Abstract
The outbreak of pneumonia caused by a new coronavirus (SARS-CoV-2) occurred in December 2019, and spread rapidly throughout the world. There have been other severe coronavirus outbreaks worldwide, namely, severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV). Because the genetic diversity of coronaviruses renders the design of vaccines complicated, broad spectrum-anti-coronavirus drugs have become a critical approach to control the coronavirus epidemic. Cyclophilin A is an important protein needed for coronavirus replication, and its inhibitor cyclosporine A has the ability to suppress coronavirus on a broad spectrum. CD147-S protein was found to be one route by which SARS-CoV-2 invades host cells, while CD147 was found to play a functional role in facilitating the infection of host cells by SARS-CoV. The CyPA/CD147 interaction may play a critical role in the ability of the SARS-CoV-2 virus to enter the host cells. However, cyclosporine A has immunosuppressive effects, so the conditions for its use as an antiviral drug are limited. As a result, cyclosporine A analogues without immunosuppressive side effects have attracted lots of interest. This review primarily discusses the drug development prospects of cyclophilin A as a therapeutic target for the treatment of coronavirus infection, especially coronavirus disease 2019 (COVID-19), and non-immunosuppressive cyclosporine analogues.
Keywords: cyclophilin A, cyclosporine A, COVID-19, SARS-CoV2
Coronavirus (CoV) is the general name of a family of viruses that is commonly found in nature [1]. These viruses are able to utilize a wide variety of host species, and because host switching is a common feature in the evolution of CoV, novel CoVs may appear at any time [2]. Currently, seven different coronaviruses (SARS-CoV, hCoV-NL63, hCoV-HKU-1, hCoV-OC43, hCoV-229E, MERS-CoV, and SARS-CoV-2) are currently reported to cause respiratory diseases in humans [[2], [3], [4]]. The December 2019 outbreak of a new coronavirus in China's Wuhan region, recently named SARS-CoV-2, belongs to the coronavirus 2B type, which shows 80% similarity to the SARS-CoV genome. There is still the possibility of continued mutation in the future [5,6]. A variety of treatments for CoVs are being developed, including immunomodulation, vaccination, CoV-specific direct-acting antivirals (DAAs), and host-specific antivirals.
Cyclophilins (Cyps) are a commonly expressed and highly conserved family of intracellular proteins which displaying peptidyl-prolyl cis-trans isomerase (PPIase) activity. Cell receptors originally found as immunosuppressive drug cyclosporine A (CsA) were found [7,8]. At this stage, CsA can suppress the coronavirus, which has raised widespread scientific research interest. This paper analyzes the developmental prospects and potential of cyclophilin inhibitors as drugs to treat coronaviruses, especially SARS-CoV-2, and provides reference for future drug research and development.
1. Introduction to Coronaviruses
Coronaviruses are enveloped viruses with a characteristic spike protein inserted into the outer membrane.Their non-fragmented single-stranded RNA genomes with plus strand orientation are about 26–32 kb long and they host seven to ten different open reading frames (ORF) [9,10]. Coronaviruses have 26 known species, including α、β、γ、δ, categorized based on sequence comparisons of entire viral genomes. There are a total of four genera, of which only α and β are disease-causing in humans [11,46].
Coronaviruses contain four major structural proteins, which include spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins, and these proteins all encoded at 3′ end ORF in the genome. Functionally, S glycoproteins are involved as the main antigens in receptor binding and cell fusion [9], while M proteins are primarily involved in germination and envelope formation, and play a significant role in subsequent viral particle assembly [12]. The spike protein is essential for viral-cell receptor binding and this implies that the M protein is essential for receptor binding [13,14]. Coronaviruses can cause respiratory and intestinal infections in animals and humans [15].
According to sequencing results, the gene sequence similarity between the SARS-CoV-2 virus and SARS-CoV is 75%–80% [17,18]. It is speculated that the SARS-CoV-2 animal host may be a bat, but SARS-CoV may also exist through an intermediate host [18]. Highly pathogenic coronavirus has been endemic three times in the past 20 years. Two highly transmitted and pathogenic diseases, including SARS-CoV and MERS-CoV, have previously caused severe respiratory epidemics worldwide [19,20].
