Abstract
Previous studies have suggested that nearly 30% of the stroke victims present with signs of depression within the first 5 years of illness. Since post-stroke depression significantly affects the patient both physically and psychologically, the emotional disturbances impede the progress and effectiveness of rehabilitation. To utilize sunlight therapy in post-stroke patients in order to improve the depression and daily activity function. This study was a single-blind design randomized controlled intervention with sunlight exposure design. The population were stroke patients with tendency to depression. Exposed to sunlight for at least 30 min per day, at least 14 days of exposure duration of 4 weeks. A total of 46 patients were 23 patients in the experimental group and the control group. The research tools include: depression status (Taiwanese depression scale, TDS), physical activity function (Barthel Index), and cognitive status (MMSE). The CONSORT guideline was used in this study. After the data was analyzed with the generalized estimating equation (GEE), significant improvement was noted among the experimental ground in 2nd post-test depression score, daily function in the first and second post-test Barthel Index score. There was no significant improvement in cognitive function. This study confirms that sunlight therapy improves the mental health of post-stroke patients with depressed mood. It also enhances daily activity and facilitates the recovery to a health state.
Keywords: Sunlight, Depression, Daily activity function, Stroke, Health sciences, Public health, Neurology, Evidence-based medicine, Clinical research, Health profession, Health promotion
Sunlight; Depression; Daily activity function; Stroke; Health sciences; Public health; Neurology; Evidence-based medicine; Clinical research; Health profession; Health promotion
1. Introduction
The 10 leading causes of death in Taiwan mainly consist of chronic diseases. Cerebrovascular disease ranked third in 2015 (Statistics Department, Ministry of Health and Welfare, 2016) and fourth in 2016 and 2017 (Statistics Department, Ministry of Health and Welfare, 2017; Statistics Department, Ministry of Health and Welfare, 2018). About 30% of cerebrovascular disease patients have been found to show signs of depression within five years after diagnosis. The psychological and physiological impacts of post-stroke depression can be described as a domino effect, as emotional distress affects the progress and effectiveness of rehabilitation. Regarding non-pharmacological treatments for depression, therapeutic methods based on light generated from instruments have been utilized in various treatments of psychological, behavioral, and emotional disorders. The promising results of these methods have led researchers to suggest that artificial light should be designed to resemble natural light. Scientific evidence has proved the benefits of sunlight on humans' health; hence, this research aimed to alleviate depression and improve the daily living activities of cerebrovascular accident patients by means of sunlight therapy.
2. Background
According to the diagnostic standards described in The Diagnostic and Statistical Manual of Mental Disorders (DSM), depression diagnosed after the occurrence of stroke is known as post-stroke depression (PSD) (Pohjasvaara et al., 1998). Patients with aphasia or a family history of mood disorders are more likely to develop PSD following a stroke that occurred in the dominant left hemisphere than after a stroke that occurred in some other region of the brain (Mitchell et al., 2017). Stroke patients with aphasia are also more likely to develop depressive tendencies that may damage the body's activities and functions within two to three weeks after stroke occurrence than those without aphasia (Wang et al., 2016). A study by Robinson and Jorge (2015) found that, for patients in acute care and rehabilitation hospitals, the incidence rates of major and minor depression were, respectively, 21.6% and 20.0%; for outpatients with a post-stroke duration ranging from three months to more than three years, the prevalence rates of major and minor depression were, respectively, 24.0% and 23.9%; and for community-dwelling stroke patients, the prevalence rates of major and minor depression were, respectively, 14% and 9%. This indicates that it is common for stroke patients to be diagnosed with depressive tendencies. After conducting a follow-up study on 175 stroke patients at three months post-stroke from 2011 and 2012, a local hospital in southern of an island state in East Asia revealed that the prevalence of depressive symptoms among the stroke patients was 30.29%. The patients' risk of developing depressive symptoms had negative correlations with their Barthel index score, which shows that depressive symptoms became more obvious following a decline in daily living activities (Chiu et al., 2014). Epidemiology shows that 30% of stroke patients suffering from depression symptoms are worthy of attention and appropriate treatment.
2.1. Light therapy
Natural and artificial light are both used to treat diseases. In artificial light therapy, a fixed spectrum is used to balance the circadian rhythm (Wu and Sung, 2010). The wavelengths, intensities, and spectrums of lamps are set to resemble those of outdoor light, and illuminance is usually set in the range of 2,000 to 10,000 lux. As the eye is exposed to light rays, the secretion time of melatonin by the pineal gland becomes adjusted, thereby balancing the circadian rhythm. In a study by Dowling et al. (2005), artificial light therapy was used to treat patients with Alzheimer's disease in long-term care facilities, and the results showed that it significantly improved the patients' insomnia, sleep disturbances, and sleepwalking problems. The use of light therapy on elderly people in rehabilitation significantly improved their depressive symptoms, which shows that light therapy is able to alleviate geriatric depression (Tsai et al., 2004). Sunlight is natural, the Sun's resources include light and heat, and light consists of visible and invisible light.
