Abstract
Background and aims
Severe Ovarian Hyperstimulation Syndrome (OHSS) forms with very aggressive clinical evolution are still common, despite prophylactic measures. Besides the Vascular Endothelial Growth Factor (VEGF), there are other angiogenic factors, like Renin-Angiotensin-Aldosterone System (RAS), that might be associated with this disorder. Our study aims to evaluate the role of VEGF and Angiotensin II (ANG II) in the development of early severe OHSS, in high risk patients under prophylactic Cabergoline therapy.
Material and Methods
We recruited 192 patients undergoing in vitro fertilization (IVF) procedures with high risk for OHSS development. Out of these, 106 patients with OHSS were enrolled in the study, of which 28 subjects had a severe form of disease (group I), and 78 patients had a mild/moderate form (group II). We collected blood and follicular fluid from our study participants and determined serum and follicular VEGF and ANG II levels using Enzyme-Linked Immunosorbent Assay (ELISA) technique.
Results
Follicular VEGF, ANG II, and serum VEGF levels were significantly higher in group I versus group II. Serum VEGF titers were 645.97 versus 548.62 (p = 0.0008), follicular VEGF titers were 2919.52 versus 1093.68 (p < 0.0001), and follicular ANG II levels were 281.64 versus 65.76 (p < 0.0001). No significant differences have been shown between the two groups for serum ANG II levels.
Conclusion
Our study results provide evidence of a OHSS phenotype that is more prone to undergo severe clinical forms of disease, despite treatments with VEGF receptor blockers, and show that ANG II appears to play a major role alongside VEGF, in the development of these severe forms of disease.
Keywords: Ovarian Hyperstimulation Syndrome; Vascular endothelial growth factor; Angiotensin, Cabergoline
Introduction
The ovarian hyperstimulation syndrome (OHSS) is defined by a massive enlargement of the ovaries (stromal edema, luteum follicular cysts, necrotic focal areas, neo-vascularization), associated with acute, massive fluid transfer from the vascular sector to the third space, on account of the increased peritoneal capillaries permeability. It is a iatrogenic induced condition and it may have severe complications that can even lead to the patient’s death. The use of Gonadotrophin Releasing Hormone (GnRh) antagonist protocol over GnRh agonists (AgGnRh) protocol, triggering ovulation with GnRH agonists and using the VEGF receptor blockers (Cabergoline), have greatly lowered the risk of complications (1,2). However, severe OHSS forms still occur and pose serious problems to practitioners in terms of finding the best therapeutic approach for these very aggressive forms of disease.
Previous studies have indicated that the major role in the OHSS pathogenesis is played by the alteration of vascular permeability induced by the Human Chorionic Gonadotropin (hCG), which is leading to massive fluid passage into the peritoneal cavity. Numerous vasoactive factors are involved in this process, such as: angiopoietin fibroblast growth factor, hypoxia inducible factor, plasminogen activator, platelet-derived growth factor, protein kinase transforming growth factor-β, VEGF receptor, urokinase-type plasminogen activator, ANG II, as well as other cytokines involved in angiogenesis (3,4). The VEGF involvement in the development of OHSS has been previously studied and is well documented. Thus, previous studies have reported increased serum VEGF levels in OHSS patients (5), increased VEGF production of granulosa cells after hCG stimulation (6), increased expression of peritoneal VEGF receptors after hCG stimulation (7). The peak VEGF-2 receptor expression was associated with a maximum increase in vascular permeability and the use of coasting (withholding the administration of FSH until estrogen levels decrease), led to a reduced expression of these receptors (8). Furthermore, the molecular mechanism through which the VEGF interferes with the remodeling of vascular endothelial cell junctions was previously described (9). The initiation of VEGF receptor blocker treatment (Cabergoline) greatly reduced the incidence of OHSS (10,11). However, there are disease forms where treatment is still ineffective, thus we assume that the pathogenetic equation of OHSS may include some other factors, which may enhance the classic pathogenetic pathways, or may indicate new ones.