At present, there is no specific treatment for coronavirus, and there is no effective treatment plan for severely ill patients. Repurposing of individual drugs, e.g. Remdisivir showed first positive effects on the duration and intensity of COVID-19. However, such drugs are far from preventing infection or disease. In deciding how to treat coronavirus infection, especially the recent COVID-19 infection, it is important and desirable to find general therapeutic targets and effective therapeutic drugs with broad-spectrum effects. Cyclophilins were identified to be essential for CoV infection. Their inhibitors restrictcoronavirus replication, providing a basis for the targeted development of pan coronavirus inhibitors.
2. Cyclophilin inhibitors
2.1. Introduction to cyclophilin
Cyclophilins (Cyps) are a commonly expressed and highly conservede class of intracellular proteins belonging to the PPlase family [21,22]. Cyps have a very important role in the replication of RNA viruses, such as influenza A viruses, HIV, HCV, and others. Cyps are known to be present in the cells of eukaryotes and prokaryote organisms [22]. There are seven main types of cyclophilins in the human body, namely cyclophilin A (CyPA), cyclophilin B (CypB), cyclophilin C (CypC), cyclophilin D (CypD), cyclophilin E (CypE), cyclophilin 40 (Cyp40), and cyclophilin NK (Cypp). They are not typically connected to each other in the human genome [23]. Of these, CyPA is the most abundant and dominant protein in the Cyps family. CyPA plays an important role in intracellular protein synthesis, folding, and transportation, as well as immunosuppression, immunomodulation, and signal conduction. However, CyPA is closely related to viral infections, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), rheumatoid arthritis, asthma, Alzheimer's disease, and cardiovascular disease [7,8]. In addition, CyPA is associated with hepatitis and fibrosis caused by viral infections such as HBV, HCV and non-alcoholic fatty liver, as well as the generation of resistance to liver cancer and liver cancer metastasis, making it a potential target for the treatment of liver disease [8].
2.2. Cyclosporine A and cyclophilins
CsA was originally discovered by Sanders in the process of screening non-cytotoxic immunosuppressants, and it was developed for use in organ transplantation [24,25]. In-depth study found that its immunosuppressive effect is due to the formation of complexes between Cyps and calcineurin (CaN), which prevent the transcription factor `Nuclear Factor of Activated T cells´ (NFAT) from regulating immune genes.
The cellular phosphatase CaN participates in the transcription process of some cytokines, such as interleukin-2 (IL-2), by activation of NFAT. Latter translocates into the nucleus upon dephosphorylation thus being able to regulate immune gene promoters. The complex between CsA, Cyps and CaN prevents dephosphorylation of NFAT inhibiting the release of immune cytokines and thus excerting immunosuppression. [[25], [26], [27], [28], [29]].
CsA is a ligand that can bind to CyPA. Upon binding, the molecular structure of CsA changes significantly compared with its unbound structure. The original hydrogen bonds in the molecules are broken, and a new hydrogen bond is formed [30]. In addition, proline-containing peptides are converted from cis to trans, so that the resulting compound can be combined with CaN, while neither CsA nor CyPA alone can bind to CaN. CsA, with a molecular size of 11 amino acid cyclopeptides, has a surface bound to CyPA's PPIase trench, as well as a surface that can bond with CaN [30] (Fig. 1).
Fig. 1.
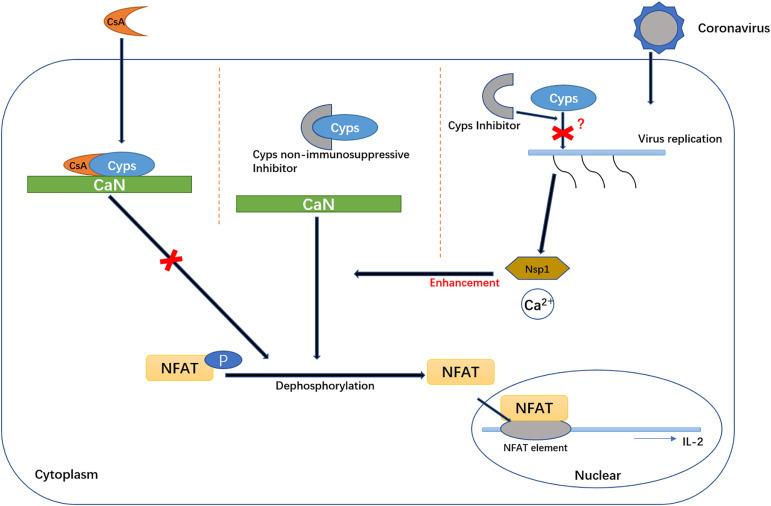
The role that CyPA plays in the NFAT pathway and coronavirus replication. CsA-CypA complex can inhibit the phosphorylation activity of CaN. CaN participates in the transcription process of some cytokines, such as IL-2, by dephosphorylation of NFAT. Nsp1 protein can enhance NFAT activities with Ca2+. CaN, calcineurin; CsA, cyclosporin A; CyPA, cyclophilin A; Nsp1, non-structure protein 1; IL-2, interleukin-2; NFAT, nuclear factor of activated T cells.
