Abstract
Dermanyssus infestation is a rural parasitic problem occurs occasionally in urban areas in people with close contact to pigeons. It can be diagnosed through clinical cutaneous symptoms in exposed body parts, nocturnal itching, and presence of mites in infested locations and can be treated by antiacaricide, environmental, and symptomatic treatments.
Keywords: Dermanyssus sp., ectoparasite, itching, pigeon, poultry
Dermanyssus infestation is a rural parasitic problem occurs occasionally in urban areas in people with close contact to pigeons. It can be diagnosed through clinical cutaneous symptoms in exposed body parts, nocturnal itching, and presence of mites in infested locations and can be treated by antiacaricide, environmental, and symptomatic treatments.

1. INTRODUCTION
Dermanyssus infestation is a well‐known parasitic problem prevalent in rural areas, particularly in individuals working in poultry industries. This paper presents a report on two patients infested with Dermanyssus mites, resulting from pigeons nesting near their home or office. The diagnosis was based on clinical‐parasitological and molecular analyses.
Dermanyssus gallinae is a hematophagous mite, reported frequently in poultry, but scarcely in pigeons. Dermanyssus gallinae also bites mammals including cats, dogs, rodents, rabbits, horses, and humans,1 where the infestation is known as gamasoidosis. It is prevalent in several parts of the world, particularly in Europe. Based on a recent epidemiological review, 83% of the European farms are infested by D gallinae. The Netherlands, Germany, and Belgium are countries with the majority of infestation incidents of Dermanyssus gallinae in poultry (94%).2 Dermanyssus gallinae has a life cycle with four stages, including larva (with six legs), protonymph (eight legs), deutonymph (eight legs), and adult (eight legs) stage.3 Among them all, the nymphs (protonymph and deutonymph) and adults are hematophagous. Females lay around 30 eggs in their lifetime. They usually feed on the bird or mammalian hosts, while males feed occasionally.4 Dermanyssus mite often shows a nocturnal activity, although it may seldom feed during the daytime. Several bacteria, viruses, filariae, and protozoa have been detected in the Dermanyssus mites. Apart from the potential vectorial role and nuisance, it is responsible for multiple economic problems that affect the meat and egg production, where it is known as poultry red mite.2, 5
2. CASE PRESENTATION
2.1. Case report 1
A 63‐year‐old man, working in the Avicenne hospital in northern suburbs of Paris, was referred to the Parasitology‐Mycology department of the hospital for intensified itching on his body, particularly on the neck, shoulders, and arms during working hours at the office. Numerous small, reddish, itchy papules were observed on his body (Figure 1A). No notion of pruritus was found before or during his presence outside his workplace. He brought some samples of an insect found in his office, which seem to be the biting bugs. Furthermore, to verify the working conditions of this patient, a visit was made by a member of Parasitology‐Mycology department, where several insects of the same species were found. The exterior side of his office led to an open area, via a window, where the pigeons nested.
Figure 1.
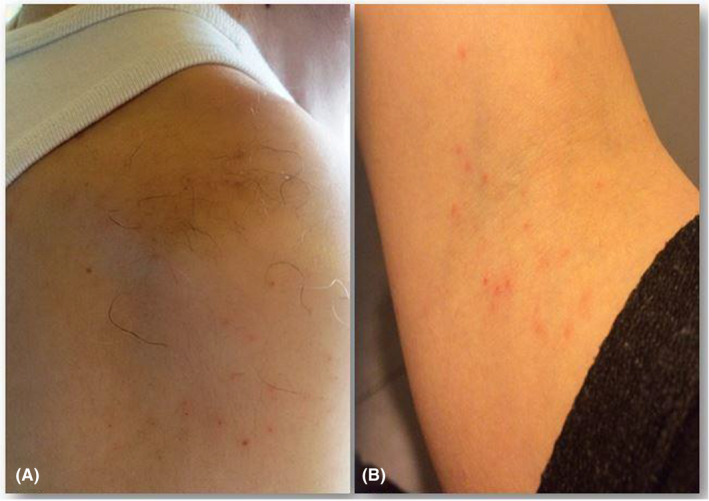
Multiple small reddish itchy papules on the right shoulder of the first patient reported as case 1 (A) and forearms of the second patient presented as case 2 (B)
2.2. Case report 2
A 34‐year‐old woman complaining of continuous intensified itching for 6 months was referred to the same department for complementary clinical‐parasitological analyses. She was residing in an apartment, where the windows opened on a terrace, in which several feral pigeons were nesting. She had never experienced pruritus earlier until the pigeons started nesting behind the terrace windows. Both of her neighbors shared similar living conditions and presented similar itching problems, particularly at night. She consulted many physicians, including a dermatologist, but it was not diagnosed accurately, causing a failure in treatment. The intensified itching was attributed to several potential factors including allergy, infestation by mites (eg, Sarcoptes scabiei), or bed bugs. Clinical examination of the patient revealed multiple groups of small erythematous itchy papules in both the forearms and arms (Figure 1B). This patient had brought some small insects found on her terrace that seems to be the biting bugs.