The Renin-Angiotensin System (RAS) is a hormone system playing major roles in controlling blood pressure and body fluid and electrolyte homeostasis. However, recent research suggests this system has consistent involvement in local processes including angiogenesis, fibrogenesis, and cellular proliferation. The complexity of the process is further increased by a great number of intermediate products proved to be biologically active (12). At the ovarian level the RAS acts via a specific local pathway, the Ovarian Renin-Angiotensin System (OVRAS), controlling the angiogenic, steroidogenic and apoptotic processes (13). The arguments to underlying RAS involvement, especially angiotensin II, at the ovarian site are diverse. Thus, messenger ribonucleic acid (mRNA) of the ANG II receptor was found in theca cells (14), while mRNA for Angiotensin I (ANG I) receptor was detected in granulosa and theca cells, or primary oocytes (15). Changes in the ANG I/ANG II receptors ratio were associated with follicular atresia or with the response to ovarian stimulation (16). The involvement of ANG II in the steroidogenesis processes was documented by the dose-dependent progesterone increase in hCG stimulated - human granulosa cell cultures, following ANG II administration (16). Moreover, previous research has indicated that the follicular fluid from patients undergoing IVF procedures contains increased levels of renin and angiotensin (ANG) (17).
This research study aims to evaluate the role of angiogenic factors VEGF and ANG II in the development of early severe OHSS in high risk patients under prophylactic VEGF receptor blockers therapy (Cabergoline).
Material and Methods
Study design and population sample
Within the Assisted Reproduction Department of the Obstetrics and Gynecology Clinic 1 (Cluj-Napoca, Romania), we conducted a panel study, between January 2016 and June 2018. All patients enrolled in the study provided written informed consent, prior to study participation and the research protocol was approved by the “Iuliu Hatieganu” University of Medicine Ethics Committee (Institutional Review Board (IRB), approval no. 221/10.05.2016).
We recruited patients undergoing IVF procedures who had clinical symptoms and biochemical tests results indicating a high risk for OHSS development. Patients were eligible for participation in the study if they had: 1) a plasma estradiol level above 3500 pg/mL on the day of hCG administration; and/or 2) developed 25 or more follicles larger than 14 mm in diameter; 3) more than 24 oocytes at pick-up; 4) symptoms such as nausea/vomiting or abdominal pain in the day of oocytes pick-up, or ascites. The criteria used for assigning patients to mild/moderate/severe OHSS were based on guidelines provided by the American Society for Reproductive Medicine (ASRM) (1). Patients less than 25 years old or over 37 years, with a body mass index (BMI) above 30 kg/m2, were not eligible for participation in the study. We decided to exclude obese patients from the study group due to the inhomogeneity of the protocol related to increased dose requirements.
We initially identified 306 patients with high risk of developing OHSS; 114 patients (out of 306) did not participate in the study, either because they were not eligible (12 patients with PCOS under 25 years, 57 patients older than 37 years and 7 obese patients), or because they refused to participate (38 patients). Out of the 192 recruited study participants, 86 patients did not develop OHSS and were excluded from the study, while 106 patients who developed OHSS were divided into two groups: group I, comprising 28 patients with severe forms of disease, and group II comprising 78 patients with mild/ moderate forms of disease (Fig. 1).
Figure 1.
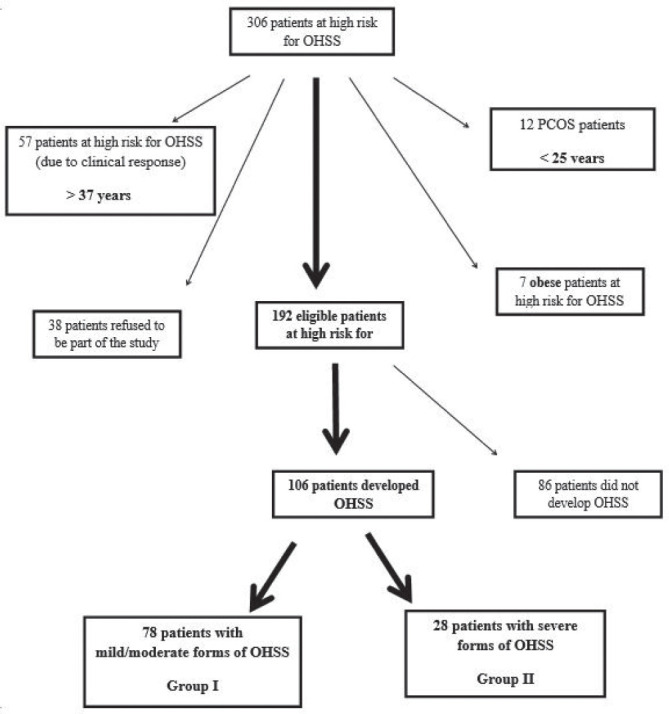
Recruitment description.
