Abstract
Phytoremediation is an important and effective tool to remove different contaminations in the soil, water and air. The main objective of this research was to evaluate the effect of phytoremediation using one species of shrubs, Nerium oleander plants, on reducing the heavy metals (HMs) contamination in the soil. The present study was carried out during 2015 in El-Dakhyla (industrial zone), Alexandria Egypt. A simple Uptake Plant Model (UPM) was used to estimate the contribution of various pathways in remediating ((Lead (Pb), Cadmium (Cd) and Zinc (Zn)) through different parts, in one of the evergreen shrubs (leaves, stem and root). These include soil-root-leaf pathway, soil-air-leaf pathway, and its deposition. The model calculations revealed that the (Root concentration Factor) log RCF of the root equals 0.5, 0.41, and 0.45, respectively. The Translocation Stem Concentration Factor (TSCF) of the upward in the xylem equals 0.85, 0.98 and 0.99. Moreover, the Bioaccumulation Factors (log BCF) of the soil is 1.32, 0.014 and 0.061. In addition, the partition coefficient of Octanol-Water (log Kow) is 4.67, 2.75, and 3.35, respectively. Therefore, one may conclude that Pb was accumulated in the root, while Cd and Zn were concentrated in the aerial parts of the Nerium oleander plant. On the other hand, Pb considered one of the heavy metals where it's movement in the plants is slower than Cd and Zn due to its molecular weight is bigger than the latter HMs.
Keywords: Phytoremediation, Simple uptake model (UPM), HMs, (Bioaccumulation factors), Nerium oleander, Agricultural soil science, Environmental science, Soil science, Environmental chemistry, Environmental pollution, Environmental toxicology, Agriculture, Toxicology
Phytoremediation, Simple uptake model (UPM), HMs, (Bioaccumulation factors), Nerium oleander; Agricultural soil science; Environmental science; Soil science; Environmental chemistry; Environmental pollution; Environmental toxicology, Agriculture, Toxicology.
1. Introduction
Environmental pollution is “the contamination of the physical and biological components of the earth/atmosphere system to such an extent that normal environmental processes are adversely affected” (Kemp, 2002). One of this environmental pollution is soil contamination as a feature of land corruption caused by the nearness of xenobiotic (human-made) synthetic substances or other change in the regular soil condition. As soil is the basic natural medium, which is liable to various poisons because of various human exercises (Al-Khashman and Shawabkeh, 2006). It has been noticed that lead, cadmium, copper, and zinc are the real metal contaminations of the roadside conditions and are discharged from years of lead enriched fuel burning, wear out of tires, spillage of oils, and erosion of batteries and metallic parts, for example, radiators etc. Phytoremediation takes the advantage of unique and selective uptake capabilities of plant root systems, together with the translocation, bioaccumulation and contamination degradation abilities of the entire plant body for the remediation process. This technology is environmentally friendly and potentially cost effective. The pollution of soils by heavy metals from automobile sources plays a serious environmental issue, also with the increasing demand for metals during industrialization and urbanization (Tangahu et al. 2011). Seaward and Mashhour (1991) found that a homogenously Nerium oleander seedling has the capability in collecting HMs on the surface of the leaf mainly from aerial sources and controlled by substratum. Aksoy and Öztürk (1997)). This due to its leaves that characterizes by its lanceolate leaves with a high cuticle thickness Ataabadi et al. (2010). It has been accounted for that Nerium oleander assumes an essential role in reducing heavy metals in nature because of its morphological and physic-compound attributes of N. oleander leave that has lanceolate leaves with high cuticle skin thickness (Houdaji et al. 2010). A simple Uptake Plant Model (UPM) systems were used to predict the contribution of various pathways in remediation three heavy metals (Pb, Cd, and Zn) during spring and autumn through different parts (leaves, stem, and root) of Nerium oleander. These include soil-root-leaf pathway, soil-air-leaf, pathway, and its deposition. The plant was represented by three functional parts (root, shoot, leaf) to interconnect, mimicking its anatomy and physiology; three phases represent abiotic factors (soil, water, and atmosphere) of the environment. The functional parts and abiotic phases are similar to other representations that can be found in a different phytoremediation modelling approach (Sundberg and Durant (2003); Ouyang and Wan (2008)).
