Abstract
Background & Aims
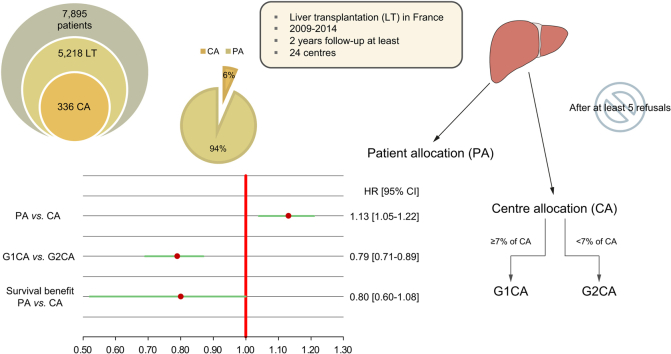
In France, liver grafts that have been refused at least 5 times can be “rescued” and allocated to a centre which chooses a recipient from its own waiting list, outside the patient-based allocation framework. We explored whether these “rescued” grafts were associated with worse graft/patient survival, as well as assessing their effect on survival benefit.
Methods
Among 7,895 candidates, 5,218 were transplanted between 2009 and 2014 (336 centre-allocated). We compared recipient/graft survival between patient allocation and centre allocation, considering a selection bias and the distribution of centre-allocation recipients among the transplant teams. We used a propensity score approach and a weighted Cox model using the inverse probability of treatment weighting method. We also explored the survival benefit associated with centre-allocation grafts.
Results
There was a significantly higher risk of graft loss/death in the centre allocation group compared to the patient allocation group (hazard ratio 1.13; 95% CI 1.05–1.22). However, this difference was no longer significant for teams that performed more than 7% of the centre-allocation transplantations. Moreover, receiving a centre-allocation graft, compared to remaining on the waiting list and possibly later receiving a patient-allocation graft, did not convey a poorer survival benefit (hazard ratio 0.80; 95% CI 0.60–1.08).
Conclusions
In centres which transplanted most of the centre-allocation grafts, using grafts repeatedly refused for top-listed candidates was not detrimental. Given the organ shortage, our findings should encourage policy makers to restrict centre-allocation grafts to targeted centres.
Lay summary
“Centre allocation” (CA) made it possible to save 6 out of 100 available liver grafts that had been refused at least 5 times for use in the top-listed candidates on the national waiting list. In this series, the largest on this topic, we showed that, in centres which transplanted most of the CA grafts, using grafts repeatedly refused for top-listed candidates did not appear to be detrimental. In the context of organ shortage, our results, which could be of interest for any country using this CA strategy, should encourage policy makers to reassess some aspects of graft allocation by restricting CA grafts to targeted centres, fostering the “best” matching between grafts and candidates on the waiting list.
Keywords: Liver transplantation, Centre allocation, Patient allocation, Patient and graft survival, Survival benefit
Abbreviations: CA, centre allocation; DCD, donation after cardiac death; DQI, donor quality index; ES, effect size; HCC, hepatocellular carcinoma; HR, hazard ratio; ICU, intensive care unit; IPTW, inverse probability of treatment weighting; LT, liver transplantation; MELD, model for end-stage liver disease; PA, patient allocation
Graphical abstract
Highlights
-
•
Centre allocation (CA) made it possible to save 6 out of 100 liver grafts.
-
•
13% higher graft loss/death for CA patients.
-
•
In transplant centres performing most CA transplants, survival was not impacted.
Introduction
In France, graft allocation is regulated by the “Agence de la Biomédecine” (ABM), which has established a national score since 2007 (https://www.agence-biomedecine.fr/). All registered candidates receive a national score which defines their priority on the national waiting list: the higher the score, the shorter the waiting time. As in most countries, this score is based on the model for end-stage liver disease (MELD) score1; the most severe patients with a high MELD score have the highest priority, according to a “sickest first” principle. That said, an increasing number of patients with a low MELD score, i.e. those with either hepatocellular carcinoma (HCC), or severe complications of cirrhosis (e.g. refractory ascites, chronic encephalopathy, portopulmonary syndrome), or other rare indications (e.g. amyloidosis, polycystic liver, neuroendocrine tumours, sclerosing cholangitis or primary biliary cholangitis), may be given additional MELD exception points,2 increasing their chance of being transplanted.
One of the major obstacles to liver transplantation (LT) is the persistent organ shortage. In France, the candidate/graft ratio rose from 2.0 in 2009 to 2.4 in 2014. To overcome this organ shortage, the criteria for donor selection have been broadened to extended donor criteria.3,4
In some cases, a graft may not be acceptable for a particular recipient and is then proposed to another recipient. However, a new refusal may occur. After at least 5 consecutive refusals, the graft is supplied to a transplant team that chooses the best candidate from its local waiting list, depending on the graft being offered. It then becomes a “centre allocation” (CA) rather than a “patient allocation” (PA) graft and may also be referred to as a “rescue allocation”. In the French allocation system, the surgeon on duty in each team is contacted by the Agency of Biomedicine. Frequently and specifically for unusual grafts, he/she always refers to a senior, either surgeon, hepatologist or intensivist. The French experience has shown that a CA can save 6 out of 100 available liver grafts. CA grafts are logically given to patients who have less access to PA grafts; they often have a low MELD score and could theoretically accept a non-optimal liver graft. Of note, grafts which have been repeatedly refused by other teams are not necessarily poor; it can be, for example, a right liver after a split, or small grafts. Unfortunately, we do not have any information about how many times and why exactly those grafts were declined. Five series with a restrictive number of patients relative to this CA strategy have been reported.[5], [6], [7], [8], [9]
In the face of liver shortages, our aim was to determine whether the survival of CA recipients/grafts was worse than that of PA recipients/grafts, taking account of selection bias. Furthermore, we explored the survival benefit offered by CA grafts by comparing grafting a patient with a CA graft without delay rather than remaining on the waiting list and possibly receiving a PA graft at a later point.