In group I, we included patients with severe signs and symptoms, such as: abdominal pain, persistent vomiting, oliguria, severe dyspnea, ascites, rapid weight gain (>1 kg in 24 h), severe hemoconcentration (hematocrit >55%), leucocyte count >25,000/mL, serum creatinine >1.6 mg/dL, Na <135 mEq/L, K >5 mEq/L, elevated liver enzymes, while in group II, we included patients with mild/moderate signs and symptoms, such as: mild abdominal discomfort, and/or mild nausea/vomiting and diarrhea, mild dyspnea and/or enlarged ovaries >6 cm, and/or ascites visible on ultrasound, and or hemoconcentration (hematocrit >41), or elevated leucocyte count (>15,000/mL). The criteria for the severity of OHSS were based on the guidelines provided by the ASRM, adapted after Navot et al., 1992 (1). In the selected study participants, prophylactic treatment with 0.5 mg Cabergoline for 7 days was started at the time of hCG administration.
OHSS treatment was adjusted according to the severity of each case and consisted in: volumetric rebalancing, administration of analgesics, antiemetics, prophylactic anticoagulation therapy, administration of albumin and, as a last therapeutic solution, culdocentesis.
Ovarian stimulation protocols, oocytes retrieval and embryo transfer
The starting gonadotrophin doses (urinary/recombinant FSH) were adjusted according to patient age, BMI, Antimullerian hormone (AMH) level, and previous IVF procedures. Patients received AgGnRh (Triptoreline, 0.1 mg) for down regulation in a long protocol, or Cetrorelix (Cetrotide, 0.25 mg or Orgalutran, 0.25 mg) in an antagonist protocol. Follicle sizes were evaluated on successive ultrasound examinations. At the time when three ovarian follicles larger than 17 mm were identified, hCG (Ovitrelle, 250 μg) was administered to enhance final oocyte maturation. At this point, patients eligible for the study received Cabergoline 0.5 mg daily for 7 days. Oocytes retrieval was performed at 34-38 h after hCG administration. The embryo transfer was performed on day three or five from oocytes retrieval, 1 to 3 embryos were transferred, and the remaining embryos were frozen. To provide the luteal phase support, progesterone was administered (Lutinus, intravaginal 100 mg three times/day). Pregnancy was diagnosed by serum Beta hCG testing at 14 days from oocytes retrieval.
Biological sampling and analysis
At the time of oocytes retrieval, we sampled 5 mL of blood by venipuncture, and 4 mL of follicular fluid from two mature follicles, carefully avoiding blood contamination. After oocyte isolation, the follicular fluid was centrifuged at 500 g for 10 min, transferred into sterile tubes, and then frozen at - 80°C until analysis.
Serum and follicular VEGF concentrations were quantified using ELISA technique (Quantikine Human VEGF Immunoassay, R&D Systems, USA). The sensitivity of the assay was 7 pg/mL. Intra- and inter-assay coefficients of variability (CV) in serum samples were 4.5 and 7%, respectively. For the detection of VEGF in the follicular fluids, a 1:4 dilution was performed using the standard diluents provided with the kit, and run in duplicate.
Serum and follicular ANG II were quantified using the ELISA technique (Cloud –Clone Corp). The minimum detectable levels were typically less than 9.27 pg/mL. Intra-Assay: CV<10%. Inter-Assay: CV<12%. The samples were prepared and tested in duplicate.
Statistical analysis
Statistical analysis was performed using OpenEpi v.3.03 and StatsDirect v.2.7.2 statistical packages to generate descriptive statistics, Pearson (r) correlation coefficients and linear regression models. To test for normal distribution, the Shapiro-Wilk test was used. For variables with normal distribution, we used the t (Student) test, and for those with non-uniform distribution or ranks, the non-parametric Mann-Whitney (U) test. The statistical significance was defined as α = 0.05 (5%), α = 0.01 (1%) or α = 0.001. To evaluate the correlation between two continuous variables, with normal (uniform) distribution, we used the Pearson (r) correlation coefficient and for variables with non-uniform distribution, the Spearman coefficient for rank correlation (ρ). The analysis of correlation coefficients followed the Colton rule. We used Excel 2010 (Microsoft Co., Redmond, Washington, USA) to generate the charts.
