Abstract
Background & Aims
Hypothermic oxygenated machine perfusion (HOPE) is a promising technique for providing oxygen to the liver during graft preservation; however, because of associated logistical constraints, addition of an oxygen transporter to static cold-storage solutions (SCS) might be easier. M101 is marine worm haemoglobin that has been shown to improve kidney preservation in the clinic when added to SCS. This study evaluated the effects of the addition of M101 to SCS on the quality of pig liver graft preservation.
Methods
Pig liver grafts were preserved using SCS, HOPE, or SCS+M101, and the liver functions were compared during cold preservation and after orthotopic allotransplantation (OLT) in pigs.
Results
During preservation of the liver grafts, mitochondrial function, ATP synthesis, antioxidant capacities, and hepatocyte architecture were better preserved, and free radical production, antioxidant activities, and inflammatory mediators were lower, with HOPE or SCS+M101 than with SCS alone. However, after 1 h of preservation, liver functions with HOPE were superior to those with SCS+M101. After 6 h of preservation and OLT, blood levels of aspartate and alanine aminotransferases and lactate dehydrogenase increased with a peak effect at Day 1 post-transplant; values were similar with HOPE and SCS+M101, and were significantly lower than those in the SCS group. At Days 1 and 3, tumor necrosis factor α levels remained lower with HOPE and SCS+M101 vs. SCS. At Day 7, liver cell necrosis and inflammation were less marked in both oxygenated groups.
Conclusions
When added to SCS, M101 effectively oxygenates liver grafts during preservation, preventing post-transplant injury; although graft performances are below those achieved with HOPE.
Lay summary
When transported between donors and recipients, even cold-stored liver grafts need oxygen to maintain their viability. To provide them with oxygen, we added a marine worm super haemoglobin (M101) to the cold-storage solution UWCS. Using a pig liver transplant model, we revealed that livers cold stored with UWCS+M101 showed improved oxygenation compared with simple cold-storage solutions, but did not reach the oxygenation level achieved with machine perfusion.
Keywords: Liver transplantation, Haemoglobin, Hepatocyte, Inflammation, Oxidative stress, Cell necrosis
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DCD, deceased after circulatory death; DGF, delayed graft function; HOPE, hypothermic oxygenated machine perfusion; INR, international normalised ratio; IRI, ischaemia-reperfusion injury; PT, prothrombin time; SCS, simple cold-storage solutions; UWCSS, University of Wisconsin cold-storage solution
Graphical abstract
Highlights
-
•
During cold storage, liver grafts lack oxygen.
-
•
Addition of the marine worm haemoglobin M101 provides oxygen during cold storage.
-
•
ATP synthesis and antioxidant activities are preserved by M101.
-
•
Reperfusion damage is reduced by M101.
Introduction
In liver transplantation, graft quality is a key factor of procedural success and long-term survival and outcome.1 Liver graft quality depends not only on its intrinsic performance at the time of harvesting, but also on the quality of preservation during transportation from donor to recipient. Core flushing and static cold storage (SCS) using an appropriate solution is the gold-standard method of preservation. Hypothermia (temperature of 4–8°C) depresses liver cell metabolism and triggers liver tissue adaptation to the anoxic environment. However, even slow metabolism requires O2 and, after a few hours' storage, O2 deprivation and the consequent dramatic reduction in ATP synthesis induces cell swelling as a result of inhibition of the membrane Na+/K+ pump,2 intracellular acidosis by anaerobic glycolysis, lactate accumulation and intracellular Ca2+ elevation, resulting in protease activation and mitochondrial respiratory channel dysfunction.3 Moreover, at revascularisation, sudden restoration of normothermia and an O2 supply in degraded tissue leads to the massive production of oxygen radical species and consequent cell membrane rupture.4 Taken together, these events are referred as ‘ischaemia-reperfusion injury’ (IRI). Although the most effective strategy is to reduce cold ischaemia time, novel conditioning methods have also been investigated to improve graft quality, including providing oxygen to the graft during cold storage.
Hypothermic oxygenated machine perfusion (HOPE) was first developed during the 1970s.5 It was applied to liver preservation in Belzer and colleagues laboratory at the University of Wisconsin during the late 1980s6 and has been shown to preserve liver grafts better and for longer compared with ischaemic cold storage.7 Recently, HOPE showed efficacy in liver transplantation8 and superiority in preserving steatotic liver grafts.9
Compared with SCS, HOPE is more expensive, requires more manpower, and can incur specific risks, such as barometric trauma. Thus, despite its effectiveness, HOPE is not universally used in clinical practice for liver transplantation. Supplying SCS solutions with an oxygen carrier would be more convenient and easier to implement. For example, adding perfluorocarbon (PFC), a high-capacity oxygen-binding compound, to the SCS solution has been shown to improve the quality of liver grafts harvested after cardiac arrest10; however, PFC equilibrates quickly with the surrounding atmosphere and rapidly loses its capacity to oxygenate the tissue. Therefore, a more efficient oxygen carrier would be desirable.
M101 (Hemarina SA, Morlaix, France) is a natural giant extracellular haemoglobin (Hb) extracted from the marine invertebrate Arenicola marina. Each Hb molecule transports 156 O2 molecules, compared with just four with human Hb. Moreover, M101 is functional over a large temperature range (4–37°C), and passively releases O2 with an oxygen gradient, providing the environment with exactly the right amount of oxygen without requiring any allosteric effector. Moreover, it has intrinsic Cu/Zn-superoxide dismutase (SOD) activity, with antioxidant effects.11
In a pig kidney transplant model, the addition of M101 to University of Wisconsin cold-storage solution (UWCSS) improved preservation, with significant benefit in both early functional recovery and outcome.12 Similar results were reported in preclinical models of lung, heart, and pancreas graft preservation.[13], [14], [15], [16] Recently, in a French clinical trial using kidney transplantation from deceased donors, M101 demonstrated safety and efficacy, drastically decreasing the rate of delayed graft function recovery from 26% to 7%.17 However, M101 performance in cold-stored liver graft remains to be studied.
