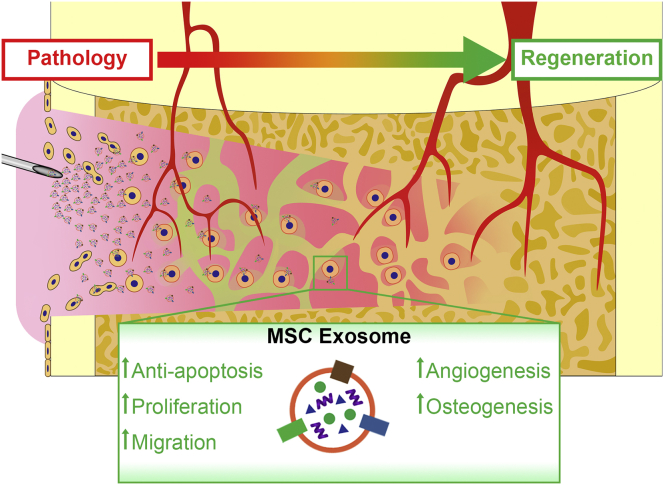
Abstract
The ability of bone for regeneration has long been recognized. However, once beyond a critical size, spontaneous regeneration of bone is limited. Several studies have focused on enhancing bone regeneration by applying mesenchymal stromal/stem cells (MSCs) in the treatment strategies. Despite the therapeutic efficacy of MSCs in bone regeneration, cell-based therapies are impeded by several challenges in maintaining the optimal cell potency and viability during expansion, storage, and final delivery to patients. Recently, there has been a paradigm shift in therapeutic mechanism of MSCs in tissue repair from one based on cellular differentiation and replacement to one based on secretion and paracrine signaling. Among the broad spectrum of trophic factors, extracellular vesicles particularly the exosomes have been reported to be therapeutically efficacious in several injury/disease indications, including bone defects and diseases. The current systematic review aims to summarize the results of the existing animal studies which were conducted to evaluate the therapeutic efficacy of MSC exosomes for bone regeneration. Following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines, the PubMed and The Cochrane Library database were searched for relevant controlled preclinical animal studies. A total of 23 studies were identified, with the total sample size being 690 rats or mice and 38 rabbits. Generally, MSC exosomes were found to be efficacious for bone regeneration in animal models of bone defects and diseases such as osteonecrosis and osteoporosis. In these studies, MSC exosomes promoted new bone formation with supporting vasculature and displayed improved morphological, biomechanical, and histological outcomes, coupled with positive effects on cell survival, proliferation, and migration, osteogenesis, and angiogenesis. Unclear-to-low risk in bias and incomplete reporting in the primary studies highlighted the need for standardization in outcome measurements and reporting. Further studies in large animal models to establish the safety and efficacy would provide useful information on guiding the design of clinical trials.
Keywords: Bone, Exosomes, Extracellular vesicles, Mesenchymal stem cells, Regeneration, Systematic review
Graphical abstract
Highlights
-
•
Therapeutic efficacy of MSC exosomes in bone regeneration was studied in 23 studies.
-
•
MSC exosomes are therapeutically efficacious in bone regeneration.
-
•
MSC exosomes improve morphological, biomechanical, and histological outcomes.
-
•
MSC exosomes promote a multifaceted mechanism in bone regeneration.
1. Introduction
The ability of bone for repair and regeneration has long been recognized [1]. It involves a complex dynamic equilibrium between breaking down of old bone and regeneration of new bone [2]. This process is essential for bone to resume its usual functions of load bearing, mobility, protection, hematopoiesis, and endocrine homeostasis [3]. However, once beyond a critical defect size, spontaneous repair and regeneration of bone is limited [4]. These can be a result of various conditions, including trauma such as fractures, degeneration such as osteoporosis, congenital deformities, tumor resections, and idiopathic conditions such as osteonecrosis [4].
These are extremely common orthopedic conditions [[5], [6], [7]]. Approximately 12.3 million individuals in the United States are expected to have osteoporosis, and approximately 150,000 hospitalizations in Australia are secondary to fractures each year [5,6]. Failure of bone regeneration in these conditions can then lead to non-union, increased propensity for fragility fractures, deformities, and chronic pain. Management of the non-union, fragility fractures, deformities, and chronic pain often requires invasive surgical management, in the form of surgical fixation, bone grafting, bone lengthening, and arthroplasties [[7], [8], [9], [10]]. Apart from the surgical risks of the procedures, these procedures also pose a huge economic burden including that of USD41 billion for fragility fractures and USD11,000 for fracture non-unions per annum [11,12].
Multiple sources of bone grafts and substitutes have been sought after as a solution to these critical-size defects [13]. Among these, autogenous bone grafts exhibit the best osteogenic potential and is usually considered the gold standard [14]. However, the limited supply of autogenous bone graft and the morbidity associated with autograft harvesting has restricted its use [14]. Allografts were therefore introduced, with advantages including its availability and lack of donor-site morbidity [15]. Despite so, the possible infection risk and reduced osteogenic and osteoinductive capabilities of allograft as a result of its sterilization and storage processes have called for the need of alternatives [15,16]. Other bone substitutes including hydroxyapatite (HAp), tricalcium phosphate (TCP), calcium sulphate, demineralized bone matrix, and bone morphogenetic proteins (BMPs) have therefore been extensively studied [16]. However, no ideal bone substitute has been identified thus far, with disadvantages of the bone substitutes having poor mechanical strength, variable rates of resorption, and limited osteogenic and osteoinductive properties [16].
Mesenchymal stromal/stem cells (MSCs) with their ease of isolation from adult tissues and extensive capacities for proliferation and differentiation into various cell lineages are presently the most tested regenerative cell type. There exist more than 1,000 registered clinical trials (https://www.clinicaltrials.gov. Accessed May 20, 2020) with their efficacy reported against a wide variety of injuries and diseases, coupled with an established safety record in human patients [17]. In bone regeneration, several clinical studies have demonstrated MSCs to be safe and efficacious for the treatment of bone defects and diseases such as osteonecrosis [[18], [19], [20], [21], [22]]. Despite their therapeutic effects, cellular MSC therapies incur significant costs and challenges as they require stringently monitored manufacturing, handling, and storage to ensure optimal viability and potency of cells needed for transplantation [23]. Although the use of MSCs for tissue repair such as bone regeneration was first predicated on the hypothesis that these cells could differentiate into osteoblasts to replace the damaged tissue, it is now apparent that MSCs secrete factors involved in cellular processes such as chemotaxis, angiogenesis, and osteogenesis to promote overall bone regeneration [24,25]. In corroboration with this hypothesis, in vitro studies demonstrated that MSCs secrete factors to promote angiogenic factor expression and tube formation of endothelial cells [26], as well as osteogenic differentiation and mineralization of MSCs [27].
Among the numerous trophic factors secreted by MSCs, extracellular vesicles (EVs) particularly the exosomes are cell-secreted bi-lipid membrane vesicles of 30–150 nm in size. These nanovesicles are commonly prepared from medium conditioned by MSCs and are defined by their endosomal origin with the expression of endosomal proteins TSG101 and ALIX and tetraspanins CD9, CD63, and CD81 [28]. They carry a rich diverse cargo of nucleic acids, proteins, and lipids that upon delivery to the recipient cells elicit biological responses reflective of the cargo contents [28]. Increasingly, MSC exosomes have replicated the wide-ranging therapeutic efficacies of MSCs in several injury and disease indications such as myocardial ischemia reperfusion injury [29], drug-induced liver injury [30], retinal laser injury [31], pulmonary hypertension [32], and graft-versus-host-disease [33,34]. MSC exosomes were also reported to be efficacious in enhancing skeletal muscle regeneration [35,36] and more recently cartilage [23,[37], [38], [39], [40]] and bone regeneration [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
The current systematic review aims to summarize the results of the existing in vivo studies which were conducted to evaluate the therapeutic efficacy of MSC exosomes for bone regeneration. In view that current EV preparations are largely heterogeneous, International Society for Extracellular Vesicles recommends the use of a collective term ‘EVs’ unless the biogenesis pathway is demonstrated [64]. Information on the size, biochemical composition, and description of cell origin and culture conditions are also encouraged to be included. In this systematic review, ‘exosome’ is used to describe a population of small EVs of 50–200 nm in size derived from MSC conditioned medium based on expression of typical markers without further demonstration of their intracellular biogenesis origin and/or purity [64,65].
2. Materials and methods
2.1. Systematic review
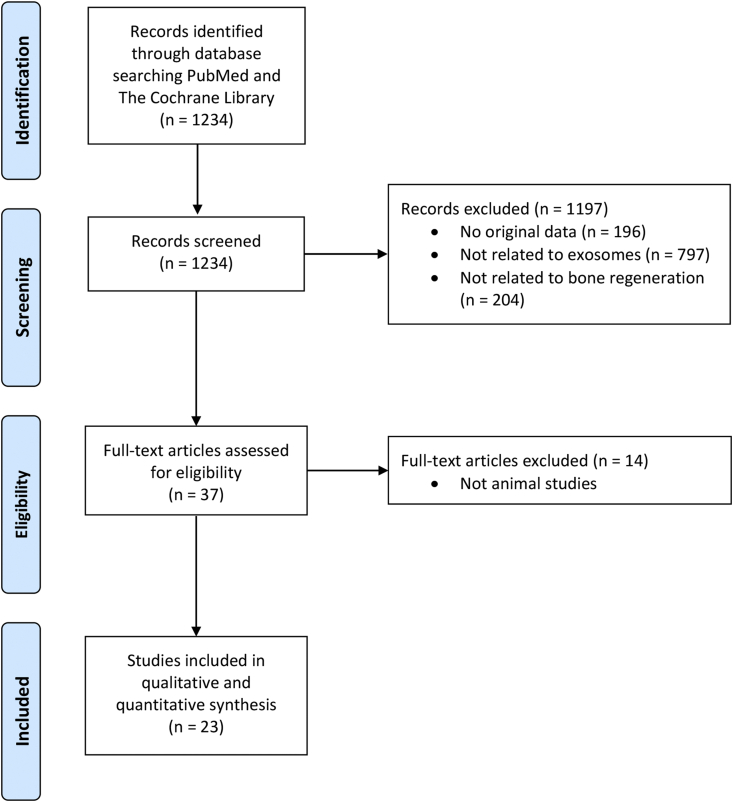
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. A search was conducted using PubMed and The Cochrane Library through April 21, 2020. The keywords used were ‘exosome(s) or secretome(s)’ and ‘cartilage or bone’. All original animal studies that reported the outcomes of MSC exosomes in bone regeneration were included. The articles were selected in two stages. First, the abstracts identified were downloaded, and the list was narrowed using the inclusion or exclusion criteria. Second, the full texts of this list were retrieved and evaluated for eligibility. The reference lists of the identified publications were hand-searched for additional relevant studies, and these were subject to the same two-stage selection (Fig. 1).
Fig. 1.
PRISMA flow diagram of the review and selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
2.2. Assessment of quality of studies
The studies included were examined for the study design. The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias assessment tool is used to assess the risk of bias for the studies [66].
