Abstract
The cyanide, haematology and histopathology profiles of white albino rats fed with ‘fufu’-based diets were monitored. The cassava tubers were subjected into different processing operations: spontaneous-soaked traditional method (SWI), grated before spontaneously-fermented without starter culture (CWI) and those grated, blanched at 65 °C for 15 min before fermented with microorganisms isolated, purified and identified from spontaneously fermented ‘fufu’ categorized into Bacteria only (BAP), Bacteria and Yeast (BYP), Yeast only (YAP), Bacteria and Mould (BMP), Mould and Yeast (MYP) and Mould only (MAP) and were oven dried and milled. The commercial ready-to-eat ‘Fufu’ (CWF) was served as control for laboratory processed samples. Albino rats of the wister strain with four (4) rats per group were fed with 50 % of Commercial vital feed (CVF) and 50% each of the remaining nine (9) “Fufu” samples in ratio 1:1 before haematology and histopathology profile of the animals were investigated. It was found out that Samples BMP and MAP had abnormal high Neutrophil (58–60; 41–42 %) lower Lymphocyte (40–43; 58–61 %), lower Packed cell volume (46–48; 45–47 %) and higher cyanide in the blood (0.004–0.006 mg/L) with sample BMP highest white blood cell (23–24 × 10⁹/L) and sample MAP least white blood cell (6.5–6.6 × 10⁹/L) when compared with other samples which were within the acceptable recommended values for animal studied for haematology and histopathology profiles. The “fufu” samples had reduced cyanide levels ranging from 0.001 mg/L to 0.006 mg/L in the blood of the animals assayed due to the processing operations used. Therefore, combined use of bacteria isolated as starter cultures in the production of odourless “Fufu” have acceptable haematology, histopathology and reduced cyanide level which suggests the suitability in terms of safety for human consumption.
Keywords: Biotechnology, Microbiology, Fufu, Starter cultures, Cyanide, Haematology, Histopathology profiles
Biotechnology; Microbiology; Fufu; Starter cultures; Cyanide; Haematology; Histopathology profiles.
1. Introduction
Cassava roots, peeled cassava roots and cassava leaves contain free and bound cyanogenic glucosides, linamarin and lotaustralin, making them not to be consumed raw. These are decomposed by linamarase, a naturally-occurring enzyme in cassava, liberating hydrogen cyanide (HCN) which are in contact with linamarase (Ngiki et al., 2014; Cardoso et al., 2005). Excess cyanide residue from improper preparation is known to cause acute cyanide intoxication, and goitres, and has been linked to ataxia (a neurological disorder affecting the ability to walk, also known as “konzo”). It has also been linked to tropical goitre, cretinism, calcific pancreatitis in humans, leading to chronic pancreatitis and cyanide poisoning as a result of long term consumption of cyanide-containing foods (Abraham et al., 2016; Egwim et al., 2013; Akyildiz et al., 2010; Bhatia, 2002). The potential toxicity due to the presence of cyanogenic glycosides can be reduced by appropriate preparation of the plant material prior to consumption as food for animals and humans (Bolariwa et al., 2016). Different processing operations including peeling, slicing soaking, grating, fermentation and cooking (e.g. boiling or baking) are usually employed to encourage break down of the cyanogenic glycosides, and subsequent removal of the liberated hydrogen cyanide (a potential toxic cyanogenic compound) to avoid getting sick and death (Egwim et al., 2013; FSANZ, 2008; Agbor-Egbe and Lape- Mbome, 2006).
One of the fermented cassava products is “Fufu”, which is also called “Akpu” in Nigeria, a fermented carbohydrate wet-paste meal from cassava (Oyewole and Sanni, 1995). Many researchers have reported and shown that different processing operations to include peeling of peels before soaking, cooking, drying and grating eliminate cyanide or reduced potential toxic cyanogenic compound to tolerable level before fermentation set in (Bolariwa et al., 2016). Ingestion/excess consumption of freshly harvested, under-processed or unprocessed cassava based diets have been reported to enzymatically breakdown of the glucoside to releasing HCN and thereby causing poisoning/toxicity may be acute and/or chronic. This toxicity is may be responsible for reduced growth rates and paralysis of the hind limbs and muscular weakness in animal (rats, pigs, goat etc (Bamidele et al., 2015; Lukuyu et al., 2014; Egwim et al., 2013; Agbor-Egbe and Lape Mbome, 2006). The cassava is processed traditionally by peeling the cassava tuber, washing, cutting into smaller pieces and steeping/soaking for 4–5 days. The fermentation process of cassava tuber further helps in detoxifying the cassava pulp of potential toxic cyanogenic compound, extend the shelf life (preservation) and impart flavour development to the end-products. The “Fufu” is slurred in boiling water, cooked and reconstituted by continuous stirring to produce stiff elastic dough eaten with flavoured sauces, soups or stews and preparation vary from locality to locality. It has been noted that “Fufu” supplies calories needed for rural life more adequately than most cassava products (IITA, 2007).
However, haematological parameters are related to the blood and blood forming organs used as good indicators in routine screening for the health and physiological status of livestock and even humans. It is an ecological and physiological interests that help in understanding the relationship of blood characteristics and environment to stimulate good performance and monitoring fed toxicity, especially with fed constituents that affect the blood as well as the health status of farm animal. Apart from the blood, it is also a commonplace in feeding trials to use some internal organs as indicators of toxicity (Waugh and Grant, 2001; Bamishaiye et al., 2009; Aro et al., 2013; Isaac et al., 2013). Abnormalities in histopathology have been associated with toxic substances in the feed as a result of metabolic rate of the organs in attempt to reduce these toxic elements or anti-nutritional factors to non-toxic metabolites (Aderemi, 2003; Obun et al., 2008; Olafadehan, 2011). The present work was designed to reveal the safety of using different starter cultures in fermentation of “Fufu” on Wister albino rats fed with the ‘fufu–based diet. The processing operations will be evaluated in order to access its cyanide level, haematology and histopathology profiles variations.