Results
Study participants demographic and clinical characteristics are described in Table 1. There were no statistical differences regarding age, BMI, the type of suppression used (agonist versus antagonist), duration of stimulation, the FSH doses required, the number of embryos transferred, or pregnancy rates. However, there were statistically significant differences, with poor clinical relevance, in favor of group I for the antral follicle count > 14 mm (22.52 versus 20.92; p = 0.03), the number of oocytes retrieved (13.07 versus 11.99; p = 0.01), estradiol levels on the day of the hCG trigger administration (4168.33 vs. 3914.94 pg/mL; p = 0.04).
Table 1.
Demographic, clinical data and biochemical test results in study participants
| Group I – OHSS severe (n=28) | Group II – OHSS mild/ moderate (n=78) | p value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± Standard Deviation (SD) | Range (Minimum – Maximum) | Median | Mean ± SD | Range (Minimum – Maximum) | Median | ||
| Age (years) | 31.00 ± 3.45 | 24-37 | 30 | 30.74 ± 3.05 | 23-38 | 29 | NSa |
| BMI (kg/m2) | 23.26 ± 1.53 | 21.7-27.9 | 23.5 | 23.75 ± 2.24 | 20.3-28.9 | 23.7 | NS |
| Type of GnRh analogue Agonist / antagonist | 12 / 16 0.75% |
34 / 44 77.27 |
NS | ||||
| Total doses of FSH (UI) | 1709.26 ± 30 | 1200-2250 | 1700 | 1817.28 ± 41 | 1150-2650 | 1850 | NS |
| Duration of stimulation (days) | 10.63 ± 1.21 | 9-13 | 10 | 10.84 ± 0.98 | 9-13 | 11 | NS |
| Estradiol concentration on the day of HCG administration (pg/mL) | 4168.33 ± 471 | 3470-5100 | 3970 | 3914.94 ± 569 | 2890-5890 | 3900 | 0.04 |
| No. of follicles > 14mm | 22.52 ± 3.0 | 18-28 | 22 | 20.92 ± 3.7 | 12-31 | 21 | 0.03 |
| No. of oocytes retrieved | 13.07 ± 2.6 | 5-16 | 13 | 11.99 ± 2.38 | 4-18 | 12 | 0.01 |
| No. of embryos transferred | 1.8 ± 0.5 | 1-3 | 2 | 1.6 ± 0.4 | 1-3 | 2 | NS |
| Clinical pregnancy (%) | 42.8% | 38.4% | NS | ||||
As regards the vasoactive parameters (Table 2), statistically significant differences were detected for serum VEGF levels (645.97 versus 548.62 pg/mL; p = 0.0008), follicular VEGF levels (2919.52 versus 1093.68 pg/mL; p < 0.0001), follicular ANG II (281.64 versus 65.76 pg/mL; p < 0.0001), but not for serum ANG II levels. Also, we found a statistically significant correlation between follicular ANG II and VEGF levels (p < 0.001) (Fig. 2).
Figure 2.
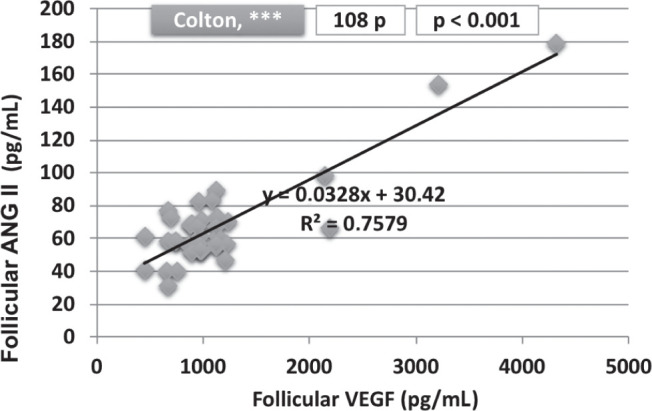
Correlation between follicular VEGF and ANG II levels.
Table 2.