Materials and methods
Materials
Two databases were chosen: one to compare the post-transplantation survival of CA vs. PA for patients and grafts, and the other to study the survival benefit in CA vs. PA patients. The former sample included grafted patients only, while the latter was larger and included all available candidates on the waiting list.
Information relative to the patients listed for LT and transplanted in France between January 4, 2009 and December 31, 2014 was obtained from the ABM (https://www.agence-biomedecine.fr/Organes). The study was conducted with the approval of the Independent Ethics Committee. Authorisation was also obtained from the French Data Protection Agency (Commission Nationale de l'Informatique et des Libertés) (approval No. 915206). All data thus collected were de-identified beforehand. Of note, in France, during the 2009–2014 period, the proportion of discarded grafts (i.e. collected but not transplanted) ranged from 5 to 7% (Table S1 provides the proportion per year and reasons why those grafts were not transplanted – it was not specified which of these grafts were proposed but discarded in the end, as this information was not available from the Agency of Biomedicine).
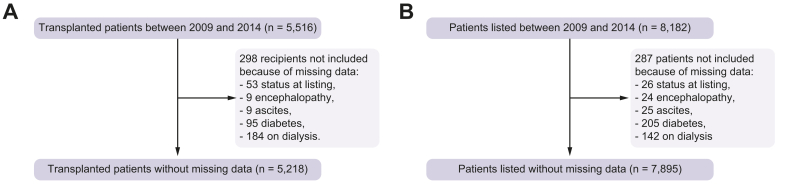
Candidates/recipients below the age of 18 years, emergencies or multiple organ transplants were not included. Candidates/recipients with incomplete covariates were not retained, as specified in the flow diagram presented in Fig. 1. Of note, no donation after cardiac death (DCD) was performed during the retained period (https://www.agence-biomedecine.fr/annexes/bilan2016/donnees/organes/01-prelevement/synthese.htm#figP1). Follow-up started at listing (or at LT, depending on the analysis retained) and ended at the onset of one of the following events: lost to follow-up, removal from the waiting list, death on the waiting list, graft loss, death after LT, or end of the study as of December 31, 2016 (or loss to follow-up, graft loss, death after LT or end of the study as of December 31, 2016, depending on the analysis retained). The outcome was defined as a “transplant strategy failure” which is a composite outcome composed of death on the waiting list, removal from the waiting list due to a worsening of the patient's condition, death after LT or graft loss (or death after LT or graft loss, depending on the analysis retained).
Fig. 1.
Flow diagram detailing missing data relative to candidates and recipients between January 2009 and December 2014.
Ultimately, 5,218 recipients from 24 centres, 336 of them being CAs (6%), were retained in the first dataset, and 7,895 patients in the second. The characteristics of recipients and donors are shown in Table 1 and in Table 2 for candidates from the second dataset.
Table 1.
Comparison of centre allocation and patient allocation recipients and donors.
| Recipient covariates | Centre allocation (n = 336) |
Patient allocation (n = 4,882) |
p value | Effect size |
|---|---|---|---|---|
| MELD score at listing | 12.97 (5.17), 12 | 18.75 (9.58), 17 | <0.001 | 0.022 |
| MELD category at listing | <0.001 | 0.105 | ||
| 6 ≤MELD ≤15 | 245 (72.92%) | 2,188 (44.82%) | ||
| 15 <MELD ≤30 | 89 (26.49%) | 1,973 (40.41%) | ||
| MELD >30 | 2 (0.6%) | 721 (14.77%) | ||
| MELD score at transplant | 12.88 (5.76), 12 | 20.69 (10.7), 19 | <0.001 | 0.032 |
| MELD category at transplant | <0.001 | 0.125 | ||
| 6 ≤MELD ≤15 | 245 (72.92%) | 1,927 (39.47%) | ||
| 15 <MELD ≤30 | 86 (25.6%) | 1,865 (38.2%) | ||
| MELD >30 | 5 (1.49%) | 1,090 (22.33%) | ||
| D-MELD | 807 (448.93), 707.5 | 1,148 (736.18), 968 | <0.001 | 0.011 |
| Age | 56.84 (7.96), 57.96 | 53.75 (10.06), 55.37 | <0.001 | 0.001 |
| Gender | <0.001 | 0.039 | ||
| Female | 60 (17.86%) | 1,216 (24.91%) | ||
| Male | 276 (82.14%) | 3,666 (75.09%) | ||
| Re-transplantation | <0.001 | 0.057 | ||
| No | 330 (98.21%) | 4,485 (91.87%) | ||
| Yes | 6 (1.79%) | 397 (8.13%) | ||
| MELD exception | <0.001 | 0.079 | ||
| No | 312 (92.86%) | 3,907 (80.03%) | ||
| Yes | 24 (7.14%) | 975 (19.97%) | ||
| Status at transplant | <0.001 | 0.073 | ||
| Home | 299 (88.99%) | 3,448 (70.63%) | ||
| Hospital | 28 (8.33%) | 721 (14.77%) | ||
| Intensive care unit | 9 (2.68%) | 713 (14.6%) | ||
| Diabetes | 0.083 | 0.024 | ||
| No | 243 (72.32%) | 3,741 (76.63%) | ||
| Yes | 93 (27.68%) | 1,141 (23.37%) | ||
| On dialysis at transplant | <0.001 | 0.051 | ||
| No | 336 (100%) | 4,680 (95.86%) | ||