2. Materials and methods
2.1. Collection of materials
Cassava tuber cultivars named TMS 320572 developed by IITA, Ibadan were distributed to farmers for planting to have good yield, less hydrogen cyanide (HCN) and early maturity (9–12 months). These cassava variety were harvested at point of maturity (November, but planted all-round the year) of 50 kg at Teaching and Research Farms located at 7.2960 °N, 5.1506° E geographic coordinates of Federal University of Technology Akure, Ondo State, Nigeria.
2.2. Chemicals and reagents
All chemicals and reagents used were of analytical grade obtained from SIGMA-ALDRICH, Germany and USA. These include: Ortho-phosphoric acid, potassium phosphate buffer (pH 7.0), 5% potassium iodide solution (w/v), EDTA, sodium hydroxide pellets, Ag (NO3)2 solution, 10% formalin saline, ethanol (95% v/v), and distilled. The water was deionized using deionizer (Dual-bed Deionizer DM-6100 Series, USA) through the process of deionization exchanges whereby total dissolved solids (TDS) are removed from water. The deionized water (DI) resins attract non-water ions and replace them with water ions, leaving a more pure water form. Deionized water was used for the preparation of all solutions. Microbiological media [Nutrient Agar (NA), deMan Rogosa Sharpe Agar (MRS) and Potato Dextrose Agar (PDA)] were L: S- Biotech, USA.
2.3. Microbiology analyses
Spontaneous fermentation of cassava tubers (TMS 320572 were carried out. The media [Nutrient Agar (NA), deMan Rogosa Sharpe Agar (MRS) and Potato Dextrose Agar (PDA)] were prepared according to manufacturer's specification for isolation. The dilution factor was at 10¯³ for saline solution. One gram each of the ‘fufu’ sample was separately homogenized in 9 ml of distilled water. Tenfold serial dilution of each sample was performed until 10−3 level of dilution was obtained. 1ml from the dilution (10−3) in each sample was pour plated on sterilized media stated above with an autoclave (Model YX-280A, Pathway Medical England) at 121 °C for 15 min and incubated aerobically for 24 h at 37 °C, anaerobically for 48 h at 37 °C and 72–120 h at 25 °C for bacterial and fungal growths respectively according to Babatuyi et al. (2019) and Fawole and Oso (2007). All microbial plates were prepared in triplicate.
2.3.1. Microbial identification
The isolated microorganisms were purified and identified according to Ochei and Kolhatkar (2008) and Bergery's manual of identification (Holt et al., 1994) by examining colonies morphology on their cultural properties followed by biochemical tests (Motility, sugar fermentation, starch hydrolysis, citrate, coagulase, gram stain, catalase and oxidase) under oil immersion lens (X100) objective lens. The fungal isolates on the other hand, were characterized by their cultural properties stained with cotton-blue lacto phenol solution and morphological observations under low power objective lens. The microorganisms were viewed using microscope (Models CHA-213 and CHB-213, Tokyo, Japan) according to Barnett and Hunter (1972).
2.3.2. Characterization of isolates
The microorganisms identified and used for the fermentation were as follows: Bacteria (Bacillus subtilis, Corynebacterium manihot, Lactobacillus plantarum and L. fermentum). Fungi (Saccharomyces cerevisiae, Candida stellata, Kloeckera apiculata (Hanseniaspora uvarum), Aspergillus niger and Penicillium notatum). These identified microorganisms were prepared and made into broth and were stored. 18h old broth culture of each of these identified microorganisms were harvested and washed three times with 0.1 M potassium phosphate buffer and centrifuged using refrigerated centrifuge (Model Harrier 18/80, Henderson Biomedical LTD MSE, UK) at 6000 rpm for 10 min. The cells were then aseptically re-suspended in 20 mL sterile 0.1M potassium phosphate buffer (pH 7.0) and standardized using a spectrophometer (Model V-721 VIS, China) at 600 nm wavelength such that 1.0 mL of the suspension contained about 2.0 x 1010 cells.
2.4. Processing of cassava tubers into “Fufu”
2.4.1. Processing of cassava tubers into mash
Fifty-two kilograms (52 kg) cassava roots were sorted, washed, peeled and re-washed before dividing into three (3) portions in which one portion was grated and blanched at 65 °C for 15 min before inoculated with the pure isolates categorized into six (6) groups as Bacteria alone (BAP), Bacteria and Yeast (BYP), Yeast alone (YAP), Bacteria and Mould (BMP), Mould and Yeast (MYP) and Mould alone (MAP). Another portion was only grated without inoculation (CWI), while the last portion was not grated but cut with knife into pieces (3–4 cm) and soaked (SWI) in 300 mL of sterilized water, making all the samples eight.
2.4.2. Preparation of “Fufu”
The portion of the cassava mash to be fermented with starter cultures was weighed to have 200 g of the mash, 300 mL of water and 30 ml broth of inocula for each group (i.e. six groups) as well as the remaining two portions (CWI and SWI) stated in section 2.4.1 above, but without addition of starter cultures/broth inocula. These three portions were put in different sterile plastic covered container and allowed to ferment. The fermentation was carried out for 3 days at 27 ± 2 °C and after which, each ‘Fufu’ samples was sieved with muslin cloth to remove the shafts. The slurry was allowed to settle to form cake; dried in a hot-air oven (Model TT-9053, Techmel & Techmel, USA, 2015) at 60 °C for 3 days and milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK) prior to animal bioassay analysis. The fermenting water of those in the first portion was changed at every 24 h for 3 consecutive days with 300 mL of sterilized water and re-inoculated with 30 mL broth of inocula for each group using McFarland as reference.