2.3. Data abstraction
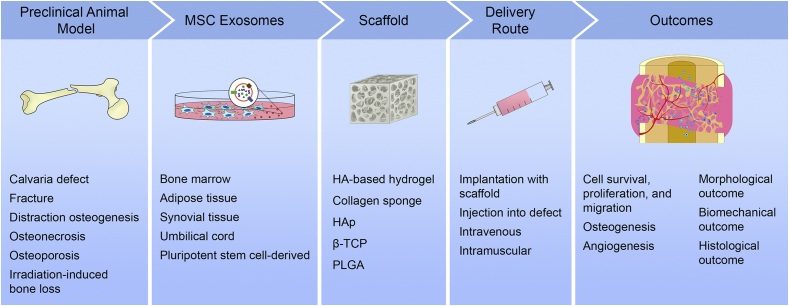
Data of each study were retrieved individually. These include the study design and outcomes of the study. Details noted regarding the study design included the sample size, the gender and species of the animals, the animal model used, the exosome characterization performed, the comparison groups within each study, and the details of the treatment for each group. Details noted for characterization of MSC exosomes included the isolation method, storage condition, size, and marker expression of the exosomes. The details of the treatment noted included the source of exosomes or control, the concentration, volume, delivery, and frequency of treatment, as well as the timing of animal euthanasia. All outcomes of the in vivo studies were noted both qualitatively and quantitatively where available. Effects of MSC exosomes on cell survival, proliferation, migration, osteogenesis, and angiogenesis were also evaluated (Fig. 2). Attempts were made to contact authors of the paper when certain details of the studies were not reported in the papers.
Fig. 2.
Key factors and outcomes identified in preclinical studies of MSC exosomes for bone regeneration. MSC, mesenchymal stem cell; HA, hyaluronic acid; HAp, hydroxyapatite; β-TCP, beta-tricalcium phosphate; PLGA, poly-lactic-co-glycolic acid.
2.4. Data analysis
Outcomes of the studies were largely evaluated qualitatively because there were insufficient quantitative data across the studies for pooling of the data quantitatively.
3. Results
3.1. Systematic review
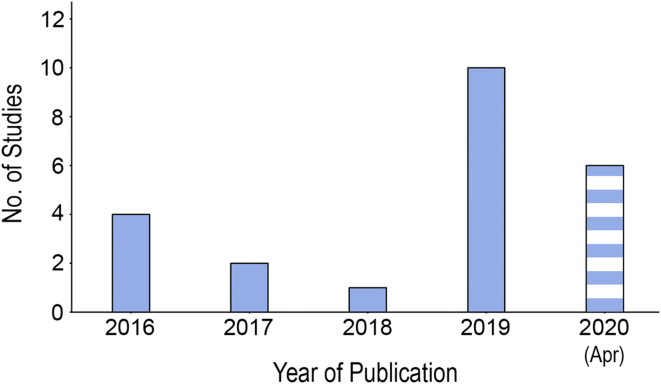
A total of 23 studies were included in the review [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. The studies were published from 2016 with a rapidly growing trend (Fig. 3). This included a sample size of 690 rats or mice and 38 rabbits [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. The number of rats or mice included in the studies by Li et al. and Yang et al. was not included in the total sample size calculated as they were not reported in their papers [49,58].
Fig. 3.
Bar chart showing the number of the published studies included in the systematic review sorted by the year of publication.
The details of each study were described in Table 1, Table 2, Table 3, Table 4. Table 1 detailed the sample size, gender and species of animals, and the animal model used. Table 2 detailed the isolation method, storage, size, and marker expression of MSC exosomes. Table 3 detailed the comparison groups, treatment, concentration, volume, scaffold, delivery route and frequency, and timing of euthanasia for the animals within each study. Table 4 detailed the summary of key therapeutic outcomes reported in each paper.
Table 1.
Summary of characteristics of animal models used.
| Author | Year | Sample size | Gender | Animal | Animal model |
|---|---|---|---|---|---|
| Chen [41] | 2019 | 36 | Male | SD rats | Calvarial defect with 5 mm diameter was constructed using a trephine bur under low-speed drilling |
| Furuta [42] | 2016 | 77 | Male | C57BL/6 mice (WT and CD9−/−) | Transverse femoral shaft fracture was generated using a C-shaped instrument by applying three-point bending |
| Guo [43] | 2016 | 60 | Female | SD rats | Glucocorticoid-induced osteonecrosis of the femoral head was achieved using intramuscular methylprednisolone injections |
| Jia [44] | 2020 | 24 | Male | SD rats | Distraction osteogenesis model was created with transverse tibial osteotomy fixed with an external fixator and distraction performed |
| Kuang [45] | 2019 | 18 | Female | SD rats | Glucocorticoid-induced osteonecrosis of the femoral head was achieved using intramuscular methylprednisolone injections |
| Li [46] | 2017 | 18 | Both | NZ rabbits | Steroid-induced osteonecrosis of the femoral head was achieved using three intramuscular methylprednisolone injections |
| Li [47] | 2018 | 24 | Male | BALB/c mice | 4 mm diameter critical-sized defect was made at the calvarium with a trephine bur |
| Li [48] | 2019 | 20 | Male | SD rats | Steroid-induced osteonecrosis of the femoral head achieved using prednisolone acetate intraperitoneal injection |
| Li [49] | 2020 | NR | Male | Nude mice (BALB/c) | 4 mm diameter critical-sized calvarial defects were created using a sterile dental drill |
| Liang [50] | 2019 | 30 | Male | SD rats | Two 5-mm diameter critical-sized calvarial defects were created on each side of the cranium using a dental trephine |
| Liao [51] | 2019 | 20 | NR | NZ rabbits | Osteonecrosis of the femoral head achieved by freezing the upper end of the femoral head using gauze dipped in liquid nitrogen and then rewarmed in warm saline |
| Liu [52] | 2017 | 40 | Male | SD rats | Osteonecrosis of the femoral head achieved by methylprednisolone acetate intramuscular injection |
| Liu [53] | 2020 | 24 | NR | Mice | Mid-diaphyseal femoral fracture was created by bone forceps |
| Qi [54] | 2016 | 27 | Female | SD rats | Two bilateral 5 mm diameter critical-sized calvarial defects were created using a dental trephine in ovariectomised rats |
| Takeuchi [55] | 2019 | 24 | Male | Wistar rats | Two critical-sized defects of 5 mm diameter were made in the calvarial bone using a trephine drill |
| Xu [56] | 2019 | 70 | NR | SD rats | Traumatic osteonecrosis of the femoral head achieved by stripping the basal periosteum and fibrous fold of the femoral neck |
| Xu [57] | 2020 | 36 | Male | SD rats | Middle femoral fracture was made using a bone forceps |
| Yang [58] | 2020 | NR | NR | SD rats | Disuse osteoporosis was induced by hind limb unloading and a head-down tail suspension to simulate immobilization |
| Zhang [59] | 2016 | 18 | NR | SD rats | Two critical-sized 5 mm diameter calvarial defects were created on each side of the cranium using dental trephine |
| Zhang [60] | 2019 | 48 | Male | Wistar rats | Transverse femoral shaft fracture created using a transverse osteotomy in the middle diaphysis of the right femur |
| Zhang [61] | 2020 | 60 | Male | Wistar rats | Transverse femoral osteotomy performed, reduced, and fixed with 0.4 mm blade at fracture site to create non-union |
| Zhou [62] | 2019 | 24 | Male | SD rats | Femoral fracture created by cutting the femoral shaft using a dental saw and aligning the fracture site via a Kirschner wire |
| Zuo [63] | 2019 | 30 | Female | SD rats | Bone loss induced by 16 Gy irradiation at the knee joint |
NR, not reported; NZ, New Zealand; SD, Sprague Dawley; C57BL/6, C57 black 6; WT, wild type; BALB/c, Bagg Albino.
Table 2.
Isolation method, storage, size, and marker expression of MSC exosomes.
| Author | Year | Origin/Source | Isolation method | Storage | Characterization | Size distribution | EV/Exosome markers |
|---|---|---|---|---|---|---|---|
| Chen [41] | 2019 | Human adipose MSC exosomes | Ultracentrifugation | NR | NTA, WB, TEM | ∼75 nm | CD9, CD63 |
| Furuta [42] | 2016 | Human BM-MSC exosomes | Ultracentrifugation | NR | WB, high-resolution frequency transmission electric-field imaging | ∼80 nm | CD9, CD81, flotillin-1 |
| Guo [43] | 2016 | Human synovial MSC exosomes | Ultrafiltration + Sucrose + Ultracentrifugation | −80°C | DLS analysis, WB, TEM | 30–100 nm | CD9, CD63, CD81, TSG101 |
| Jia [44] | 2020 | Rat BM-MSCs | Ultracentrifugation | −80°C | TRPS analysis, TEM, WB | 60–130 nm | CD9, CD63, TSG101 |
| Kuang [45] | 2019 | Human Wharton's jelly UC-MSC exosomes | exoEasy Maxi Kit, Qiagen | −80°C | DLS analysis, TEM, WB | 30–100 nm | CD9, CD63, CD81, TSG101 |
| Li [46] | 2017 | Rabbit BM-MSC exosomes | Total Exosome Isolation Kit, Invitrogen | NR | TRPS analysis, TEM, WB | 75–150 nm | CD9, CD63, CD81 |
| Li [47] | 2018 | Human adipose stem cell exosomes | Ultracentrifugation | NR | NTA, TEM, WB | 33–177 nm | CD9, CD63 |
| Li [48] | 2019 | Human UC-MSC exosomes | Ultracentrifugation | NR | TEM, WB | 50–80 nm | CD9, CD63, CD81 |
| Li [49] | 2020 | Human iliac bone marrow MSC exosomes Human jawbone marrow MSC exosomes |
Ultracentrifugation | NR | NTA, TEM, WB | ∼100 nm | CD63, ALIX |
| Liang [50] | 2019 | Human BM-MSC exosomes | Ultracentrifugation | −80°C | TRPS analysis, TEM, WB | 80–182 nm | CD9, CD63, TSG101, GM130 |
| Liao [51] | 2019 | Rabbit BM-MSC exosomes | Ultracentrifugation | NR | NTA, TEM, WB | 90–150 nm | CD63, TSG101, HSP70 |
| Liu [52] | 2017 | Human iPSC-MSC exosomes | Ultracentrifugation + Ultrafiltration | NR | TRPS analysis, TEM, WB | 50–100 nm | CD8, CD63, CD81 |
| Liu [53] | 2020 | Human UC-MSC exosomes | Ultrafiltration + Sucrose + Ultracentrifugation | −80°C | NTA, TEM, WB | 50–150 nm | CD9, CD63, CD81, TSG101 |
| Qi [54] | 2016 | Human iPSC-MSC exosomes | Ultrafiltration + Sucrose + Ultracentrifugation | NR | TRPS analysis, WB | 50–150 nm | CD9, CD63, CD81 |
| Takeuchi [55] | 2019 | Human BM-MSC exosomes | Ultracentrifugation | −80°C | NTA, TEM, WB | 80–100 nm | CD9, CD63, CD81 |
| Xu [56] | 2019 | Human BM-MSC exosomes | Total Exosome Isolation Kit, Invitrogen | NR | NTA, TEM, WB | 30–100 nm | CD9, CD63, CD81 |
| Xu [57] | 2020 | Rat BM-MSC exosomes | Ultrafiltration + Sucrose + Ultracentrifugation | −80°C | NTA, TEM, Flow cytometry | 50–150 nm | CD63, CD81 |
| Yang [58] | 2020 | Human UC-MSC exosomes | Ultracentrifugation | −80°C | NTA, TEM, WB | 100–150 nm | CD9, CD63, CD81, ALIX |
| Zhang [59] | 2016 | Human iPSC-MSC exosomes | Ultrafiltration + Sucrose + Ultracentrifugation | −80°C | TRPS analysis, TEM, WB | 50–150 nm | CD9, CD63, CD81 |
| Zhang [60] | 2019 | Human UC-MSC exosomes | Ultracentrifugation | NR | NTA, TEM, WB | ∼100 nm | CD9, CD63, CD81 |
| Zhang [61] | 2020 | Rat BM-MSC exosomes | Ultracentrifugation | NR | NTA, TEM, WB | ∼122 nm | CD9, CD63, CD81 |
| Zhou [62] | 2019 | Human UC-MSC exosomes | Ultracentrifugation | NR | TEM, WB | 30–100 nm | CD9, CD63, CD81 |
| Zuo [63] | 2019 | Rat BM-MSC exosomes | Ultracentrifugation | −80°C | TEM, WB | 40–100 nm | CD63, CD81, TSG101 |
NR, not reported; MSC, mesenchymal stem cells; BM-MSC, bone marrow mesenchymal stem cell; UC-MSC, umbilical cord mesenchymal stem cell; ESC-MSC, embryonic stem cell-derived mesenchymal stem cell; EV, extracellular vesicle; iPSC-MSC, induced pluripotent stem cell-derived mesenchymal stem cells; DLS, dynamic light scattering; NTA, nanoparticle tracking analysis; TEM, transmission electron microscopy; TRPS, tunable resistive pulse sensing, WB, western blotting.