| Yes | 0 (0%) | 202 (4.14%) | ||
| ABO group | <0.001 | 0.032 | ||
| A | 154 (45.83%) | 2,205 (45.17%) | ||
| AB | 5 (1.49%) | 228 (4.67%) | ||
| B | 24 (7.14%) | 555 (11.37%) | ||
| O | 153 (45.54%) | 1,894 (38.8%) | ||
| Hepatocellular carcinoma | <0.001 | 0.161 | ||
| No | 112 (33.33%) | 3,177 (65.08%) | ||
| Yes | 224 (66.67%) | 1,705 (34.92%) | ||
| Decompensated cirrhosis | <0.001 | 0.112 | ||
| No | 278 (82.74%) | 2,949 (60.41%) | ||
| Yes | 58 (17.26%) | 1,933 (39.59%) | ||
| Non-cirrhotic liver disease | 0.01 | 0.032 | ||
| No | 332 (98.81%) | 4,697 (96.21%) | ||
| Yes | 4 (1.19%) | 185 (3.79%) | ||
| BMI at listing | 26.44 (4.67) | 25.88 (4.85) | <0.001 | <0.001 |
| Encephalopathy | <0.001 | 0.06 | ||
| No | 274 (81.55%) | 3,431 (70.28%) | ||
| Yes | 62 (18.45%) | 1,451 (29.72%) | ||
| Ascites | <0.001 | 0.063 | ||
| No | 185 (55.06%) | 2,064 (42.28%) | ||
| Yes | 151 (44.94%) | 2,818 (57.72%) | ||
| Waiting time (in days) | 204.78 (192.56), 162.5 | 189.8 (218.1), 123 | <0.001 | <0.001 |
| ABO compatibility | <0.001 | 0.037 | ||
| Compatible | 7 (2.08%) | 91 (1.86%) | ||
| Identical | 328 (97.62%) | 4,791 (98.14%) | ||
| Incompatible | 1 (0.3%) | 0 (0%) | ||
| Ischaemia time (in minutes)† | 536.38 (230.83), 520 | 490.36 (237.67), 468 | <0.001 | <0.001 |
| Follow-up | 1,281 (790.50), 1,326 | 1,353 (829.34), 1,322 | 0.15 | <0.001 |
| Primary non-function∗ | 9 (2.68%) | 112 (2.29%) | – | – |
| Graft loss∗ | 23 (6.85%) | 337 (6.9%) | – | – |
| Death∗ | 81 (24.11%) | 1,081 (22.14%) | – | – |
| Causes of death | 0.50 | 0.029 | ||
| Graft†† | 4 (4.94%) | 73 (6.75%) | ||
| Organ failure# | 14 (17.28%) | 152 (14.06%) | ||
| Recurrence of the initial liver disease | 12 (14.81%) | 121 (11.19%) | ||
| Chronic rejection | 0 (0%) | 8 (0.74%) | ||
| Cancer‡ | 16 (19.75%) | 156 (14.43%) | ||
| Infections | 11 (13.58%) | 187 (17.30%) | ||
| Cardiovascular | 5 (6.17%) | 131 (12.12%) | ||
| Non-infectious complications¶ | 1 (1.23%) | 43 (3.98%) | ||
| Suicide | 0 (0%) | 3 (0.28%) | ||
| Other | 18 (22.22%) | 207 (19.15%) | ||
| Donor covariates | Centre allocation (n = 336) |
Patient allocation (n = 4,882) |
p value | Effect size |
|---|---|---|---|---|
| ABO group | <0.001 | 0.036 | ||
| A | 156 (46.43%) | 2,211 (45.29%) | ||
| AB | 1 (0.3%) | 198 (4.06%) | ||
| B | 22 (6.55%) | 513 (10.51%) | ||
| O | 157 (46.73%) | 1,960 (40.15%) | ||
| Gender | 0.41 | 0.011 | ||
| Female | 160 (47.62%) | 2,204 (45.15%) | ||
| Male | 176 (52.38%) | 2,678 (54.85%) | ||
| Age | 62.53 (18.37), 64 | 54.87 (18.25), 57 | <0.001 | 0.011 |
| ICU stay (in days) | 3.31 (5.27), 2 | 3.28 (3.76), 2 | 0.514 | <0.001 |
| Liver type | 0.059 | 0.026 | ||
| Total | 327 (97.32%) | 4,630 (94.84%) | ||
| Split | 9 (2.68%) | 252 (5.16%) | ||
| Cause of death | 0.186 | 0.018 | ||
| Anoxia | 31 (9.23%) | 617 (12.64%) | ||
| Cerebrovascular accident | 220 (65.48%) | 2,952 (60.47%) | ||
| Trauma | 75 (22.32%) | 1,182 (24.21%) | ||
| Other | 10 (2.98%) | 131 (2.68%) | ||
| MDRD creatinine clearance: lowest (ml/min/1.73 m2) | 72.87 (48.12), 67.21 | 74.12 (33.51), 71.37 | 0.03 | <0.001 |
| Length | 167.38 (10.95), 168 | 169.41 (9.97), 170 | <0.001 | <0.001 |
| DQI | 1.96 (0.5), 1.83 | 1.85 (0.45), 1.76 | <0.001 | <0.001 |
| DQI group at risk | <0.001 | 0.04 | ||
| Group 0: 1.0 <DQI <1.58 | 91 (27.08%) | 1,639 (33.57%) | ||
| Group 1: 1.58 <DQI <2.35 | 159 (47.32%) | 2,408 (49.32%) | ||
| Group 2: DQI >2.35 | 86 (25.6%) | 835 (17.1%) |
For quantitative covariates, the results are shown as a mean (SD), median, and for qualitative covariates as number (percentage). Student's t test, Wilcoxon-Mann-Whitney test, the χ2 test or Fisher's exact test were used when appropriate. For each test, an ES was also reported. For χ2 and Fisher's exact tests, was calculated (i.e. magnitude of the ES; small 0.1≤ES<0.3; medium 0.3≤ES<0.5 and large ES>0.5), and for the Student's t and Wilcoxon-Mann-Whitney tests, r2 and Cohen's r2 were determined (i.e. magnitude of the ES; small 0.01≤ES<0.09; medium 0.09≤ES<0.25 and large ES>0.25), respectively. †Ischaemia time was calculated in 334 centre allocation recipients and 4,837 patient allocation recipients. ∗See Cox model in the Results section. ††Primary non-functioning graft; Hyperacute rejection; Acute rejection; Vascular complication; Biliary complications; Haemorrhage; Other causes. #Mainly multiple organ failure (82%). ‡Hepatocellular carcinoma: 6/16 and 33/156, respectively. ¶Mainly haemorrhage (72%).