Here, we present an experimental study of M101 added to the SCS solution. Cold-stored livers with or without added M101 were compared vs. livers preserved using HOPE. Preservation method performance was first assessed in vitro, during cold storage, and then in an allogeneic pig OLT model.
Materials and methods
Animals
Thirty female Large White/Landrace × Piétrain pigs (15 donors and 15 recipients) were obtained from the French National Research Institute for Agriculture, Food, and the Environment (INRAE, Saint-Gilles, France). All experiments were performed in accordance with the European Union regulations for animal experimentation (directive 2010/63/EU and INRAE facility agreement n°A35-622), and conformed to the ARRIVE guidelines of the NC3Rs. The Regional Ethics Committee in Animal Experiment of Brittany validated the entire procedure described in this paper (n°2015042811522667, individual authorisation for DVL, n°35-88). Experimented surgeons (KB and PA) performed all the surgical interventions with the assistance of INRAE-trained staff for humane pig handling, anaesthesia, and analgesia.
Animals were 3 months old, weighing 35–40 kg. Just before and after surgery, the pigs were housed in individual pens (150×80×60 cm) and had free access to water. Toys (e.g. chains and balloons) were provided to enrich their environment and fulfil their natural disposition to play. The room was maintained at 24.0 ± 0.3°C with a 13:11-h light–dark cycle combining both daylight and artificial lighting. The animals were fed daily a pelleted diet comprising 40% pea, 15% corn, 14.46% barley, 13.92% wheat, 13.56% soybean meal, 0.68% calcium carbonate, 0.58% mono-calcic phosphate, 0.3% vegetable oil, 0.3% vitamin complement, and 0.24% salt (net energy: 2.15 MCal/kg of feed). The daily feed ration before surgery corresponded to the usual standards for the age and species. The week after surgery, this ration was progressively resumed according to the status of the animals and caretakers' insight.
Twenty four hours before surgery, the animals were fasted with free access to water. After slight sedation by intramuscular injection of ketamine 15 mg/kg (Imalgene 1000; Merial, Lyon, France), all animals were anaesthetised by spontaneous mask ventilation (Isoflurane 2–3% v/v; Baxter, France). Once the pharyngotracheal reflex was abolished, tracheal intubation was performed and general anaesthesia was prolonged with controlled ventilation (mixed air and oxygen 60%). The marginal vein of one ear was catheterised to allow fluid (Ringer Lactate 250 ml/h, Baxter) and anaesthetic drug administration. Controlled ventilation was maintained throughout the surgical procedure; the fraction of inspired oxygen (FiO2) was maintained above 60%, and SpO2 saturation on pulse oximetry (3800 Pulse Spo2 Oximeter, Datex-Ohmeda, France) was maintained above 98%. Per operative analgesia was obtained by intravenous fentanyl infusion (one vial of 10 ml, 0.05 mg/ml; Renaudin, France) at 1.4 ml/min until the end of the procedure. In transplanted animals, 10 mg morphine hydrochloride was also injected subcutaneously for analgesia.
At the end of the experiments (i.e. immediately after aortic cross clamping in donors and at Day 7 after blood sampling in recipients), animals were euthanised under general anaesthesia by intravenous overdose bolus of T61 (embutramide + mebezonium).
Liver procurement and preservation
Graft harvesting, cold washout, and back-table preparation were performed as previously described.18 Briefly, livers were harvested after in situ flushing through the aorta (1 L) and portal vein (1 L) using 4–8°C Belzer UWCSS (Bridge to Life Ltd, London, UK). The liver was then quickly detached from its vessels and ligaments. The hepatic artery and portal vein were retrieved in continuity with the coeliac trunk and superior mesenteric vein, respectively, and the infrahepatic vena cava was left long and cut below the ostia of the renal vein, which were tightened. The graft was then transferred to a back-table and placed in a sterile plastic bag in a basin on ice (for cold-stored grafts) or in the perfusion cassette (for machine-perfused grafts). The graft was then placed posterior surface facing upward, exposing the pedicle for an ex situ second flush through the portal vein before preservation using one of the three study methods.
Study groups
Three cold-preservation methods were compared: (i) static cold storage (SCS group, N = 10): the ex situ second flush was performed through the portal vein with 1 L cold (4°C) UWCSS alone. Livers were then stored floating in the effluent solution; (ii) static cold storage with added M101 (SCS+M101 group, N = 10): the ex situ flush was performed through the portal vein with 1 L cold (4°C) UWCSS supplemented with 1 g M101. The vial containing the M101 (HEMO2life®; Hemarina) was stored at −20°C, allowed to thaw at room temperature for 2 h before use, then carefully retrieved from the vial using a sterile glass syringe and injected into the 1 L of UWCSS under sterile conditions immediately before reflush. Livers were then stored floating in the effluent solution; and (iii) HOPE group (N = 10): The ex situ flush was performed through the portal vein with 1 L cold (4°C) Belzer UW® Machine Perfusion Solution (UWMPS). One supplementary litre of UWMPS was added directly to the cassette. The experimental perfusion machine was designed in the INRAE workshops and inspired by the machine used by F.O. Belzer's group at the University of Wisconsin.6 Briefly, the cassette was sterile and closed, infusion was provided by a roller pump through the portal vein only, and oxygenation was provided by continuous oxygen flow (1 L/min) delivered to the surface of the solution. A pressure sensor was placed at the entrance of the portal vein and the perfusion pressure, which depends on intrahepatic vascular resistance, regulated the perfusion flow.19 Maximum perfusion pressure was set at 5 mmHg and the flow capped at 200 ml/min. In all perfused livers, the flow reached its maximum (200 ml/min) in under 5 min, while the pressure remained steady between 3 mmHg and 5 mmHg.