Nine insect samples collected by both the patients and also by a parasitologist, from the office of the first patient, were morphologically identified under a microscope, using the identification key of.6 In addition, for a definite insect identity, the morphological identification was coupled with molecular analysis. The DNA of the insect was extracted using Chelex 10%,7 which was then subjected to a conventional PCR, using a primer pair, targeting the mitochondrial cytochrome oxidase 1 (COI) gene.8 Amplicons were analyzed via electrophoresis in 1.5% agarose gel containing ethidium bromide as a staining agent. Moreover, they were directly sequenced in both directions, using the same primers used for PCR. The sequences obtained were edited using BioEdit v7.0.0 software9 and were compared with homologous sequences in the GenBank, by means of Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov/BLAST). The specimens were identified at the species level, based on ≥99% identity with sequences deposited in the GenBank. In order to evaluate the intraspecific variability, phylogenetic relationships, and polymorphisms within the Dermanyssus species, COI sequences of the mites were aligned against the GenBank sequences. Phylogenetic analysis was carried out using MEGA v.6 software. The inferred phylogenetic tree of Dermanyssus mites obtained in this study, together with sequences coming from the GenBank, was constructed based on the Neighbor‐Joining (NJ) method, with bootstrap values determined by 1000 replicates.
The collected samples were morphologically identified as Dermanyssus mites (Figure 2). In addition, a fragment containing 675 base pairs of the COI gene was amplified by conventional PCR. Based on the sequencing results, these strains demonstrated more than 99% identity with Dermanyssus sp. sequences deposited in the GenBank (AM921875). Furthermore, the sequences obtained in the present study were deposited in the GenBank and referenced by accession numbers of AH364013‐AH364016. The phylogenetic analysis showed high congruence to the COI tree, in which all taxa were clustered into the similar species complex (Figure 3).
Figure 2.
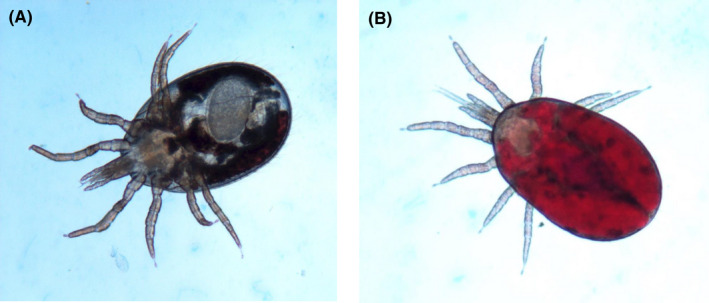
Microscopic illustration of Dermanyssus samples (A, collected from the first patient and B, from the second patient) processed in the present study
Figure 3.