DQI, donor quality index; ES, effect size; ICU, intensive care unit; MELD, model for end-stage liver disease.
Table 2.
Main characteristics of candidates on the waiting list for liver transplantation.
| Candidate characteristics (n = 7,895) | |
|---|---|
| MELD score at listing | 17.7 (9), 16 |
| Age at listing | 54.2 (9.9), 55.8 |
| Hepatocellular carcinoma | 2,953 (37.4%) |
| Female | 1,925 (24.4%) |
| Re-transplantation | 602 (7.6%) |
| MELD exception | 1,190 (15.1%) |
| Diabetes | 1,871 (23.7%) |
| On dialysis | 278 (3.5%) |
| Status at listing | |
| At home | 5,944 (75.3%) |
| Hospital | 1,071 (13.6%) |
| ICU | 880 (11.1%) |
| Decompensated cirrhosis | 2,896 (36.7%) |
| Non-cirrhotic liver disease | 283 (3.6%) |
| Body mass index | 25.9 (4.9), 25.3 |
| Encephalopathy | 2,301 (29.1%) |
| Ascites | 4,431 (56.1%) |
For quantitative covariates, the result is shown as a mean (SD), median, and for qualitative covariates as number (percentage).
ICU, intensive care unit; MELD, model for end-stage liver disease.
In France, all donors are by definition cared for in intensive care units (ICUs). The length of stay in an ICU is the number of nights spent there, rather than the number of days, so a stay of 0 day is possible. Recipients presenting with cirrhosis of the liver, without HCC or non-HCC liver cancer, an MELD score ≥16 and Child-Pugh B or C were considered as having decompensated cirrhosis. Patients without cancer or cirrhosis were considered to be suffering from non-cirrhotic liver disease. MELD exceptions were identified and resulted in extra points while on the waiting list.2 Finally, grafts were qualified using the donor quality index (DQI) developed from the French database.10 Indeed, neither the donor risk index nor the Eurotransplant donor risk index have been validated for the French database.11
Methods
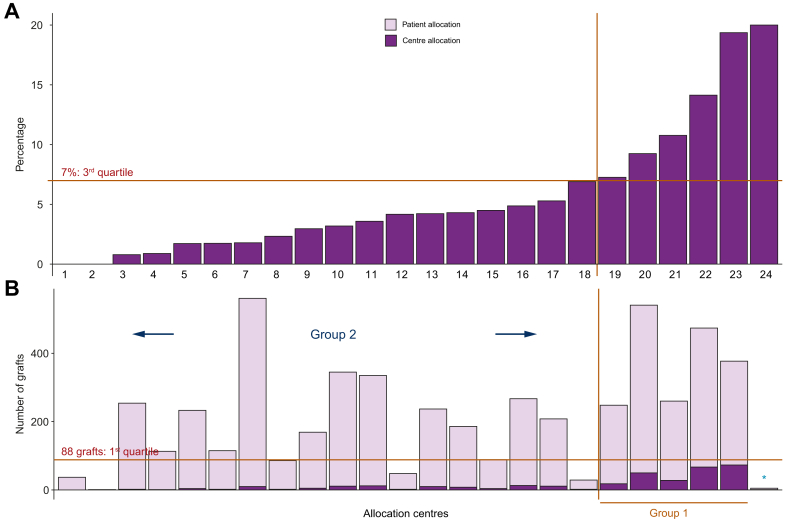
A comparison of CA and PA recipients was performed. To deal with any potential selection bias, we used a propensity score approach and a weighted Cox model using the inverse probability of treatment weighting (IPTW) method to compare recipient and graft survival between PA and CA (see supplementary information for more details). We completed our analysis by considering the ratio of CA transplants performed by each team. Indeed, some teams did no such transplants (2 centres) or only a few CA transplants (Fig. 2). The third quartile of the distribution was 7% (i.e. 7% of CA transplants were performed out of the total number of transplants over the period from 2009 to 2014). Two groups were therefore created according to this threshold:
-
•
Group 1: teams performing 7% or more of CA transplants,
-
•
Group 2: teams performing less than 7% of CA transplants.
Fig. 2.
Definition of the centre allocation groups based on 2 criteria.
(A) Definition of the threshold retained to delineate Groups 1 and 2 according to the 3rd quartile of the distribution of the percentages of centre allocation transplants performed by the 24 liver transplantation centres. Six centres can be seen above the 7% threshold on the right-hand side. NB. Two centres did not perform any centre allocation transplants during the study period. (B) Numbers of centre allocation and patient allocation grafts performed by each centre. The 1st quartile of the distribution was retained to qualify a threshold for the total number of transplants performed during the study period (corresponding to 88 procedures). NB. The centre marked with an asterisk moved from Group 1 to Group 2 because its total number of transplants was below the threshold for the number performed during the study period (i.e. 1st quartile).
In each group, PA and CA subgroups of patients were defined as G1PA, G1CA, G2PA, and G2CA, respectively.
It should be noted that the conduct of more than 88 LTs (i.e. first quartile of the distribution of the number of grafts) during the period from 2009 to 2014 was considered to be a condition of belonging to Group 1. Indeed, some teams only performed a limited number of LTs during that period. Fig. 2 shows a summary of how the centre allocation groups were constructed (i.e. Groups 1 and 2) according to their percentage of CA LTs and the numbers of CA and PA grafts. Five centres performed more than 7% of the CA grafts and more than 88 LTs between 2009-2014 (Fig. 2: centres 19, 20, 21, 22 and 23). One centre (∗ or the 24th centre in Fig. 2) was not included in Group 1 because of its small number of LTs (5 during the study period).