2.2. Association between sunlight and health
Since vitamin D plays a key role in protecting the kidneys and the cardiovascular system, the lack of sunlight or vitamin D is often associated with high blood pressure, heart attacks, and stroke. Vitamin D has potential anti-inflammatory effects on relevant cardiovascular diseases (such as diabetes, high blood pressure, heart failure, peripheral vascular disease, and atherosclerosis) and chronic kidney disease. Therefore, the level of vitamin D can be regarded as an important indicator for predicting cardiovascular diseases (Lai and Fang, 2014; Wang et al., 2018).
Exposure to sunlight for 5–30 min, 2 to 3 times weekly, allows the production of hormones that help maintain a sufficient amount of vitamin D required by the body (World Health Organization, 2020). However, due to strong ultraviolet (UV) rays and overly high temperatures, it is recommended that individuals avoid outdoor activities during the two to three hours before noon, and that they take note of the day's UV index. Preventive measures should be taken as well, such as applying sunscreens with SPFs of 15 or higher; wearing appropriate sweat-absorbing, quick-drying, and breathable clothing; and wearing sunglasses to protect the eyes (Lin and Liao, 2016; Holick et al., 2011).
At present, there have only been a few academic randomized controlled trial (RCT) studies that actually utilized sunlight therapy to treat PSD patients. In a prospective study by the Daemen et al. (2014) and Danish researchers West et al., 2017a, West et al., 2017b, since bedridden stroke patients were indoors and could not be exposed to the natural daytime variation, naturalistic lighting that mimicked the natural daytime spectrum variation was installed in the experimental unit, while standard artificial lighting was installed in the control unit. The depression scale results of both groups were compared after at least 14 days of light exposure, and statistically significant differences were observed in the experimental group in terms of the improvement of depressive symptoms.
In this study, interventional sunlight treatment will be applied to stroke patients to explore their effectiveness in improving depression and daily activity functions.
3. Methods
As a pre-post-intervention experimental research study involving two groups, a single-blind trial was adopted for the participants, who were recruited via purposive sampling. Participants who met the eligibility criteria of this research were randomly divided into two groups by drawing numbers: those who drew odd numbers were the experimental group, while those who drew even numbers were the control group. A structured questionnaire was administered to the participants to collect and analyze their basic data. Based on the sample size, and in accordance with the methods described by Søndergaard et al. (2006), the effect size was calculated by using the means and standard deviations of the pre-post-test depression scales of the two groups. The estimated effect size was 0.20; the α error was 0.05; the power (1-β error) was 0.8; and the validity was 0.80. 42 participants were originally estimated to be divided into the two groups.
The study was approved by the Local Bioethiommission, No 2017-09-001CC; clinicaltrials.gov =ID: NCT04036565.
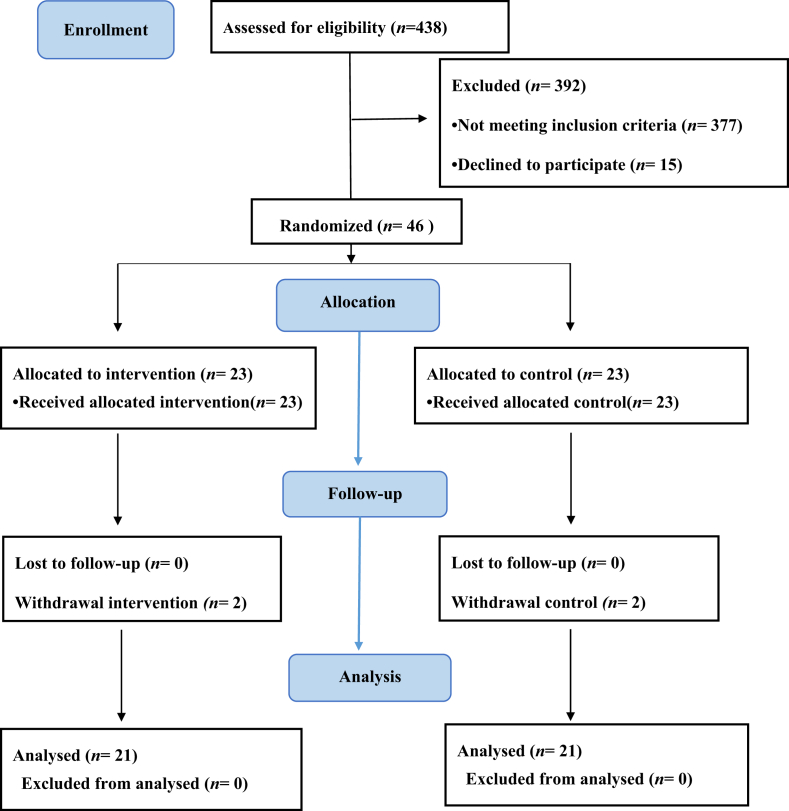
This study was conducted from October 2017 to April 2018. In order to avoid large climate-related variations, the participants had to be one-month post stroke patients who, after being discharged, mainly convalesced in their homes in northern of an island state in East. In retrospect, 438 potential participants were screened from September 2017 to January 2018, of whom 377 failed to meet the eligibility criteria and 15 were unwilling to participate; thus, there were 46 participants in this study. The participants were divided into two groups of equal numbers by means of simple randomization (lottery: single is experimental group, double is control). The experimental group participated in sunlight therapy, while the control group received standard care. Throughout the intervention period, two participants from each group had dropped out of this study, indicating a dropout rate of 9%. As shown in Figure 1, a total of 42 participants, 21 in the experimental group and 21 in the control group, completed the six to eight-week study period.