2.3. Study of cyclophilin as a therapeutic target for coronavirus
CyPA plays an extremely important role in HCV, human papillomavirus (HPV), HIV-1, and bovine pox virus [31]. As a ligand of CyPA, it has been found that CsA can prevent the replication of most CoVs by inhibiting cyclophilin proteins, including SARS-CoV, hCoV-229E, and hCoV-NL63, as well as the avian infectious bronchitis virus [4,[32], [33], [34], [35]].
CyPA is a potential drug target for coronaviruses [33]. Brunn's team at LMU University in Germany reported that the N protein of SARS-CoV binds closely to CyPA in humans, and that inhibiting the binding of two proteins by CsA inhibits the replication of hCoV-229E [4,35,36]. In 2011, it was reported that CsA inhibits CoV infection [32]. In the same year, the Brunn team demonstrated that the host's Cyps were a possible target for CoV, and also noted that non-immunosuppressive cyclosporine derivatives may have the potential to become a panCoV inhibitor [33]. Later, in 2014, the Brunn team further confirmed that the low-host CyPA could prevent the replication of hCoV-NL63. In a recent study, Alisporivir, which is a non-immunosuppressive CyPA inhibitor, was reported to reduce SARS-CoV-2 RNA production in vitro with an EC50 of 0.46 ± 0.04 μM and Alisporivir inhibited a post-entry step in the life cycle of SARS-CoV-2 [45].
In vitro, CsA can inhibit the replication of almost all coronavirus genera in a dose-dependent manner [33,37]. Cell culture experiments have found that CsA can strongly inhibit the viral replication of SARS-CoV, MERS-CoV, hCoV-229E, poultry infectious bronchitis virus, and mouse hepatitis virus [33], but only showed significant blocking in the early stages of replication [32]. Blocking coronavirus replication requires a higher CsA concentration (16 μM) than other RNA viruses (0.5–3 μM), indicating that the CoV is less sensitive to CsA treatment [32]. As an effective and panCoV inhibitor, CsA and its analogues have good research and development prospects.
2.4. CD147 in coronavirus infection
An extracellular matrix metalloproteinase inducer (CD147/EMMPRIN) is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily [11]. CD147 is involved in tumor development, plasmodium invasion and virus infection [[12], [13], [14]]. CD147 can interact with cyclophilins as a signaling receptor and mediate the signaling and chemotactic activities of extracellular CyPA [15,16].
CD147 plays a critical role in the infection of various viruses in the human body, such as HIV, HBV, HCV, and KSHV. Related studies have shown that, during HIV-1 infection, the CyPA of the host cells incorporates the nascent virus through interaction with HIV-1 Gag protein. As the virus matures and releases the Gag protein, CyPA becomes redistributed on the surface of the virus and mediates HIV-1's adhesion to target cells by interacting with protein receptors expressed by host cells. The combination of CD4 and chemokine receptors on the host cell promotes the fusion of the virus and the cell membrane, and eventually causes the virus to invade the host cell. The CD147 molecule of the host cell can promote infection of the host cell by the HIV-1 virus through interaction with the virus-associated CyPA.
During SARS-CoV infection of host cells, the CD147 molecule works via a mechanism similar to that in HIV-1 infection: interaction with CyPA. CD147-antagonist peptide 9, which has a high binding rate to HEK293 cells, has an inhibitory effect on SARS-CoV. Because SARS-CoV and SARS-CoV-2 have similar characteristics, Chen et al. and colleagues investigated the possible function of CD147 in the virus entry to host cells. It was reported that blocking CD147 on host cells shows inhibition on SARS-CoV-2 and that CD147 has a critical role in promoting the infection of host cells by the virus. Surface plasmon resonance by using Biacore analysis confirmed the interaction between CD147 and S protein (SP) [17]. In earlier studies, mediated through CyPA bound to N protein of SARS-CoV, CD147 plays a role in facilitating infection of host cells by SARS-CoV [18,19]. There are currently clinical trials using CD147 antibodies, including Meplazumab, which efficiently improved the recovery of patients with COVID-19 in a favorable safety dose [20]. This evidence shows that CD147 can be a novel route for the infection of SARS-CoV-2 as well as a novel target for COVID-19 treatment [21]. CyPA may play a pivotal role in with CD147 in SARS-CoV-2 infection, and CyPA inhibitors may inhibit the infection of SARS-CoV-2 by blocking the infection route.