Table 3.
Summary of animal studies and treatment parameters.
| Author | Year | Group/treatment | Concentration | Volume | Delivery route | Frequency | Euthanasia |
|---|---|---|---|---|---|---|---|
| Chen [41] | 2019 | 1. HA/HAp/HP hydrogel loaded with exosomes isolated from miR-375-overexpressing human adipose MSCs | 50 μg/mL | 20 μL + 250 μL hydrogel | Implantation | At day 0 | 3 days, 2, 4 and 8 weeks |
| 2. HA/HAp/HP hydrogel loaded with exosomes isolated from human adipose MSCs expressing the control vector | 50 μg/mL | 20 μL + 250 μL hydrogel | Implantation | At day 0 | 3 days, 2, 4 and 8 weeks | ||
| 3. HA/HAp/HP hydrogel | NA | 250 μL | Implantation | At day 0 | 3 days, 2, 4 and 8 weeks | ||
| 4. No treatment | NA | NA | NA | NA | 3 days, 2, 4 and 8 weeks | ||
| Furuta [42] | 2016 | 1. Injection of human BM-MSC exosomes into CD9−/− mice | NR | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks |
| 2. Injection of human BM-MSC exosomes into WT mice | NR | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 3. Injection of human BM-MSC CM into CD9−/− mice | NR | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 4. Injection of human BM-MSC CM into WT mice | NR | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 5. Injection of human BM-MSC CM (exosome-depleted) into CD9−/− mice | NA | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 6. Injection of human BM-MSC CM (exosome-depleted) into WT mice | NA | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 7. Injection of PBS into CD9−/− mice | NA | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 8. Injection of PBS into WT mice | NA | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| 9. Injection of exosomes derived from the human osteosarcoma cell line into CD9−/− mice | NR | 100 μL | Injection into fracture site | At day 1 and 8 | 6 weeks | ||
| Guo [43] | 2016 | 1. Exosomes from human synovial MSCs | 1 × 1011 particles | 200 μL | Intravenous injection | At day 0 | 3 days, 6 weeks |
| 2. PBS | NA | 200 μL | Intravenous injection | At day 0 | 3 days, 6 weeks | ||
| 3. Normal group | NA | NA | NA | NA | 3 days, 6 weeks | ||
| Jia [44] | 2020 | 1. Exosomes from rat BM-MSCs | 1 × 1010 particles | 100 μL | Injection into distraction gap | Weekly | 7 weeks |
| 2. PBS | NA | 100 μL | Injection into distraction gap | Weekly | 7 weeks | ||
| Kuang [45] | 2019 | 1. Exosomes from human Wharton's jelly UC-MSCs | NR | 100 μg | Intramuscular injection | Daily for 5 weeks | 10 weeks |
| 2. Exosomes from human Wharton's jelly UC-MSCs and miR-21 antagomir | NR | 100 μg | Intramuscular injection | Daily for 5 weeks Weekly for miR-21 |
10 weeks | ||
| 3. miR-21 agomir | NR | 10 μM | Intramuscular injection | Weekly | 10 weeks | ||
| 4. No treatment | NA | NA | NA | NA | 10 weeks | ||
| 5. Normal group | NA | NR | Intramuscular injection | Daily for 5 weeks | 10 weeks | ||
| Li [46] | 2017 | 1. Exosomes from rabbit BM-MSCs transfected with WT HIF-1α adenovirus | 80 μg/mL | 0.5 mL | Injection into femoral head | At day 0 | 6 weeks |
| 2. Exosomes from rabbit BM-MSCs transfected with mutant HIF-1α adenovirus | 80 μg/mL | 0.5 mL | Injection into femoral head | At day 0 | 6 weeks | ||
| 3. PBS | NA | NR | Injection into femoral head | At day 0 | 6 weeks | ||
| Li [47] | 2018 | 1. PLGA/pDA scaffold immobilised with exosomes from human adipose stem cells | 1 μg/μL | 250 μL | Implantation | At day 0 | 6 weeks |
| 2. PLGA/pDA scaffold | NA | NA | Implantation | At day 0 | 6 weeks | ||
| 3. PLGA scaffold | NA | NA | Implantation | At day 0 | 6 weeks | ||
| Li [48] | 2019 | 1. HyStem-HP hydrogel with exosomes from human UC-MSCs | 100 μg/150 μL | 150 μL | Injection into femoral head | At day 0 | 3 weeks |
| 2. No treatment | NA | NA | NA | NA | 3 weeks | ||
| Li [49] | 2020 | 1. PLGA scaffold loaded with human BM-MSCs from ilium cultured with exosomes from human BM-MSCs from ilium | NR | NR | Implantation | At day 0 | 12 weeks |
| 2. PLGA scaffold loaded with human BM-MSCs from ilium cultured with exosomes from human BM-MSCs from jawbone | NR | NR | Implantation | At day 0 | 12 weeks | ||
| 3. PLGA scaffold loaded with exosomes from human BM-MSCs from ilium | NR | NR | Implantation | At day 0 | 12 weeks | ||
| 4. PLGA scaffold loaded with exosomes from human BM-MSCs from jawbone | NR | NR | Implantation | At day 0 | 12 weeks | ||
| 5. PLGA scaffold | NA | NA | Implantation | At day 0 | 12 weeks | ||
| Liang [50] | 2019 | 1. HAp scaffold with exosomes from human BM-MSCs | 0.5 μg/μL | 200 μL | Implantation | At day 0 | 8 weeks |
| 2. HAp scaffold with exosomes from human BM-MSCs preconditioned with DMOG | 0.5 μg/μL | 200 μL | Implantation | At day 0 | 8 weeks | ||
| 3. HAp scaffold with PBS | NA | 200 μL | Implantation | At day 0 | 8 weeks | ||
| Liao [51] | 2019 | 1. Exosomes from rabbit BM-MSCs transfected with miR-122-5p negative control | NR | NR | Intravenous injection | At day 0 | 8 weeks |
| 2. Exosomes from rabbit BM-MSCs transfected with miR-122-5p agomir | NR | NR | Intravenous injection | At day 0 | 8 weeks | ||
| Liu [52] | 2017 | 1. Exosomes from human iPSC-MSCs | 1 × 1010 or 1011 particles/mL | 100 μL | Intravenous injection | At day 0 | 3 weeks |
| 2. Control medium | NA | 100 μL | Intravenous injection | At day 0 | 3 weeks | ||
| 3. No treatment | NA | NA | NA | NA | 3 weeks | ||
| 4. Normal group | NA | NA | NA | NA | 3 weeks | ||
| Liu [53] | 2020 | 1. Exosomes from human UC-MSCs cultured in normoxic condition | 1 μg/μL | 200 μL | Injection near fracture site | At day 0 | 7 days |
| 2. Exosomes from human UC-MSCs cultured in hypoxic condition | 1 μg/μL | 200 μL | Injection near fracture site | At day 0 | 7 days | ||
| 3. PBS | NA | 200 μL | Injection near fracture site | At day 0 | 7 days | ||
| Qi [54] | 2016 | 1. β-TCP scaffold with exosomes from human iPSC-MSCs | 100 μg/scaffold | NA | Implantation | At day 0 | 8 weeks |
| 2. β-TCP scaffold with exosomes from human iPSC-MSCs | 200 μg/scaffold | NA | Implantation | At day 0 | 8 weeks | ||
| 3. β-TCP scaffold | NA | NA | Implantation | At day 0 | 8 weeks | ||
| Takeuchi [55] | 2019 | 1. Atelocollagen sponge with exosomes from human BM-MSC CM | NR | 30 μg | Implantation | At day 0 | 2, 4 weeks |
| 2. Atelocollagen sponge with exosomes from human BM-MSC CM and anti-VEGFA antibody | NR | 30 μg | Implantation | At day 0 | 2, 4 weeks | ||
| 3. Atelocollagen sponge with human BM-MSC CM | NR | NR | Implantation | At day 0 | 2, 4 weeks | ||
| 4. Atelocollagen sponge with PBS | NA | NR | Implantation | At day 0 | 2, 4 weeks | ||
| 5. No treatment | NA | NA | NA | NA | 2, 4 weeks | ||
| Xu [56] | 2019 | 1. Exosomes from human BM-MSCs | NR | 400 μg | Intramuscular injection | At day 1 | 6 weeks |
| 2. Exosomes from human BM-MSCs with negative control | NR | 400 μg | Intramuscular injection | At day 1 | 6 weeks | ||
| 3. Exosomes from human BM-MSCs with miR-224-3p mimic | NR | 400 μg | Intramuscular injection | At day 1 | 6 weeks | ||
| 4. Exosomes from human BM-MSCs with inhibitor negative control | NR | 400 μg | Intramuscular injection | At day 1 | 6 weeks | ||
| 5. Exosomes from human BM-MSCs with miR-224-3p inhibitor | NR | 400 μg | Intramuscular injection | At day 1 | 6 weeks | ||
| 6. No treatment | NA | NA | NA | NA | 6 weeks | ||
| 7. Normal group | NA | NA | NA | NA | 6 weeks | ||
| Xu [57] | 2020 | 1. Exosomes from 4-week old rat BM-MSCs | 1 μg/μL | 200 μL | Injection near fracture site | At day 0 | 2, 3, 4 weeks |
| 2. Exosomes from 72-week old rat BM-MSCs | 1 μg/μL | 200 μL | Injection near fracture site | At day 0 | 2, 3, 4 weeks | ||
| 3. PBS | NA | 200 μL | Injection near fracture site | At day 0 | 2, 3, 4 weeks | ||
| Yang [58] | 2020 | 1. Exosomes from human UC-MSCs | NR | NR | Intramuscular injection | NR | 8 weeks |
| 2. Exosomes from human UC-MSCs and miR-1263 inhibitor | NR | NR | Intramuscular injection | NR | 8 weeks | ||
| 3. miR-1263 mimics | NR | NR | Intramuscular injection | NR | 8 weeks | ||
| 4. PBS | NA | NR | Intramuscular injection | NR | 8 weeks | ||
| 5. Normal control | NA | NA | Intramuscular injection | NA | 8 weeks | ||
| Zhang [59] | 2016 | 1. β-TCP scaffold with exosomes from human iPSC-MSCs | 5 × 1011 particles/mL | 100 μL | Implantation | At day 0 | 8 weeks |
| 2. β-TCP scaffold with exosomes from human iPSC-MSCs | 1 × 1012 particles/mL | 100 μL | Implantation | At day 0 | 8 weeks | ||
| 3. β-TCP scaffold with PBS | NA | 100 μL | Implantation | At day 0 | 8 weeks | ||
| Zhang [60] | 2019 | 1. HyStem-HP hydrogel with exosomes from human UC-MSCs | 100 μg/mL | NR | Injection near fracture site | At day 0 | 2 weeks |
| 2. HyStem-HP hydrogel with exosomes from HEK293 cells | 100 μg/mL | NR | Injection near fracture site | At day 0 | 2 weeks | ||
| 3. HyStem-HP hydrogel with PBS | NA | NR | Injection near fracture site | At day 0 | 2 weeks | ||
| Zhang [61] | 2020 | 1. Exosomes from rat BM-MSCs | 1 × 1010 particles in 100 μL | 100 μL | Injection into fracture site | At day 0 | 8, 14, 20 weeks |
| 2. Exosome-depleted CM from rat BM-MSCs | NA | 100 μL | Injection into fracture site | At day 0 | 8, 14, 20 weeks | ||
| 3. PBS | NA | 100 μL | Injection into fracture site | At day 0 | 8, 14, 20 weeks | ||
| Zhou [62] | 2019 | 1. HyStem-HP hydrogel with exosomes from human UC-MSCs | 1 μg/μL | 100 μL | Injection into fracture site | At day 0 | 2, 3 weeks |
| 2. HyStem-HP hydrogel with PBS | NA | 100 μL | Injection into fracture site | At day 0 | 2, 3 weeks | ||
| 3. No treatment | NA | NA | NA | NA | 2, 3 weeks | ||
| Zuo [63] | 2019 | 1. Irradiation and treatment with exosomes from rat BM-MSCs | 1.6 mg/kg | 400 μL | Intravenous injection | At day 0 | 4 weeks |
| 2. Irradiation and treatment with rat BM-MSCs | 1 × 106 cells | 400 μL | Intravenous injection | At day 0 | 4 weeks | ||