Ten livers were studied per group: five for ex situ analysis during preservation and five for transplantation.
Sampling, biochemical determination, and electron microscopy in ex situ experiments
Liver biopsies using a 4-mm sterile puncher were taken from different lobes at 30 min, 60 min, 120 min, 180 min, 360 min, and 540 min of cold preservation. Samples were snap-frozen in liquid nitrogen and stored at −80°C awaiting analysis. For analysis, frozen tissue samples were homogenised (100 mg per 1 ml PBS); intrahepatic ATP content was measured on a bioluminescence assay kit (Sigma Aldrich, St Quentin Fallavier, France); tissue tumor necrosis factor alpha (TNFα) levels were quantified on porcine ELISA (BD Bio-sciences, San Diego, CA, USA); lipid peroxidation, as an indirect measure of oxidative stress induced by reactive oxygen species, was determined by quantification of malondialdehyde (MDA) using a thiobarbituric acid reactive substances assay kit (Interchim, Montluçon, France); catalase and SOD activities were determined on colorimetric and spectrophotometric assay, respectively (Interchim, Montluçon, France).
For electron microscopy, tissue samples were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer for at least 4 h at room temperature or overnight at 4°C. Subsequently, after rinsing in the same buffer, samples were post-fixed for 1 h with 1% osmium tetroxide solution diluted in 0.1 M cacodylate buffer, rinsed, dehydrated through a graded acetone series, and embedded in Epon® resin (Silmid, France). Finally, samples were polymerised at 60°C for 48 h. From the resin-embedded tissue blocks, ultra-thin (80 nm) sections were cut using a Leica UC7 ultramicrotome (Leica Geosystems, Vienna, Austria), mounted on 300-mesh thin-bar nickel support grids (EMS, Hatfield, PA, USA) and stained with uranyl acetate and lead citrate. Grids were observed using a Jeol JEM-1400 transmission electron microscope (Jeol Ltd, Tokyo, Japan) at an accelerating voltage of 120 kV, equipped with an Orius 1000 camera (Gatan Inc., Pleasanton, CA, USA). Images were taken from three different livers for each condition and, for each liver, four different areas were investigated. An accurate comparison of tissue ultrastructural alterations on the same pictures were conducted by three different observers independently (AB, AC, and BT).
Allogeneic liver orthotopic transplantation experiments
Livers were cold-preserved for 6 h. Grafts were retrieved from the preservation solution 20 min before the estimated time of revascularisation, for ex situ preparation and implantation.
All recipient animals (N = 15) underwent the same surgical procedure, using the cuff technique.20 Briefly, before laparotomy, the left internal jugular vein was freed, ready for later introduction of the portojugular shunt. After median laparotomy, the native liver was freed from its attachments and its vessels were taped. Immediately before retrieval of the native liver, the liver graft was removed from the preservation container and prepared on a back-table on a layer of sterile crushed ice. The suprahepatic vena cava was shortened right above a thin diaphragm collar and the diaphragmatic veins were stitched; the infrahepatic vena cava and portal vein were hemmed around a homemade Teflon cuff adapted to vessel diameter (internal diameter, 5–10 mm); the coeliac trunk was sectioned 5 mm below the origin of the splenic artery and the gastric artery was obliterated. In the recipient, the native liver was retrieved and a passive veno-venous shunt was established between the portal vein and left internal jugular vein. During the anhepatic phase, 250 ml of 9% saline was infused through the peripheral venous line to maintain stable haemodynamic conditions. The graft liver was implanted orthotopically with end-to-end anastomoses between the suprahepatic inferior vena cava, portal vein (cuff technique), infrahepatic inferior vena cava (cuff technique), and hepatic artery (separate stiches), in that order. Liver grafts were reperfused with the portal blood after completion of the portal vein anastomosis. Bile ducts were also connected by end-to-end anastomosis. Just before clamping off, 40 ml of 4.2% sodium bicarbonate and 4 ml of 10% calcium gluconate were intravenously administered to the recipient.
At the end of the transplant procedure, a central silicone catheter was introduced through the left internal jugular vein and externalised at the posterior surface of the neck of the animal for daily blood sampling. After each blood sample, the intravenous line was rinsed with 0.5 ml of heparinised serum and kept closed with a sterile three-way stopcock wrapped in a plaster.
Recipients were extubated immediately after surgery and placed back in their cage with heat lamps. Analgesia was given by subcutaneous injection of 0.3 mg/kg of morphine. Water was provided ad libitum and feeding was allowed on postoperative Day 1. After transplantation, no immunosuppression treatment was implemented.
Sampling, biochemical determination, and histological studies in transplanted animals
Transplanted animals were studied until Day 7 post-transplant. Blood samples were taken 1 h and 3 h after transplantation for assessment of TNFα plasma levels, then every morning between 07:00 h and 08:00 h from Day 1 to Day 7. Hepatocellular injury (i.e. membrane rupture) was evaluated using AST, ALT, and lactate dehydrogenase (LDH) plasma levels. Liver function was assessed using total bilirubin (tBil), prothrombin time (PT), factor V (FV) level, and the international normalised ratio (INR). Systemic inflammation and macrophage activation were assessed using TNFα blood levels. AST, ALT, LDH, and tBil were measured by photometry using a multiparametric analyser (Cobas 8000, Roche, France). PT, FV, and INR were analysed using a multiparametric analyser (STAR Max, Stago, France). TNF-α levels were quantified by means of a porcine ELISA kits (BD Bio-sciences).