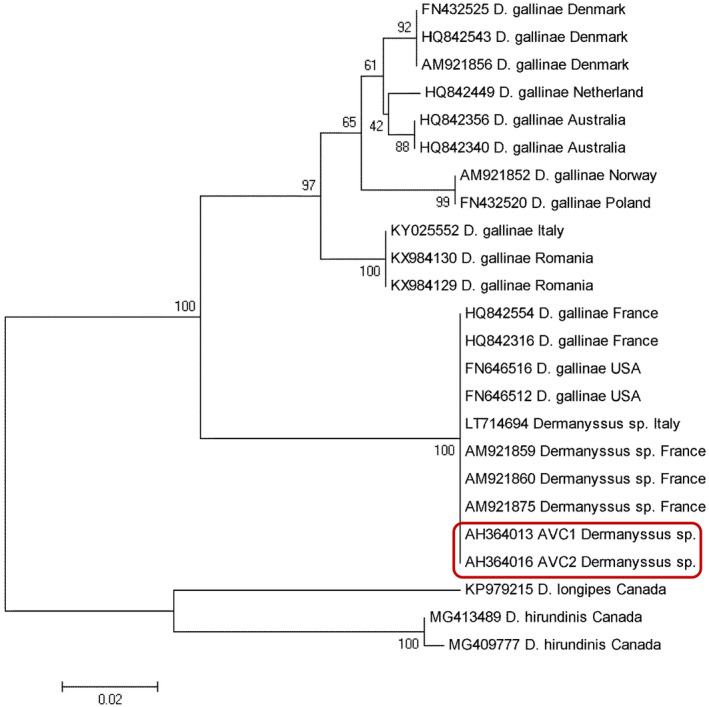
Neighbor‐Joining phylogenetic tree constructed based on COI gene sequences of Dermanyssus species obtained in the present study (highlighted in red) together with those deposited in GenBank
3. DISCUSSION
Dermanyssus infestation is a known parasitic problem, prevalent in rural areas, particularly in individuals working in the poultry industries. However, in urban areas, it is an underestimated disorder, which may occur occasionally in individuals who may come in close contact with pigeons. The identification of clinical manifestations resulting from Dermanyssus bites and the clinical disorders caused by them can be difficult for physicians who are not familiar with these mites. This condition may appear complicated due to the similarity of its symptoms to other diseases. However, the presence of localized reddish itchy papules in exposed parts of the body, particular itching schedule (nocturnal), no history of disease, or infection in either of the patients before the mentioned period, together with the presence of mites in infested locations, suggests the presence of the causative agent (Tables 1 and 2). In addition, this mite species may be confused with another Dermanyssoid mite, Ornithonyssus sylviarum, which often shares the same host and environmental conditions and can cause similar clinical manifestations.10 However, these mites can be easily distinguished from one another with the help of morphological characteristics of the adult stage. In the present study, microscopic and molecular examinations were performed for accurate identification of the mites. In addition, patients in this study were particularly infested with this species as the feral pigeons nested either just behind the windows of the office (first case) or on the flat terrace (second case). Feral pigeons are peridomestic birds, live around human habitations, and are sometimes also raised as pet birds in houses. They host several pathogens, although human infestations with mites from pigeons have rarely been reported in the urban areas.11 Until now, 109 different pathogenic agents have been reported in the pigeons that can potentially infect humans. Among them, six agents including Chlamydophila psittaci, Histoplasma capsulatum, Cryptococcus neoformans, Aspergillus sp., Candida sp., and Toxoplasma gondii can be transmitted to humans.12 Furthermore, several ectoparasites like Argas reflexus, Columbicola columbae, Menopon gallinae, Lipeurus caponis, Goniocotes gallinae, Menacanthus stramineus, Goniodes gigas, and Pseudolynchia canariensis have been reported from the feral pigeons.1, 13 Consequently, they are an important source of infections for several zoonotic endo‐ and ectoparasites, which are in close contact with humans.
Table 1.
Discriminative clinical characters associated with Dermanyssus mites comparing to other hematophagous arthropods biting
| Criteria | Dermanyssus | Mosquito | Biting midge, Sandfly | Louse | Tick | Flea | Bed bug | |
|---|---|---|---|---|---|---|---|---|
| Clinical manifestations | Pruritic dermatitis, erythematous, small maculopapular or papulovesicular lesions, itching | Red itchy papules | Small reddish swollen bump | Small painful red spot or skin rash, inflammation and irritation with blue spots or small spots of blood on the skin | Volcanic papule with central hole, reddish wheal or plaque | Small itchy bumps surrounded by reddish inflamed skin, macule, papule, nodule | Macule, papule, nodule, vesicle, bullae, erythematous and pruritic symptoms, allergy, systematic reaction | |
| Bite spot diagnosis | Bite feeling | Painless | Painless | Painful | Painful | Painless | Painful | Painless |
| Bite spot pattern | Sporadic and separated | Sporadic and separated | Sporadic and separated | Cluster of separated small red bumps | separated and sporadic | Cluster of 2, 3 or more bites | Zigzag or straight line | |
| Bitting time | Nocturnal | Evening and night | Evening and night | Any time | Any time | Any time | Nocturnal | |
| Location | Exposed area | Exposed area | Exposed area | Throughout body particularly on the scalp, neck, and shoulders | Exposed area | Lower extremities, rarely upper body | Exposed area | |
Table 2.