| 3. Irradiation and no treatment | NA | NA | NA | NA | 4 weeks | ||
| 4. No irradiation and no treatment (4 weeks) | NA | NA | NA | NA | 4 weeks | ||
| 5. No irradiation and no treatment (day 0) | NA | NA | NA | NA | day 0 |
NR, not reported; NA, not applicable; MSCs, mesenchymal stem cells; BM-MSC, bone marrow mesenchymal stem cell; UC-MSCs, umbilical cord mesenchymal stem cells; iPSC-MSCs, induced pluripotent stem cell-derived mesenchymal stem cells; HEK293, human embryonic kidney 293; SD, Sprague Dawley; WT, wild type; PLGA, poly-lactic-co-glycolic acid; pDA, poly-dopamine; PLGA/pDA, PLGA scaffold coated with poly-dopamine; HAp, Hydroxyapatite; HA/HAp/HP, hydrogel based on thiol-modified hyaluronan, hydroxyapatite and thiol-modified heparin; HyStem-HP, hydrogel based on thiol-modified hyaluronan, gelatin and heparin; β-TCP, Beta-tricalcium phosphate; PBS, phosphate-buffered saline; CM, conditioned medium; DMOG, dimethyloxaloylglycine; HIF-1α, hypoxia inducible factor-1α; VEGFA, vascular endothelial growth factor A.
Table 4.
Summary of key therapeutic outcomes.
| Author | Year | In vivo outcomes | In vitro outcomes |
|---|---|---|---|
| Chen [41] | 2019 | In a rat calvaria defect model, rats treated with miR-375-overexpressing human adipose MSC exosomes loaded in HA/HAp/HP hydrogel showed the greatest quantity of new bone formation with more mature osteoid, displayed the greatest increase in BV/TV and BMD, and had the most intense staining of OCN and BMP-2 and the least of IGFBP3, compared with rats treated with exosomes from MSCs expressing the control vector, followed by rats with hydrogel only. | Exosomes from miR-375-overexpressing human adipose MSCs showed more potent effects than that of control MSCs in osteogenic differentiation of human BM-MSCs, with significantly higher ALP activity, calcium deposition, and osteogenic gene marker expression. These osteogenic effects were reduced in the presence of IGFBP3. |
| Furuta [42] | 2016 | Injections of human BM-MSC exosomes and CM into the fracture site enhanced callus formation and bony union in both CD9−/− mice and WT mice. However, bony union was not enhanced by injection of exosome-depleted CM in both CD9−/− mice and WT mice, with significantly longer time to union than those injected with exosomes and CM. Unlike MSC exosomes, the human OS exosomes failed to promote callus formation. | Using a Bio-Plex system, SDF-1, MCP-1, and MCP-3 levels in MSC exosomes were significantly lower than the levels in CM and exosome-depleted CM. Assay of miRNAs further revealed that miR-4532, miR125b-5p, and miR338-3p were more abundantly expressed in MSC exosomes relative to human OS exosomes or exosome-depleted CM. |
| Guo [43] | 2016 | In rat model of glucocorticoid-induced osteonecrosis, rats treated with exosomes from human synovial MSCs had significantly reduced osteonecrotic changes as compared with rats treated with PBS. Rats treated with exosomes also had significantly improved bone structural parameters including increased BV/TV, Tb·Th and Tb·N, and decreased Tb·Sp as compared with PBS-treated rats. Histologically, osteonecrotic rats treated with exosomes displayed decreased TUNEL+ apoptotic cells, increased Ki67+ proliferative cells, and enhanced osteogenesis marked by increased OCN staining, as compared with PBS-treated rats. | Human synovial MSC exosomes showed proliferative and antiapoptotic effects on BM-MSCs against dexamethasone-induced apoptosis. |
| Jia [44] | 2020 | Rats treated with exosomes from rat BM-MSCs had more callus in distraction gaps with improved BV/TV and BMD, greater ultimate load, and greater energy to failure compared with rats treated with PBS. | Rat BM-MSC exosomes enhanced proliferation and osteogenic differentiation of BM-MSCs in a dose-dependent manner. |
| Kuang [45] | 2019 | Rats treated with exosomes from human Wharton's jelly UC-MSCs had well-arranged trabecular bone, significantly improved bone structural parameters including increased BV/TV, Tb·Th and Tb·N, and decreased Tb·Sp compared with rats treated with saline. Rats treated with exosomes had increased AKT phosphorylation as compared with rats treated with saline. | Human Wharton's jelly UC-MSC exosomes exhibited anti-apoptotic and proliferative effects on MLO-Y4 osteocytes through the miR-21-PTEN-AKT pathway. These effects were partly abolished in the presence of miR-21 inhibitor. |
| Li [46] | 2017 | In a rabbit model of osteonecrosis of the femoral head, animals treated with HIF-1α overexpressing BM-MSC exosomes had reduced abnormality on MRI, enhanced trabecular tissue generation with more newly formed cartilage and bone, lesser necrotic and adipose tissues and higher density of CD31+ micro-vessels, as compared with rabbits treated with WT exosomes or rabbits treated with PBS. | Relative to the WT exosomes, HIF-1α overexpressing MSC exosomes had a significantly higher HIF-1α protein level and were more potent in enhancing osteogenic differentiation of BM-MSCs, as well as migration and tube formation of HUVECs in a dose-dependent manner. |
| Li [47] | 2018 | In a mouse calvarial defect model, PLGA/pDA scaffold immobilized with human adipose stem cell exosomes promoted more new bone formation with more intense staining for RUNX2 and OCN than that of PLGA/pDA and PLGA scaffolds, possibly through enhanced mobilization of endogenous SSEA-4+/CD45- MSCs. | Exosomes from human adipose stem cells enhanced cellular migration, proliferation, and osteogenic differentiation of human BM-MSCs. |
| Li [48] | 2019 | In rat model of osteonecrosis of the femoral head, rats treated with human UC-MSC exosomes showed callus formation with more obvious trabecular structure, fewer enlarged adipocytes, significantly reduced TUNEL+ apoptotic cells, and elevated expressions of CD31, BMP-2, and VEGF as compared with rats without treatment. | NR |
| Li [49] | 2020 | Nude mice treated with human BM-MSCs cultured with MSC exosomes had significantly more new bone formation compared with rats treated with MSC exosomes alone. Mice treated with iliac MSCs cultured with exosomes from jawbone marrow MSCs had more new bone formation with significantly higher BV/TV and BMD than mice treated with iliac MSCs cultured with iliac bone marrow MSC exosomes. | Exosomes from jawbone marrow MSCs exhibited more potent effects than that of iliac bone marrow MSCs on osteogenic differentiation of iliac bone marrow MSCs, with significant increase in ALP and alizarin red staining, and upregulation of osteogenic marker (ALP, OSX and RUNX2) expression. Blocking the secretion of exosomes using siRNA for Rab27 inhibited the effect of jawbone marrow MSCs. |
| Liang [50] | 2019 | In a rat critical-sized calvarial defect model, rats treated with exosomes from human BM-MSCs preconditioned with DMOG had more new bone formation, higher BV/TV, and BMD, more new vessel formation with increased staining for CD31, as compared with rats treated with exosomes from BM-MSCs or rats treated with PBS. | Exosomes from DMOG-stimulated BM-MSCs had more potent effects on migration and tube formation of HUVECs than that from native MSC exosomes, partly through PTEN downregulation and AKT/mTOR activation. |
| Liao [51] | 2019 | In a rabbit model of osteonecrosis of the femoral head, rabbits treated with miR-122-5p overexpressing MSC exosomes showed reduced necrosis, decreased number of cavities, and displayed significant improvements in the bone parameters including BMD, relative TBV and MTPT of the femoral head, and increased density of CD31+ microvessels, as compared with rabbits treated with control exosomes. | MiR-122-5p enhanced proliferation and differentiation of osteoblasts (hFOB1.191) by negatively regulating Sprouty2 and activating the RTK/Ras/MAPK pathway. |
| Liu [52] | 2017 | In a rat model of osteonecrosis of femoral head, rats treated with human iPSC-MSC exosomes at 1 × 1011 particles/ml had reduced osteonecrosis, improved bone structure parameters including increased BV/TV, bone surface area over bone volume, Tb·Th and Tb·N, and enhanced angiogenesis with increased vessel number and volume, microvessel density and expression of VEGFR2 and CD31, compared with rats treated with 1 × 1010 particles/ml of exosomes, followed by rats with no treatment. | Human iPSC-MSC exosomes at higher concentration of 1 × 1011 particles/ml was more potent than 1 × 1010 particles/ml in enhancing proliferation, migration, and tube formation of HUVECs. These angiogenic effects of MSC exosomes were inhibited in the presence of LY294002, implicating the involvement of PI3K/AKT signaling in exosome-induced angiogenesis. |
| Liu [53] | 2020 | In a mouse femoral fracture model, mice treated with exosomes from human UC-MSCs cultured in hypoxic (1% O2) condition showed significantly larger callus volume over tissue volume, greater vascularity with higher blood vessel volume and number and proliferative Ki67+CD31+ endothelial cells in the fracture site, than those treated with exosomes from MSCs cultured in normoxic (21%) condition or mice treated with PBS. These effects of hypoxic MSC exosomes were partly abrogated in the presence of miR-126 inhibitor that resulted reduced callus volume, decreased blood vessel number and Ki67+CD31+ cells. | Hypoxic MSC exosomes promoted proliferation, migration, and tube formation of HUVECs. miR-126 was found upregulated in exosomes from hypoxic MSCs, possibly through HIF-1α, and mediated enhanced angiogenesis through a SPRED1/Ras/Erk pathway. |