2.5. Chemical analyses
2.5.1. Determination of total cyanide level
The cyanide content was determined according to the method of AOAC (2015). Four grams of each of the “Fufu” samples was soaked in a mixture of 40 mL distilled water and 2 mL of Ortho-phosphoric acid. The “Fufu” sample was thoroughly mixed, stoppered and left overnight at room temperature of 28 °C ± 2 °C to set all the bounded hydrocyanic acid free. The resulting mixture (“Fufu” sample) was transferred into distillation flask and a drop of heptane (antifoaming agent) was added with broken chips to prevent ‘bumping’. The flask was fitted to other distillation apparatus and distillation was carried out with Pyrex™ Regular Cyanide Distillation Apparatus (Model 3350C Fisher Scientific, USA). 45 mL of the distillate was collected in the receiving flask containing 4 ml of distilled water and 0.1 g of sodium hydroxide pellets. The distillate was then transferred into 50 mL volumetric flask and made up to the mark level with distilled water. Distillate water (20 mL) was collected into a conical flask and 1.6 mL of 5% potassium iodide solution (w/v) was added and titrated against 0.01M Ag (NO3)2 solution. The blank was also titrated until the end point was indicated by a constant faint, but permanent turbidity.
| (i) |
2.5.2. Animal bioassay
2.5.2.1. Feeding treatments on albino rats of the wister strain
Forty (40) white albino rats of two (2) weeks old weighing 30.41–30.90 g were obtained from the Animal House Unit of the Department of Microbiology, Federal University of Technology, Akure. They were fed with growers feed (vital feed®) to acclimatize at temperature 22 ± 3 °C for seven (7) days. They were divided into ten (10) groups; with four (4) rats per group and were kept in clean plastic cages and maintained under standard laboratory conditions (temperature 22 ± 3 °C; photo period, 12 h natural light and 12 h dark; humidity, 40–45%) (Lawal et al., 2015). Four groups out of the ten groups were fed with 100 % of these CVF, CWI, SWI and CWF, while the other six groups were fed with 50 % of CVF and each of the other “Fufu” samples left at 50%, 60%, 70%, 80% and 90% respectively. The animals were monitored for seven (7) days during which they were examined daily for acceptability and weight gain or loss. CVF and each “Fufu” at ratio (50:50) which had the best weight gain was used to feed forty (40) albino rats for another twenty-one (21) days before the rats were examined and compared with CVF sample for haematology and histopathology profiles.
2.5.2.2. Haematological analysis
The albino rats were fasted overnight with access to water ad libitum and sacrificed under chloroform anaesthesia, where 99.8% chloroform was poured into an airtight enclosed container and the rats were allowed to inhale the vapour for a period of time until they began to feel dizzy, weak and unconscious. The blood samples were collected randomly from the four (4) albino rats in each group using the methods of Ahamefule et al. (2006) by puncturing the jugular vein and 2 ml of blood was collected from free flow of blood into labelled sterile universal bottles containing Ethylene-diamine-tetra-acetic (EDTA) and analysed for complete white blood cell count and differential count (Eosinophil, monocyte, lymphocyte, neutrophil and erythrocyte sedimentation rate). The analysis was carried out at the Federal University of Technology Akure, Health Centre.
2.5.2.3. Histopathological assay
Histopathological examination of the kidney, liver and ileum of the cassava fed albino rats were carried out by fixing the tissues in a large fixative (10% formalin saline) and tissues were embedded in paraffin, sectioned and stained with haematoxylin and eosin using standard methods. The slides were read and the photomicrograph was taken at a resolution power Hex400 according to the method of Ebuehi and Mbara (2011).
2.5.3. Statement of animal rights
The study was approved by the Ethical Committee for Laboratory Animals of Agriculture and Agricultural Technology, Akure Nigeria (FUTA/SAAT/2019/001). The experiments on the animals were conducted in accordance with the force laws and regulations as regards animal use and care as contained in the Canadian Council on Animal Care Guidelines and Protocol Review (CCAC, 1993).
2.6. Statistical analysis
Data were subjected to analysis of variance using SPSS (IBM version.21.0, SPSS Inc., Quarry Bay, Hong Kong) and present as mean (±SD). Comparisons between difference groups was done using Analysis of Variance (ANOVA) and Duncan's Multiple Range Test (DMRT). Values of p < 0.05 were considered as statistically significant as described by Yalta and Talha (2008).
3. Results and discussion
3.1. Microorganisms used for fermentation
The category of microorganisms isolated and identified for the fermentation of ‘Fufu’ are both bacteria and fungi. The bacteria are: B. subtilis, Corynebacterium manihot, Lactobacillus plantarum and L. fermentum, while the fungi are: Saccharomyces cerevisiae, Kloeckera apiculata (Hanseniaspora uvarum), Candida stellata, Aspergillus niger and Penicillium notatum.
The dynamics of microorganisms' selection as starter cultures in the fermentation of ‘Fufu’ allowed the interaction of the microorganism (s) to add value to it such as to improve the nutrition (i.e. lactic acid bacteria and Bacillus subtilis during break down of some enzymatic activities to release nutritional compounds), impart both flavour (moulds) and aroma (yeasts) such as Corynebacterium manihot and categories of moulds and yeasts already mentioned above. During fermentation, the microbial cultures breakdown the cell wall of the cassava root by the enzymes secreted from the microbial metabolic activities to hydrolyse polysaccharide into sugar and other by products to include alcohol. The yeasts secreted secondary metabolites to inhibit growth of mycotoxin-producing moulds (Alexandraki et al., 2013; Tamang et al., 2015). The moulds produce both intracellular and extracellular proteolytic and lipolytic enzymes that influence the flavour and texture of the ‘Fufu’. Furthermore, the enzymatic activities degrade the antinutritional factors (tannin, oxalate and phytate etc.) to enable bioavailability of minerals to the body.