2. Data and methodology
The present study was carried out in El- Dakhyla district in Alexandria city, Egypt (Figure 1) during spring and autumn 2015. It considered as an industrial area, which includes one of the largest oil company for Petroleum Maintenance (Alexandria Petroleum) in the province of Alexandria. It represents one of the main sources of emissions of many pollutants in the area. It is located at latitude 31.18 ᵒN, longitude 29.94 ᵒE and elevation 23ft. It is characterized by an average daily temperature 62ᵒF and 73ᵒF during spring and autumn, respectively.
Figure 1.
Dakhyla Region (Google image).
2.1. Experiment description
First March 2015, six Nerium oleander plants were used in the experimental field with 50–60 cm height and 20–25 leaves. It gently geminated individually in plastic pots (30 cm diameter) filled with a 7 kg mixture sand and clay at the ratio of 1:1 by volume in one of the nurseries in Alexandria. Then, the plants were planted in the two locations in Alexandria (3 plants for each zone).The plants were watered during the experiment using field capacity. The sample of soils were collected, and plants were harvested in plastic bags after 30 days to transfer it to the lab during both seasons spring and autumn 2015 (Naira et al., 2019) (Thus, the duration of the experiment was 120 day).The biomass (aboveground and roots) were measured as mixed referring to all the plants and the trial of the experiment started with mass (6 kg). After that, each part of the plants (shoots and roots) has been analyzed separately to know the amount of HMs. Moreover, it was noticed that the highest HMs that caused air pollution in Alexandria are (Pb, Cd, and Zn). These results are similar to what Ashraf (2002) who found that some results of different air pollutants measurements at selected zones over Alexandria governorate.
The chemical constituents of the soil have been collected and analyzed as described by Jackson (2005) in Table 1.
Table 1.
Chemical analysis of the used mixture soil for the first sucessive season 2015.
| Characteristics | Values | Units |
|---|---|---|
| EC | dSm−1 | 1.52 |
| PH | 7.91 | |
| Sand | gkg−1 | 596.4 |
| Silt | gkg−1 | 141.3 |
| Clay | gkg−1 | 262.30 |
| Cd | mg/kg | 0.33 |
| Pb | mg/kg | 6.13 |
| Zn |
mg/kg |
8.18 |
|
Soluble cations | ||
| Ca++ | meq/l | 3.2 |
| Mg++ | meq/l | 3.0 |
| Na+ |
meq/l |
6.3 |
|
Soluble anions | ||
| HCO3- | meq/l | 3.3 |
| Cl- | meq/l | 6.5 |
| SO2- | meq/l | 2.2 |
2.1.1. Vegetative growth parameters
Koller (1972) method used to record plant height (cm), number of leaves per plant, leaves dry weight per plant (g), area of leaf (cm2), stem diameter (cm), stem dry weight (g), root length (cm) and root dry weight (g).
2.1.2. Chemical analysis
Determination of (Pb, Cd and Zn) content in plants was taken the following steps: (a) drying plant samples (leaves, stem, and roots) at 70 °C in an oven; (b) extraction of HMs; according to Piper (1947) method; (c) determination of HMs concentration using an atomic absorption spectrophotometer.
Available HMs in soil samples was extracted by using DTPA solution according to Lindsay and Norvell (1978) and was determined by Inductively Coupled Plasma Spectrometry.
Transfer factor (TF) was calculated according to the relation of the ratio of the concentration of HMs in the shoots to their concentration in the soil Chen (2004). The transfer factor is a value used in evaluation studies on the impact of routine or accidental releases of a pollutant in the environment.