Figure 1.
Flow chart of participants in the study.
3.1. Eligibility criteria
Participants had to be verified one-month post-stroke patients in stable condition, with an NIHSS score of not more than 15 (minor or moderate stroke). Participants also had to meet at least two of the DSM-IV diagnosis criteria for minor depression, dejection, or dysthymia, and had to be conscious and capable of expressing themselves. After being discharged, participants mainly convalesced in their homes in northern of an island state in East. Participants had to be aged 20 or above, able to converse in or be literate in Mandarin or Taiwanese, and agree, after being asked to provide consent, to participate in this study.
3.2. Exclusion criteria
Patients with a NIHSS score higher than 16 (severe stroke); patients with stroke caused by cancer metastasis; stroke patients with cancer comorbidity; and patients who were on dialysis, pregnant, mentally-challenged, or suffering from severe cognitive disabilities.
3.3. Sunlight therapy intervention
The sun had to be bright and visible during the day, with an illuminance of 10,000 lux and above (Chang et al., 2011). Participants were required to employ appropriate protective measures when directly exposing their forearms or calves under the sun for an accumulated minimum of 30 min per day, for a total of 14 days in four weeks.
3.4. Research tools
3.4.1. Depressive state (Taiwanese depression scale, TDS)
The use of TDS as the research tool of this study was approved by Dr. Yu Lee on April 14, 2017. The TDS's internal consistency reliability was 0.90; its sensitivity was 89%; and its specificity was 92% (Lee et al., 2000).
3.4.2. National institute of health stroke scale (NIHSS)
The Chinese version of the NIHSS was adopted in this study to assess neurological damage. The internal consistency of the NIHSS (Cronbach's α) was 0.92 (Sun et al., 2006); stroke severity was categorized as minor (NIHSS = 0 to 6), moderate (NIHSS = 7 to 15), and severe (NIHSS = 16 to 38) (Lee et al., 2012; Chang and Tseng, 2003).
3.4.3. Physical mobility (Barthel Index)
The Barthel Index was adopted in this study to assess the progress of rehabilitation among stroke patients. The internal consistency Kappa value was 0.9, and the correlation coefficient was 0.98 (Loewen and Anderson, 1988).
3.4.4. Cognitive functions (Mini-mental state examination, MMSE)
The inter-rater reliability of the MMSE was 0.83; the test-retest reliability was 0.89–0.98 (Guo et al., 1988).
The participants in the experimental and control groups were subjected to a pre-test measurement after they were recruited. For the experimental group, the first post-test measurements were taken right after the participants completed the sunlight therapy (two to four weeks after the start of the intervention), and the second post-test measurements were taken one month after the intervention was completed (six to eight weeks after the start of the intervention). For the control group, the first and second post-test measurements were taken two and six weeks, respectively, after they started receiving standard care.
Throughout the study, luminosity monitoring was obtained from the Central Weather Bureau's Observation Data Inquiry System (http://e-service.cwb.gov.tw/HistoryDataQuery/index.jsp) to calculate sunshine duration. In order to check the completeness of data records in the two groups, participants were followed-up during their check-ups or by means of phone calls or house visits.
4. Results
The results from the test of homogeneity between the experimental group and control group showed that except the stroke location is statistically significant differences (p = 0.048) in both groups, as 12 participants in the experimental group (52%) had a stroke that mainly occurred in their right hemisphere, whereas 9 participants (29%) in the control group had a stroke that mainly occurred in their left hemisphere. There were no statistically significant differences in terms of demography and outcome indicators.
The Pearson statistical analysis method was adopted in this study to check the correlation between the stroke patients' independent variables (basic attributes) and dependent variables (NIHSS score, TDS score, MMSE score, and Barthel index score). Details are shown in Table 1.
Table 1.