The 4 subgroups (G1PA, G1CA, G2PA, G2CA) were then included in the weighted Cox model, making it possible to consider the interaction between the treatment effect and the transplant centre effect (measured as a percentage of CA transplants at a 7% threshold).
Survival benefit: Sequential stratification method
The survival benefit associated with a CA graft was estimated using sequential stratification derived from the method described in Schaubel et al.[12], [13], [14], [15] This method essentially reorganises the observed data, and as close as possible reproduces the conditions of a randomised controlled trial (see supplementary information). The aim was to determine in a given patient, whether it was better to be grafted without delay with a CA graft rather than remaining on the waiting list and possibly later receiving a PA graft.
All analyses were performed using R software, version 3.3.0.16
Results
Comparison between PA and CA
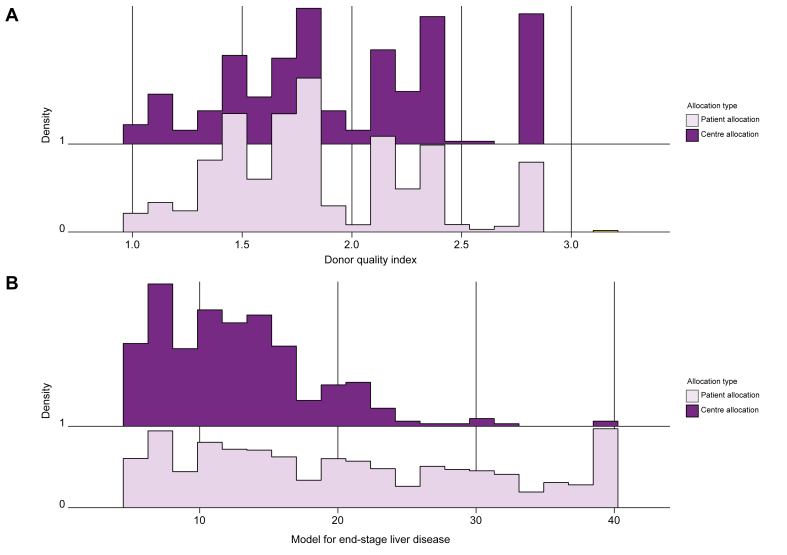
Table 1 provides the comparison between PA and CA groups for recipients and donors, respectively. Fig. 3 gives the distribution of DQI as well as the MELD score according to CA and PA groups. For CA grafts, donors were older, DQIs higher and cold ischaemia times longer than for PAs. These CA grafts mostly belonged to the higher DQI group at risk (25.6% vs. 17.1% for CA and PA, respectively, p <0.001, effect size [ES] = 0.054). The distribution of CA grafts was heterogeneous among the 24 centres. Two of the 24 centres did not use any CA grafts (Fig. 2, centres 1 and 2).
Fig. 3.
Distribution of donor quality index and model for end-stage liver disease scores.
(A) Donor quality index and (B) model for end-stage liver disease score per allocation type: centre allocation (dark purple) and patient allocation (light purple). (The abscissa reflects the density of distribution).
Propensity score and weighted Cox model
After matching, the results of all tests (Student's t or Wilcoxon-Mann-Whitney and χ2 tests) were non-significant. Thus, the 4 covariates (age, gender, MELD score at transplant and HCC) could be considered as being well balanced after adjustment on the propensity score.
Using the propensity score and IPTW method, a weighted Cox model was fitted and adjusted to the covariates (i.e. for recipients: re-transplantation, MELD exceptions, status at transplant (hospital, ICU, home), diabetes, on dialysis at LT, decompensated cirrhosis, non-cirrhotic liver disease, body mass index, encephalopathy, ascites, waiting time and ABO compatibility; for donors: height and DQI10). No inter-regional effect was identified. The hazard ratio (HR) corresponding to the CA vs. PA groups was 1.13 (95% CI 1.05–1.22). There was therefore a significant rise of 13% in the graft loss/death risk among the recipients of CA grafts vs. PA grafts.
Analysis in Groups 1 and 2 in terms of PA and CA categories
One explanation for the significant survival difference between PA and CA groups may have been the quality of the grafts, which appeared to be poorer in the CA group than in the PA group (Table 1, Fig. 3). Nevertheless, as noted, the distribution of CA grafts was heterogeneous, so we completed our analysis by taking account of the ratio of CA transplants performed by each team. There was no significant survival difference between G1PA and G1CA (HR 0.97; 0.87–1.09; p = 0.66) and between G2PA and G1CA (HR 0.95; 0.86–1.05; p = 0.35). This means that in Group 1 (i.e. teams performing more than 7% of CA transplants), the survival of CA recipients and grafts was preserved. Meanwhile, we observed a significant difference in survival between G1CA and G2CA (HR 0.79; 95% CI 0.71–0.89; p <0.01). Thus, within the CA groups, teams performing more than 7% of CA transplants (i.e. Group 1) achieved a significantly higher survival rate of 21% than Group 2 (teams performing fewer than 7% of CA transplants). Moreover, there was a difference in survival between G2PA and G2CA (HR 0.76; 95% CI 0.68–0.85; p <0.01) but no difference between G2PA and G1PA (HR 0.98; 0.88–1.09; p = 0.71).
In order to explore this survival difference, we then compared Group 1 and Group 2 within the CA groups (Table 3). Teams belonging to the G1CA subgroup had more patients on the waiting list than G2CA teams (on average, 723 vs. 333 listed patients in 2009–2014, respectively; p = 0.03 with a large ES = 0.26). They transplanted 36% of all the grafts during the study period, and 70% of CA grafts. Moreover, they transplanted grafts of poorer quality, as determined by a higher DQI, than those in Group 2 (Table 3).
Table 3.
