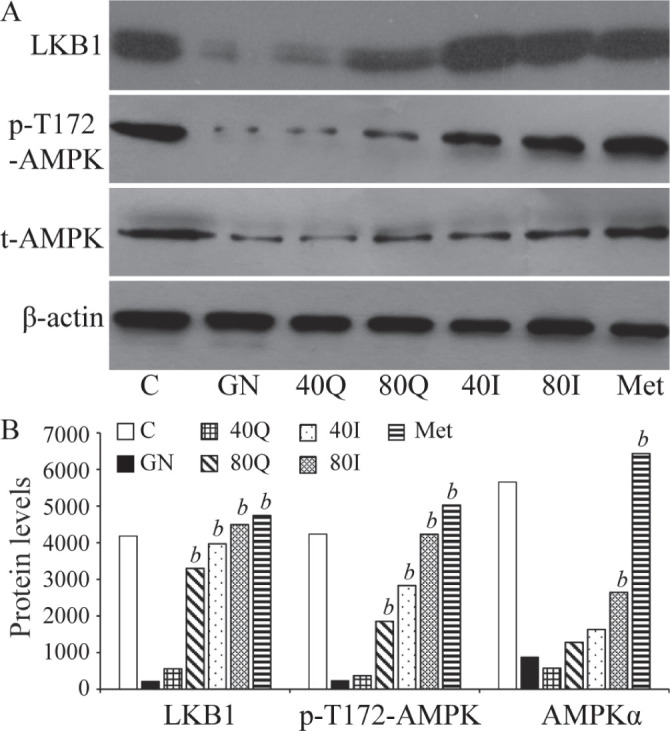
Comparison of AMPKα and pT172-AMPKα levels in each group of hepatocytes.
DISCUSSION
In recent years, the development of medicinal plants with hypoglycemic effects has attracted much attention, and more and more people are working hard to find safe and effective drugs to prevent diabetes and its complications from natural plants. Current research has confirmed that Okra extract can reduce blood glucose (2), consistent with our previous research results. However, since various flavonoids and polysaccharides contained in Okra extract have been confirmed to have hypoglycemic effects (10, 11), we propose that some major components of Okra can be studied in vitro so as to further explore their mechanism of reducing blood glucose. Isoquercitrin and quercetin are two major flavonoids of Okra (12). This study has observed that these two main flavonoids can in vitro inhibit gluconeogenesis in hepatocytes, and the hypoglycemic effect, mechanism, and pathway are similar to MET, which supports our hypothesis. Although the two substrates are different flavonoids extracted from Okra, isoquercitrin is a glycoside derivative of quercetin by the link between 3-OH and glucose and shares the same parent nucleus structure of flavonoids (2-phenyl chromogenic ketone) with quercetin. That structural homology of isoquercitrin and quercetin might be related to the mechanism that the two different extracted substrates showed similar effects on hepatic gluconeogenesis.
We successfully induced the hepatocytic gluconeogenesis model using lactic acid and pyruvic acid. The results showed that both quercetin and isoquercitrin can significantly reduce the glucose production in hepatocytes, accompanied by a significant decrease in the expressions of PEPCK and G6Pase, suggesting that these flavonoids can reduce hepatic gluconeogenesis by inhibiting the transcription of key enzyme genes. At present, it has been found that quercetin and isoquercitrin have hypoglycemic effects, and possible mechanisms include antagonizing oxidative stress and insulin resistance (13, 14), inhibiting intestinal α-glucosidase (15, 16), increasing endogenous glucagon-like peptide-1 (GLP-1) level (17), etc., suggesting that quercetin and isoquercitrin have independent roles in regulating glucose metabolism and improving insulin secretion. Our results are consistent with those of other scholars on the hypoglycemic effects of quercetin and isoquercetin.
We further explored the molecular mechanism of these two flavonoids. The results showed that quercetin and isoquercetin up-regulated LKB1 gene and protein expression in hepatocytes, and increased Thr172 phosphorylated AMPKα significantly, which was similar to the effect of metformin on hepatocytes. The results showed that the mechanism of quercetin and isoquercetin inhibiting hepatic gluconeogenesis was by up-regulation of LKB1 to promote AMPKα Thr172 phosphorylation. There was no significant change in total AMPKα gene level, suggesting that quercetin and isoquercetin were mainly modified at protein level through LKB1 regulation of AMPK.