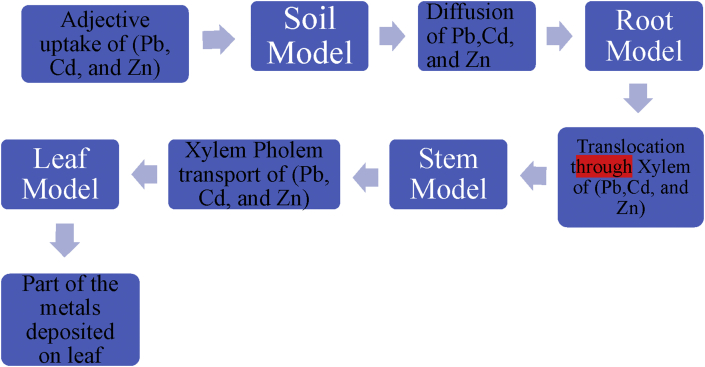
2.2. The mathematical model (phytoremediation uptake model)
A Simple Uptake Plant Model (UPM) systems used to predict the contribution of various pathways in remediation (Pb, Cd and Zn) through different parts (leaves, stem, and root) in one of the evergreen shrubs Nerium oleander to interconnect, mimicking its anatomy and physiology; three phases represent abiotic factors (soil, water, atmosphere) of the environment. This model includes three pathways that are a soil-root-leaf pathway, soil-air-leaf pathway, and its deposition. This was like other representation can be found in a different phytoremediation modeling approach Sundberg and Durant (2003); Ouyang and Wan (2008).
Simple Uptake Model is represented as Adriano, 2001, Aksoy and Öztürk, 1997, Al-Khashman and Shawabkeh, 2006, Chen, 2004, Dettenmaier, 2008, Houdaji et al., 2010, Jackson, 2005, Jadia and Fulekar, 2009, Kabata-Pendias and Pendias, 2001, Kemp, 2002, Koller, 1972, Legind et al., 2011, Lindsay and Norvell, 1978, Liu, 2007, Manivasagaperumal, 2011, Mouchet, 2008, Naira et al., 2019, Oliva and Espinosa, 2007, Ouyang and Wan, 2008, Paquin, 2002, Piper, 1947, SAS Institute, 2002, Seaward and Mashhour, 1991, Snedecor and Cochran, 1989, Standard institution and Iran industrial research,, Sundberg and Durant, 2003, Tangahu et al., 2011, Trapp, 2002, Verma and Dubey, 2003 includes four types of model which are:
2.2.1. Soil model
The BCF (Bioaccumulation factors) approach is easy to implement, the limitation of this approach is that its lack of precision greatly impairs its usefulness as a tool for risk evaluation and decision-making. For this reason, Mouchet (2008) found that there were other parameters used beside the BCF, as Distribution Coefficient (Kd) between water and dry soil.
The concentration of HMs in water is determined by collecting of samples (the water that was used to irrigate the plants in the pots and soil) in polyethylene bottles washed out and were soaked in acid (2+1HCl) for 24 h and then, were rinsed with distilled water for three times. All of the samples were treated with acid to achieve pH ≈ 2, and then in the laboratory, the samples concentrated 10 to 1 volume at 80 °C and were kept in the refrigerator for final analysis, according to standard methods manuals (Standard institution and Iran industrial research). Then, HMs (Pb, Cd and Zn) concentrations (a total of 234 heavy metal concentrations) were measured using flame atomic absorption spectroscopy device calibrated with standard solutions. Then the calculations are present as its equation is represented by:
| (1) |
Where:
CM: Concentration of mass plant.
Kd: Distribution coefficient of HMs between dry soil and water.
Cw: Concentration of HMs in water.
| (2) |
Where:
Cw: Concentration of HMs in water.
Csoil: Concentration of HMs in soil.
Kws: Partition coefficient of HMs between two phase water and soil.
2.2.2. Root model
It represented by the following equations:
Mass balance: Flux in –Flux out is given by:
| (3) |
Where:
Cw: Concentration of HMs in water.
Csoil: Concentration of HMs in soil.
Kd: Distribution coefficient
Concentration divided by plant mass:
| (4) |
Where:
Cxy: Concentration of the HMs inside the xylem.
Cr: Concentration of HMs in root.
Krw: Partition coefficient of HMs in two phases root zone and water.
Paquin (2002) found that steady state was important as the chemistry of the rhizospheric solution to which the plant is really exposed differs from the chemistry of the bulk soil solution and must be quantified properly. On the other side, the level of accumulation at the site of action (the biotic ligand) is assumed to be related to the toxicological responses.
Set to steady state and solve for Cr
| (5) |
| (6) |
Where:
Q: Flow of water in the soil (ml/kg).
K: Rate of change in time.
M: Plant mass.