The Pearson correlation analysis between basic attributes and pre-tests of variables.
| Variable | NIHSS |
TDS |
MMSE |
Barthel Index |
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | .131 | .387 | .506 | .000∗∗ | .114 | .451 | .110 | .468 |
| Gender | .028 | .852 | -.417 | .004∗∗ | .256 | .086 | -.067 | .659 |
| Source of income | .115 | .447 | .170 | .258 | -.371 | .011∗ | -.042 | .780 |
| Education level | -.015 | .923 | -.048 | .750 | .345 | .019∗ | .037 | .808 |
| Marital status | .073 | .631 | -.100 | .508 | -.385 | .008∗∗ | -.163 | .278 |
| Living condition | -.254 | .088 | -.122 | .419 | -.122 | .421 | .166 | .270 |
| Religion | -.084 | .581 | -.066 | .663 | .161 | .285 | .039 | .795 |
| Chronic disease | .166 | .271 | .285 | .055 | -.348 | .018∗ | -.097 | .520 |
| Chronic disease medication | .057 | .705 | .254 | .089 | -.176 | .241 | .022 | .886 |
| Stroke times | -.070 | .643 | -.172 | .254 | -.197 | .189 | .084 | .579 |
| stroke pattern | .300 | .043∗ | .278 | .061 | .101 | .503 | -.255 | .087 |
| stroke location | -.083 | .584 | -.001 | .996 | .005 | .973 | .142 | .347 |
| Stroke durations | .131 | .387 | .506 | .000∗∗ | .114 | .451 | .110 | .468 |
| Aphasia | .416 | .004∗∗ | .155 | .304 | -.127 | .401 | -.333 | .024∗ |
| Rehabilitation | .577 | .000∗∗ | .305 | .039∗ | -.136 | .369 | -.459 | .000∗∗ |
| Rehabilitation frequency | .405 | .049∗ | .156 | .465 | -.097 | .651 | -.487 | .016∗ |
Pearson Correlation; ∗p < 0.05, ∗∗p < 0.01.
Sunlight therapy was implemented as an intervention in this study, and generalized estimating equations (GEE) were used to analyze and compare the pre-test and the two post-test results between the two groups. The pre-test measurement was taken on the day the experimental group and control group received sunlight therapy and standard care, respectively. The first post-test measurement was taken two to four weeks after sunlight therapy and standard care had concluded; the second post-test measurement was taken six to eight weeks after sunlight therapy and standard care had concluded. Improvement efficacies of dependent variables (TDS score, MMSE score, NIHSS score, and Barthel index score) were analyzed. In terms of patients' basic attributes, statistically significant differences were observed between degree of depression improvement and stroke duration (95% CI [.070–.158], p = .000), age (95% CI [.044–.239], p = .005), and gender (95% CI [-.869– -2.744], p = .000). In addition, there were statistically significant differences between patients' basic attributes and rehabilitation frequencies (95% CI [.115–5.523], p = .041), and between patients' degree of daily living activities improvement and, respectively, rehabilitation conditions (95% CI [-59.619– -14.899], p = .001) and rehabilitation frequencies (95% CI [-20.761– -2.867], p = .010).
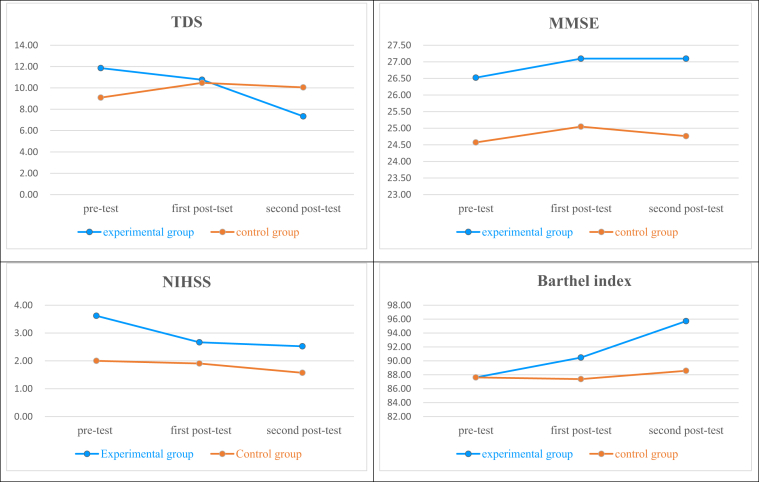
The analysis of depression improvement efficacies after sunlight therapy revealed that there were no statistically significant differences between the pre-test and first post-test results of the experimental group (95% CI [-.802–3.564], p = .215). There were statistically significant group by time interactions between the pre-test and the second post-test results of the experimental group (95% CI [-10.495– -.457], p = .032), indicating that the effects of sunlight therapy on emotional responses may be delayed, and were not immediately effective. The analysis of cognitive function improvement efficacies after sunlight therapy showed that there were no statistically significant differences between the pre-test and first post-test results of the experimental group (95% CI [-.179–1.131], p = .154) and the control group (95% CI [-748–1510], p = .508), indicating that sunlight therapy had not improved cognitive functions. The analysis of stroke severity (NIHSS score) improvement efficacies after sunlight therapy revealed that there were statistically significant group by time interactions between the pre-test and the first post-test results of the experimental group (95% CI [-1.535– -.179], p = .013); however, there were no statistically significant differences between the pre-test and the second post-test results (95% CI [-1.442–.018], p = .092), indicating that initially, there were remarkable improvements in stroke severity after the implementation of sunlight therapy, but that subsequently, the improvements became unremarkable. The progress of improvement may have been restricted by the low stroke severity of patients, as well as the process of natural healing. On the other hand, stroke severity was closely related to daily living activities (Barthel index score). Analysis of daily living activities improvement efficacies after sunlight therapy revealed that in the experimental group, there were statistically significant group by time interactions between the pre-test and the first post-test results (95% CI [.068–6.123], p = .045); and between the pre-test and the second post-test results (95% CI [.606–13.679], p = .033), indicating that there were significant improvements in patients' daily living activities after receiving sunlight therapy, which could be related with their proactive participation in rehabilitation as well (as shown in Table 2 and Figure 2).