PEPCK and G6pase are key enzymes of hepatic gluconeogenesis, and the regulation of their transcriptional activity is dependent on upstream AMPK. AMPK is recognized as an “energy susceptor”, is activated by LKB1 through the phosphorylation of α-subunit threonine residue (Thr172) (18), and can prevent the entry of gluconeogenesis regulator, transducer of regulated CREB (TORC2) into the nucleus, so it can inhibit a variety of PEPCK and G6Pase activating factors, such as peroxisome proliferator activated receptor γ coactivator-1α (PGC-1α), forehead transcription factor O1 (FOXO1), or hepatic nuclear factor 4α (HNF4α), thus reducing hepatic gluconeogenesis and lowering the blood glucose concentration (19, 20). Recent studies have found that the hypoglycemic effects of quercetin and isoquercetin are related to the activation of AMPK. Ahn et al. also have found that quercetin can up-regulate the levels of phosphorylated AMPK and its substrate acetyl-CoA carboxylase (ACC) (21). Eid et al. have found that quercetin could up-regulate glycogen synthase in hepatocytes, inhibit G6Pase and improve glucose metabolism in skeletal muscle cells and hepatocytes cultured in vitro (22). Zhou et al. have found that isoquercitrin can enhance the phosphorylation of AMPK (23). We observed that quercetin and isoquercitrin can inhibit hepatocytic gluconeogenesis in vitro, which is related to the upregulation of LKB1 and AMPK phosphorylation, and is consistent with other scholars.
The deacetylation of LKB1 and the formation of complex are important to activate AMPKα. Although the overall level of LKB1 has increased, more studies are needed to investigate the role of acetylation/deacetylation of LKB1 in the regulation of hepatic gluconeogenesis by quercetin and isoquercitrin in the future. In addition, this study could not explain how quercetin and isoquercetin up-regulate LKB1. Further studies are needed to determine whether quercetin and isoquercetin, the two different substances, activate the LKB1-AMPKα pathway through the same receptor or site, thus showing the co-action of hepatic gluconeogenesis. We need to study the activity level and activation mechanism of LKB1, and the downstream signal molecular changes of PEPCK and G6Ppase regulated by AMPKα, such as FOXO1, PGC-1a, HNF4 alpha, TORC2, etc. to fully explain the molecular mechanism of quercetin and isoquercetin regulating blood glucose, so as to provide more evidence for the hypoglycemic function of Okra and explore more potential hypoglycemic plant monomers.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by Scientific Research Project of Shanghai Health and Family Planning Commission (No. 201840345), Project of Health and Family Planning Commission, Putuo District, Shanghai (No. 15-PT-05).
References
- 1.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosrozadeh M, Heydari N, Abootalebi M. The Effect of Abelmoschus esculentus on Blood Levels of Glucose in Diabetes Mellitus. Iran J Med Sci. 2016;41(3):S63. [PMC free article] [PubMed] [Google Scholar]
- 3.Thanakosai W, Phuwapraisirisan P. First identification of α-glucosidase inhibitors from okra (Abelmoschus esculentus) seeds. Nat Prod Commun. 2013;8(8):1085–1088. [PubMed] [Google Scholar]
- 4.Peng CH, Lin HC, Lin CL, Wang CJ, Huang CN. Abelmoschus esculentus subfractions improved nephropathy with regulating dipeptidyl peptidase-4 and type 1 glucagon-like peptide receptor in type 2 diabetic rats. J Food Drug Anal. 2019;27(1):135–144. doi: 10.1016/j.jfda.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erfani Majd N, Tabandeh MR, Shahriari A, Soleimani Z. Okra (Abelmoscus esculentus) improved islets structure, and down-regulated PPARs gene expression in pancreas of high-fat diet and streptozotocin-induced diabetic rats. Cell J. 2018;20(1):31–40. doi: 10.22074/cellj.2018.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CN, Wang CJ, Lin CL, Lin HT, Peng CH. The nutraceutical benefits of subfractions of Abelmoschus esculentus in treating type 2 diabetes mellitus. PLoS One. 2017;12(12):e0189065. doi: 10.1371/journal.pone.0189065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan S, Zhang Y, Sun Q, Yu L, Li M, Zheng B, Wu X, Yang B, Li Y, Huang C. Extract of okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice. Nutr Biochem. 2014;25(7):702–709. doi: 10.1016/j.jnutbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed Pharmacother. 2019;1(11):947–957. doi: 10.1016/j.biopha.2018.12.127. [DOI] [PubMed] [Google Scholar]