At Day 7 post-transplant, immediately after animal sacrifice, autopsy was performed, with two wedge biopsies of the liver graft for pathological examination. Liver tissue samples were fixed in 4% formaldehyde at room temperature, embedded in paraffin, and cut into 5 μM slices. Slides were stained with haematoxylin–eosin–saffron (HES). Histological damage blind assessment was performed by an experienced liver pathologist (BT). The histological severity of the liver damage was graded using Suzuki's score, as recently reported.21 It comprised three parameters of hepatic IRI: sinusoidal congestion, vacuolisation of hepatocyte cytoplasm, and parenchymal necrosis. Each parameter was graded numerically as follows: 0 = none; 1 = minimal; 2 = mild; 3 = moderate; and 4 = severe. The same criteria were utilised in the graduation of vacuolisation, and for necrosis, the numerical graduation was as follows: 0 = nonnecrotic cells; 1 = single cell necrosis; 2 = <30% necrosis; 3 = <60% necrosis; and 4 = >60% necrosis. Inflammation in the centrilobular and portal areas, which is not considered in the Suzuki's score, was also graded as follows: 0 = none; 1 = minimal; 2 = mild; 3 = moderate; and 4 = severe.
Statistical analyses
A size sample of five animals per group was considered the minimum required to guarantee sufficient statistical power for this study, in terms of the physiological parameters considered as major judgment criteria (i.e. enzyme activity, inflammation markers, and histological criteria). The sample size was validated by an independent ethics committee and was coherent with previously published papers in this thematic in the pig model.12
Data were expressed as the mean ± standard error of the mean for five animals per group. Differences between the groups were tested using a two-way ANOVA followed by a Tukey's multiple comparisons test when the overall comparison of groups was significant. A p value <0.05 was considered to be significant. Analyses were performed using Graph Pad Prism software (version 6.0; Graph Pad, San Diego, CA, USA).
Results
Liver study during preservation
Mitochondrial function and ATP synthesis were improved with SCS+M101 and HOPE
Given that ATP stock reflects the preservation of mitochondrial function and, thus, the ability of the liver to recover after IRI, ATP was quantified in liver samples at various time points during preservation. As shown in Figure 1, whichever the preservation method, ATP levels declined after 20 min of preservation, but were significantly lower with SCS alone than with either SCS+M101 or HOPE at 1 h (2.9 ± 0.7 μmol/mg vs. 9.9 ± 3.1 μmol/mg and 9.6 ± 1.7 μmol/mg protein, respectively), 3 h (1.8 ± 0.8 μmol/mg vs. 4.9 ± 1.6 μmol/mg and 6.3 ± 1.4 μmol/mg protein, respectively), and 9 h (0.8 ± 0.3 μmol/mg vs. 2.4 ± 0.6 μmol/mg and 6.4 ± 2.8 μmol/mg protein, respectively). At the end of preservation, ATP levels were significantly higher in HOPE-preserved livers than in livers preserved with SCS+M101.
Fig. 1.
Progression of tissue ATP concentration according to liver cold preservation time.
Data are presented for the three groups: (i) SCS; (ii) SCS with M101 added to the UWCSS (SCS+M101); and (iii) HOPE with the UW machine perfusion solution. Bars represent SEM. ∗p <0.05; ∗∗p <0.01 (ANOVA + Tukey's test). HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; UWCSS, University of Wisconsin cold-storage solution.
Parallel to ATP depletion in the SCS group, electron microscopy showed marked disintegration of hepatic cellular architecture compared with the SCS+M101 and HOPE groups (Fig. 2). After 9 h of preservation vs. T0 (immediately before cold storage or perfusion), cell impairment was detected in all preserved livers, but ultrastructural changes were particularly marked in the SCS group, with swollen and less dense mitochondria and disappearance of cristae. In the HOPE and SCS+M101 groups, mitochondrial ultrastructure was also altered, with loss of matrix and impaired outer membrane integrity; however, in both oxygenated groups, typical mitochondrial morphology persisted, especially in HOPE-preserved livers, in which the outer membrane of mitochondria and cristae were better preserved.
Fig. 2.
TEM pictures of pig liver tissue.
Data are presented before (A,E) and after 9 h preservation during SCS (B,F), SCS with M101 added to the UWCSS (SCS+M101) (C,G), and HOPE (D,H). Low magnification (scale bar: 5 μm) of liver cells (A–D) and high magnification (scale bar: 0.5 μm) (E–H) of intracellular mitochondrial ultrastructure. White arrows and solid-black arrows indicate cristae alteration and loss of outer mitochondrial membrane integrity, respectively. HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; TEM, transmission electron microscopy; UWCSS, University of Wisconsin cold-storage solution.
To shed light on the mechanisms involved in liver ATP production, mitochondrial complex activities were evaluated. In the SCS group, complex I, II, and IV activity was preserved throughout the experimental period (Fig. 3). By contrast, in the HOPE group, the activity of complexes I and IV of the respiratory chain was greater than in the SCS and SCS+M101 groups.
Fig. 3.
Activities of mitochondrial complexes I, II and IV according to liver cold preservation time.
Data are presented for the three groups: during SCS, SCS with M101 added to the UWCSS (SCS + M101), and HOPE with the UW machine perfusion solution (bars represent SEM). ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001 (ANOVA + Tukey's test). HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; UWCSS, University of Wisconsin cold-storage solution.