Table 2.
Analyze and compare the pre-test and the two post-tests results between the two groups by generalized estimating equations.
| TDS |
MMSE |
NIHSS |
Barthel Index |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Intercept | 9.095 | 1.5355 | .000∗∗ | 24.571 | 1.3745 | .000∗∗ | 2.000 | .7408 | .007∗∗ | 87.619 | 5.9344 | .000∗∗ |
| Group | ||||||||||||
| Experimental group vs Control group | 2.762 | 2.2938 | .229 | 1.952 | 1.5623 | .211 | 1.619 | .9954 | .104 | -4280E-15 | 7.7755 | 1.000 |
| Time | ||||||||||||
| Pre-test vs second post-test | .952 | 1.4685 | .517 | .190 | .3409 | .576 | -.429 | .2083 | .040∗ | .952 | .9885 | .355 |
| Pre-test vs first post-test | 1.381 | 1.1137 | .215 | .476 | .3341 | .154 | -.095 | .2511 | .704 | -.238 | .7124 | .738 |
| Interaction | ||||||||||||
| Experimental group ∗ second post test | -5.476 | 2.5609 | .032∗ | .381 | .5761 | .508 | -.667 | .3954 | .092 | 7.413 | 3.3350 | .033∗ |
| Experimental group∗ first post-test | -2.476 | 1.7266 | .152 | .095 | .5003 | .849 | -.857 | .3459 | .013∗ | 3.095 | 1.5448 | .045∗ |
Generalized Estimating Equations(GEE); ∗p < 0.05, ∗∗p < 0.01.
Figure 2.
Indicated the change in two groups in pre-test, first post-test and second post-test after sunlight therapy.
5. Discussion
As shown in Table 3, Total of 20 patients were depression symptoms in these two age groups which 45 to 64 years and 65 years above. An analysis of the distribution of depressive symptoms showed that there were 11 patients aged between 45 to 64 years with TDS scores of 9 or above, which indicates that their depression had reached a critical level. In an analysis of the relevant PSD risk factors for middle-aged to elderly patients. The results of this study were similar to those of Alajbegovic et al. (2014) and Chiu et al. (2014). Their results were highly in accordance with the results of this study, indicating that middle aged and elderly patients were more likely to develop post-stroke depressive tendencies. Since stroke is the main cause of acquired disability, the fear of being disabled is more of a concern among stroke patients than the fear of death (Tsui, 2016; Qian and Hsu, 2018). The role reversal from being someone others rely on to someone dependent on others (due to disabilities) may be the cause of depression among middle-aged and elderly groups.
Table 3.
The chi-square analysis the TDS (Depression Level) with the age and gender on the pre-test.
| Variable | TDS N (%) |
p | |||
|---|---|---|---|---|---|
| 0~8 (without depression) |
9~14 (the distribution of depressive symptoms) |
15~18 (depression reached a critical level) |
>=19 (major depression) |
||
| Age (year) | .391 | ||||
| <=44 | 4(66.7) | 1(16.7) | 1(16.7) | 0 | |
| 45-64 | 6(35.3) | 5(29.4) | 1(5.9) | 5(29.4) | |
| >=65 | 14(60.9) | 5(21.7) | 2(8.7) | 2(8.7) | |
| Gender | .030∗ | ||||
| Female | 7(35) | 4(20) | 3(15) | 6(30) | |
| Male | 17(65.4) | 7(26.9) | 1(3.8) | 1(3.8) | |
Chi-square: ∗p < 0.05.
As shown in Table 1, indicating that females had more depressive symptoms than males. These results were in line with those of Kwa et al. (1996). Alajbegovic et al. (2014) also observed that depression was significantly more prevalent among females than males. These differences may be attributed to the roles women play in their families or work and their perceived feminine traits, which makes them more prone to developing depressive emotions when compared to men.