Comparison within centre allocation subgroups of teams performing more than 7% centre allocation transplants and those performing fewer than 7% centre allocation transplants.
| Group 1 centre allocation (n = 236) |
Group 2 centre allocation (n = 100) |
p value | Effect size | |
|---|---|---|---|---|
| Gender (female) | 44 (18.64%) | 16 (16%) | 0.672 | 0.023 |
| Re-transplantation | 2 (0.85%) | 4 (4%) | 0.067 | 0.084 |
| MELD exception | 17 (7.2%) | 7 (7%) | 1 | <0.001 |
| Status at transplant | 0.089 | 0.084 | ||
| Home | 209 (88.56%) | 90 (90%) | ||
| Hospital | 23 (9.75%) | 5 (5%) | ||
| Intensive care unit | 4 (1.69%) | 5 (5%) | ||
| Diabetes | 65 (27.54%) | 28 (28%) | 1 | <0.001 |
| On dialysis | 0 (0%) | 0 (0%) | – | – |
| Hepatocellular carcinoma | 161 (68.22%) | 62 (63%) | 0.423 | 0.044 |
| Decompensated cirrhosis | 41 (17.37%) | 17 (17%) | 1 | <0.001 |
| Hepatic disease no cirrhotic | 3 (1.27%) | 1 (1%) | 1 | <0.001 |
| Encephalopathy | 40 (16.95%) | 22 (22%) | 0.349 | 0.051 |
| Ascites | 104 (44.07%) | 47 (47%) | 0.708 | 0.02 |
| ABO compatibility | 0.026 | 0.097 | ||
| Compatible | 2 (0.85%) | 5 (5%) | ||
| Identical | 233 (98.73%) | 95 (95%) | ||
| Incompatible | 1 (0.42%) | – | ||
| Model for end-stage liver disease | 12.78 (5.44), 12 | 13.14 (6.48), 12 | 0.913 | <0.001 |
| Age | 56.83 (7.66), 57.94 | 56.87 (8.67), 58 | 0.687 | <0.001 |
| BMI | 26.46 (4.77), 26.17 | 26.4 (4.44), 26.27 | 0.929 | <0.001 |
| Waiting time (in days) | 201.33 (163.04), 157 | 212.92 (249.55), 164.50 | 0.638 | <0.001 |
| Ischaemia time (in minutes)† | 539.63 (251.37), 507 | 528.78 (174.43), 534 | 0.677 | 0.001 |
| Follow-up | 1,315 (805.32), 1,332 | 1,200 (752.15), 1,318 | 0.26 | 0.004 |
| Primary non-function∗ | 3 (1.27%) | 6 (6%) | – | – |
| Graft loss∗ | 18 (7.63%) | 5 (5%) | – | – |
| Death∗ | 56 (23.73%) | 25 (25%) | – | – |
| Cause of death | 0.71 | 0.093 | ||
| Graft†† | 2 (3.6%) | 2 (8%) | ||
| Organ failure# | 9 (16.07%) | 5 (20%) | ||
| Recurrence of the initial liver disease | 8 (14.29%) | 4 (16%) | ||
| Cancer‡ | 11 (19.64%) | 5 (20%) | ||
| Infections | 9 (16.07%) | 2 (8%) | ||
| Cardiovascular | 5 (8.93%) | 0 (0%) | ||
| Non-infectious complications¶ | 1 (1.79%) | 0 (0%) | ||
| Other | 11 (19.64%) | 7 (28%) | ||
| DQI group at risk | 0.116 | 0.08 | ||
| 0 | 57 (24.15%) | 34 (34%) | ||
| 1 | 113 (47.88%) | 46 (46%) | ||
| 2 | 66 (27.97%) | 20 (20%) | ||
| Donor height | 166.75 (10.02), 166 | 168.86 (12.8), 170 | 0.042 | 0.012 |
| DQI | 2 (0.49), 2 | 1.85 (0.51), 1.76 | <0.01 | 0.021 |
For quantitative covariates, the results are shown as a mean (SD), median, and for qualitative covariates as number (percentage). Student's t test, the Wilcoxon-Mann-Whitney test and the χ2 test or Fisher's exact test were used when appropriate. For each test, an ES was also reported. For χ2 and Fisher's exact tests, was calculated (i.e. magnitude of the ES; small 0.1≤ES<0.3; medium 0.3≤ES<0.5 and large ES>0.5), and for the Student's t and Wilcoxon-Mann-Whitney tests, r2 and Cohen's r2 were determined (i.e. magnitude of the ES; small 0.01≤ES<0.09; medium 0.09≤ES<0.25 and large ES>0.25), respectively. †Ischaemia time was calculated in 234 G1CA. ∗See Cox model in the Results section. ††Primary non-functioning graft; Hyperacute rejection; Acute rejection; Vascular complications; Biliary complications; Haemorrhage; other causes. #Mainly multiple organ failure (93%). ‡Hepatocellular carcinoma: 4/11 and 2/5, respectively. ¶Haemorrhage (100%).
DQI, donor quality index; ES, effect size; ICU, intensive care unit; MELD, model for end-stage liver disease.
Survival benefit: Sequential stratification method
Among the 346 CA grafts, 319 were matched according to the matching covariates. The characteristics of candidates are shown in Table 2.
CA recipients displayed a non-significant survival benefit (i.e. HR 0.80; 95% CI 0.60–1.08; p = 0.14) compared to patients who remained on the waiting list and possibly received a PA graft at a later point. We then verified the assumptions of the model. We tested the consistency of and 500 simulations were computed. The results obtained were similar to those mentioned (HR 0.78; 95% CI 0.53–1.16: p = 0.22). When graphically verified, the normality assumption was consistent. We also studied the hypothesis that the HR remained constant over time. The HR for liver transplants performed during the first year on the waiting list was 0.83 (95% CI 0.62–1.13), while it was 0.64 (95% CI 0.34–1.21) thereafter.
It should be noted that the HR was non-significant in the G1CA group (0.81; 95% CI 0.59–1.13; p = 0.22) and in the G2CA group (0.78; 95% CI 0.50–1.20; p = 0.25).