Free radical production, antioxidant activity, and inflammatory mediators were reduced with SCS+M101 and HOPE
To evaluate the impact of preservation strategy on oxidative stress, MDA levels and catalase and SOD activities within liver tissue were analysed throughout the preservation period. As shown in Figure 4A, MDA levels increased over time in all groups, but were lower with SCS+M101 and HOPE than with SCS alone. After 60 min of preservation, HOPE did better than SCS+M101. In addition, SOD activity was significantly higher with SCS+M101 and HOPE (Fig. 4B) than with SCS. Catalase activity was higher in both oxygenated preservation groups than with SCS, although HOPE did better than SCS+M101 throughout the preservation period (Fig. 4C). TNFα concentrations increased markedly over time in the SCS group and remained significantly higher than with SCS+M101 or HOPE throughout preservation (Fig. 4D).
Fig. 4.
Progression of tissue concentration or activity of different indicators of the hepatic function/status.
(A) MDA, (B) SOD, (C) catalase, and (D) TNFα during SCS, SCS with M101 added to the UWCSS (SCS + M101), and HOPE with the UW machine perfusion solution (bars represent SEM). ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001 (ANOVA + Tukey's test). HOPE, hypothermic oxygenated machine perfusion; MDA, malondialdehyde; SCS, static cold storage; SOD, superoxide dismutase; TNF, tumor necrosis factor; UWCSS, University of Wisconsin cold-storage solution.
Post-transplant liver function
Animal weights ranged between 30 kg and 32 kg. Cold ischaemia time (327 ± 18 min, 323 ± 16 min, and 345 ± 14 min) and anhepatic phase duration (29 ± 6 min, 35 ± 7 min, and 34 ± 4 min) were similar in SCS, SCS+M101, and HOPE groups, respectively. All animals survived transplantation and were euthanised at Day 7.
Hepatocellular damage was lower in SCS+M101 and HOPE livers
To evaluate hepatocellular injury, release of ALT, AST, and LDH was determined postoperatively from Day 1 to Day 7. Enzyme release peaked at Day 1, then slowly returned to normal by Day 4. Blood levels at postoperative Day 1 were significantly higher after SCS than with SCS+M101 or HOPE, but there was no further significant difference between groups from postoperative Day 2 to 7 (Fig. 5A).
Fig. 5.
Evolution of blood concentrations of different indicators of the hepatic function and/or status.
(A) AST, (B) ALT, (C) LDH, (D) factor 5 (FV: percentage of normal value), (E) INR, and (F) total bilirubin blood concentrations from Day 1 to Day 7 post-transplantation (bars represent SEM). ∗p <0.05, ∗∗p <0.01, ∗∗∗∗p <0.0001 vs. HOPE, §§p <0.05 vs. SCS + M101, §§§§p <0.01 vs. SCS + M101 (ANOVA + Tukey's test). ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOPE, hypothermic oxygenated machine perfusion; INR, international normalised ratio; LDH, lactate dehydrogenase; SCS, static cold storage.
Liver synthesis capacity was similar in all groups
Prothrombin time (INR) and factor V values had similar profiles in all groups (Fig. 5B). Total bilirubin concentrations were normal in all groups until postoperative Day 4, and then slightly increased until sacrifice at Day 7 with no difference between groups.
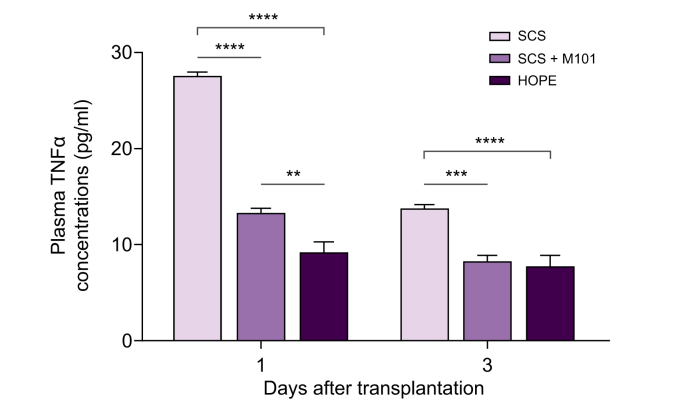
Plasma TNFα concentrations were significantly lower in SCS+M101 and HOPE groups at both 1 day and 3 days post-transplant than in the SCS group (Fig. 6).
Fig. 6.
Plasma levels of TNFα 1 h and 3 h after the end of liver transplantation.
Animals were transplanted using liver grafts preserved using SCS (5 animals), SCS with M101 added to the UWCSS (SCS+M101) (five animals), or HOPE with the UW machine-perfusion solution (5 animals) (bars represent SEM). ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001 (ANOVA + Tukey's test). HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; TNF, tumour necrosis factor; UWCSS, University of Wisconsin cold-storage solution.
Histological damage was more pronounced with SCS than with SCS+M101 or HOPE
HES staining revealed significant morphological changes in Suzuki scores. The lower levels of congestion, vacuolisation, and necrosis were observed in the SCS+M101 and HOPE groups (SCS group vs. SCS+M101 group: 4.2 ± 0.7 vs. 2.4 ± 0.7, p <0.05; SCS group vs. HOPE group: 4.2 ± 0.7 vs. 1.8 ± 0.3, p <0.01) (Fig. 7).
Fig. 7.
Suzuki score at Day 7 post-transplantation.
Animals were transplanted using liver grafts preserved using SCS, SCS with M101 added to the UWCSS (SCS+M101), or HOPE with the UW machine-perfusion solution (bars represent SEM). ∗p <0.05, ∗∗p <0.01 (ANOVA + Tukey's test). HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; UWCSS, University of Wisconsin cold-storage solution.