- 9.Bule M, Abdurahman A, Nikfar S, Abdollahi M, Amini M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem Toxicol. 2019;1(25):494–502. doi: 10.1016/j.fct.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Zhao Y, Wu Q, John A, Jiang Y, Yang J, Liu H, Yang B. Structure characterisation of polysaccharides in vegetable "okra" and evaluation of hypoglycemic activity. Food Chem. 2018;2(42):211–216. doi: 10.1016/j.foodchem.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Huang CN, Wang CJ, Lee YJ, Peng CH. Active subfractions of Abelmoschus esculentus substantially prevent free fatty acid-induced β cell apoptosis via inhibiting dipeptidyl peptidase-4. PLoS One. 2017;12(7):e0180285. doi: 10.1371/journal.pone.0180285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia F, Zhong Y, Li M, Chang Q, Liao Y, Liu X, Pan R. Antioxidant and Anti-Fatigue Constituents of Okra. Nutrients. 2015;7(10):8846–8858. doi: 10.3390/nu7105435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayachandran M, Zhang T, Ganesan K, Xu B, Chung SSM. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signalling pathway in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2018;8(29):112–120. doi: 10.1016/j.ejphar.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Fadul E, Nizamani A, Rasheed S, Adhikari A, Yousuf S, Parveen S, Gören N, Alhazmi HA, Choudhary MI, Khalid A. Anti-glycating and anti-oxidant compounds from traditionally used anti-diabetic plant Geigeria alata (DC) Oliv. & Hiern. Nat Prod Res. 2019:1–9. doi: 10.1080/14786419.2018.1542388. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Ouyang K, Jiang Y, Yang Z, Hu W, Xiong L, Wang N, Liu X, Wang W. Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on α-glucosidase activity. Int J Biol Macromol. 2017;98:829–836. doi: 10.1016/j.ijbiomac.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Renda G, Sari S, Barut B, Šoral M, Liptaj T, Korkmaz B, Özel A, Erik İ, Şöhretoğlu D. α-Glucosidase inhibitory effects of polyphenols from Geranium asphodeloides: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Bioorg Chem. 2018;8(1):545–552. doi: 10.1016/j.bioorg.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Gaballah HH, Zakaria SS, Mwafy SE, Tahoon NM, Ebeid AM. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed Pharmacother. 2017;9(2):331–339. doi: 10.1016/j.biopha.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 18.Voss CM, Pajęcka K, Stridh MH, Nissen JD, Schousboe A, Waagepetersen HS. AMPK Activation Affects Glutamate Metabolism in Astrocytes. Neurochem Res. 2015;40(12):2431–2442. doi: 10.1007/s11064-015-1558-5. [DOI] [PubMed] [Google Scholar]
- 19.Ren Z, Xie Z, Cao D, Gong M, Yang L, Zhou Z, Ou Y. C-Phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistance hepatocytes. Food Funct. 2018;9(5):2829–2839. doi: 10.1039/c8fo00257f. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Lv X, Li J, Meng Z, Wang Q, Chang W, Li W, Chen L, Liu Y. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol Cell Endocrinol. 2012;363(1-2):122–130. doi: 10.1016/j.mce.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373(4):545–549. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 22.Eid HM, Nachar A, Thong F, Sweeney G, Haddad PS. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag. 2015;11(41):74–81. doi: 10.4103/0973-1296.149708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Yoshitomi H, Liu T, Zhou B, Sun W, Qin L, Guo X, Huang L, Wu L, Gao M. Isoquercitrin activates the AMP-activated protein kinase (AMPK) signal pathway in rat H4IIE cells. BMC Complement Altern Med. 2014;1(4):42. doi: 10.1186/1472-6882-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]