| Qi [54] | 2016 | In a rat osteoporotic calvarial defect model, rats treated with 200 μg human iPSC-MSC exosomes in β-TCP scaffold had more newly formed bone with higher BV/TV and BMD, greater neovascularization with higher vessel area and number, and more intense staining for OCN and OPN, as compared with rats treated with 100 μg exosomes or scaffold alone. | Human iPSC-MSC exosomes at higher concentration of 200 μg/ml was more potent than 100 μg/ml in enhancing proliferation and osteogenic differentiation of osteoporotic rat BM-MSCs. |
| Takeuchi [55] | 2019 | In a rat calvaria defect model, significantly higher amounts of newly formed bone, with increased staining for OCN, VEGF, CD31+ cells and CD44+ cells were noted in rats treated with human BM-MSC exosomes or MSC CM, as compared with rats treated with PBS or no treatment. These effects of MSC exosomes on angiogenesis and bone regeneration were abolished in the presence of anti-VEGFA antibody | MSC exosomes enhanced cellular migration, osteogenic differentiation, and expression of angiogenesis and osteogenesis-related genes in human BM-MSCs. These effects of MSC exosomes were inhibited in the presence of anti-VEGFA antibody. |
| Xu [56] | 2019 | In a rat model of traumatic osteonecrosis of femoral head, human BM-MSC exosomes with miR-224-3p inhibition ameliorated osteonecrosis, with increased bone viability, coupled with increased protein levels of FIP200 and VEGF, compared with the other groups. | miR-224-3p present in MSC exosomes was found downregulated in osteonecrosis of the femoral head. Downregulated miR-224-3p expression enhanced angiogenesis by promoting proliferation, migration, and tube formation of HUVECs through positive regulation of FIP200. |
| Xu [57] | 2020 | In a rat femoral fracture model, rats treated with exosomes from 4-week-old rat BM-MSCs showed more newly formed callus, increased BV/TV, and increased gene expression levels of RUNX2, ALP, and Col I, as compared with rats treated with exosomes from 72-week-old rat bone marrow MSCs or PBS. | Exosomes from 4-week-old rat BM-MSCs were more potent than that from 72-week-old rat BM-MSCs in enhancing osteogenic differentiation of MSCs. The miR-128-3p was found upregulated in exosomes from 72-week-old rat BM-MSCs and negatively regulated osteogenesis through Smad5 inhibition. |
| Yang [58] | 2020 | In a rat model of disuse osteoporosis, rats treated with human UC-MSC exosomes showed improvements in bone histology and structural parameters including increased BV/TV, Tb·Th and Tb·N, and decreased Tb·Sp, and reduced apoptosis of bone marrow MSCs with decreased Mob1 and increased YAP expression, as compared with rats treated with PBS. These effects of MSC exosomes were partly recapitulated with miR-1263 mimics but abrogated in the presence of miR-1263 inhibitor. | Exosomal miR-1263 could bind to 3′untranslated region of Mob1. The inhibition of Mob1 could activate YAP expression and inhibition of Hippo signaling pathway that reversed the apoptosis of BM-MSCs induced by hind limb unloading. |
| Zhang [59] | 2016 | In a rat calvarial defect model, rats treated with β-TCP scaffold containing 1 × 1012 particles/ml of human iPSC-MSC exosomes had more newly formed bone with higher BV/TV and BMD, and more intense staining for OCN, compared with rats treated with β-TCP scaffold with 5 × 1011 particles/ml of exosomes, followed by rats treated with scaffold alone. | Human iPSC-MSC exosomes promoted migration, proliferation, and osteogenic differentiation of human BM-MSCs, with the higher concentration of 1 × 1012 particles/ml having a more potent effect than 5 × 1011 particles/ml. The osteogenic effects of MSC exosomes were inhibited in the presence of LY294002, implicating the involvement of PI3K/AKT signaling in exosome-induced osteogenesis. |
| Zhang [60] | 2019 | In a rat fracture model, rats treated with human UC-MSC exosomes delivered in HyStem-HP hydrogel showed larger callus width and volume, improved radiographic scores and bone structural parameters including increased BMD and BV/TV, enhanced vascularization with increased vessel volume and CD31+ vessels, as well as significantly enhanced maximum load at failure and bending stiffness, as compared with rats treated with HEK293 exosomes or PBS in hydrogel. | Human UC-MSC exosomes enhanced proliferation, migration, and tube formation of HUVECs. These angiogenic effects of MSC exosomes on HUVECs were abrogated by siRNA silencing of HIF-1α. |
| Zhang [61] | 2020 | In a rat fracture non-union model, rats treated with rat BM-MSC exosomes showed accelerated bone healing with more new bone formation, higher radiographic score and BV/TV, increased expression of angiogenesis-related markers including CD31, VEGF, and HIF-1α, and osteogenesis markers including OPN and OGN, BMP-2, Smad1, and RUNX2, and overall improved fracture healing score compared with rats treated with exosome-depleted CM or PBS. | Rat BM-MSC exosomes enhanced proliferation and migration of MC3T3-E1Cs and HUVECs, and tube formation of HUVECs. Inhibition of BMP signaling with LDN-193189 or noggin suppressed exosome-induced osteogenesis of MC3T3-E1Cs with reduced mineralization and decreased levels of BMP-2, Smad1, and RUNX2. |
| Zhou [62] | 2019 | In a rat femoral fracture model, rats treated with human UC-MSC exosomes loaded in HyStem-HP hydrogel had significant fracture healing with upregulated expression levels of β-catenin and Wnt3a and osteogenic marker genes including Col I, OPN, and RUNX2 at the fracture site compared with rats treated with PBS-loaded hydrogel or left untreated. | NR |
| Zuo [63] | 2019 | In a rat irradiation bone loss model, irradiated rats treated with BM-MSCs or derived exosomes exhibited improvements in bone histology and structural parameters including BMD, BV/TV, Tb·Th, Tb·N, and Conn·D compared with rats with irradiation and no treatment. | Rat BM-MSC exosomes alleviated oxidative damage, enhanced DNA repair, rescued the inhibition of proliferation, restored the balance in osteogenic and adipogenic differentiation, and decreased the senescence-associated protein expression of BM-MSCs following irradiation. |
NR, not reported; OCN, osteocalcin; OPN, osteopontin; OGN, osteoglycin; BMP-2, bone morphogenetic protein-2; IGFBP3, insulin-like growth factor binding protein 3; OS, osteosarcoma; CM, conditioned medium; PBS, phosphate buffered saline; BMD, bone mineral density; BV/TV, bone volume per tissue volume; Tb·Th, trabecular thickness; Tb·N, trabecular number; Tb·Sp, trabecular separation; TBV, trabecular bone volume; MTPT, mean trabecular plate thickness; Conn·D, connective density; TRAP, tartrate-resistant acid phosphatase; α-SMA, α-smooth muscle actin; AKT, protein kinase B; CD31, cluster of differentiation 3; DMOG, dimethyloxaloylglycine; YAP, yes associated protein; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2; FIP200, family kinase interacting protein of 200 kDa; HIF-1α, hypoxia-inducible factor 1α; RUNX2, runt-related transcription factor 2; iPSC; induced pluripotent stem cell; MSCs, mesenchymal stem cells; BM-MSC, bone marrow mesenchymal stem cell; UC-MSC, umbilical cord mesenchymal stem cell; ESC-MSC, embryonic stem cell-derived mesenchymal stem cell; iPSC-MSCs, induced pluripotent stem cell-derived mesenchymal stem cells; ALP, alkaline phosphatase; Col 1, type I collagen; VEGFA, vascular endothelial growth factor A; MAPK, mitogen-activated protein kinase; RTK, receptor tyrosine kinase; hFOB, human fetal osteoblasts; OS, Osteosarcoma; HEK293, human embryonic kidney 293; HUVECs, human umbilical vein endothelial cells.
3.2. Assessment of quality of studies
The detailed assessment of the quality of each study was described in Table 5. All studies had low risk for selection bias secondary to baseline characteristics, detection bias secondary to random outcome assessment, attrition bias, reporting bias, and other bias [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. All studies reported some form of baseline characteristics for the animals used, such as the gender, species or age of the animals used, and were therefore assigned low risk for the domain of baseline characteristics [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. All the studies also reported some form of quantitative outcome that was presumably averaged across all the animals studied; therefore, they were assigned as low risk for detection bias in terms of random outcome assessment and for reporting bias [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. None of the animals were reported to have died before the conclusion of the studies, and therefore, the studies were assigned as low risk for attrition bias [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. Seventeen out of 23 studies reported that the allocations of groups were random and were therefore assigned as low risk, though the randomization process was not clearly specified in all but one study [[43], [44], [45], [46], [47], [48],50,[52], [53], [54], [55], [56], [57], [58], [59],61,63]. The other six studies were classified as unclear risk for the sequence generation bias [41,42,49,51,60,62]. Eight studies reported the housing environment of the animals and were therefore classified as low risk for the random housing domain [41,46,51,52,55,56,58,62], whereas the rest of the studies were classified as unclear risk [[42], [43], [44], [45],[47], [48], [49], [50],53,54,57,[59], [60], [61],63]. Only 1 study reported blinding of the assessors when assessing for the outcomes and were therefore classified as low risk for blinding under detection bias [52], whereas the rest of the studies were classified as unclear risk [[42], [43], [44], [45], [46], [47], [48], [49], [50], [51],[53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. None of the studies reported on allocation concealment and blinding for performance bias; therefore, these were assigned as unclear risk for all studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
Table 5.