CyPA/CD147 interaction is also related to other diseases. The pathophysiological relevance of CyPA-CD147 interactions to inflammatory processes has been studied in many animal models. Studies on synovial macrophages in patients with rheumatoid arthritis have reported that the expression of CyPA and CD147 can be found [22]. CyPA/CD147 interactions can be a novel target for anti-inflammatory therapeutics [23]. CyPA/CD147 in cardiac remodeling are also important. CyPA and CD147 have higher levels of expression in pancreatic cancer tissues, in which CyPA is found in a dose-dependent manner. This proliferation of pancreatic cancer cells can be effectively blocked by CD147 antibody [25].
Cyclophilin inhibitors can not only inhibit the replication of SARS-CoV intracellularly, but also may inhibit the infection of SARS-CoV into host cells via interacting with CD147.
2.5. Prospects for non-immunosuppressive cyclophilin inhibitors
As an immunosuppressive drug, CsA has a broad spectrum of antiviral effects [8,36], but its immunosuppressive properties can lead to adverse side effects during antiviral therapy. As a result, several non-immunosuppressive cyclophilin inhibitors based on CsA structures are being developed (Table 1 ), and these non-immunosuppressive CyPA inhibitors separate their function of PPIase inhibition from their immunosuppressive function. The side chains of the modified CsA molecule can develop non-immunosuppressive analogues, such as Alisporivir, NIM811, SCY-635, sangliferins, and STG-175. In order to better understand the structural diversity of CyPA inhibitors, more non-immunosuppressive CsA derivatives need to be developed. Known co-crystal structures provide the basis for drug optimization of non-immunosuppressive cyclophilin inhibitors.
Table 1.
Structure and current research status of some cyclophilin inhibitors.
| Name | Structure | Corporation | Indication | Activity | Clinical trial stage |
|---|---|---|---|---|---|
| CsA | 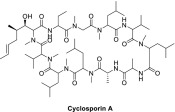 |
Norvatis | Adult, non-immunocompromised patients with severe, recalcitrant, plaque psoriasis who have failed to respond to at least one systemic therapy (e.g., PUVA, retinoids, or methotrexate) and in patients for whom other systemic therapies are contraindicated or cannot be tolerated | CsA inhibits the replication of many coronaviruses. Treatment of infected cells with 16 μM CsA strongly reduced viral and reporter gene expression of SARS-CoV–GFP, the amount of dsRNA in infected cells, and the viral titer in culture supernatants (by >3 logs) [32]. | Indication mentioned previously: On market Hepatitis C viral infection: Phase IV completed |
| Alisporivir | 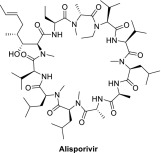 |
Norvatis | Hepatitis C; chronic hepatitis C | Alisporivir block SARS-CoV and MERS-CoV replication. Combination treatment with alisporivir and ribavirin increases the anti-MERS-CoV activity in vitro[39]. | Gastrointestinal disorders: Preclinical Chronic hepatitis C virus infection/hepatitis C viral infection: Phase III completed |
| NIM811 | 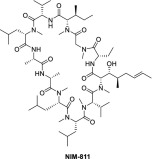 |
Novartis | Hepatitis C; HIV-1 infection | Induces a dose-dependent reduction of HCV RNA in the replicon cells with an IC50 of 0.66 μM at 48 h [40]. | Chronic hepatitis C genotype-1 relapse: Phase II completed |
| SCY-635 |  |
SCYNEXIS, Inc. | Hepatitis C; hepatitis B | Show inhibition at 0.07, 0.08, and 0.15 μM, respectively, in the HCV subgenomic replicon system [41]. | Hepatitis C: Phase II Hepatitis B: No development reported |
| STG-175 |  |
S & T Global | Hepatitis B | STG-175 inhibits at a nanomolar range the replication of all HCV genotypes: GT1a (EC50 = 13.5 nM), GT1b (EC50 = 15.1 nM), GT2a (EC50 = 11.5 nM), GT3a (EC50 = 38.9 nM) and GT4a (EC50 = 15.2 nM) [42]. | Hepatitis B: Preclinical |
| CRV431 |
 |