Discussion
In this series, the largest on this topic, we showed that CA grafts conferred a 13% increased risk of death/graft loss compared to PAs in general. However, in the transplant centres that performed most CA transplants, which were also the centres with larger waiting lists and that performed more LTs, patient and graft survival were not impacted by the graft type (CA vs. PA). Thus, the results of a centre-oriented offer did not appear different from a patient-oriented offer, given the current organization of the LT network in France. Thus, centre allocation made it possible to save 6 out of 100 available liver grafts that had been refused at least 5 times for use in the top-listed candidates on the national waiting list.
CA grafts have been used in other countries; however, our results are not in accordance with the literature. In the Eurotransplant group, after 3 refusals a graft is qualified as a rescue allocation. In this context, the studies by Doenecke et al.,5 Schemmer et al.6 and Mossdorf et al.,7 performed in small groups of patients, did not reveal any significant difference in survival between CA and PA. Indeed, in Eurotransplant, more than 20% of LTs are considered as CA, whereas in France this subpopulation only accounts for 6% of LTs. Giretti et al.8 conducted a single centre study in France that involved 354 LTs, only 33 of which were CA transplants. Patient survival did not differ between CA and PA grafts. In these 3 small series,5,6,8 graft and recipient survivals were compared using a log-rank test without adjustment, matching, or even weighting, in order to prevent any selection bias.
Comparing CA and PA post-transplantation raw survival is not appropriate since recipients and grafts were different between the 2 groups. Moreover, CA grafts were preferentially allocated to candidates with mild liver failure (i.e. low MELD patients) and HCC. In order to render the 2 recipient groups comparable, a propensity score and a weighted Cox model based on the IPTW method were used.
In a study conducted in the United Kingdom between 2011-2015, in which up to 20% of liver graft offers were not used for transplantation (the median refusal rate was 4 centres per liver), Marcon et al.9 compared patient and graft survival between CA and PA separately for the donation after brain death and DCD liver cohort (206 CA patients). After performing a matching to correct for potential key confounders and using a log-rank test, they identified no significant difference in post-transplant graft and patient survival.
In a complementary analysis within the PA group, we did not find any significant survival difference between teams performing more than 7% CA transplants (i.e. G1PA) and the other teams (G2PA). By contrast, within the CA groups, we showed that teams which performed more than 7% CA transplants (G1CA) achieved better patient/graft survival than the other teams (G2CA). Under the current French system, there are no specific rules concerning the matching of graft donors and candidates, except for blood group. However, in the 5 teams that more frequently perform CA grafts (70% of CA grafts), local matching of these grafts appeared to be favourable and tended to support the more general idea that the matching between donors and recipients could improve LT outcomes.17 Of note, their waiting lists are bigger than those of other teams and enable optimal matching. It is also plausible that for these grafts, the experience gained in the macroscopic evaluation of graft quality might also be better in teams with higher transplant volumes.
Finally, using a sequential stratification approach, we evaluated the survival benefit procured by CA grafts when compared to waiting for a PA graft on the waiting list. The survival benefit of receiving a CA graft did not appear significant compared to remaining on the waiting list and possibly later receiving a PA graft. This can be interpreted as CA grafts not conferring a worse survival benefit than PA grafts. This was the case in both teams using CA grafts (i.e. G1CA and G2CA).
The strength of our study concerned the methodology we adopted. We developed a propensity score to take account of any selection bias and used this propensity score in a weighted Cox model alongside the IPTW method, as well as adjusting for covariates that influenced post-transplant survival. This method enabled the inclusion of all patients who underwent LT during the study period. We then used a sequential stratification model to evaluate the survival benefit between CA and PA grafts, which included all candidates on the waiting list.
Nevertheless, our study has some limitations. The covariates were chosen a priori, and the set selected was not comprehensive. Moreover, the causes for graft refusal (i.e. causes and number of times the grafts have been refused) were not available as they were not reliably collected in the database of the French Agency of Biomedicine, which is in charge of graft allocation at the national level. Thus, a team might have refused a graft for purely logistical reasons. The reasons why a graft is discarded might be different from one team to another. However, when a graft was also consecutively refused by 4 other teams, logistical reasons seemed unlikely. In Henri Mondor hospital (1 of the centres included in our analysis) between January 2011 and April 2015, in a small series of 64 CA grafts, causes of graft refusals were exclusively medical in 20.3% of cases; exclusively logistical in 4.6%; and mixed medical and logistical in 75%.18 We can assume a similar distribution in our study. CA grafts were refused for PA cases on the basis of standard variables, apart from an important one: the macroscopic aspect of the graft, which could only be assessed by the harvesting team. In our study, graft biopsies were seldom performed when appropriate, but were not in fact reported. Moreover, with only 336 CA LTs compared to 4,882 PA LTs, our results should be interpreted with caution and a lack of power cannot be omitted, especially when dealing with subgroups. Nor did we observe any significant survival benefit. Indeed, we saw large confidence intervals due to the relatively limited number of index patients. In terms of assuming consistency over time, we found a slight difference as a function of the year of LT, and the confidence interval also appeared large. A larger number of patients and/or longer follow-up would be useful to improve the accuracy of such an approach.
In centres which transplanted most of the CA grafts, using grafts repeatedly refused for top-listed candidates did not appear to be detrimental. In the context of organ shortage, our results, which could be of interest for any country using this CA strategy, should encourage policy makers to reassess some aspects of graft allocation by restricting CA grafts to targeted centres, fostering the “best” matching between grafts and candidates on the waiting list. It is a first step that warrants further investigations in other settings.
Financial support
This work was carried out in the context of the “Optimatch” study funded by the French Ministry of Health within the framework of the national Clinical Research Hospital Program.