Serum and follicular VEGF and ANG II levels measured in study participants
| Group I – OHSS severe (n=28) | Group II – OHSS mild moderate (n=78) | p value | |||||
| Mean ± SD | Range (Minimum – Maximum) | Median | Mean ± SD | Range (Minimum – Maximum) | Median | ||
| Serum VEGF (pg/mL) | 645.97 ± 129.68 | 399.99-854.35 | 642.12 | 548.62 ± 126.46 | 321.90-879.92 | 551.84 | 0.0008 |
| Follicular VEGF (pg/mL) | 2919.52 ± 1692.40 | 768-6213 | 2976 | 1093.68 ± 675.40 | 452-4321 | 896 | < 0.0001 |
| Serum ANG II (pg/mL) | 59.79 ± 16.78 | 36.46-95.60 | 54.84 | 56.35 ± 18.06 | 29.84-85.23 | 56.31 | NSa |
| Follicular ANG II (pg/mL) | 281.64 ± 181.47 | 57.89-649.65 | 234.23 | 65.76 ± 25.21 | 21.38-178.56 | 60.47 | < 0.0001 |
NS = no significance.
Discussion
The focus of this study was to evaluate the main contributors to the development of severe clinical forms of OHSS. Our study results provide support for the association of severe OHSS and the number of follicles larger than 14 mm, the number of oocytes retrieved and the level of estradiol on the day of retrieval, as well as for the clear involvement of VEGF and ANG II in the development of these severe forms, as they were associated with higher follicular levels of either VEGF, ANG II, or both.
The increase in serum and follicular levels of VEGF and ANG II that we found in severe forms may indicate that in addition to the increase in the number of follicles, the follicular behavior might be impaired. At the time of oocytes retrieval, none of the patients had any signs of hemoconcentration/hypovolemia that could have triggered the renal production of ANG II. At the same time, the association of much elevated ANG II levels with severe disease forms may indicate the existence of OHSS variants, where direct antagonism of VEGF receptor is not sufficient to block the syndrome. Thus, we can speculate the implication of either a mechanism of additional potentiation of VEGF - ANG II line, or a distinct line of action of ANG II, in essence, indicating a particular phenotype of OHSS.
Starting from the terminology of the syndrome and considering the pathogenetic data that are describing the OHSS, we can assume that OHSS is a polymorphic disorder with incompletely understood mechanisms of action. The angiogenic factors play a central role in the unrolling of peri-ovulatory physiological events, and implicitly, in the development of this syndrome. The complexity of this process is due to the large number of actors involved, to the mechanisms developed for their biological validation, and to the numerous connections between these factors. Severe OHSS forms are a distinct group of diseases not only from the clinical implications point of view, but also from a pathogenetic perspective. Thus, previous study results have suggested that the development of these forms is not only the result of the excessive activation of a single pathogenetic pathway, but it also involves several pathogenetic pathways. If the implication of VEGF in the development of OHSS is indisputable, the part this factor is playing in triggering these severe forms is less clear. In this context, many studies have indicated that the development of severe forms of disease requires the implication of adjuvant factors, such as certain VEGF (19) or VEGF receptor polymorphisms (VEGFR1-519 and VEGF-405) (20), cytokines (interleukin (IL) 2, IL6, IL8) or cytokine signaling (SOCS) proteins (21) and, last but not least, certain factors involved in angiogenesis (Pigment Epithelium-Derived Factor) (PEDF) (22). This is also the case of severe OHSS forms occurring in patients undergoing VEGF receptor blockers therapy. The most recent Cochrane review (2) reports a vigorous decrease in the number of OHSS cases, but a much lower impact on the severity of the syndrome.
The association between the RAS and OHSS was less investigated and there is only modest quality data available from case reports. The identification of increased ANG II, renin, pro-renin levels was initially considered to be associated with hypovolemia but, then, a growing body of literature reported that higher levels of ANG II and renin were found in ascites fluid than in serum (23, 24), thus proving evidence for the direct implication of the OVRAS system in the development of this pathology. Medication designed to block the RAS system in patients with OHSS, such as ANG Converting Enzyme (ACE) inhibitors or ANG II receptor antagonists, failed to completely shut down the process (25). Recent studies describe RAS as a complex system arranged on two main axes: one represented by the ACE/ANG II/ANG I receptor, and the other ACE 2/ANG 1-7/MAS receptor (26). The intermediate products ANG 1-7 have been shown to be biologically active, which renders it more difficult to quantify the process and to manage and control this system with appropriate medication (27). This study is, to the best of our knowledge, one of the first studies in our country showing elevated ANG II levels in the follicular fluid collected from OHSS patients.