3.2. Cyanide level in the blood
There was no deposit of cyanogenic glycosides in the blood of the albino rats in all the samples. The values ranged between (0.001 and 0.006 mg/L). This could be attributed to effective different processing operations used which opened up the surface area of the cassava tuber to allow breakdown of cyanogenic glycoside. This was further explained according to Hernadez et al. (1995) and Carlsson et al. (1999) that the amount of the cyanogenic glycosides ingested could be absorbed and excreted intact in their urine. The fraction unabsorbed can be enzymatically converted to hydrogen cyanide (HCN) by microorganisms in the gastrointestinal tract (GIT). Rats excreted intact linamarin from their blood by urinating intact absorbed dose and less than half (½) of orally ingested linamarin was converted to cyanide and hence, about one-fourth (¼) was excreted unchanged.
3.3. Haematology parameters
The leucocyte differential counts (neutrophil, lymphocyte, monocyte, eosinophil and basophil) and erythrocyte sedimentation rate (ESR) are shown in Table 1. The monocytes, eosinophil, basophil and ERS values of the samples ranged between 0.00 ± 0.00 and 0.03 ± 0.00 % which are within normal range for Wister rat (0–0.06 %) which justified the efficacy of the processing methods in reducing the toxic HCN in the samples to a tolerance level or non-toxic level. This indicates that the rats were in good health. The function of the ESR is to indicate the presence of infection in a living system especially the infection that affects protein content which constitutes the antibody. Adverse effect of protein content depresses the body immune thereby producing negative effect on the animal or human's PCV which is usually seen in immune-deficient patients. The PCV level of the albino rats fed with the “Fufu” samples produced with different starter cultures and commercial vital feed (CVF) are shown in Table 1. The PCV counts ranged between 47.00 ± 1.00 % and 55.53 ± 0.50 %, while samples BMP and MAP showed the least PCV values between 47.00 ± 1.00 % and 46.00 ± 0.10% respectively. If the feeding continues, the PCV of those wister albino rats may tend towards being anaemic which may encourage erythrocytes destruction or hindering erythrocyte products as reported by Joshi et al. (2002); Omitoyin and Ajibade (2014) in the work of Adeosun et al. (2019). The PCV of the Wister rats fed with other samples are still within normal range (18–48%) for albino rats when compared with control. Their values ranged between 55 and 56% for the period of feeding. The higher PCV in the other “Fufu” samples could be a reflection of efficient detoxification of cassava mash by different processing treatments given to have prevented interference/impurity in the blood production. Adequate PCV level in the body indicates the absence of normocytic anaemia. This is in correlation with the nutritional status of animals and adequate protein in the diet (Unigwe et al., 2016).
Table 1.
Haematological Parameters of Wister White Albino Rats Fed with Fufu-based diets.
| PARAMETER | ∗REF. | CVF | BAP | BYP | YAP | CWI | SWI | BMP | MYP | MAP | CWF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ∗∗Cyanide in blood (mg/L) | <0.50 mcg/ml | 0.000 ± 0.00d | 0.0015 ± 0.00c | 0.0017 ± 0.00c | 0.0020 ± 0.00c | 0.0015 ± 0.00c | 0.0035 ± 0.00b | 0.0050 ± 0.00a | 0.0045 ± 0.00ab | 0.0048 ± 0.00a | 0.0040 ± 0.00ab |
| PCV (%) | 18–48 | 55.53 ± 0.50a | 53.00 ± 0.00b | 52.50 ± 0.29ab | 52.00 ± 1.00ab | 51.00 ± 1.00cd | 50.50 ± 0.05d | 47.00 ± 1.00e | 50.50 ± 0.50d | 46.00 ± 0.10e | 50.00 ± 1.00d |
| WBC per mm³) | 4400–14800 | 14100 ± 0.10b | 13220 ± 0.26d | 11200 ± 0.20e | 10650 ± 0.05f | 10150 ± 0.05g | 10050 ± 0.05g | 23500 ± 0.50a | 13550 ± 0.05d | 6550 ± 0.05h | 10050 ± 0.05g |
| Neutrophil (%) | 13–36 | 18.50 ± 0.50cd | 19.50 ± 0.50c | 16.50 ± 0.50e | 15.50 ± 0.50ef | 14.50 ± 0.50f | 12.00 ± 1.00g | 59.00 ± 1.00a | 18.00 ± 1.00d | 41.50 ± 0.50b | 12.00 ± 1.00g |
| Lymphocyte (%) | 61–86 | 80.50 ± 0.50a | 78.50 ± 0.50b | 81.00 ± 1.00a | 70.50 ± 0.50d | 75.50 ± 0.50c | 76.50 ± 0.50c | 41.50 ± 1.50f | 77.00 ± 1.00bc | 59.50 ± 1.50e | 75.50 ± 0.50c |
| Eosinophil (%) | 00–06 | 0.00 ± 0.00c | 0.02 ± 0.00a | 0.01 ± 0.00abc | 0.01 ± 0.00abc | 0.01 ± 0.00abc | 0.00 ± 0.00c | 0.01 ± 0.01ab | 0.00 ± 0.00bc | 0.00 ± 0.00bc | 0.01 ± 0.00abc |
| Basophil (%) | 00–02 | 0.00 ± 0.00e | 0.00 ± 0.00e | 0.03 ± 0.00a | 0.02 ± 0.00bc | 0.00 ± 0.00e | 0.01 ± 0.00de | 0.01 ± 0.00cd | 0.02 ± 0.01ab | 0.01 ± 0.00cd | 0.01 ± 0.00cd |
| Monocyte (%) | 00–01 | 0.01 ± 0.00de | 0.01 ± 0.00de | 0.02 ± 0.01ab | 0.02 ± 0.00bc | 0.01 ± 0.00cd | 0.01 ± 0.00cd | 0.00 ± 0.00e | 0.03 ± 0.00a | 0.00 ± 0.00e | 0.00 ± 0.00e |
| ESR% | 00–05 | 0.01 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.01 ± 0.09a | 0.01 ± 0.00b | 0.01 ± .0.00b | 0.01 ± 0.00b | 0.02 ± 0.00b | 0.01 ± 0.00b | 0.00 ± 0.00b |
Values on the same row with different superscripts are statistically different (P < 0.05) Mean ± SD.