KM: Rate of change in mass of the plant.
Ksw: Partition coefficient of HMs in two phases soil and water.
2.2.3. Stem model
Briggs and Sculpher (1998); Dettenmaier (2008) found that stem model calculated according to xylem phloem translocation in the following equation:
| (7) |
Where:
TSCF: Translocation stem concentration factor.
2.2.4. Leaf model
The following equations represent the calculation of this model:
| (8) |
Where:
BCF: Bioaccumulation factors.
Kow: Partition coefficient of HMs between two phase octanol (organic compound) and water.
Outflux:
| (9) |
Where:
Q: Flow of the water from the soil to the root (ml/kg).
Mr: Mass of the root.
Kws: Partition coefficient of HMs between two phase water.
Cs: Concentration of HMs in soil.
Kr: Rate of change in the weight of the root.
Influx to leaves:
| (10) |
Where:
CL: Concentration of HMs in leaves.
K: Rate of change in days.
Q: Flow of water through the stem to the leaves (ml/kg).
Ml: Mass of leaves.
| (11) |
Where:
Kaw: Partition coefficient of HMs between two phase air and water.
Ca: Concentration of HMs in air sample.
Cw: Concentration of HMs in water.
2.3. Statistical analysis
The experimental data were subjected to analysis of variance (ANOVA) using the SAS program, SAS Institute (SAS Institute, 2002). The means of the individual factors and their interactions were compared with L.S.D test at 5% level of probability according to Snedecor and Cochran (1989). While, the UPM was developed and analyzed using the Microsoft Excel (see Figure 2).
Figure 2.
Diagram of Phytoremediation Uptake Model (UPM) in which the system composed of two section: ground zone (which consists of soil and root), above zone (which consists of stem, leaf, and atmosphere).
3. Results and discussion
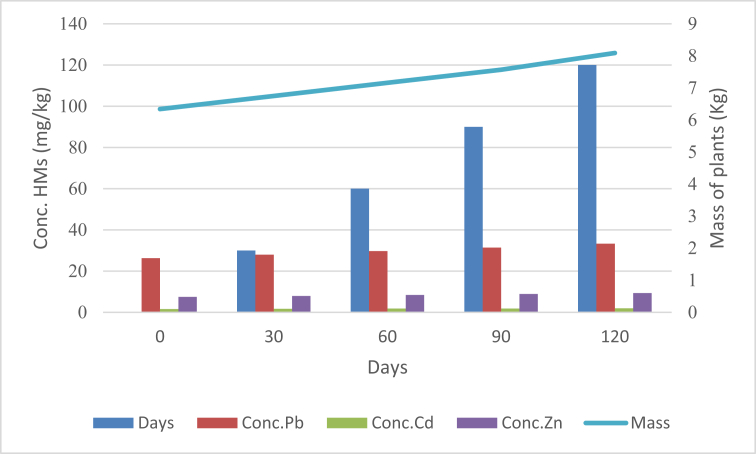
3.1. Rate of change in plant mass and HMs concentration
Figure (3) shows a comparison between rate of change in plant mass and (Pb, Cd and Zn) concentration every 30 days during different growth stages through 2015. This interrelation showed that the concentration of HMs was increasing with time comparable to the mass of plants that was very small. This result agreed with the finding of other studies that heavy metals uptake and their accumulation were affected by time changes (Liu (2007)). On the other side, Jadia and Fulekar (2009) suggested that increasing in the concentration of HMs with decreases in the biomass of the plants might be due to low protein formation, resulting in inhibition of photosynthesis, as well as hampered carbohydrate translocation (Manivasagaperumal (2011)).
Figure 3.
Rate of change in mass of plant and concentration of HMs.