Portal and centrilobular inflammatory infiltrate levels appeared greater with SCS and HOPE compared with SCS+M101. However, the scores did not show significant differences between groups (SCS group vs. SCS+M101 group: 6.4 ± 1.1 vs. 5.6.4 ± 0.5, NS; SCS group vs. HOPE group: 6.4 ± 1.1 vs. 6.2 ± 0.3, NS) (Fig. 8). None of the groups showed ischaemic-type biliary ductules lesions.
Fig. 8.
Histological features of livers 7 days after transplantation.
Animals were transplanted using liver grafts preserved using SCS, SCS with M101 added to the UWCSS (SCS+M101), or HOPE with the UW machine-perfusion solution. HES staining. Original magnification ×200. Line I shows centrolobular inflammatory infiltrate, and line II shows portal inflammatory infiltrate. HES, haematoxylin–eosin–saffron; HOPE, hypothermic oxygenated machine perfusion; SCS, static cold storage; UWCSS, University of Wisconsin cold-storage solution.
Discussion
The aim of this study was to evaluate the benefit of adding a novel biological oxygen carrier, M101, to the UWSCS solution during preservation of pig liver grafts. Livers preserved with M101 added to the SCS solution were compared with livers preserved either under anoxic conditions with SCS alone, or by HOPE. Both during hypothermic preservation and after transplantation, livers preserved with SCS+M101 or HOPE showed better outcomes than those preserved by SCS alone. These results show that oxygen, added to the solution, is crucial in improving the efficacy of preservation solutions, as do the hydroxyethyl starch, high-molecular-weight sugars, and antioxidants contained in the UWSCS.
Oxygen can be delivered continuously using an infusion machine. Although several machine perfusion techniques are now available, including normothermic perfusion, we chose to compare SCS+M101 with HOPE because the latter was the most widely used perfusion technique in clinical practice at the time the project was set. In that case, the oxygen dissolves in the preservation solution and is released and used by the cells via a mechanism that remains poorly understood. Alternatively, oxygen can be delivered as an initial charge bound to transporter (e.g. bound on haemoglobin, similar to M101) and released physiologically (i.e. gradually along a concentration gradient, the level of which depends on the needs of the irrigated tissue16).
To show the effectiveness of static preservation with added M101 (SCS+M101), we first studied the behaviour of liver grafts ex vivo throughout the preservation period. In the SCS-alone group, during cold preservation, tissue ATP concentrations markedly decreased in the first hour. This previously described phenomenon22 reflects a switch from aerobic glycolysis via the Krebs cycle to anaerobic glycolysis via the lactate pathway. In SCS+M101 preserved liver or with HOPE, tissue ATP levels also fell after 1 h of storage, but were significantly higher than with SCS alone.
This difference can be explained by the difference in the available oxygen level in the solution rather than an alteration of mitochondrial function. Indeed, we showed that, after 1 h of storage, the activities of complexes I, II, and IV were equally preserved in the SCS and SCS+M101 groups, and that the structural alteration of the mitochondria, more marked in the SCS group, did not appear until after 9 h of preservation. This suggested that, in SCS-preserved livers, ATP depletion was due mainly to reduced ATP synthase (complex V) activity. This hypothesis is in line with previous studies showing that ischaemia mainly impairs ATP synthase activity,23,24 via the degradation of complex V.25 Thus, the significant prevention of ATP depletion observed with SCS+M101 and HOPE compared with SCS suggests some preservation of ATP synthase activity. Alternatively, the superiority of HOPE over SCS+M101 regarding ATP synthesis could also result from better preservation of complex I and IV activities (possibly because of higher protection against oxidative stress and lower TNFα release) as well as permanent reload of O2 allowed by fluid recirculation.
The superiority of HOPE preservation over SCS+M101 can be explained by the continuous supply of oxygen by recirculation of the solution during storage with the machine whereas, with SCS+M101, oxygen availability might decrease with consumption. Although M101 has been shown to be reloadable with O2 at the interface between the solution and the air, the solution does not recirculate into the sinusoids as it does with HOPE. Another explanation for the superior performance of HOPE is the elimination of cell catabolism products and toxic oxygen derivatives, because machine perfusion rinses the hepatic sinusoids.26
A maintained ability to synthesise ATP in M101 cold-stored livers and with HOPE could also explain the lower tissue levels of MDA and TNFα: liver preservation in the presence of oxygen might enhance antioxidant strategies, such as increased catalase or SOD activities. Again, the slightly better results obtained with HOPE than with SCS+M101 might be related to the continuous capillary network rinsing in perfused livers.
The ultimate test in evaluating the effectiveness of any preservation protocol is the successful transplantation of the preserved organs. We used a pig transplant model because it is a validated and well-recognised model, in which we have experience.18 Although it is usually used in murine models, we preferred cuffed anastomoses to standard vascular anastomosis to shorten the anhepatic phase and splanchnic venous flow obstruction, which are crucial episodes in the pig liver transplant model. We also chose to use a short, 6-h cold ischaemia time so as to be below the 8-h limit beyond which the solutions and the cold ischaemia preservation technique are associated with significant deterioration of liver cells and impaired survival in the pig liver transplant model.18,27 We might have been able to detect more notable differences between the groups if we had used a more extreme preclinical model, such as a deceased after circulatory death (DCD) model, or a prerecovery period of warm ischaemia. However, the goal of our preliminary study was to focus on the ability of M101 to provide oxygen (and allow aerobic metabolism) to the liver tissues rather than showing its capacity to prolong hypothermic preservation or ameliorate the quality of preservation in stress conditions. Therefore, we think that, rather than being a method to prolong cold storage, SCS+M101 should be a method to improve preservation conditions in a limited time of preservation. This limit has yet to be found.