Differential diagnosis criteria to discriminate Dermanyssus mites infestation
| Disease/Disorder | Symptoms |
|---|---|
| Dermanyssus bites | Localized pruritic dermatitis, erythematous, small maculopapular or papulovesicular lesions in exposed parts of body |
| Insects bites | Bumps in bite spots, swelling, itching in exposed parts of body |
| Human scabies |
Intense itching over most of the body especially at night leading to scratching and excoriations, skin rash appearing as red bumps, burrows, or pimple‐like irritations |
| Acute urticaria | Eruption of itching papules, usually systemic |
| Impetigo | Bacterial infection of the skin resulting in tiny blisters |
| Eczema | Skin inflammation with itchy, red, cracked, and rough lesions |
| Contact dermatitis | Inflammation of skin due to direct allergen or irritant contact |
| Folliculitis | Inflammation of hair follicles |
On the other side, Dermanyssus species have been documented to be involved in the transmission of several bacteria (Erysipelothrix rhusiopathiae, Pasteurella multocida, Chlamydia psittaci, Borrelia bugdorferi, Salmonella sp, Spirocheta spp., and Rickettsia spp.), viruses (West Nile virus, Fowl pox virus, Newcastle virus, and encephalitis viruses), filariae, and protozoa, which are responsible for animal and human diseases.14, 15 Some analyses have ascertained its role in experimental transmissions of some pathogenic agents in birds.16, 17 Nevertheless, no evidence attesting Dermanyssus species as a biological vector of human diseases is available. In the present study, neither of the two patients showed any clinical symptoms corresponding to bacterial, viral, or parasitic infection. Based on the phylogenetic analysis, Dermanyssus species identified in this study demonstrated a high degree of genetic similarity with other Dermanyssus mites reported from Europe, such as that in France18, 19 and Italy,20 as well as in the USA21 (Figure 3).
Considering the vast host variation among Dermanyssus species, 22 some treatment approaches are rather host‐dependent. Consequently, accurate species identification would help select the proper method(s) for treatment. One of the first actions preventing human Dermanyssus infestation relies on the treatment of the birds and poultry, including proper chemical and nonchemical control management.3 In the present case, antipigeon devices were installed behind the patient's windows to prevent the pigeon nesting as the first step. Dermanyssus mites in infested office and home were eliminated, by the hospital ecology department or by the patient herself, respectively, using an acaricide. Patients were advised to wash their clothes and linens at 60°C. To treat clinical symptoms and relieve the itching, an antiseptic (Hexomedine, two times per day) and an antihistamine (Cetirizine, 10 mg/d for 2 weeks) were prescribed. The patients were referred to the Parasitology department once again for follow‐up visit two weeks post‐treatment, and no clinical symptoms were observed in the patients then.
4. CONCLUSION
Although Dermanyssus mite infestation is a parasitic problem prevalent in rural areas, particularly in individuals working in the poultry industries, it can occasionally occur in urban areas as well. In the urban cities, it usually affects individuals who remain in close contact with feral pigeons, the peridomestic birds. This paper presents two case reports of patients infested by Dermanyssus mites due to nesting of the feral pigeons either behind the office windows (first case) or on the flat terrace (second case). Consequently, the pigeons are a potentially important source of infections by several zoonotic endo‐ and ectoparasites including Dermanyssus mites.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
MA and AI: conceived and designed the study; MA, SB, AM, and AI: drafted and edited the manuscript; MA, SB, AM, and AI: participated in data analyses. MA, SB, AM, JB, HBR, and AI: conducted the data collection; all authors: read and approved the final predraft manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr Christiane Bruel (Agence régionale de santé, Île‐de‐France), Mr Stéphane Mantelet, and Mrs Samira Dziri (Parasitology‐Mycology and Bacteriology‐Virology departments of Avicenne hospital) for their valuable helps dedicated to this investigation.