Risk of bias assessed using SYRCLE risk of bias assessment tool.
| Author | Year | Selection bias |
Performance bias |
Detection bias |
Attrition bias |
Reporting bias |
Other |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias | ||
| Chen [41] | 2019 | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Furuta [42] | 2016 | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Guo [43] | 2016 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Jia [44] | 2020 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Kuang [45] | 2019 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Li [46] | 2017 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Li [47] | 2018 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Li [48] | 2019 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Li [49] | 2020 | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Liang [50] | 2019 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Liao [51] | 2019 | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Liu [52] | 2017 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Liu [53] | 2020 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Qi [54] | 2016 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Takeuchi [55] | 2019 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Xu [56] | 2019 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Xu [57] | 2020 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Yang [58] | 2020 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zhang [59] | 2016 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zhang [60] | 2019 | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zhang [61] | 2020 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zhou [62] | 2019 | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Zuo [63] | 2019 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
3.3. Animal models
Six types of animal models were used across the 23 studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. These included seven studies which used critical-size calvaria defect models [41,47,49,50,54,55,59], seven studies which used osteonecrosis models [43,45,46,48,51,52,56], six studies which used fracture models [42,53,57,[60], [61], [62]], two studies which used osteoporosis models [54,58], one study that used a distraction osteogenesis model [44], and one study that used irradiation-induced bone loss model [63]. Qi et al. employed both the critical-size bone defect and osteoporosis in their animal model and was therefore counted in both types of models [54]. Regardless of the models, all studies demonstrated therapeutic efficacy in bone regeneration with MSC exosomes [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
3.4. Source of exosomes
Seventeen studies used human MSC exosomes [[41], [42], [43],45,[47], [48], [49], [50],[52], [53], [54], [55], [56],[58], [59], [60],62], four studies used rat MSC exosomes [44,57,61,63], and two studies used rabbit MSC exosomes [46,51]. Sources of human exosomes included bone marrow MSCs [42,49,50,55,56], adipose MSCs [41,47], synovial MSCs [43], umbilical cord MSCs [45,48,53,58,60,62], and pluripotent stem cell-derived MSCs [52,54,59], whereas rat and rabbit exosomes were solely from bone marrow MSCs [44,46,51,57,61,63]. Regardless of the source of exosomes, all studies demonstrated therapeutic efficacy with MSC exosomes in bone regeneration [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
In comparing the different sources of exosomes, Furuta et al. added exosomes derived from human osteosarcoma cell line as a comparison group to exosomes derived from human bone marrow MSCs [42]. They noted that while exosomes from human bone marrow MSCs could promote callus formation at the fracture site 10 days after fracture, exosomes from the human osteosarcoma cell line failed to promote callus formation [42]. Similarly, Zhang et al. noted superior therapeutic effects of human umbilical cord MSC exosomes over that of exosomes derived from human embryonic kidney 293 (HEK293) cells on fracture healing [60]. In that study, rats treated with human umbilical cord MSC exosomes showed accelerated fracture healing with larger callus formation, increased bone volume and mineral density, and enhanced vascularization with increased CD31+ vessels, as compared with rats treated with exosomes from HEK293 cells [60]. Two other studies compared the MSC exosomes of different anatomical origin and age [49,57]. Li et al. comparatively evaluated the MSC exosomes derived from jawbone and iliac bone marrow and found that the bone marrow MSC exosomes from the jawbone were more efficacious in promoting osteogenesis of transplanted iliac MSCs in bone regeneration [49]. In another study, Xu et al. compared between exosomes derived from MSCs of young vs. old rats and noted in a femoral fracture model that rats treated with exosomes from 4-week-old rat bone marrow MSCs showed more newly formed callus, increased bone volume, and increased gene expression levels of runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and type I collagen, as compared with rats treated with exosomes from 72-week-old rat bone marrow MSCs or phosphate buffered saline (PBS) [57].
3.5. Isolation and characterization of exosomes
Most of the studies employed ultracentrifugation-based methods for isolation of EVs/exosomes [[41], [42], [43], [44],[47], [48], [49], [50], [51], [52], [53], [54], [55],[57], [58], [59], [60], [61], [62], [63]]. Three studies used commercially available exosome isolation kits [45,46,56]. For characterization, nanoparticle tracking analysis, transmission electron microscopy, and western blotting were frequently being performed for analysis of size distribution, morphology, and presence of exosome-associated markers, respectively. Despite some variability among the studies, the isolated MSC exosomes generally appeared as bi-lipid membrane structures under transmission electron microscope, displayed a size distribution range of approximately 30–150 nm, and possessed exosome-associated markers such as endosomal proteins TSG101 and ALIX, and tetraspanins CD9, CD63, and CD81 [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. It is however important to note that the EV/exosome preparations were largely heterogenous with variable level of undetermined purity that could influence the outcomes in downstream functional studies. Ten studies [[43], [44], [45],50,53,55,[57], [58], [59],63] reported storage of the isolated exosomes at −80°C until use. Although the period of storage and the use of any cryo-preservatives were not specified, the storage did not appear to affect the functions of the isolated MSC exosomes in those studies. This observation suggests that MSC exosomes may hold promise for a cell-free off-the-shelf therapeutic strategy.
3.6. Concentration of exosomes
A wide range of concentration of exosomes were employed across the different studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. Of these, three studies compared the therapeutic efficacy with regard to the concentration of exosomes [52,54,59]. In regeneration of critical-size calvaria defects, Qi et al. compared between 100 and 200 μg of exosomes, whereas Zhang et al. compared between 5 × 1011 and 1 × 1012 particles/mL of exosomes blotted onto β-TCP scaffolds [54,59]. Both studies similarly identified that the amount of newly formed bone, bone mineral density (BMD), bone volume fraction, area of neovascularization, amount of newly formed vessels, percentages of tetracycline, alizarin red and calcein labeling, as well as extent of osteocalcin (OCN) and osteopontin staining were proportional to the concentration of exosomes, with higher concentrations of exosomes demonstrating greater therapeutic efficacy [54,59]. Similarly, in a rat model of osteonecrosis of the femoral head, rats treated with MSC exosomes at 1 × 1011 particles/mL had reduced necrotic tissues, increased new bone formation with improved structural parameters, and enhanced vascularization with increased expression of vascular endothelial growth factor receptor 2 and CD31 compared with rats treated with 1 × 1010 particles/mL of exosomes, followed by rats with no treatment [52]. Although a higher dose/concentration of exosomes is generally in favor of relatively better bone regeneration, none of the studies have demonstrated the optimal concentration of exosomes for their animal models [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
3.7. Delivery of exosomes
All the studies delivered the exosomes using either scaffolds, injections, or both [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. A variety of biomaterial scaffolds, ranging from hyaluronic acid (HA)-based hydrogels and collagen sponges to HAp and TCP ceramics have been studied as carriers for delivery of exosomes [41,[47], [48], [49], [50],54,55,59,60,62]. Various routes of administration that ranged from implantation of scaffold with exosomes to direct intravenous or intramuscular injections of exosomes have also been explored [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
All the studies that compared between scaffold delivery of exosomes and scaffolds alone reported superior therapeutic outcomes in bone regeneration with scaffold delivery of exosomes [41,47,49,50,54,55,59,60,62]. It was noted that the addition of MSC exosomes into scaffolds promoted more extensive neovascularization [50,54,55,60] and robust bone formation with improved histological and structural parameters [41,47,49,50,54,55,59,60,62].
In terms of frequency of treatment, there are several studies that applied single scaffold implantation with exosomes [41,[47], [48], [49], [50],54,55,59,60,62] or single injection of exosomes [43,46,[51], [52], [53],56,57,61,63]. There are also studies that applied multiple injections [42,44,45]. None of the studies have however compared the efficacy of different biomaterial scaffolds or varying exosome treatment frequencies for bone regeneration in their animal models. Clearly, an optimal delivery method and/or frequency of exosome treatment remain to be determined, which will require optimization for a specific bone injury/disease indication.
3.8. Morphological outcomes
Morphological analyses were mainly performed by micro-computed tomography (micro-CT) or X-ray examination. Majority of the studies reported the morphological outcomes of bone regeneration with MSC exosomes [[41], [42], [43], [44], [45], [46], [47],[49], [50], [51], [52], [53], [54], [55],[57], [58], [59], [60], [61], [62], [63]]. Morphologically, rats, mice or rabbits treated with MSC exosomes demonstrated new bone formation, with improvements in the bone structural parameters such as increased BMD, increased bone volume (BV), increased bone volume over total volume (BV/TV), increased trabecular thickness (Tb·Th), increased trabecular number (Tb·N), and decreased trabecular separation (Tb·Sp) after exosome treatment [[41], [42], [43], [44], [45], [46], [47],[49], [50], [51], [52], [53], [54], [55],[57], [58], [59], [60], [61], [62], [63]].
3.9. Biomechanical outcomes
Biomechanical outcomes were reported in two studies [44,60]. Jia et al. used a distraction osteogenesis model and loaded the tibia of the rats treated in the anterior-posterior direction at a loading rate of 1 mm/min until failure, and it was noted that rats treated with exosomes had greater ultimate load and greater energy to failure as compared with rats treated with PBS [44]. Zhang et al. employed a rat fracture model and performed biomechanical testing using a quasistatic 3-point bending test where a bending load was applied at a crosshead speed of 1.5 mm/min until fracture, and it was noted that as early as 2 weeks posttreatment, the MSC exosome group had significantly enhanced maximum load at failure and bending stiffness as compared with the control groups [60].
3.10. Histological outcomes
Histological outcomes following exosome treatment were reported in 21 studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56],[58], [59], [60], [61],63]. In calvaria defect model, the presence of newly formed bone tissue was generally observed in the center and along the defect margins in rats or mice treated with exosomes [41,47,49,50,54,55,59]. Osteoblast-like cells and infiltration of neutrophils were noted in the defect margins in the early stages, with the formation of bone bridge with osteocytes and vasculature in the later stage [55]. This was as opposed to the presence of fibrous tissues in the control groups [41,47,49,50,54,55,59]. Among these, three studies performed trichromatic sequential fluorescent labeling to facilitate identification of the newly formed bone, and groups treated with MSC exosomes were found to demonstrate greater percentages of tetracycline, alizarin red, and calcein labeling than those groups treated with scaffolds only [50,54,59].
In the osteonecrosis models, rats and rabbits treated with exosomes also demonstrated well-organized and denser trabecular bone, increased number of osteocytes distributed in the trabeculae, increased presence of proliferative cells, decreased number of vacuum clefts or empty lacunae, reduced amounts of necrotic tissues with decreased number of apoptotic cells, and lesser adipose tissues [43,45,46,48,51,52,56]. This stands in contrast to animals in the control groups which had mostly necrotic tissue with large amounts of osteolysis accompanied by disorganized and sparse trabecular bone [43,45,46,48,51,52,56].
In the fracture models, there was enhanced callus formation and bony union in rats or mice treated with exosomes as compared with the control groups [42,53,60,61]. The newly formed bone in the exosome groups demonstrated accelerated formation of hypertrophic chondrocytes and woven bone as opposed to the formation of substantial fibrous tissue in the control groups [42].
In the osteoporotic model, rats treated with exosomes had enhanced bone healing irrespective of inhibition by osteoporosis [54,58]. Additionally, the new bone formed was found to be profoundly remodeled [54,58]. In contrast, control rats without exosome treatment demonstrated bone loss with minimal mineralization and bone formation [54,58].