Follicular production of cytokines/angiogenic factors is altered during ovarian stimulation procedures and VEGF is one of the factors with the most significant increase. Moreover, local hypoxia associated with disorders such as endometriosis (28) or PCOS (29), or other types of follicular dysfunctions described in poor responder patients (30), may induce elevated follicular VEGF levels. Both high and low follicular VEGF levels were reported in OHSS patients (31,32), thus confirming the polymorphic feature of this disease. Our results are consistent with those reported in previous studies. The mild forms of OHSS showed a certain tendency towards slightly elevated VEGF levels, however low levels were also recorded. On the other hand, high follicular levels were found almost exclusively in patients who developed severe forms of OHSS, specifically in patients with elevated ANG II levels. The association between the increased follicular production of VEGF and the elevated concentrations of ANG II has not been previously evaluated in OHSS patients, but both the activation of ANG II receptor and the increased production of ANG II, as a result of endothelial cell cultures exposure to VEGF, have been reported in oncological diseases (33,34).
From a clinical point of view, patients with this OHSS phenotype (characterized by increased follicular levels of VEGF and ANG II) might be eligible for a more aggressive therapeutic approach, starting from increasing the Cabergoline doses, associating other drugs, postponing embryo transfer to a subsequent cycle, and eventually, even using ACE inhibitors.
The evaluation of follicular milieu without having specific monograms is not currently helpful for clinical decision making. However, on long-term, there is an urgent need to characterize this space in order to facilitate a much more consistent evaluation of follicular processes, which have an impact on oocyte quality and on the initiation of disorder such as PCOS and OHSS.
Our study has a number of limitations. It was a prospective study in nature, but we measured ANG II and VEGF levels at only a single time point, and have not monitored their levels in time. Another concern is that we have done an evaluation of the whole follicular milieu based on the extrapolation of the ANG II and VEGF levels measured in the follicular fluid extracted from three follicles, fact that may have entailed a risk of error. Also, the assessment of the full roles that the vasoactive factors are playing in the development of the severe forms of OHSS would have required a more extensive investigation into the control systems and into the factors that modulate vasoactive factors activity, which we have not performed here. Another important limitation of our study was that we did not include a control group in our assessment. Having a control group perhaps would have brought more insight into this pathogenetic process. Another concern related to this analysis was the limited statistical power, which may have restricted our ability to detect modest associations.
In conclusion, our study results provide evidence of an OHSS phenotype which is more prone to undergo severe clinical forms of disease, despite treatments with VEGF receptor blockers, and show that ANG II appears to play a major role alongside VEGF in the development of these severe forms of disease. These data underscore the need for a more extensive investigation (larger prospective cohort studies) to collect essential information necessary to create the framework for a selective approach of these patients, with further decrease in OHSS morbidity, and to enable the opening of new research perspectives targeting the individualization of these disease forms, based on pathogenetic criteria.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.American Society for Reproductive Medicine: Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106:1634–1647. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews. 2017;1.:CD012103. doi: 10.1002/14651858.CD012103.pub2. Art. No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels With Inflammatory Processes. Endocr Rev. 2019;40(2):369–416. doi: 10.1210/er.2018-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manau D, Fabregues F, Penarrubia J, Creus M, Carmona F, Casals G, Jiménez W, Balasch J. Vascular endothelial growth factor levels in serum and plasma from patients undergoing controlled ovarian hyperstimulation for IVF. Hum Reprod. 2007;22:669–675. doi: 10.1093/humrep/del427. [DOI] [PubMed] [Google Scholar]