3.2. Root model
The results obtained from the preliminary analysis of root model are summarized in the following sections:
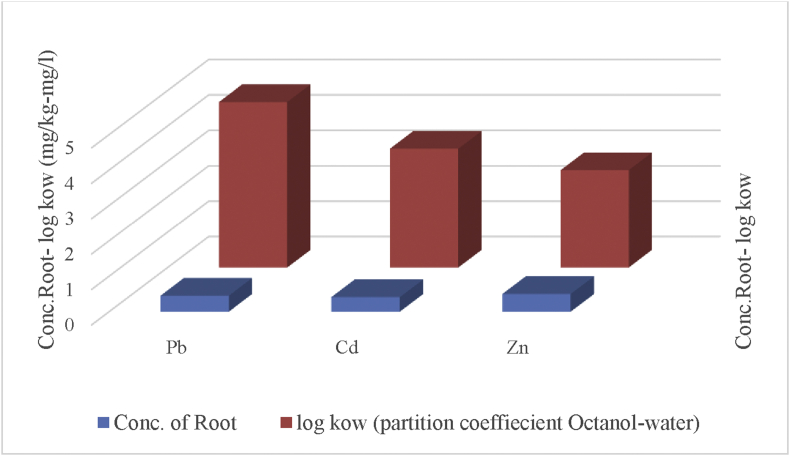
3.2.1. Relation between concentration of HMs in roots and partition coefficient (octanol-water)
Figure (4) shows low interrelation between the concentration of (Pb, Cd, and Zn) in the root and its partition coefficient in the two phases (Octanol-Water). The obtained results show higher distribution of heavy metals (Pb, Cd, and Zn) between the two Phases (octanol-water) than in the root. These results are in agreement with those obtained by Oliva and Espinosa (2007) that high amount of Cd and Zn were distributed in most of the transect due to especial kind of industrial complexes and soils that encourage presence both in soil then to the plant: CF=(Mplant/Msoil).
Figure 4.
Relation between log kow and concentration root for HMs.
3.2.2. Partition constant root to water in their equilibrium
Figure (5) summarizes the significant interrelation between partition constant root to water of (Pb, Cd, and Zn) (Krw) and their equilibrium concentration of HMs in both phases (Kow). As the concentration of (Pb, Cd, and Zn) and their distribution between the two phases (Root-water) was higher than its distribution between the two phases (octanol-water). Furthermore, the most important statement on heavy metals contamination is greater affinity of surface soils to accumulate also highly variable in soil horizons due to different soil processes Adriano (2001). Although, regarding, the content of the three metals (Pb, Cd, and Zn) in roots (Verma and Dubey, 2003) reported that in the roots HMs are binding to cell walls and vacuoles, or by extracellular precipitation, which prevents it from entering the cytoplasm, avoiding the toxic effects of heavy metals on the cytosol, so HMs became easily uptake by plant root, but very low amount of it transfers to above ground plant parts natural concentration of lead in plant tissues is lower than 10 mg in kg dry weight, so threshold value of this metal in plant was assumed 10 ppm.
Figure 5.
Partition constant root to water and their equilibrium.
3.2.3. Relation between bioaccumulation factor and partition coefficient of HMs
Figure (6) describes the relation between both the bioaccumulation factor and partition coefficient of Pb, Cd and Zn were very high in the soil as BCF. The observed results show that the distribution of (Pb, Cd, and Zn) between two phases (Octanol-water) was little higher than Bioaccumulation of (Pb, Cd, and Zn). Mouchet (2008) noted that the total trace element concentration in soil that calculated from a strong acid extraction is used to construct the BCF (Bioaccumulation factor) ratio. The partition coefficient (Kow) described the distribution of Pb, Cd, and Zn between the two phases. These results were obtained according to Adriano (2001) who observed that the accumulation of HMs contamination is greater affinity to surface soils. In the same time, there is a highly variable in soil horizons due to different soil processes.
Figure 6.
Bioaccumulation factors and partition coefficient of HMs.