CVF: Commercial vital feed, BAP: Bacteria only, BYP: Bacteria and Yeasts, YAP: Yeasts only, CWI: Grating method without inoculum, SWI: Soaking method without inoculum, BMP: Bacteria and Moulds, MYP: Moulds and Yeasts, MAP: Moulds only, CWF: Commercial ready-to-eat Fufu”, PCV: Packed Cell Volume, ESR: Erythrocyte Sedimentation Rate.
The white blood cells (WBC) of the albino rats are shown in Table 1. The “Fufu” samples BMP and MAP had the highest (23500 WBC per mm³) and lowest (6550 WBC per mm³) values when compared with the control (14100 per mm³) and with other samples respectively. This could be as a result of red blood cell (RBC) fighting back by secretion of the antibodies (a blood protein) produced in the body’ defensive mechanisms to counteract with antigen (substances recognizes as alien) as an indication of infection. It was reported that toxic/foreign substance in feed tends to suppress haemopoietin tissues with consequent production of lower WBC, while significant WBC value may be associated with microbial infection or presence of antigens or foreign proteins in the circulatory system (Eroschenko, 2000; Ahamefule et al., 2006). The albino rats produced more WBC in response to the relatively high microbial metabolic activities to release toxic substance from the diet of those fed in combination of moulds (i.e. samples BMP and MAP), trying to defend against infectious agent. This could have caused malfunctioning of kidney and other organs by invasive of these foreign bodies (Adeosun et al., 2019).
The Neutrophil of ‘Fufu’ samples BMP (59.00 ± 1.0 %) and MAP (41.50 ± 0.50 %) had abnormal higher values when compared with the control (18.50 ± 0.50 %) and other samples. They ranged within reference value (13–36 %) for albino rats. The variations from the value of those samples with higher and lowest neutrophil resulted in low intake of the feed. This could be due to high metabolic level of the fungi (mould) present in ‘Fufu’ samples BMP and MAP. The amino acid imbalance in the body can possibly be a deficiency in methionine, which might have exerted a physiological influence on the ACTH (Adrenocorticotropic hormone) release to bring about the changes in the leukocyte counts similar with bacterial infection (Akinmutimi, 2004). Likewise, lower lymphocytes were recorded in ‘Fufu’ samples BMP (41.50 ± 1.50 %) and MAP (59.5 ± 1.50 %) when compared with the control (80.5 ± 0.50 %) and other samples; which were within the reference range (61–86 %) for albino rats. There may be an indication of disease of immune system known as lymphocytopenia (abnormal low level of lymphocyte in the blood). This usually occurred when the B-lymphocytes that produces antibiotics are not secreted enough due to several factors to include metabolites from microbial activities from samples BMP and MAP. This was seen in the rats fed with ‘Fufu’ samples having moulds in combination which may be due to mycotoxins produced from Aspergillus niger and Penicillium notatum that could cause food poisoning.
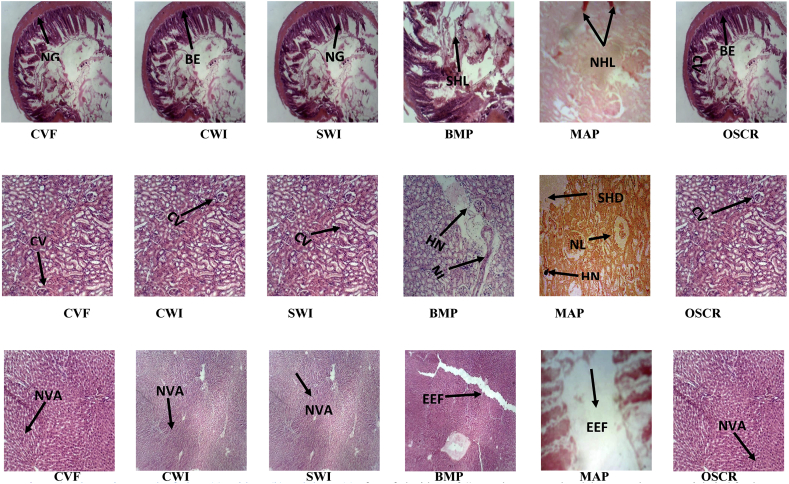
3.4. Histopathological structures
The Liver histological assessment is shown in Figure 1a. Bilirubin is a diagnostic marker of liver and blood disorders, and the end product of the breakdown of haemoglobin according to Singh et al. (2011). When there is damage to the liver cell, there is impairment of bilirubin excretion. This caused accumulation of bilirubin in the blood and extracellular fluid to alter the integrity of the hepatocytes and affect liver function (Ihedioha et al., 2019).
Figure 1.
Photomicrograph of Liver (a), Kidney (b) and Ileum (c) of rats fed with 'Fufu' inoculated with starter cultures, without inoculation, and control at 400XMAG respectively. OSCR: Other starter culture representatives (BAP, BYP, YAP and MYP); CWI: Grating method without inoculum; SWI: Soaking method without inoculum; BMP: Bacteria and Moulds; MAP: Moulds only; CWF: Commercial “Fufu” in powdery form; CV: central vein; HN: hepatic necrosis; NL: necrotic lesion; SHD: Severe hepatocellular damage; NG: Normal glomeruli; BE: brush-like edges present in tubules; NHL: necrotic hemorrhagic lesion; SHL: severe hepatocellular lesion; NVA: Normal vein architecture; EEF: Enlongated epithelial villi.