- 5.Pietrowski D, Szabo L, Sator M, Just A, Egarter C. Ovarian hyperstimulation syndrome is correlated with a reduction of soluble VEGF receptor protein level and a higher amount of VEGF-A. Hum Reprod. 2012;27(1):196–199. doi: 10.1093/humrep/der349. [DOI] [PubMed] [Google Scholar]
- 6.Herr D, Fraser H M, Konrad R, Holzheu I, Kreienberg R, Wulff C. Human chorionic gonadotrophin controls luteal vascular permeability via vascular endothelial growth factor by down-regulation of a cascade of adhesion proteins. Fertil Steril. 2013;99:1749–1758. doi: 10.1016/j.fertnstert.2013.01.120. [DOI] [PubMed] [Google Scholar]
- 7.McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV Jr, Connolly DT. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet. 1994;344:235–236. doi: 10.1016/s0140-6736(94)93001-5. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Velasco JA, Zuniga A, Pacheo A, Gomez A, Simon C, Remohi J, Pellicer A. Coasting acts through downregulation of VEGF gene expression and protein secretion. Hum Reprod. 2004;19:1530–1538. doi: 10.1093/humrep/deh298. [DOI] [PubMed] [Google Scholar]
- 9.Gavard J, Gutking JS. VEGF controls endothelial- cell permeability by promting the beta arestin-dependant endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1239. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 10.Ferrero H, García-Pascual CM, Gómez R, Delgado-Rosas F, Cauli O, Simón C, Gaytan F, Pellicer A. Dopamine receptor 2 activation inhibits ovarían vascular endothelial growth factor secretion in vitro: implications for treatment of ovarían hyperstimulation syndrome with dopamine receptor 2 agonists. Fertil Steril. 2014;101:1411–1418. doi: 10.1016/j.fertnstert.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Youssef MA, Van Wely M, Hassan MA, Al-Inany HG, Mochtar M, Khattab S, van der Veen F. Can dopamine agonists reduce the incidence and severity of OHSS in IVF/ICSI treatment cycles? A systematic review and meta-analysis. Hum Reprod Update. 2010;16:459–466. doi: 10.1093/humupd/dmq006. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond). 2012;123(5):273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbo A, Ávila J, Naftolin F. The Ovarian Renin-Angiotensin System (OVRAS): A Major Factor in Ovarian Function and Disease. Reprod Sci. 2016;23(12):1644–1655. doi: 10.1177/1933719116672588. [DOI] [PubMed] [Google Scholar]
- 14.Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95(1):176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 15.Pena O, Palumbo A, Gonzalez-Fernandez R, Hernandez J, Naftolin F, Avila J. Expression of angiotensin II type 1 (AT1) and angiotensin II type 2 (AT2) receptors in human granulosa-lutein (GL) cells: correlation with infertility diagnoses. Fertil Steril. 2010;93(5):1601–1608. doi: 10.1016/j.fertnstert.2009.03.092. [DOI] [PubMed] [Google Scholar]
- 16.Morris RS, Francis MM, Do YS, Hsueh WA, Lobo RA, Paulson RJ. Angiotensin II (AII) modulation of steroidogenesis by luteinized granulosa cells in vitro. J Assist Reprod Genet. 1994;11(3):117–122. doi: 10.1007/BF02332088. [DOI] [PubMed] [Google Scholar]
- 17.Malamitsi-Puchner A, Sarandakou A, Baka SG, Tziotis J, Rizos D, Hassiakos D, Creatsas G. Concentrations of angiogenic factors in follicular fluid and oocyte-cumulus complex culture medium from women undergoing in vitro fertilization: association with oocyte maturity and fertilization. Fertil Steril. 2001;76:98–101. doi: 10.1016/s0015-0282(01)01854-4. [DOI] [PubMed] [Google Scholar]
- 18.Kahnberg A, Enskog A, Brännström M, Lundin K, Bergh C. Prediction of ovarian hyperstimulation syndrome in women undergoing in vitro fertilization. Acta Obstet Gynecol Scand. 2009;88:1373–1381. doi: 10.3109/00016340903287482. [DOI] [PubMed] [Google Scholar]