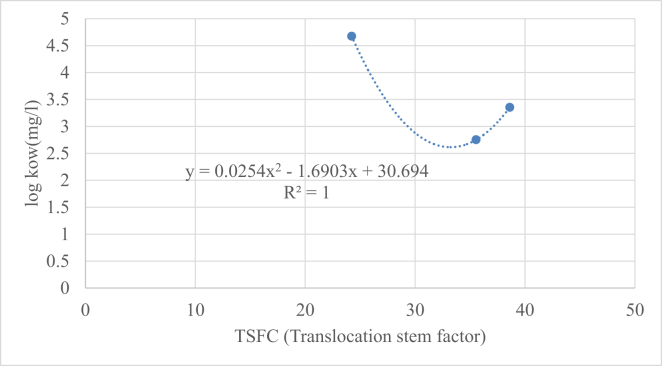
3.3. Stem model
Figure (7) shows a highly significant positive regression of the concentration of HMs toward the phase of octanol-water than toward the stem. This agrees with the results obtained from Kabata-Pendias and Pendias (2001) who found that the concentration factor (CF) values of Pb were sufficiently lower than Cd and Zn. Regarding the content of Zn in the bask, Adriano (2001) who reported that Nerium oleander was higher as compared to background values because of the influence of industrial emissions. Although, the relative bioavailability of Pb was about 5 times higher than Cd in the studied soils the concentration factor (CF) values of Pb were sufficiently lower than Cd, indicating lower translocation of Pb from soil to plant leaves and the plants are characterized as unpolluted of Pb Kabata-Pendias and Pendias (2001). Also, Wind direction affects the distribution of Cd and Zn only in the transect where most industrial complexes and soils: CF= (Mplant/Msoil) and reveals the behavior about the pollutants in plants Oliva and Espinosa (2007). The data obtained show that there was a high match between TSCF (translocation stem factor) and log (Kow) partition coefficient of Octanol and water as the value of R2 was 1. These results confirmed with the previous studies (Santhi et al. (2001), Van Liew et al. (2003); (Moriasi et al. (2007)), who regarded that when the values of R2 were greater than 0.5, therefore it was acceptable.
Figure 7.
Regression correlation between TSCF and log Kow of HMs.
3.4. Leaf model
What stands out in this Figure (8) is the third step, which is the wide range of (Pb, Cd, and Zn) concentration in the leaf model. It shows that the distribution of HMs concentration between Octanol and water phase (Kow) was high compatible to the concentration of (Pb, Cd, and Zn) in leaf and its distribution between air and water (Kaw) due to the translocation factor from the stem to the leaf was higher than that of root to stem and leaf. This finding was in a good agreement with results obtained from wheat grown in soil amended with industrial sludge by Bose and Bhattacharyya (2008). Concerning the content of HMs in leaves, Kabata-Pendias and Pendias (2004) indicated translocation and bioaccumulation factors reflect and the capacity of N. oleander to exclude (Pb, Cd and Zn) from aerial parts.
Figure 8.
Uptake of HMs in leaves from soil.
4. Conclusion
The Nerium oleander plants were able to stabilize HMs (Pb, Cd, and Zn) in the soil making them less bioavailable from the soil. The phytoremediation uptake model predicted the uptake of (Pb, Cd, and Zn) in different parts of Nerium oleander plants. Therefore, it can be used in estimation of accumulation of (Pb, Cd, and Zn) in soil, root stem, and leaves. One may conclude that the concentration of Cd and Zn were greater in the root than the aerial parts. Thus, the effects of phytoremediation using Nerium oleander plants to reduce HMs (Pb, Cd, and, Zn) presented in soils of El-Dakhyla region in Alexandria city, Egypt has been evaluated. In addition, a simple uptake model (UPM) has been developed to estimate the uptake of HMs in the different parts of plant.