In this preclinical transplant model, the most demonstrative marker was the plasma intracytoplasmic enzyme peak that reflects the level of necrosis and apoptosis of hepatic cells (i.e. mainly hepatocytes) and, indirectly, the performance of the storage solutions and modalities. Enzyme release was lower in the SCS+M101 and HOPE groups compared with SCS alone at 24 h post-transplant, a difference that faded rapidly and disappeared at 72 h, reflecting the regenerative capacity of the hepatic parenchyma. Such an effect was not observed on liver synthesis and excretion capacity, which were similar between all experimental groups. In all three groups, bilirubin levels increased from Day 4 post-transplant, possibly reflecting a graft rejection process, favoured by the absence of immunosuppressive therapy in this model.
Morphological analysis, performed either by electron or optical microscopy, is known to be subjective; however, in the present study, the pathologist operated blindly and found higher hepatocyte necrosis, cytoplasmic vacuolisation, and sinusoidal congestion levels in the SCS-alone group than in livers preserved in the presence of oxygen, whether supplied by M101 or by the perfusion machine. In some cases, periportal inflammation appeared more marked in livers preserved by HOPE, possibly because of the continuous infusion into the portal network and perfusion pressure damage to the endothelium. This raises the question whether a lower flow or a lower pressure perfusion with HOPE should be mandatory. There are currently no data that reflect the optimal parameters of hypothermic organ perfusion.
In this experimental study, one drawback was that two different solutions were used in the SCS and HOPE groups. We chose to use UWCSS for cold storage and UWMPS for perfusion to better reflect clinical practice. Even if UWMPS might be suitable to cold store livers, this has not yet been documented, and UWCSS remains the gold standard for cold storage in liver transplantation.28 Another drawback is that here are no medico-economic data available regarding the potential benefit of M101 in liver transplantation. However, in the field of kidney transplantation, a medico-economic study (called OXYOP2) is currently comparing the costs of kidney transplantation depending on whether the graft is HOPE or SCS+M101 preserved. This study is the continuation of the OXYOP trial,17 which showed that the rate of patients necessitating at least one post-transplant dialysis was significantly lower in SCS+M101-preserved kidneys compared with HOPE-preserved kidneys (7% vs. 26%, p = 0.038). As a comparison to this 19% difference in delayed graft function (DGF) rates, an international randomised, controlled trial, comparing HOPE or SCS in deceased-donor kidney transplantation29 reported DGF rates of 20.8% and 26.5% in SCS and HOPE groups, respectively. This 5.7% difference could have largely covered the excessive costs induced by HOPE.
Conclusion
In conclusion, we evaluated the performance of a static cold-storage solution supplemented with a highly effective oxygen carrier, and showed that this protocol was effective to provide oxygen to the graft during preservation. HOPE was even more effective than M101 supplementation, but this needs to be weighed against the cumbersomeness and cost of infusion machines, which require continuous manpower presence and know-how, and are associated with a risk of vascular trauma in organ grafts that might compromise the outcome of transplantation. M101 (HEMO2life®; Hemarina) provides a valuable alternative option in the field of organ preservation, complementary to the range of available modalities, and enabling tailored organ preservation solutions depending on the quality of the graft and the time available to transport the graft from the donor to the recipient.
Financial support
The study was supported by CORECT (Comité de la Recherche Clinique et Translationnelle), Centre Hospitalier et Universitaire de Rennes, France. M101 (HEMO2Life®) was provided by Hemarina Quimper France. UWCS- and UWMP-solutions were provided by Bridge to Life (Europe) Ltd, London, UK.
Authors' contributions
KB, PA, and DVL designed the study and performed the experiments. KB and AC wrote the manuscript. BT performed the histological studies. IBM and AC performed the tissue measurements. CB performed the plasma measurement. AB performed the electronic microscopy studies. EB designed and set up the hypothermic machine perfusion. ED, FZ, and DVL designed the study and revised the manuscript.
Conflict of interest
FZ is founder of, and holds stock in, Hemarina, the company that produces HEMO2life®. ED is an employee of Hemarina. The other authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
Hemo2Life® (M101) used in this research was kindly supplied by Hemarina. The Belzer UW cold storage and Belzer MPS machine perfusion solutions used in this research were kindly supplied by Bridge to Life. The support of Galia Links and Mike Kappler from Bridge to Life is appreciated. We thank Bernard Fromenty for his help in interpreting mitochondrial activities. We are grateful to Peter Tucker for language-editing assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100119.