- 19.Ghasemi N, Dehghani Firouzabadi R, Ahmadi S. Association of −460C/T and +405 G/C polymorphisms of vascular endothelial growth factor gene and susceptibility to ovarian hyperstimulation syndrome. Int J Reprod Biomed (Yazd). 2017;15:87–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Nouri K, Haslinger P, Szabo L, Sator M, Schreiber M, Schneeberger C, Pietrowski D. Polymorphisms of VEGF and VEGF receptors are associated with the occurrence of ovarian hyperstimulation syndrome (OHSS)-a retrospective case-control study. J Ovarian Res. 2014;13:54. doi: 10.1186/1757-2215-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orvieto R, Dratviman-Storobinsky O, Lantsberg D, Haas J, Mashiach R, Cohen Y. Interleukin-2 and SOCS-1 proteins involvement in the pathophysiology of severe ovarian hyperstimulation syndrome--a preliminary proof of concept. J Ovarian Res. 2014;7:106. doi: 10.1186/s13048-014-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-Joseph H, Ben-Ami I, Ron-El R, Shalgi R, Chuderland D. Pigment epithelium-derived factor regulation by human chorionic gonadotropin in granulosa cells. Reproduction. 2016;151(2):179–185. doi: 10.1530/REP-15-0478. [DOI] [PubMed] [Google Scholar]
- 23.Delbaere A, Bergmann PJ, Gervy-Decoster C, Staroukine M, Englert Y. Angiotensin II immunoreactivity is elevated in ascites during severe ovarian hyperstimulation syndrome: implications for pathophysiology and clinical management. Fertil Steril. 1994;62(4):731–737. doi: 10.1016/s0015-0282(16)56997-0. [DOI] [PubMed] [Google Scholar]
- 24.Navot D, Margalioth EJ, Laufer N, Birkenfeld A, Relou A, Rosler A, Schenker J. G. Direct correlation between plasma renin activity and severity of the ovarian hyperstimulation syndrome. Fertil Steril. 1987;48(1):57–61. doi: 10.1016/s0015-0282(16)59290-5. [DOI] [PubMed] [Google Scholar]
- 25.Ata B, Yakin K, Alatas C, Urman B. Dual renin-angiotensin blockage and total embryo cryopreservation is not a risk-free strategy in patients at high risk for ovarian hyperstimulation syndrome. Fertil Steril. 2008;90:531–536. doi: 10.1016/j.fertnstert.2007.07.1309. [DOI] [PubMed] [Google Scholar]
- 26.Scotti L, Abramovich D, Pascuali N, de Zúñiga I, Oubina A, Kopcow L, Lange S, Owen G, Tesone M, Parborell F. Involvement of the ANGPTs/Tie-2 system in ovarian hyperstimulation syndrome (OHSS) Mol. Cell. Endocrinol. 2013;365:223–230. doi: 10.1016/j.mce.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Cavallo IK, Dela Cruz C, Oliveira ML, Del Puerto HL, Dias JA, Lobach VN, Casalechi M, Camargos MG, Reis AM, Santos RA, Reis FM. Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Hum Reprod. 2017;32(6):1318–1324. doi: 10.1093/humrep/dex072. [DOI] [PubMed] [Google Scholar]
- 28.Fujii EY, Nakayama M, Nakagawa A. Concentrations of receptor for advanced glycation end products, VEGF and CML in plasma, follicular fluid, and peritoneal fluid in women with and without endometriosis. Reprod Sci. 2008;15:1066–1074. doi: 10.1177/1933719108323445. [DOI] [PubMed] [Google Scholar]
- 29.Artini PG, Ruggiero M, Parisen Toldin MR, Monteleone P, Monti M, Cela V, Genazzani AR. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum Fertil (Camb). 2009;12:40–44. doi: 10.1080/14647270802621358. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia C, Genazzani AD, Regnani G, Primavera MR, Petraglia F, Volpe A. Perifollicular Doppler flow and follicular fluid vascular endothelial growth factor concentrations in poor responders. Fertil Steril. 2000;74(4):809–812. doi: 10.1016/s0015-0282(00)01517-x. [DOI] [PubMed] [Google Scholar]
- 31.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, Simón C. The pathogenesis of ovarian hyperstimulation syndrome: in vivo studies investigating the role of interleukin-1beta, interleukin-6, and vascular endothelial growth factor. Fertil Steril. 1999;71(3):482–489. doi: 10.1016/s0015-0282(98)00484-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang TH, Horng SG, Chang CL, Wu HM, Tsai YJ, Wang HS, Soong YK. Human Chorionic Gonadotropin-Induced Ovarian Hyperstimulation Syndrome is associated with up-regulation of Vascular Endothelial Growth Factor. Journal Clin Endocrinol Metab. 2002;87(7):3300–3308. doi: 10.1210/jcem.87.7.8651. [DOI] [PubMed] [Google Scholar]
- 33.Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, Nomura S, Kikkawa F. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer. 2006;94(4):552–560. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S, Kikkawa F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11(7):2686–2694. doi: 10.1158/1078-0432.CCR-04-1946. [DOI] [PubMed] [Google Scholar]