Twelve studies evaluated the extent of neovascularization with exosome treatment during bone regeneration [42,46,48,[50], [51], [52], [53], [54], [55], [56],60,61]. Immunohistochemical staining for vascular markers, including CD31, vascular endothelial growth factor (VEGF), and α-smooth muscle actin (α-SMA) for vascular endothelial cells and vessels, and Microfil perfusion followed by micro-CT analysis were frequently performed in these studies to assess neovascularization during bone regeneration [42,46,48,[50], [51], [52], [53], [54], [55], [56],60,61]. In these studies, rats, mice, or rabbits treated with exosomes showed enhanced neovascularization histologically as compared with the control groups [42,46,48,[50], [51], [52], [53], [54], [55], [56],60,61]. This is evidenced by the increased tissue vascularity and abundance of newly formed vessels, along with increases in vessel volume, number, density, thickness, and branches in the fracture sites or bone defects in the animals treated with exosomes [42,46,48,[50], [51], [52], [53], [54], [55], [56],60,61].
3.11. Cell survival, proliferation, and migration
Upon bone injury/disease, there is perturbation of tissue homeostasis with excessive inflammation resulting in cellular apoptosis, matrix degradation, and bone loss. Enhancing cell survival, proliferation, and migration are therefore fundamentally important to restore the cell number and function to facilitate bone repair and regeneration. For instance, in a rat model of glucocorticoid-induced osteonecrosis, human synovial MSC exosomes alleviated bone loss and apoptosis, as demonstrated by improvements in bone histology and microstructure, along with decrease in terminal dUTP nick end labeling (TUNEL)+ apoptotic cells and parallel increase in Ki67+ proliferative cells [43]. Similarly, Kuang et al. observed therapeutic efficacy of human umbilical cord MSC exosomes in osteonecrosis and attributed the antiapoptotic and prosurvival effects of MSC exosomes to miR-21-mediated PTEN (phosphatase and tensin homolog) downregulation and AKT (protein kinase B) phosphorylation [45]. In a rat model of disuse osteoporosis, human umbilical cord MSC exosomes reportedly improved the bone histological and structural parameters including increased BV/TV, Tb·Th, and Tb·N and decreased Tb·Sp and reduced apoptosis of bone marrow MSCs with Mob1 suppression, resulting in increased expression of yes-associated protein and inhibition of Hippo signaling pathway, as compared with PBS-treated rats [58]. These effects of MSC exosomes were partly recapitulated with miR-1263 mimics but abrogated in the presence of miR-1263 inhibitor [58].
These in vivo findings were supported by in vitro findings for cell survival, proliferation, and migration. Seventeen studies reported positive effects of MSC exosomes on proliferation and/or migration of a wide variety of cell types including osteoblasts, osteocytes, MSCs, and endothelial cells in vitro [[43], [44], [45], [46], [47],[50], [51], [52], [53], [54], [55], [56],[58], [59], [60], [61],63]. Among these, 3 studies also observed antiapoptotic effects of MSC exosomes on osteocytes and bone marrow MSCs [43,45,58]. Collectively, MSC exosomes exert potent effects on cell survival, proliferation, and migration to promote bone regeneration.
3.12. Osteogenesis
As earlier mentioned, all studies reported therapeutic efficacy of MSC exosomes in bone regeneration, as demonstrated in their morphological, biomechanical, and/or histological outcomes [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. In understanding the process of bone development and formation, commonly known as osteogenesis, several studies have also further investigated the role of MSC exosomes in osteogenesis in cell culture studies [41,44,47,49,51,54,55,57,59,61,63].
Consistent with the therapeutic outcome in bone regeneration in vivo, MSC exosomes were observed to enhance osteogenic differentiation of cell types such as bone marrow MSCs, osteoblasts, and osteocytes in vitro, with increases in ALP activity, mineralization and expression of osteogenesis-related markers such as OCN and RUNX2 [41,44,47,49,54,55,57,59,61,63].
In understanding the mechanism of action of MSC exosomes in osteogenesis and bone regeneration, six studies reportedly identified mitogen-activated protein kinase, phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), BMP/Smad, and Wnt/β-catenin signaling pathways in regulating exosome-induced osteogenesis [51,57,59,[61], [62], [63]]. For instance, Zhang et al. reported that human iPSC-MSC exosomes promoted osteogenic differentiation of human bone marrow MSCs, with the higher concentration of 1 × 1012 particles/mL, having a more potent effect than 5 × 1011 particles/mL. The osteogenic effects of MSC exosomes were inhibited in the presence of LY294002, implicating the involvement of PI3K/AKT signaling in exosome-induced osteogenesis [59]. In another study, rat bone marrow MSC exosomes promoted osteogenic differentiation and mineralization of MC3TE-E1 osteoblasts, but these effects were abolished by BMP-2 inhibitors noggin and LDN193189, implicating the role of BMP/Smad signaling pathway in exosome-induced osteogenesis [61]. Indeed, the identification of multiple signaling pathways activated in exosome-induced osteogenesis and bone regeneration is not surprising and could be attributed to the rich protein cargo of MSC exosomes carrying >850 proteins including transforming growth factor-β, insulin growth factor, platelet-derived growth factor and growth differentiation factor [28,67,68].
3.13. Angiogenesis
Osteogenesis is closely related to vascularization through communication between vascular endothelial cells and osteoblasts, and adequate vascularization is critical for promoting bone regeneration. Twelve studies investigated the effects of MSC exosomes on angiogenesis and observed positive correlation with enhanced osteogenesis during bone regeneration [42,46,48,[50], [51], [52], [53], [54], [55], [56],60,61]. Angiogenic factors including VEGF and hypoxia-inducible factor-1α (HIF-1α) were found elevated along with increase in CD31+ endothelial cells and vessel formation that led to more new bone formation at the defect site [48,52,55,56,61].
The role of HIF-1α/VEGF pathway is well-established in angiogenesis. On this note, HIF-1α and VEGF were identified as important angiogenic regulators of MSC exosomes in bone regeneration [46,55,60]. Zhang et al. demonstrated that human umbilical cord MSC exosomes enhanced proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVECs). These angiogenic effects of MSC exosomes on HUVECs were abrogated by siRNA silencing of HIF-1α [60]. To enhance the angiogenic effects of MSC exosomes, Li et al. showed that HIF-1α overexpressing MSC exosomes had a relatively higher level of exosomal HIF-1α protein and were more potent than the wild-type MSC exosomes in enhancing osteogenic differentiation of bone marrow MSCs, as well as migration and tube formation of HUVECs [46]. In a rabbit model of osteonecrosis of the femoral head, animals treated with HIF-1α overexpressing MSC exosomes had enhanced trabecular tissue regeneration with more newly formed cartilage and bone, lesser necrotic and adipose tissues, and higher density of CD31+ microvessels, as compared with rabbits treated with wild-type MSC exosomes [46]. Separately, Takeuchi et al. [55] investigated the role of VEGF in MSC exosome-induced angiogenesis and noted that while rats treated with exosomes had increased amounts of newly formed bone, rats treated with exosomes and angiogenesis inhibitor (anti-VEGFA antibody) had significantly decreased amounts of new bone formed, which was even lower than rats treated with PBS [55]. Evidently, in the presence of anti-VEGFA, there was suppressed angiogenesis with decreased numbers of VEGF+ and CD31+ endothelial cells, reduced mobilization of endogenous MSCs as detected by CD44 and reduced bone formation [55]. Collectively, these findings suggest the role of HIF-1α and VEGF in angiogenesis critical for effective bone regeneration [46,55,60].
MicroRNAs such as miR-126 and miR-224-3p have also reportedly regulated the angiogenic effects of MSC exosomes in bone regeneration [53,56]. For instance, Liu et al. identified miR-126 as a candidate molecule mediating the angiogenic effects of MSC exosomes in bone regeneration [53]. In their study, miR-126 was upregulated in exosomes possibly through the actions of HIF-1α in human umbilical cord MSCs cultured in hypoxic (1% O2) condition [53]. Relative to exosomes from MSCs cultured in normoxic (21% O2) condition, hypoxic MSC exosomes enriched with miR-126 demonstrated a more potent angiogenic effect in bone regeneration, with increased numbers of proliferative Ki67+CD31+ endothelial cells and blood vessels and more callus formation in a mouse femoral fracture model [53]. Inhibition of miR-126 was found to partially abolish these angiogenic effects of hypoxic MSC exosomes in bone regeneration [53]. In another study, Xu et al. found another molecule miR-224-3p present in bone marrow MSC exosomes to be downregulated in osteonecrosis of the femoral head [56]. In that study, MSC exosomes with miR-224-3p inhibition ameliorated osteonecrosis by enhancing angiogenesis, coupled with upregulation of focal adhesion kinase family interacting protein of 200 kDa (FIP200) and VEGF. Consistently, downregulated miR-224-3p expression enhanced proliferation, migration, and tube formation of HUVECs through upregulation of FIP200 [56].
4. Discussion
The principal finding of this systematic review is that MSC exosomes are therapeutically efficacious in bone regeneration. This therapeutic efficacy of MSC exosomes in bone regeneration is demonstrated by the formation of new bone with supporting vasculature and improved morphological, biomechanical, and histological outcomes, in the studies reviewed [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
Six different animal models of bone defects and diseases were used in these studies to evaluate the therapeutic efficacy of MSC exosomes for bone regeneration. These included animal models of calvaria defects, fractures, distraction osteogenesis, osteoporosis, osteonecrosis, and irradiation-induced bone loss that reflect the various clinical conditions that could result in bone defects or bone loss, including trauma, degeneration, congenital deformities, tumor resections and idiopathic conditions [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. Through this systematic review, it was noted that irrespective of the models used, MSC exosomes were found to exhibit prolific therapeutic efficacy in bone regeneration for the various bone defects and diseases, and no adverse effects associated with the use of MSC exosomes were reported from those studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
Despite the therapeutic efficacy of MSC exosomes, several factors/variables have been identified to likely influence the outcome of intervention with the use of MSC exosomes for bone regeneration. These factors included primarily the source and dosage/concentration of MSC exosomes. With regards to the source of exosomes, the anatomical origin and developmental age of the tissues for isolation of MSCs reportedly influenced the potency of MSC exosomes for bone regeneration [49,57]. The culture condition of MSCs also affects the potency of isolated exosomes. By means of preconditioning with small molecules such as dimethyloxaloylglycine [50] or hypoxia stimulation [53], these studies demonstrated that such culture manipulation of MSCs could enhance the potency of MSC exosomes in angiogenesis and henceforth promote bone regeneration. Alternatively, genetic manipulation/modification of MSCs by overexpressing certain factors such as HIF-1α [46] or miRNAs such as miR-375 [41], and miR-122-5p [51] also proved to be a viable strategy to investigate the role of the specific factor or miRNA, in efforts to engineer MSC exosomes to possess the desired therapeutic properties for bone regeneration.
The dosage/concentration of MSC exosomes represented another factor or variable that would need to be considered and ideally tested. Although higher concentrations of MSC exosomes were demonstrated to be more efficacious in enhancing bone regeneration [52,54,59], the optimal concentration of exosomes remains to be determined. Similarly, the use of scaffold, delivery route, and frequency of treatment were factors/variables identified that would likely influence the therapeutic outcome of MSC exosomes in bone regeneration and need to be carefully considered, if not ideally tested. However, none of the studies have systematically evaluated these variables to determine the optimal scaffold, delivery route, and/or frequency of exosome treatment, which would likely require optimization for a specific bone defect/disease.