Correlations between basic attributes and degree of depression: Stroke duration and rehabilitation conditions positively correlated with depression, which was in agreement with the results obtained by Robinson and Price (1982). Cheng et al. (2005) concluded that there was no statistically significant correlation between stoke location and depression among six-months post-stroke patients, which was in agreement with the results of this study. The results of this study were also not in agreement with those of Mitchell et al. (2017) and Wang et al. (2016), who reported that stroke patients with aphasia were more likely to develop depression than those without aphasia. This may have been due to differences in the depression assessment tools used. As depression and rehabilitation were positively correlated. In relation to stroke severity, there were statistically significant and positive correlations between stroke type and respectively, aphasia, rehabilitation conditions, and rehabilitation frequencies. These results were in line with those of Szu et al. (2017), who found that, among stroke patients, the need to undergo rehabilitation was significantly correlated to stroke severity. This indicates that as stroke severity increases, the corresponding comorbidities such as aphasia or physical disabilities will increase the number and frequency of rehabilitation sessions. Therefore, depression among stroke patients is related to their stroke severity.
Correlations between basic attributes and cognitive functions: Lo et al. (2014) suggested that cognitive functions are positively correlated with educational level, which was in line with the results of this study, and indicated that patients' educational level affects their MMSE scores, as patients with higher educational levels had better cognitive functions. With respect to marital status, single patients had better cognitive functions, and in this study, all the single patients were aged between 24 and 45 years. The presence of chronic diseases was also related to cognitive functions in this study, as patients with chronic diseases had lower cognitive functions. In a study by Chen et al. (2016), comorbidities resulting from the poor management of chronic diseases had statistically significant impacts on cognitive functions. Some researchers suggested that the decline in cognitive functions is not only a sign of normal aging, but could also be due to organ or functioning damages caused by diseases (Dai et al., 1999). Therefore, the results of this study were in accordance with those obtained by other researchers.
Correlations between rehabilitation and daily living activities: The Barthel index was adopted in this study to measure and assess the daily living activities of stroke patients. The results showed that there were negative correlations between daily living activities and respectively, aphasia, rehabilitation conditions, and rehabilitation frequency. The daily living activities of patients with aphasia were worse than those without aphasia; the daily living activities of patients in rehabilitation were worse than those not in rehabilitation; and the daily living activities of patients with high rehabilitation frequency were worse than those with low rehabilitation frequency. This indicates that stroke patients who require higher rehabilitation training frequencies suffer from more severe acquired physical damage, which worsens their daily living activities. Hsien et al. (2015) concluded that proactive rehabilitation implemented throughout the post-stroke golden recovery period was able to decrease the loss of activity and promote improvement in daily living activities among patients. Their conclusions were in accordance with the results of this study, but they also suggested that hemorrhagic stroke patients had better improvements than ischemic stroke patients, which was not observed in this study. This may be due to the low number of hemorrhagic patients in this study, which only accounted for around 10% or so of the total sample, such that the improvement efficacies were not significant.
Post-intervention GEE analyses of the basic attributes and the degree of depression improvement of the two groups showed that the experimental group, the degree of depression improvement seen at six to eight weeks after the end of sunlight therapy was related to stroke duration, age, gender, and rehabilitation frequency.
The one month after the end of sunlight therapy showed that there were depression improvements statistically significant.The effects of sunlight therapy on emotional responses may be delayed and not immediately apparent. However, there is little literature on the implementation of sunlight therapy to improve depression among stroke patients. In a similar study, West et al., 2017a, West et al., 2017b utilized the design of naturalistic lighting that mimicked the natural daytime spectrum variation in stroke patients' wards and showed that its effect one month after sunlight therapy on stroke patients depression improvement was statistically significant.
In relation to improvements in daily living activities, after the experimental group had received the 14-day sunlight therapy, that their daily living activities (Barthel index score) had improved. However, since the improvements in daily living activities are correlated with stroke severity (NIHSS score), the first and second post-test NIHSS scores among patients in the experimental group were significantly better than their pre-test scores, which may also have been due to natural healing. On the other hand, the improvements in daily living activities (Barthel index score) and stroke severity (NIHSS score) among patients in the control group were relatively gradual and were not statistically significant.
6. Conclusion
In terms of nursing practice, the results of this study revealed that the incidence of developing PSD was 47.8%, whereas a study by Chiu et al. (2014) showed that the PSD prevalence was 30.29%. This indicates that there has been an increase in PSD among stroke patients; hence it is not uncommon for stroke patients to develop depressive emotions and disorders. A subsequent analysis of age and gender showed that the degree of depression was higher among middle-aged and older patients than young patients, and that females had more depressive symptoms than males. These findings demonstrate the severity of depression among stroke patients, which is a problem that affects the patients themselves and their families or caretakers, and also has impacts on their post-stroke rehabilitation and daily living activities during the recovery period. The utilization of non-invasive sunlight therapy can be implemented in moderation to improve patients' emotions, promote health and mobility, alleviate stroke severity, increase levels of vitamin D in the blood, and reduce the severity of osteoporosis (Sato et al., 2003). Recently, West et al. (2019) designed a randomized controlled trial and showed that the serotonin levels in stroke patients in rehabilitation had increased after receiving naturalistic light therapy, which stabilized their emotions. Indeed, it is recommended that clinical care professionals should provide relevant health education to their patients to improve their emotional health.