Therefore, the albino rats fed with ‘Fufu’ samples BMP and MAP showed there was hepatocellular damage, because proteins are normally synthesized by the liver and when there is inhibition of protein synthesis, there is an indication of disruption and dissociation of polyribosome from endoplasmic reticulum. The lyses of liver occur when there is an infection until it becomes plasmolyzed (shrinkage of liver). This infection could occurred during the metabolic activities of the microorganisms in the substrate as previously reported by researchers especially the fungi (Mould- Aspergillus niger and Penicillin notatum). This could be seen in the diet of ‘Fufu’ samples having combination of moulds (BMP and MAP) even though they contributed to the organoleptic attributes of the products (Chávez et al., 2011). This hepatocellular damage is a pointer that there may be high release of intracellular aspartate aminotransferase (AST), alanine transferase (ALT), serum alkaline phosphatase (SALP) and lactate dehydrogenase (LDH) enzymes (Babalola et al., 2016). These enzymes are useful as quantitative marker to check the extent of hepatocellular damage according to Bhadauria et al. (2007). The toxic hepatitis with hyperplasia and microvascular caused changes in the hepatocytes as reported by Boby and Indira (2004) who worked on long-term studies of toxicity and carcinogenicity on weanling inbred Wistar strain albino rats.
The kidney histological assessment is shown in Figure 1b. The normal glomeruli, the basal apical nuclei along the normal lumen were peculiar to all the samples fermented in combination of bacteria and yeasts characterized by ‘Fufu’ samples BAP, BYP and YAP. This trend could suggest that there was no kidney damage due to HCN or other anti-nutrients in the diets possibly due to its tolerable level of metabolic activities of the microorganisms such as lactic acid bacteria (LAB) was able to suppress and co-ordinate activities of other inoculated microorganisms such as Bacillus subtilis by producing amylase enzymes that is necessary to breakdown starch to sugars needed for the growth of other fermenting microorganisms such as Corynebacterium manihot and some fungi to be responsible for the flavour and odour development (Oyewole and Odunfa, 1990) and high acidic level involved in the fermented ‘Fufu’ samples was sufficient to inhibit the growth of pathogenic and spoilage microorganisms in the substrate during fermentation (Ewanfo et al., 2017). The major fermenting microorganisms present in the substrate played the role of improving food safety apart from enhancing bio-availability of nutrients and bio-preservative effects such as lactic acid bacteria, Corynebacterium manihot, and Saccharomyces cerevisiae just to mention few (Tamang et al., 2016; Unigwe et al., 2016). Samples BMP and MAP showed lesion which could be due to toxic damage by alkaline phosphatase (ALPase) activity resulting in the suppression of tubular re-absorption (Bhadauria et al., 2007; Ihedioha et al., 2019). The lesions observed from the organ of the rats could be as a result of the effect of the diet composition consisting of bacteria and moulds which combined with the degree of the severity of the lesions. This explained the impact of safety of diet on human/animal's health and was in agreement with Laleye et al. (2016) that the degree of the severity of the lesions could explain the impact the supplements (diet) had on arrest of the situation of the disease condition. Likewise, there was also cellular vacuolization, degeneration and necrosis with similar report on short term toxicological studies on cyanogenic glycosides on rats by Oyewole and Olayinka (2009).
The Ileum histological assessment is shown in Figure 1c. The albino rats fed with ‘Fufu’ samples BMP and MAP showed that there was enlargement of the epithelium of villi (EEV) which was due to inability of the rats to absorb food leading to lower rate of absorption of food to have caused emaciation. This result concord with the result on an infection in the ileum on short term toxicological studies of cyanogenic glycosides in rats by Oyewole and Olayinka (2009).
4. Conclusion
The present study has revealed the safety of ‘Fufu’ fermented with different starter cultures in combinations. The cyanide level in all the samples were drastically reduced and those samples fermented with mixed cultures of bacteria and yeasts gave acceptable and safe ‘Fufu’ products as they pose no side effect (s) when their toxicological profile were examined. Moreover, samples fermented with moulds only and those in combination of moulds with bacteria (BMP and MAP) respectively did not yield safe products. However, ‘Fufu’ samples fermented with only bacteria (BAP) could be recommended, followed by sample grated without inoculum (CWI) and as well as sample with bacteria and yeasts (BYP). All the ‘Fufu’ samples were devoid of characterized ‘Fufu’ odour due to change of the fermenting water.
Declarations
Author contribution statement
Babatuyi, C. Y.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Boboye, B. E.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fagbemi, T. N.: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Enujiugha, V. N.: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abraham K., Buhrke T., Lampen A. Bioavailability of cyanide after consumption of a single foods containing high levels of cyanogenic glycosides: a crossover study in humans. Arch. Toxicol. 2016;90(3):559–574. doi: 10.1007/s00204-015-1479-8. ISSN: 1432-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeosun O.1., Adedokun M.A., Ajibade A.O., Balogun J.O. Paper Utilization and Haematological changes of fish fed African star apple (Chrysophyllum albidum) seed meal. Afr. J. Food Sci. 2019;13(9):203–209. [Google Scholar]