Akhoundi M, Brun S, Marteau A, Debédat J, Ben Romdhane H, Izri A. Occasional human infestations by feral pigeons' ectoparasites: Two case reports. Clin Case Rep. 2020;8:1255–1260. 10.1002/ccr3.2764
REFERENCES
- 1. Haag‐Wackernagel D, Bircher AJ. Ectoparasites from feral pigeons affecting humans. Dermatology. 2010;220:82‐92. [DOI] [PubMed] [Google Scholar]
- 2. Thomas E, Chiquet M, Sander B, Zschiesche E, Flochlay AS. Field efficacy and safety of fluralaner solution for administration in drinking water for the treatment of poultry red mite (Dermanyssus gallinae) infestations in commercial flocks in Europe. Parasite Vectors. 2017;10:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sparagano O, George D, Harrington D, Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Ann Rev Entomol. 2014;59:447‐466. [DOI] [PubMed] [Google Scholar]
- 4. Chauve C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): Current situation and future prospects for control. Vet Parasitol. 1998;79:239‐245. [DOI] [PubMed] [Google Scholar]
- 5. Flochlay AS, Thomas E, Sparagano O. Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg‐laying industry in Europe. Parasite Vectors. 2017;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moss WW. An illustrated key to the species of the acarine genus Dermanyssus (Mesostigmata: Laelapoidea: Dermanyssidae). J Med Entomol. 1968;5:67‐84. [DOI] [PubMed] [Google Scholar]
- 7. Musapa M, Kumwenda T, Mkulama M, et al. A simple chelex protocol for DNA extraction from Anopheles spp. J Visual Exp. 2013;71:3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oines O, Brännström S. Molecular investigations of cytochrome c oxidase subunit I (COI) and the internal transcribed spacer (ITS) in the poultry red mite, Dermanyssus gallinae, in northern Europe and implications for its transmission between laying poultry farms. J Med Vet Entomol. 2011;25:402‐412. [DOI] [PubMed] [Google Scholar]
- 9. Hall TA. Bioedit: a user‐friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95‐98. [Google Scholar]
- 10. Di Palma A, Giangaspero A, Cafiero MA, Germinara GS. A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasite Vectors. 2012;5:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cafiero MA, Camarda A, Circella E, Galante D, Lomuto M. An urban outbreak of red mite dermatitis in Italy. Int J Dermatol. 2009;48:1119‐1121. [DOI] [PubMed] [Google Scholar]
- 12. Haag‐Wackernagel D. Human diseases caused by feral pigeons. Adv Vert Pest Manage. 2006;4:31‐58. [Google Scholar]
- 13. Saikia M, Bhattacharjee K, Sarmah PC, Deka DK, Mushahary D. Prevalence of ectoparasitic infestation of pigeon (Columba livia domestica) in Assam. India. J Entomol Zoolog Studies. 2017;5:1286‐1288. [Google Scholar]
- 14. Valiente Moro C, De Luna CJ, Tod A, Guy JH, Sparagano OA, Zenner L. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol. 2009;48:93‐104. [DOI] [PubMed] [Google Scholar]
- 15. De Luna CJ, Arkle S, Harrington D, George DR, Guy JH, Sparagano OA. The poultry red mite Dermanyssus gallinae as a potential carrier of vector‐borne diseases. Ann New York Acad Sci. 2008;1149:255‐258. [DOI] [PubMed] [Google Scholar]
- 16. Brännström S, Hansson I, Chirico J. Experimental study on possible transmission of the bacterium Erysipelothrix rhusiopathiae to chickens by the poultry red mite, Dermanyssus gallinae. Exp Appl Acarol. 2010;50:299‐307. [DOI] [PubMed] [Google Scholar]
- 17. Valiente Moro C, Chauve C, Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329‐336. [DOI] [PubMed] [Google Scholar]
- 18. Roy L, Dowling AP, Chauve CM, Buronfosse T. Delimiting species boundaries within Dermanyssus Dugès, 1834 (Acari:Dermanyssidae) using a total evidence approach. Mol Phylogend Evol. 2009;50:446‐470. [DOI] [PubMed] [Google Scholar]
- 19. Roy L, Buronfosse T. Using mitochondrial and nuclear sequence data for disentangling population structure in complex pest species: a case study with Dermanyssus gallinae . PLoS ONE. 2011;6:e22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pezzi M, Leis M, Chicca M, Roy L. Gamasoidosis caused by the special lineage L1 of Dermanyssus gallinae (Acarina: Dermanyssidae): A case of heavy infestation in a public place in Italy. Parasitol Int. 2017;66:666‐670. [DOI] [PubMed] [Google Scholar]
- 21. Roy L, Dowling APG, Chauve CM, Buronfosse T. Diversity of phylogenetic information according to the locus and the taxonomic level: an example from a parasitic mesostigmatid mite genus. Int J Mol Sci. 2010;11:1704‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roy L, Chauve CM. Historical review of the genus dermanyssus Dugès, 1834 (acari: mesostigmata: dermanyssidae). Parasite. 2007;14:87‐100. [DOI] [PubMed] [Google Scholar]