Hepion Pharmaceuticals | Hepatitis B | In a transgenic mouse model, CRV431 (10 mg/kg/day) reduced HBV DNA levels by only 13% relative to the vehicle group, the dose (50 mg/kg) reduced the mean HBV DNA level by 91% [44]. | Hepatitis B: Phase I/II Hepatitis D; liver cancer; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis: Preclinical Coronavirus infections; hepatitis C; HIV-1 infections: Discontinued |
| CPI-431-32 |  |
Ciclofilin Pharmaceuticals Inc | Hepatitis B | Effectively inhibits HIV-1/HCV co-infection; also shows efficacy against drug-resistant HIV-1 and HCV variants. A daily CPI-431-32 dose of 0.5 μM is used. [43]. | Hepatitis B; neurological disorders; reperfusion injury: Preclinical-discontinued HIV-1, HCV: Preclinical A study in healthy volunteers and patients with chronic hepatitis B: Phase I: Active, not recruiting |
It was reported that multiple derivatives of non-immunosuppressive CsA were effective in inhibiting hCoV-229E replication, indicating that they could be a candidate drug for the treatment of CoV infection in humans [4,36]. CsA and its derivatives interrupt the interaction between CyPA and CaN proteins [38]. The study also found that in cells, CyPA inhibitors - alisporivir can inhibit the replication of MERS and SARS CoVs, and in cell-based infection models, ribavirin further enhances alisporivir's antiviral effect [39].
3. Conclusions and prospects
In the last 20 years, from SARS-CoV in 2002, to MERS-CoV in 2009, to the recent SARS-CoV-2 epidemic, there have been three outbreaks of severe infectious diseases caused by CoVs, which require high attention. In recent years, the results of genomic sequencing and bioinformatics for these CoVs have enabled the creation of screening models, which have been used to develop small molecule drugs and vaccines with different mechanisms of action, such as protease inhibitors, nucleic acid synthesis inhibitors and polymerase inhibitors. However, there have been no potent and specific drugs developed for coronaviruses.
As an important intracellular protein in RNA viruses, Cyps have been shown to play an important part in virus replication. In addition, CyPA is considered to be a potential drug target for CoVs, and its inhibitory ligand CsA has been reported to inhibit the replication process of several CoVs. However, as an immunosuppressant inhibitor, the side effects caused by CsA in clinical applications cannot be ignored. The development of non-immunosuppressive inhibitors based on CsA's structure has begun to attract attention.
A variety of non-immunosuppressive cyclophilin inhibitors are currently being developed, such as alisporivir, SCY-635, NIM811, and CRV431. CyPA target affinity for these inhibitors has been extensively validated in HCV. Furthermore, some studies have found that many of these inhibitors can effectively inhibit the replication of hCoV-229E, indicating its potential as a treatment for human CoV infection.
Management of inflammation is important for COVID-19 treatment, and non-immunosuppressive CsA inhibitors can be used in combination with anti-inflammation drugs in clinical settings. For minor cases of COVID-19 without inflammation syndrome, non-immunosuppressive CsA inhibitors could be a feasible part of treatment.
There is still no complete validation of the inhibitory effect of non-immunosuppressive cyclophilin inhibitors and therapies on coronaviruses, especially COVID-19. However, as a highly promising class of drugs, non-immunosuppressive CyPA inhibitors will attract more attention.
Funding
The current study was supported by the Science and Technology Commission of Shanghai (18ZR1403900) (D. Zhu), the National Natural Science Foundation of China (81872895) (D.Z.).
Conflict of interest statement
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript. The authors declare no competing financial interests.
Author contributions
C.L. and D.Z. drafted the manuscript. A.v.B edited the manuscript. D. Z conceptualized and oversaw the process and wrote the manuscript.
Acknowledgement
We thank Hao Zhang from China State Institute of Pharmaceutical Industry for suggestions in chemical structure.