Supplementary data
References
- 1.Feng S., Goodrich N.P., Bragg-Gresham J.L., Dykstra D.M., Punch J.D., DebRoy M.A. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 2.Belzer F.O., Southard J.H. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre J.V., Cheung J.Y. Effects of metabolic acidosis on viability of cells exposed to anoxia. Am J Physiol. 1985;249:C149. doi: 10.1152/ajpcell.1985.249.1.C149. [DOI] [PubMed] [Google Scholar]
- 4.McCord J.M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 5.Claes G., Aarell M., Brunius U. Kidney preservation with continuing perfusion. Nord Med. 1970;84:923. [PubMed] [Google Scholar]
- 6.Pienaar B.H., Lindell S.L., Van Gulik T., Southard J.H., Belzer F.O. Seventy-two-hour preservation of the canine liver by machine perfusion. Transplantation. 1990;49:258. doi: 10.1097/00007890-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 7.D'Alessandro A., Southard J.H., Kalayoglu M., Belzer F.O. Comparison of cold storage and perfusion of dog livers on function of tissue slices. Cryobiology. 1986;23:161. doi: 10.1016/0011-2240(86)90007-6. [DOI] [PubMed] [Google Scholar]
- 8.Garrera J.V., Henry S.D., Samstein B., Reznik E., Musat C., Lukose T.I. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–169. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 9.Kron P., Schlegel A., Mancina L., Clavien P.A., Dutkowski P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol. 2018;68:82–91. doi: 10.1016/j.jhep.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Martins P.N., Berendsen T.A., Yeh H., Bruinsma B.G., Izamis M.L., Op Den Dries S. Oxygenated UW solution decreases ATP decay and improves survival after transplantation of DCD liver grafts. Transplantation. 2019;103:363–370. doi: 10.1097/TP.0000000000002530. [DOI] [PubMed] [Google Scholar]
- 11.Rousselot M., Delpy E., Drieu La Rochelle C., Lagente V., Pirow R., Rees J.-F. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol J. 2006;1:333–345. doi: 10.1002/biot.200500049. [DOI] [PubMed] [Google Scholar]
- 12.Thuillier R., Dutheil D., Trieu M.T.N., Mallet V., Allain G., Rousselot M. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. Am J Transplant. 2011;11:1845–1860. doi: 10.1111/j.1600-6143.2011.03614.x. [DOI] [PubMed] [Google Scholar]
- 13.Glorion M., Polard V., Favereau F., Hauet T., Zal F., Fadel E. Prevention of ischemia-reperfusion lung injury during static cold preservation by supplementation of standard preservation solution with HEMO2life® in pig lung transplantation model. Artif Cells Nanomed Biotechnol. 2018;46:1773–1780. doi: 10.1080/21691401.2017.1392315. [DOI] [PubMed] [Google Scholar]
- 14.Teh E.S., Zal F., Polard V., Menasché P., Chambers D.J. HEMO2life® as a protective additive to Celsior solution for static storage of donor hearts prior to transplantation. Artif Cells Nanomed Biotechnol. 2017;45:717–722. doi: 10.1080/21691401.2016.1265974. [DOI] [PubMed] [Google Scholar]
- 15.Lemaire F., Sigrist S., Delpy E., Cherfan J., Peronet C., Zal F. Beneficial effects of the novel marine oxygen carrier M101 during cold preservation of rat and human pancreas. J Cell Mol Med. 2019;23:8025–8034. doi: 10.1111/jcmm.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thuillier R., Delpy E., Matillon X., Kaminsky J., Kasil A., Soussi D. Preventing acute kidney injury during transplantation: the application of novel oxygen carriers. Expert Opin Investig Drugs. 2019;28:643–657. doi: 10.1080/13543784.2019.1628217. [DOI] [PubMed] [Google Scholar]
- 17.Le Meur Y., Badet L., Essig L., Thierry A., Büchler M., Drouin S. First-in-human use of a marine oxygen carrier (M101) for organ preservation: a safety and proof-of-principle study. Am J Transplant. 2020;20:1729–1738. doi: 10.1111/ajt.15798. [DOI] [PubMed] [Google Scholar]
- 18.Audet M., Alexandre E., Mustun A., David P., Chenard-Neu M.P., Tiollier J. Comparative evaluation of Celsior solution vs. Viaspan in a pig liver transplantation model. Transplantation. 2001;71:1731–1735. doi: 10.1097/00007890-200106270-00005. [DOI] [PubMed] [Google Scholar]
- 19.Compagnon P., Clément B., Campion J.P., Boudjema K. Effect of hypothermic machine perfusion on rat liver function depending on the route of perfusion. Transplantation. 2001;72:606–614. doi: 10.1097/00007890-200108270-00008. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson N.V., Sundberg R., Lindell S., Kalayoglu M., Southard J.H., Belzer F.O. A simplified technique for transplantation of the canine liver. Acta Chir Scand. 1988;154:511–516. [PubMed] [Google Scholar]
- 21.Saeed W.K., Jun D.W., Jang K., Chae Y.J., Lee J.S., Kang H.T. Does necroptosis have a crucial role in hepatic ischemia-reperfusion injury? PLoS One. 2017;12:e0184752. doi: 10.1371/journal.pone.0184752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAnulty J.F., Southard J.H., Belzer F.O. Comparison of the effects of adenine-ribose with adenosine for maintenance of ATP concentrations in 5-day hypothermically perfused dog kidneys. Cryobiology. 1988;25:409–416. doi: 10.1016/0011-2240(88)90048-x. [DOI] [PubMed] [Google Scholar]
- 23.Caraceni P., Bianchi C., Domenicali M., Maria Pertosa A., Maiolini E., Parenti Castelli G. Impairment of mitochondrial oxidative phosphorylation in rat fatty liver exposed to preservation-reperfusion injury. J Hepatol. 2004;41:82–88. doi: 10.1016/j.jhep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Sammut I.A., Thorniley M.S., Simpkin S., Fuller B.J., Bates T.E., Green C.J. Impairment of hepatic mitochondrial respiratory function following storage and orthotopic transplantation of rat livers. Cryobiology. 1998;36:49–60. doi: 10.1006/cryo.1997.2063. [DOI] [PubMed] [Google Scholar]
- 25.Moon K.H., Hood B.L., Mukhopadhyay P., Rajesh M., Abdelmegeed M.A., Kwon Y.I. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Alessandro A.M., Southard J.H., Love R.B., Belzer F.O. Organ preservation. Surg Clin North Am. 1994;74:1083–1095. [PubMed] [Google Scholar]
- 27.Boudjema K., Lindell S.L., Southard J.H., Belzer F.O. The effects of fasting on the quality of liver preservation by simple cold storage. Transplantation. 1990;50:943–948. doi: 10.1097/00007890-199012000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Adam R., Delvart V., Karam V., Ducerf C., Navarro F., Letoublon C. Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395–406. doi: 10.1111/ajt.13060. [DOI] [PubMed] [Google Scholar]
- 29.Moers C., Smits J.M., Maathuis M.H., Treckmann J., van Gelder F., Napieralski B.P. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.