- Aderemi F.A. Effect of enzyme supplemented cassava root sievate (CRS) in cassava based diet on some visceral organs of pullet chicks. In: Olatunji E.A., Ayanwale B.A., Shiawoya E.L., Aremu A., editors. Proceedings of the 8th Annual Conference of Animal Science Association of Nigeria. 2003. pp. 25–27. September, 2003, Minna, Nigeria. [Google Scholar]
- Agbor-Egbe T., Lape M.I. The effects of processing techniques in reducing cyanogen levels during the production of some Cameroonian cassava foods. J. Food Compos. Anal. 2006;19:354–363. [Google Scholar]
- Ahamefule F.O., Eduko G.O., Usman A., Amaefule K.U., Obua B.E., Oguike S.A. Blood biochemistry and haematology of weaner rabbits fed sundried, ensiled and fermented cassava peel based diets. Pakistan J. Nutr. 2006;5(3):248–253. [Google Scholar]
- Akinmutimi A.H. Ph.D Thesis Michael Okpara University of Agriculture Umudike; Nigeria: 2004. Evaluation of Sword Bean (Canvalia Gladiate) as an Alternative Feed Resources for Boiler Chickens. [Google Scholar]
- Akyildiz B., Kurtoğlu S., Kondolot M., Tunç A. Cyanide poisoning caused by ingestion of Apricot seeds. Ann. Trop. Paediatr. 2010;30(1):39–43. doi: 10.1179/146532810X12637745451951. ISSN: 1465-3281. [DOI] [PubMed] [Google Scholar]
- Alexandraki V., Tsakalidou E., Papadimitriou K., Holzapfel W. Commission on Genetic Resources for Food and Agriculture Background Study Paper. Vol. 65. 2013. Status and trends of the conservation and sustainable use of micro-organisms in food processes; pp. 1–160. [Google Scholar]
- AOAC . Official Methods of Analysis of the Analytical Chemists International. twenty-first ed. 2015. Association of official and analytical chemists. Gaithersburg, Maryland, USA. [Google Scholar]
- Aro S.O., Ogunwale F.F., Falade O.A. Proc. Of the 18th Annual Conference of Animal Science Association of Nigeria. 2013. Blood viscosity of finisher cockerel fed dietary inclusions of fermented cassava tuber wastes; pp. 74–77. [Google Scholar]
- Babalola T.O., Oyawale F.E., Adejumo I.O., Bolu S.A. Effects of dietary fish oil replacement by vegetable oil on the serum biochemical and haematological parameters of African catfish (Heterobranchus longifilis) fingerlings. Iran. J. Fish. Sci. 2016;15(2):775–788. [Google Scholar]
- Babatuyi C.Y., Akinyede A.I., Enujiugha V.N. Physicochemical, Microbiological and Sensory Qualities of Milk Extract from Three Varieties of Tiger nut during Storage. Food Sci. Qual. Manag. 2019;84:1–8. [Google Scholar]
- Bamidele O.P., Fasogbon M.B., Oladiran D.A., Akande E.O. Nutritional composition of fufu analog flour produced from Cassava root (Manihot esculenta) and Cocoyam (Colocasia esculenta) tuber. J. Food Sci. Nutr. 2015;3(6):597–603. doi: 10.1002/fsn3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamishaiye E., Muhammad N., Bamishaiye O. Haematological parameters of albino rats fed on tiger nuts (Cyperus esculentus) tuber oil meat based diet. Internet J. Nutr. Wellness. 2009;10(1):58–62. [Google Scholar]
- Barnett H.L., Hunter B.B. third ed. Burgess Publishing Company, Minneapolis; Minnesota: 1972. Illustrated Genera of Imperfect Fungi; p. 214. [Google Scholar]
- Bhadauria M., Nirala S.K., Shukla S. Hepatoprotective efficacy of Propolis Extract: a biochemical and histopathological approach. Iran. J. Pharmacol. Ther. 2007;6(2):145–154. [Google Scholar]
- Bhatia E. Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations. J. Gastroenterol. 2002;123:1020–1025. doi: 10.1053/gast.2002.36028. [DOI] [PubMed] [Google Scholar]
- Boby R.G., Indira M. Effect of co-administration of cassava Manihot esculenta (Crantz) rich diet and alcohol in rats. Indian J. Physiol. Pharmacol. 2004;48(1):41–50. [PubMed] [Google Scholar]
- Bolarinwa I.F., Oke M.O., Olaniyan S.A., Ajala A.S. A Review of Cyanide Glycolysis in Edible Plants. Publisher by Intech Open; 2016. Toxicology- new aspects to this conundrum. Chapter 8; pp. 171–191. [Google Scholar]
- Cardoso A.P., Mirione E., Ernesto M., Massaza F. Processing of cassava roots to remove cyanogens. J. Food Compos. Anal. 2005;18:451–460. [Google Scholar]
- Carlsson L., Mlingi M., Juma A., Ronsquist G., Rosling H. Metabolic fates in humans of linamarin in cassava flour ingested as stiff porridge. J. Food Chem. Toxicol. 1999;37:307–312. doi: 10.1016/s0278-6915(99)00015-0. [DOI] [PubMed] [Google Scholar]
- CCAC . Canadian Council on animal care. In: Olfert E.D., Cross B.M., McWilliams A.A., editors. second ed. Vol. 1. CCAC; Ontario: 1993. (Guide to the Care and Use of Experimental Animals). [Google Scholar]
- Chávez R., Fierro F., García-Rico R.O., Laich F. Chapter 5, Penicillium Spp. As Ripening Agents in the Elaboration of Cheese and Meat Products. Mycofactories. 2011. Mold-fermented foods; pp. 73–98. [Google Scholar]
- Delwatta S.L., Gunatilake M., Baumans V. Reference values for selected hematological, biochemical and physiological parameters of sprague-Dawley rats at the animal House, faculty of medicine, University of colombo, Sri Lanka. Animal Model Exp. Med. 2018;1:250–254. doi: 10.1002/ame2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebuehi O.A.T., Mbara K.C. Biochemical studies of iron fortified gari fed to phenyl hydrazine-induced anaemic rats. Am. J. Food Technol. 2011;6(6):472–482. [Google Scholar]