Declarations
Author contribution statement
Naira Ibrahim, Gamal El Afandi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adriano D.C. second ed. Springer-Verlag; New York: 2001. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals. [Google Scholar]
- Aksoy A., Öztürk E. Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean. Sci. Total Environ. 1997;205(2-3) [Google Scholar]
- Al-Khashman O.A., Shawabkeh R.A. Metals distribution in soils around the cement factory in southern Jordan. Environ. Pollut. 2006;140:387–394. doi: 10.1016/j.envpol.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Ataabadi M. Mobarakeh steel company; 2010. Heavy Metals Biomonitoring by Plants Grown in an Industrial Area of Isfahan. [Google Scholar]
- Bose S., Bhattacharyya A.J.C. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. 2008;70(7):1264–1272. doi: 10.1016/j.chemosphere.2007.07.062. [DOI] [PubMed] [Google Scholar]
- Briggs A., Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- Chen Y. The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. 2004;19(10):1553–1565. [Google Scholar]
- Dettenmaier E.M. Chemical hydrophobicity and uptake by plant roots. 2008;43(2):324–329. doi: 10.1021/es801751x. [DOI] [PubMed] [Google Scholar]
- Houdaji M., Ataabadi M., Najafi P. Biomonitoring of airborne heavy metal contamination. In: Khare M., editor. Air Pollution – Monitoring, Modeling, Health and Control. 2010. p. 221. [Google Scholar]
- Jackson M.L. UW-Madison Libraries Parallel Press; 2005. Soil Chemical Analysis: Advanced Course; p. 930. [Google Scholar]
- Jadia C.D., Fulekar M.J. Phytoremediation of heavy metals: recent techniques. Bulletin. 2009;8(6):427–440. 135. [Google Scholar]
- Kabata-Pendias A., Pendias H. third ed. CRC Press; Boca Raton, Fla: 2004. Trace Elements in Soils and Plants. [Google Scholar]
- Kemp D. Routledge; 2002. The Environment Dictionary; pp. 4–480. [Google Scholar]
- Koller H.J. Leaf area-leaf weight relationships in the soybean canopy 1. 1972;12(2):180–183. [Google Scholar]
- Legind C.N. Dynamic plant uptake model applied for drip irrigation of an insecticide to pepper fruit plants. 2011;67(5):521–527. doi: 10.1002/ps.2087. [DOI] [PubMed] [Google Scholar]
- Lindsay W.L., Norvell W.A.J.S. Development of a DTPA soil test for zinc, iron, manganese, and copper 1. 1978;42(3):421–428. [Google Scholar]
- Liu J.X. An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. 2007;19(12):4111–4119. doi: 10.1105/tpc.106.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivasagaperumal R. Effect of zinc on germination, seedling growth and biochemical content of cluster bean (Cyamopsis tetragonoloba (L.) Taub) Curr. Bot. 2011;2(5):11–15. www.scholarjournals.org [Google Scholar]
- Mouchet F. Évaluation de la contamination de plantes potagères cultivées dans un environnement potentiellement pollué: contexte actuel et propositions d’outils opérationnels. 2008. www.m.elewa.org 7(3): 203-208.
- Naira A.A., Inas Z.A., Ashraf A.Z., Nader A.E. Bioremediation of heavy metals by using some shrubs in three different locations of Alexandria city. (B) N. Oleander plant. Alexandria Science Exchange Journal. 2019;40:99–114. [Google Scholar]
- Oliva S.R., Espinosa A.F. Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. 2007;86(1):131–139. [Google Scholar]
- Ouyang Z., Wan B.J.J. Modeling of moisture diffusion in FRP strengthened concrete specimens. 2008;12(4):425–434. [Google Scholar]
- Paquin P.R. The biotic ligand model: a historical overview. 2002;133(1-2):3–35. doi: 10.1016/s1532-0456(02)00112-6. [DOI] [PubMed] [Google Scholar]
- Piper J.J. The fate of heparin in rabbits after intravenous injection: filtration and tubular secretion in the kidneys. 1947;3(4):373–384. doi: 10.1111/j.1600-0773.1947.tb02666.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute . 2002. SAS User Guide and Program 20 Version 9.0.38. cary, NC27513. [Google Scholar]
- Seaward M.R.D., Mashhour M.A. Oleander (Nerium oleander L.) as a monitor of heavymetal pollution. In: Ozturk A.M., Erdem U., Gork G., editors. Urban Ecology. Izmir,(Turkey) Ege University Press; 1991. pp. 48–61. [Google Scholar]
- Snedecor G., Cochran W. eighth ed. Edition. Iowa State University Press; ISSN: 1989. Statistical Methods; pp. 1076–9986. [Google Scholar]
- Standard institution and Iran industrial research Physical and chemical characteristics of drinking water. https://www.iso.org/member/1803.html (Standard number 1053)
- Sundberg D.C., Durant Y.G. Latex particle morphology, fundamental aspects: a review. 2003;11(3):379–432. [Google Scholar]
- Tangahu B.V., Sheikh Abdullah S.R., Basri H. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011:2011. [Google Scholar]
- Trapp S. Dynamic root uptake model for neutral lipophilic organics. Environ. Toxicol. Chem. 2002;21:203–206. [PubMed] [Google Scholar]
- Verma S., Dubey R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655. [Google Scholar]