Declarations
Author contribution statement
Wang Su-Jen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chen Miao-Yen: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at clinicaltrials.gov under the registration number NCT04036565.
References
- Alajbegovic A., Djelilovic-Vranic J., Alajbegovic S., Nakicevic A., Todorovic L., Tiric-Campara M. Post stroke depression. Med. Arch. 2014;68(1):47. doi: 10.5455/medarh.2014.68.47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.C., Tseng M.C. Costs of acute care of first-ever ischemic stroke in Taiwan. Stroke. 2003;34(11):e219–e221. doi: 10.1161/01.STR.0000095565.12945.18. [DOI] [PubMed] [Google Scholar]
- 2021.Chang C.Y., Li C.J., Cheng C.W. Application of hybrid solar light collectors and artificial light sources on interior lighting. J. Design. 2011;16(2):45–60. [Google Scholar]
- Chen H.H., Huang H.L., Lin C.M., Wang H.L. Association between metabolic syndrome and cognitive impairment among community-dwelling elderly in Taiwan. Taiwan Geriatr. Gerontol. 2016;11(4):262–277. [Google Scholar]
- Cheng Y.Y., Chou C.L., Cheng S.P., Liu T.J., Chan R.C., Wong W.J. Risk factors of post-stroke depression in middle-aged adults and the elderly. Taiwan J. Phys. Med. Rehabil. 2005;33(3):149–159. [Google Scholar]
- Chiu S.Y., Tsao L.L., Tsai T.Y. Related factors for depressive symptoms among patients with stroke-A hospital-based study in southern Taiwan. Chinese J. Occup. Med. 2014;21(2):73–80. [Google Scholar]
- Dai Y.T., Yip P.K., Huang G.S., Lou M.F. Cognitive function of elderly patients. Formos. J. Med. 1999;3(3):279–286. [Google Scholar]
- Daemen E.M.L., Flinsenberg I.C.M., Van Loenen E.J., Cuppen R.P.G., Rajae-Joordens R.J.E. Adaptable healing patient room for stroke patients. Methods Inf. Med. 2014;53(5):406–415. doi: 10.3414/ME13-02-0032. [DOI] [PubMed] [Google Scholar]
- Dowling G.A., Hubbard E.M., Mastick J., Luxenberg J.S., Burr R.L., Van Someren E.J. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer's disease. Int. Psychogeriatr. 2005;17(2):221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N.W., Liu H.C., Wang P.F., Liao K.K., Yan S.H., Lin K.P.…Hsu T.C. Chinese version and norms of the mini-mental state examination. Taiwan J. Phys. Med. Rehabil. 1988;16:52–59. [Google Scholar]
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P.…Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Hsien H.H., Liou W.C., Yang Y.H. Post-acute care improves daily activity functions of stroke patients: an effectiveness analysis. Taiwan Geriatr. Gerontol. 2015;10(3):159–171. [Google Scholar]
- Kwa V.I., Limburg M., de Haan R.J. The role of cognitive impairment in the quality of life after ischaemic stroke. J. Neurol. 1996;243(8):599–604. doi: 10.1007/BF00900948. [DOI] [PubMed] [Google Scholar]
- Lai Y.H., Fang T.C. Recent advances in vitamin D. J. Intern. Med. Taiwan. 2014;25:7–14. [Google Scholar]
- Lee Y., Yang M.J., Lai T.J., Chiu N.M., Chau T.T. Development of the Taiwanese depression questionnaire. Chang Gung Med. J. 2000;23:688–694. [PubMed] [Google Scholar]
- Lee H.C., Chang K.C., Huang Y.C. Hospital-based primary ischemic stroke three-year mortality and initial brain stroke severity studies. Stroke. 2012;19(2):10–11. [Google Scholar]
- Lin T.C., Liao Y.C. The impact of sunlight exposure on the health of older adults. J. Nurs. 2016;63(4):116–122. doi: 10.6224/JN.63.4.116. [DOI] [PubMed] [Google Scholar]
- Lo H.H., Lee L.H., Lee H.C., Hsu N.L. A survey of depression and its related factors of the veterans' in longterm care facilities. J. Health Architect. 2014;1(3):68–76. [Google Scholar]
- Loewen S.C., Anderson B.A. Reliability of the modified motor assessment scale and the Barthel index. Phys. Ther. 1988;68(7):1077–1081. doi: 10.1093/ptj/68.7.1077. [DOI] [PubMed] [Google Scholar]
- Mitchell A.J., Sheth B., Gill J., Yadegarfar M., Stubbs B., Yadegarfar M., Meader N. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatr. 2017;47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T., Leppävuori A., Siira I., Vataja R., Kaste M., Erkinjuntti T. Frequency and clinical determinants of poststroke depression. Stroke. 1998;29(11):2311–2317. doi: 10.1161/01.str.29.11.2311. [DOI] [PubMed] [Google Scholar]