- Egwim E., Musa A., Abubakar Y., Mainuna B. Nigerian Indigenous Fermented Foods: Processes and Prospects. NTECH; 2013. Mycotoxin and food safety in developing countries. Chapter 7; pp. 153–180. [Google Scholar]
- Eroschenko V.P. ninth ed. Lippincolt Williams and Wilkins; USA: 2000. Di Fiore’s Atlas of Histology with Functional Correlations. [Google Scholar]
- Ewanfo I.J., James I.M., Ugueri U. American microbiological quality of commercially ready-to-eat fufu sold in Benin city, Nigeria. J. Food Nutr. Publ. 2017;2(5):26–30. [Google Scholar]
- Fawole M.O., Oso B.A. Spectrum Books Ltd; Ibadan: 2007. Laboratory Manual of Microbiology; pp. 11–24. [Google Scholar]
- Food Standards of Australia. New Zealand (FSANZ) 2008. Proposal P1002 Hydrocyanic Acid in Ready-To-Eat Cassava Chips Approval Report; pp. 1–137. [Google Scholar]
- Hernadez T., Lundquist P., Lourdes O., Perez-Crisha R., Rodreiguez E., Rosling H. Fate in human dietary intake of cyanogenic glycosides from roots of sweet cassava consumed in Cuba. J. Nat. Toxins. 1995;3:114–117. doi: 10.1002/nt.2620030210. [DOI] [PubMed] [Google Scholar]
- Holt J.G., Krieg N.R., Sneath P.H., Stanley J.J., Williams S.T. ninth ed. Waverly press; 1994. Bergey’s Manual of Determinative Bacteriology; pp. 45–56. [Google Scholar]
- Ihedioha T.E., Odo R.I., Onoja U.S., Chiwetalu C.E., Ihedioha J.I. Effects of methanolic tuber extract of Cyperus esculentus Linn (tiger nuts) on sub-acute liver damage in albino rats. African J. Pharm. Pharmacol. 2019;13(15):236–243. [Google Scholar]
- IITA Cassava processing and utilization. Int. Inst. Trop. Agric. 2007;28:26–28. [Google Scholar]
- Isaac L.J., Abah G., Akpan B., Ekaete I.U. Haematological properties of different breeds and sexes of rabbits. Proc. of the 18th Annual Conf. of Anim. Sci. Asoc. of Nig. 2013:24–27. [Google Scholar]
- Joshi P.K., Bose M., Harish D. Changes in the blood of Clariasbatrachus (Linn) exposed to Mercuric chloride. J. Ecotoxicol. Environ. Mon. 2002;122:119–222. [Google Scholar]
- Laleye S.A., Aderiye B.I., David O.M. Hypolipidemic activity of fermented Sorghum (Sorghum bicolor L. Moench) gruel (ogi) in hypercholesterolemic rats (Rattus nervegicus) Int. J. Biochem. Res. Rev. 2016;9(1):1–15. Article no.IJBcRR.19659 ISSN: 2231-086X, NLM ID: 101654445. [Google Scholar]
- Lawal B., Shittu O.K., Abubakar A.N., Haruna G.M., Sani S., Ossai P.C. Haematopoietic effect of methanol extract of Nigerian honey bee (Apismellifera) propolis in mice. J. Coastal Life Med. 2015;3(8):648–651. [Google Scholar]
- Lukuyu B., Okike I., Duncan A., Beveridge M., Blümmel M. International Livestock Research Institute; Nairobi, Kenya: 2014. Use of Cassava in Livestock and Aquaculture Feeding Programs. ILRI Discussion Paper 25. [Google Scholar]
- MSD Veterinary Manual . 2019. Overview of Cyanide Poisoning- Toxicology Veterinary Manual. [Google Scholar]
- Ngiki Y.U., Igwebuike J.U., Moruppa S.M. Utilization of cassava products for poultry feeding: a review. Int. J. Sci. Technol. 2014;2(6):48–59. [Google Scholar]
- Obun C.O., Olafadehan O.A., Ayanwale B.A., Inuwa M. Growth, carcass and organ weight of finisher broilers fed differently processed Detarium microcarpum seed meal. Livest. Res. Rural Dev. 2008;20(8):1–7. [Google Scholar]
- Ochei J., Kolhatkar A. McGraw-Hill; New York, NY, USA: 2008. Medical Laboratory Science: Theory and Practice. [Google Scholar]
- Olafadehan O.A. Haematological parameters, serum constituents and organ development of growing rabbits as affected by feeding of processed cassava peels. Anim. Nutr. Feed Technol. 2011;11:41–51. [Google Scholar]
- Omitoyin B.O., Ajibade A.O. Haematological evaluation of Clariasgariepinus (Burchell) juveniles exposed to lethal concentrations of the latex of Calotropisprocera (Ait) J. Org. Agric. Environ. 2014;2:141–145. [Google Scholar]
- Oyewole O.B., Odunfa S.A. Characterization and distribution of lactic acid bacteria in cassava retting during “Fufu” production. J. Appl. Bacteriol. 1990;68:145–152. [Google Scholar]
- Oyewole O.I., Olayinka E.T. Hydroxocobalamin (Vit. B 12a) effectively reduced extent of cyanide poisoning arising from oral amygdalin ingestion in rats. J. Toxicol. Environ. Health Sci. 2009;1(1):8–11. [Google Scholar]
- Oyewole O.B., Sanni L.O. Constraints in traditional cassava processing – the case of “Fufu” production. In: Agbor-Egbe T., Brauma A., Griffon T., Treche O.R.S.T.O.M., editors. Cassava Food Processing. 1995. pp. 523–529. France. [Google Scholar]
- Singh A., Bhat T.K., Sharma O.P. Clinical biochemistry of hepatotoxicity. J. Clin. Toxicol. 2011;S4:1–19. [Google Scholar]
- Tamang J.P., Thapa N., Tamang B., Rai A., Chettri R. 2015. Health Benefits of Fermented Foods and Beverage. Chapter 1: Microorganisms in Fermented Foods and Beverages; pp. 1–109. [Google Scholar]
- Tamang J.P., Shin D.D., Jung S., Chae S. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016;7:1–29. doi: 10.3389/fmicb.2016.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unigwe C.R., Marire B.N., Omeke B.C.O., Abonyi F.O., Oladipo T.A., Adebayo D.M. Effects of maize-replaced fermented cassava peels and enzyme- supplemented diet on haematology and serum biochemistry of cross-bred female pigs. Int. J. Adv. Res. Biol. Sci. 2016;3(6):198–208. [Google Scholar]
- Waugh A., Grant A. ninth ed. Churchill, Livingstone, an imprint of Elsevier Science, Ltd; 2001. Anatomy and Physiology in Health and Illness; pp. 59–70. [Google Scholar]
- Yalta A., Talha The accuracy of statistical distributions in Microsoft Excel 2007. Comput. Stat. Data Anal. 2008;52:4579–4586. [Google Scholar]