Authors’ contributions
Participated in research design: Audrey Winter, Jean-Pierre Daurès, Paul Landais, Cyrille Feray. Participated in the writing of the paper: Audrey Winter, Jean-Pierre Daurès, Paul Landais, Cyrille Feray. Participated in the performance of the research: Audrey Winter, Jean-Pierre Daurès, Paul Landais. Participated in data analysis: Audrey Winter, Jean-Pierre Daurès, Paul Landais. Participated in interpretation of data: Audrey Winter, Jean-Pierre Daurès, Paul Landais, Daniel Azoulay, Maria Disabato, Philippe Compagnon, Corinne Antoine, Christian Jacquelinet, Cyrille Feray. Final approval of the version to be published: Audrey Winter, Jean-Pierre Daurès, Paul Landais, Daniel Azoulay, Maria Disabato, Philippe Compagnon, Corinne Antoine, Christian Jacquelinet, Cyrille Feray.
Data availability
The data that support the findings of this study are available upon request from the “Agence de la Biomédecine” (https://www.agence-biomedecine.fr/demande-acces-donnees-cristal).
Conflict of interest
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
Our warmest thanks go to Mrs Vicky Hawken for her constructive and sensitive editorial assistance. We also thank all the professionals who contributed to gathering the information in the LT Cristal database, and members of the OPTIMATCH Hospital Clinical Research Programme.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100118.
Contributor Information
Audrey Winter, Email: audrey.winter89@gmail.com.
Cyrille Féray, Email: cyrille.feray@aphp.fr.
Supplementary data
References
- 1.Kamath P.S., Wiesner R.H., Malinchoc M., Kremers W., Therneau T.M., Kosberg C.L. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 2.Francoz C., Belghiti J., Castaing D., Chazouillères O., Duclos-Vallée J.-C., Duvoux C. Model for end-stage liver disease exceptions in the context of the French model for end-stage liver disease score-based liver allocation system. Liver Transplant. 2011;17(10):1137–1151. doi: 10.1002/lt.22363. [DOI] [PubMed] [Google Scholar]
- 3.Alkofer B., Samstein B., Guarrera J.V., Kin C., Jan D., Bellemare S. Extended-donor criteria liver allografts. Semin Liver Dis. 2006;26(03):221–233. doi: 10.1055/s-2006-947292. [DOI] [PubMed] [Google Scholar]
- 4.Barshes N., Horwitz I., Franzini L., Vierling J., Goss J. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7(5):1265–1270. doi: 10.1111/j.1600-6143.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 5.Doenecke A., Scherer M.N., Tsui T.-Y., Schnitzbauer A.A., Schlitt H.-J., Obed A. “Rescue allocation offers” in liver transplantation: is there any reason to reject “unwanted” organs? Scand J Gastroenterol. 2010;45(12):1516–1517. doi: 10.3109/00365521.2010.510577. [DOI] [PubMed] [Google Scholar]
- 6.Schemmer P., Nickkholgh A., Gerling T., Weitz J., Büchler M.W., Schmidt J. Rescue allocation for liver transplantation within Eurotransplant: the Heidelberg experience: rescue allocation in liver transplantation. Clin Transplant. 2009;23:42–48. doi: 10.1111/j.1399-0012.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- 7.Mossdorf A., Kalverkamp S., Langenbrinck L., Ulmer T.F., Temizel I., Neumann U. Allocation procedure has no impact on patient and graft outcome after liver transplantation. Transpl Int. 2013;26(9):886–892. doi: 10.1111/tri.12144. [DOI] [PubMed] [Google Scholar]
- 8.Giretti G., Barbier L., Bucur P., Marques F., Perarnau J.-M., Ferrandière M. Recipient selection for optimal utilization of discarded grafts in liver transplantation. Transplantation. 2018;5(102):775–782. doi: 10.1097/TP.0000000000002069. [DOI] [PubMed] [Google Scholar]
- 9.Marcon F., Schlegel A., Bartlett D.C., Kalisvaart M., Bishop D., Mergental H. Utilization of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation. 2018;102(5):e211–e218. doi: 10.1097/TP.0000000000002127. [DOI] [PubMed] [Google Scholar]
- 10.Winter A., Féray C., Audureau E., Azoulay D., Antoine C., Daurès J.-P. A donor quality index for liver transplantation: development, internal and external validation. Scientific Rep. 2018;8(8):9871–9884. doi: 10.1038/s41598-018-27960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter A., Féray C., Audureau E., Écochard R., Jacquelinet C., Roudot-Thoraval F. External validation of the donor risk index and the Eurotransplant donor risk index on the French liver transplantation registry. Liver Int. 2017;37(8):1229–1238. doi: 10.1111/liv.13378. [DOI] [PubMed] [Google Scholar]
- 12.Schaubel D., Sima C., Goodrich N., Feng S., Merion R. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8(2):419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaubel D.E., Wolfe R.A., Port F.K. A sequential stratification method for estimating the effect of a time-dependent experimental treatment in observational studies. Biometrics. 2006;62(3):910–917. doi: 10.1111/j.1541-0420.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaubel D.E., Kalbfleisch J.D. Assessing the effect on survival of kidney transplantation with higher-risk donor kidneys. In: Lawless J.F., editor. Statistics in Action: A Canadian Outlook. A Chapman & Hall Book; London: 2014. pp. 209–224. [Google Scholar]
- 15.Winter A., Feray C., Antoine C., Azoulay D., Daurès J.-P., Landais P. Matching graft quality to recipient's disease severity based on the survival benefit in liver transplantation. Scientific Rep. 2020 doi: 10.1038/s41598-020-60973-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 17.Briceño J., Ciria R., de la Mata M. Donor-recipient matching: myths and realities. J Hepatol. 2013;58(4):811–820. doi: 10.1016/j.jhep.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Azoulay D., Disabato M., Gomez-Gavara C., Feray C., Salloum C., Ngonggang N. Liver transplantation with “Hors Tour” allocated versus standard MELD allocated grafts: single-center audit and impact on the liver pool in France. World J Surg. 2020;44(3):912–924. doi: 10.1007/s00268-019-05271-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the “Agence de la Biomédecine” (https://www.agence-biomedecine.fr/demande-acces-donnees-cristal).