- Qian J., Hsu L.C. Development and prospect of acute ischemic stroke therapy. Clin. Med. 2018;81(5):296–301. [Google Scholar]
- Robinson R.G., Price T.R. Post-stroke depressive disorders: a follow-up study of 103 patients. Stroke. 1982;13(5):635–641. doi: 10.1161/01.str.13.5.635. [DOI] [PubMed] [Google Scholar]
- Robinson R.G., Jorge R.E. Post-stroke depression: a review. Am. J. Psychiatr. 2015;173(3):221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- Sato Y., Metoki N., Iwamoto J., Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in stroke patients. Neurology. 2003;61(3):338–342. doi: 10.1212/01.wnl.0000078892.24356.90. [DOI] [PubMed] [Google Scholar]
- Søndergaard M.P., Jarden J.O., Martiny K., Andersen G., Bech P. Dose response to adjunctive light therapy in citalopram-treated patients with post-stroke depression. Psychother. Psychosom. 2006;75(4):244–248. doi: 10.1159/000092895. [DOI] [PubMed] [Google Scholar]
- Statistics Department, Ministry of Health and Welfare . 2016. 2015 Mortality Cause Statistics.http://www.mohw.gov.tw/cht/DOS/Statistic.aspx?f_list_no=312&fod_list_no=6201 Retrieved from. [Google Scholar]
- Statistics Department, Ministry of Health and Welfare . 2017. 2016 Mortality Cause Statistics.http://www.mohw.gov.tw/cp-3250-33598-1.html Retrieved from. [Google Scholar]
- Statistics Department, Ministry of Health and Welfare . 2018. 2017 Mortality Cause Statistics.https://www.mohw.gov.tw/cp-16-41794-1.html Retrieved from. [Google Scholar]
- 2022.Sun T.K., Chiu S.C., Yeh S.H., Chang K.C. Assessing reliability and validity of the Chinese version of the stroke scale: scale development. Int. J. Nurse. Studies. 2006;43(4):457–463. doi: 10.1016/j.ijnurstu.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Szu L.Y., Hsieh S.I., Tseng S.M., Huang T.H. The determinants of dysphagia in patients with stroke during hospitalized rehabilitation. J. Nurs. 2017;64(3):43–55. doi: 10.6224/JN.000039. [DOI] [PubMed] [Google Scholar]
- Tsai Y.F., Wong T.K., Juang Y.Y., Tsai H.H. The effects of light therapy on depressed elders. Int. J. Geriatr. Psychiatr. 2004;19(6):545–548. doi: 10.1002/gps.1125. [DOI] [PubMed] [Google Scholar]
- Tsui Y.K. Treatment of acute ischemic stroke in the arterial artery - introduction, current status, and prospects. Stroke. 2016;23(3):11–14. [Google Scholar]
- Wang C., Wang S., Shi Y., Yang Y., Zhang N., Wu S.…Zhu M. Impact of aphasia upon the prevalence of depressive symptoms and functional outcomes among stroke survivors: a prospective cohort study. Eur. Neuropsychopharmacol. 2016;26:S660–S661. [Google Scholar]
- Wang Q., Zhu Z., Liu Y., Tu X., He J. Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. 2018;8(2) doi: 10.1002/brb3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A., Jennum P., Simonsen S.A., Sander B., Pavlova M., Iversen H.K. Impact of naturalistic lighting on hospitalized stroke patients in a rehabilitation unit: design and measurement. Chronobiol. Int. 2017:1–11. doi: 10.1080/07420528.2017.1314300. [DOI] [PubMed] [Google Scholar]
- West A., Simonsen S., Zielinsky A., Cyril N., Schønsted M., Sander B.…Iversen H. 2017. The Effect of Circadian Light on Depressive Mood in Post Stroke Patient during Admission for Rehabilitation. [Google Scholar]
- West A.S., Sennels H.P., Simonsen S.A., Schønsted M., Zielinski A.H., Hansen N.C.…Iversen H.K. The effects of naturalistic light on diurnal plasma melatonin and serum cortisol levels in stroke patients during admission for rehabilitation: a randomized controlled trial. Int. J. Med. Sci. 2019;16(1):125. doi: 10.7150/ijms.28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. The Known Health Effects of UV.http://www.who.int/uv/faq/uvhealtfac/en/index1.html Retrieved from. [Google Scholar]
- Wu M.C., Sung H.C. The application of light therapy in geriatric care. Tzu Chi Nurs. J. 2010;9(3):63–70. [Google Scholar]