References
- 1.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A., Chan J.F.W., Azhar E.I., et al. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Brunn A., Ciesek S., Von Brunn B., et al. Genetic deficiency and polymorphisms of cyclophilin A reveal its essential role for Human Coronavirus 229E replication. Curr Opin Virol. 2015;14:56–61. doi: 10.1016/j.coviro.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu D., Wang Z., Zhao J.-J., et al. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat Med. 2015;21(6):572–580. doi: 10.1038/nm.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naoumov N.V. Cyclophilin inhibition as potential therapy for liver diseases. J Hepatol. 2014;61(5):1166–1174. doi: 10.1016/j.jhep.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Song Z., Xu Y., Bao L., et al. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilianski A., Baker S.C. Cell-based antiviral screening against coronaviruses: developing virus-specific and broad-spectrum inhibitors. Antiviral Res. 2014;101:105–112. doi: 10.1016/j.antiviral.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paules C.I., Marston H.D., Fauci A.S. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 12.Tseng Y.-T., Wang S.-M., Huang K.-J., et al. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J Biol Chem. 2010;285(17):12862–12872. doi: 10.1074/jbc.M109.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munster V.J., Koopmans M., Van Doremalen N., et al. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 17.Hui D.S., I Azhar E., Madani T.A., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlman S. Another Decade, Another Coronavirus. N Engl J Med. 2020;382(8):760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Wit E., Van Doremalen N., Falzarano D., et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su S., Wong G., Shi W., et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Wilde A.H., Pham U., Posthuma C.C., et al. Cyclophilins and cyclophilin inhibitors in nidovirus replication. Virology. 2018;522:46–55. doi: 10.1016/j.virol.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olejnik P., Nuc K. Cyclophilins – proteins with many functions. Postepy Biochem. 2018;64(1):46–54. doi: 10.18388/pb.2018_104. [DOI] [PubMed] [Google Scholar]
- 23.Wang P., Heitman J. The cyclophilins. Genome Biol. 2005;6(7):226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heusler K., Pletscher A. The controversial early history of cyclosporin. Swiss Med Wkly. 2001;131(21–22):299–302. doi: 10.4414/smw.2001.09702. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney Z.K., Fu J., Wiedmann B. From chemical tools to clinical medicines: nonimmunosuppressive cyclophilin inhibitors derived from the cyclosporin and sanglifehrin scaffolds. J Med Chem. 2014;57(17):7145–7159. doi: 10.1021/jm500223x. [DOI] [PubMed] [Google Scholar]
- 26.Colombani P.M., Robb A., Hess A.D. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science (New York, NY) 1985;228(4697):337–339. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber S.L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber S.L., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 29.Peel M., Scribner A. Cyclophilin inhibitors as antiviral agents. Bioorg Med Chem Lett. 2013;23(16):4485–4492. doi: 10.1016/j.bmcl.2013.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu J., Tjandra M., Becker C., et al. Potent nonimmunosuppressive cyclophilin inhibitors with improved pharmaceutical properties and decreased transporter inhibition. J Med Chem. 2014;57(20):8503–8516. doi: 10.1021/jm500862r. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Wilde A.H., Zevenhoven-Dobbe J.C., Van Der Meer Y., et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfefferle S., SCHöPF J., KöGL M., et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7(10):e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y., Sato Y., Sasaki T. Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J Gen Virol. 2017;98(2):190–200. doi: 10.1099/jgv.0.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma-Lauer Y., Zheng Y., Malešević M., et al. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antiviral Res. 2020;173:104620. doi: 10.1016/j.antiviral.2019.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigro P., Pompilio G., Capogrossi M.C. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4(10):e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka Y., Sato Y., Osawa S., et al. Suppression of feline coronavirus replication in vitro by cyclosporin A. Vet Res. 2012;43(1):41. doi: 10.1186/1297-9716-43-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carbajo-Lozoya J., Ma-Lauer Y., Malešević M., et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014;184:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Wilde A.H., Falzarano D., Zevenhoven-Dobbe J.C., et al. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma S., Boerner J.E., Tiongyip C., et al. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob Agents Chemother. 2006;50(9):2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins S., Scorneaux B., Huang Z., et al. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother. 2010;54(2):660–672. doi: 10.1128/AAC.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallay P.A., Chatterji U., Bobardt M.D., et al. Characterization of the Anti-HCV Activities of the New Cyclophilin Inhibitor STG-175. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallay P.A., Bobardt M.D., Chatterji U., et al. The Novel Cyclophilin Inhibitor CPI-431-32 Concurrently Blocks HCV and HIV-1 Infections via a Similar Mechanism of Action. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallay P., Ure D., Bobardt M., et al. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofitc l., Brillet R., Berry F., et al. Antimicrob Agents Chemother. 2020:1–10. doi: 10.1128/AAC.00876-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