While the exact mechanisms by which MSC exosomes exerted their therapeutic effects for bone regeneration remain to be elucidated, it could be noted from this systematic review of 23 studies that MSC exosomes work through a multifaceted mechanism in bone regeneration by enhancing cell survival, proliferation, and migration, and promoting osteogenesis and angiogenesis (Fig. 4) [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. This multifaceted mechanism of action of MSC exosomes in bone regeneration could be attributed to the rich diverse cargo of nucleic acids, proteins, and lipids [67,69]. Given the well-established immunomodulatory properties of MSCs, previous transcriptomics and proteomics profiling of MSC EVs/exosomes have also revealed an enrichment of growth factors and cytokines involved in immunomodulation [[70], [71], [72], [73]]. However, in the 23 studies reviewed [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]], none have examined the immunomodulatory effects of MSC exosomes on bone regeneration. This clearly reflects the nascent state of our understanding of the therapeutic mechanisms of MSC exosomes, particularly their immunomodulatory activities and mechanisms in bone repair and regeneration, that would require further investigation.
Fig. 4.
MSC exosomes alleviate the pathological processes in bone injury/disease to promote regeneration through a multifaceted mechanism of enhancing cell survival, proliferation and migration, and promoting osteogenesis and angiogenesis. MSC, mesenchymal stem cell.
Indeed, it could be concluded that while MSC exosomes were promising therapeutic agents, the research on the efficacy of MSC exosomes on bone regeneration is still in its infancy. All the studies included in the review were conducted on small animals including rats, mice, and rabbits, and none of the studies have managed to progress to large animal studies [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. The identification of the key factors/variables that could influence the therapeutic outcome of MSC exosomes and the improved understanding of the mechanism of action of these therapeutic exosomes on bone regeneration, as summarized in this timely review, would undoubtedly shed light to facilitate the planning and execution of further studies to advance the research in this field [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
Additionally, the review has identified that despite the presence of guidelines intended to improve the reporting of research using animals, such as the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, the reporting of the methodology in many of the studies could still be greatly improved [66,74]. This was because while most of the studies included in the review appeared to be methodologically sound, the SYRCLE risk of bias assessment tool had classified all the studies to have unclear risk for some of the domains analyzed owing to the lack of proper reporting of the methodology in the publications [66,74]. The review then highlighted and emphasized the importance of the adherence to the ARRIVE guidelines in future publications to improve the credibility and reliability of these studies and to allow other researchers to stand on the shoulders of previous researchers to advance the field of MSC exosomes in bone regeneration.
Our systematic review faced several limitations. First, the level of evidence of the review was limited by the quality of the studies included. The lack of proper documentation of the methodology of the studies, including the randomization process, the housing environment of the animals, the allocation concealment, and the blinding for performance and outcome assessment had rendered the studies to be at risk of bias according to the SYRCLE risk of bias assessment tool. As such, more detailed documentation of the methodology should be performed in future studies to improve the credibility and reliability of the studies. Second, the power of the review was also limited by the fact that the research on therapeutic efficacy of MSC exosomes for bone regeneration was still in its infancy, as the studies are largely limited to small animal models. Further efforts to progress to clinically relevant large animal models and ultimately to clinical studies would therefore be useful to advance the field of MSC exosomes for bone regeneration. Third, there was insufficient evidence across studies to support a particular source and/or concentration of exosomes, or a specific scaffold and/or delivery route over the others, that would likely depend on the specific bone/disease indication, and further studies would need to be performed to address these factors/variables. Finally, the relative heterogeneity in the assessment and reporting of outcomes in the various studies precluded more rigorous analysis of the studies. Owing to the lack of uniform reporting of the outcomes, especially quantitatively, further analysis in the form of pooling of the results via meta-analysis could not be performed in the current review. Therefore, it would be vital for further efforts to be made to standardize the reporting of the methodology and outcomes of these studies, so that more robust conclusions can be drawn from the studies.
5. Conclusion
In this review, we systematically assessed the existing preclinical animal studies and broadly demonstrated the effectiveness of MSC exosomes for bone regeneration in animal models of bone defects and diseases such as osteonecrosis and osteoporosis. The findings obtained support the basis for clinical translation of MSC exosomes as a ready-to-use cell-free MSC therapeutic for bone regeneration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
This work was supported by grants from the National University of Singapore (R221000114114) and National Medical Research Council Singapore (NMRC/CIRG/1480/2017 and MOH-CNIG17nov-0002).
Contributor Information
S.H.S. Tan, Email: sharon_sh_tan@nuhs.edu.sg.
J.R.Y. Wong, Email: wmapjwo@ucl.ac.uk.
S.J.Y. Sim, Email: zchajys@ucl.ac.uk.
C.K.E. Tjio, Email: calvin.tjio@mohh.com.sg.
K.L. Wong, Email: francis_kl_wong@nuhs.edu.sg.
J.R.J. Chew, Email: dencrjj@nus.edu.sg.
J.H.P. Hui, Email: doshuij@nus.edu.sg.
W.S. Toh, Email: dentohws@nus.edu.sg.
References
- 1.Galen The usefulness of parts of the body. Clin. Orthop. Relat. Res. 1997:3–12. doi: 10.1097/00003086-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Grabowski P. Physiology of bone. Endocr. Dev. 2015;28:33–55. doi: 10.1159/000380991. [DOI] [PubMed] [Google Scholar]
- 4.Walmsley G.G., Ransom R.C., Zielins E.R., Leavitt T., Flacco J.S., Hu M.S., Lee A.S., Longaker M.T., Wan D.C. Stem cells in bone regeneration. Stem Cell Rev. Rep. 2016;12:524–529. doi: 10.1007/s12015-016-9665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W., Doubeni C.A., Epling J.W., Jr., Kemper A.R., Kubik M., Landefeld C.S., Mangione C.M., Phipps M.G., Pignone M., Silverstein M., Simon M.A., Tseng C.W., Wong J.B. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. J. Am. Med. Assoc. 2018;319:2521–2531. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 6.Ekegren C.L., Edwards E.R., de Steiger R., Gabbe B.J. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int. J. Environ. Res. Publ. Health. 2018;15:2845. doi: 10.3390/ijerph15122845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson A.J., Mont M.A., Tsao A.K., Jones L.C. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin. Orthop. Relat. Res. 2014;472:617–623. doi: 10.1007/s11999-013-3220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson A.H.R.W., Robiati L., Jalal M.M.K., Tsang S.T.J. Non-union: indications for external fixation. Injury. 2019;50(Suppl 1):S73–S78. doi: 10.1016/j.injury.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 9.Birch J.G. A brief history of limb lengthening. J. Pediatr. Orthop. 2017;37(Suppl 2):S1–S8. doi: 10.1097/BPO.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari M., Swiontkowski M. Management of acute hip fracture. N. Engl. J. Med. 2017;377:2053–2062. doi: 10.1056/NEJMcp1611090. [DOI] [PubMed] [Google Scholar]
- 11.Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European union: medical management, epidemiology and economic burden. A report prepared in collaboration with the international osteoporosis foundation (IOF) and the European federation of pharmaceutical industry associations (EFPIA) Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hak D.J., Fitzpatrick D., Bishop J.A., Marsh J.L., Tilp S., Schnettler R., Simpson H., Alt V. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald A.S., Boden S.D., Goldberg V.M., Khan Y., Laurencin C.T., Rosier R.N., American Academy of Orthopaedic Surgeons The committee on biological implants, bone-graft substitutes: facts, fictions, and applications. J. Bone Jt. Surg. Am. 2001;83-A(Suppl 2 Pt 2):98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 15.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. Am. 2002;84:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Sohn H.S., Oh J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019;23:9. doi: 10.1186/s40824-019-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernigou P., Auregan J.C., Dubory A., Flouzat-Lachaniette C.H., Chevallier N., Rouard H. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int. Orthop. 2018;42:2563–2571. doi: 10.1007/s00264-018-3916-9. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P., Dubory A., Homma Y., Guissou I., Flouzat Lachaniette C.H., Chevallier N., Rouard H. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int. Orthop. 2018;42:1639–1649. doi: 10.1007/s00264-018-3941-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Qu Z., Yin X., Shang C., Ao Q., Gu Y., Liu Y. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: a three-year follow-up study. Mol. Med. Rep. 2016;14:4209–4215. doi: 10.3892/mmr.2016.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebergall M., Schroeder J., Mosheiff R., Gazit Z., Yoram Z., Rasooly L., Daskal A., Khoury A., Weil Y., Beyth S. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol. Ther. 2013;21:1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo-Cardiel G., López-Echaury A.C., Saucedo-Ortiz J.A., Fuentes-Orozco C., Michel-Espinoza L.R., Irusteta-Jiménez L., Salazar-Parra M., González-Ojeda A. Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent. Traumatol. 2017;33:38–44. doi: 10.1111/edt.12303. [DOI] [PubMed] [Google Scholar]
- 23.Tan S.S.H., Tjio C.K.E., Wong J.R.Y., Wong K.L., Chew J.R.J., Hui J.H.P., Toh W.S. Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Eng. B Rev. 2020 doi: 10.1089/ten.TEB.2019.0326. [DOI] [PubMed] [Google Scholar]
- 24.Meirelles L. da S., da Silva Meirelles L., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Hofer H.R., Tuan R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016;7:131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaw S.Y.M., Kaneko T., Zaw Z.C.T., Sone P.P., Murano H., Gu B., Okada Y., Han P., Katsube K.I., Okiji T. Crosstalk between dental pulp stem cells and endothelial cells augments angiogenic factor expression. Oral Dis. 2020 doi: 10.1111/odi.13341. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Wang B., Sun Y., Wu T., Liu Y., Zhang J., Lee W.Y., Pan X., Chai Y., Li G. Human fetal mesenchymal stem cell secretome enhances bone consolidation in distraction osteogenesis. Stem Cell Res. Ther. 2016;7:134. doi: 10.1186/s13287-016-0392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toh W.S., Lai R.C., Zhang B., Lim S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018;46:843–853. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai R.C., Arslan F., Lee M.M., Sze N.S.K., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M., Pasterkamp G., de Kleijn D.P.V., Lim S.K. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Tan C.Y., Lai R.C., Wong W., Dan Y.Y., Lim S.K., Ho H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu B., Shao H., Su C., Jiang Y., Chen X., Bai L., Zhang Y., Li Q., Zhang X., Li X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016;6:34562. doi: 10.1038/srep34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G., Sdrimas K., Fernandez-Gonzalez A., Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B., Yeo R.W.Y., Lai R.C., Sim E.W.K., Chin K.C., Lim S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy. 2018;20:687–696. doi: 10.1016/j.jcyt.2018.02.372. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Yin Y., Lai R.C., Tan S.S., Choo A.B.H., Lim S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cell. Dev. 2014;23:1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N., Akimoto T., Higashi Y., Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Bier P. Berenstein, Kronfeld N., Morgoulis D., Ziv-Av A., Goldstein H., Kazimirsky G., Cazacu S., Meir R., Popovtzer R., Dori A., Brodie C. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018;174:67–78. doi: 10.1016/j.biomaterials.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Wong K.L., Zhang S., Wang M., Ren X., Afizah H., Lai R.C., Lim S.K., Lee E.H., Hui J.H.P., Toh W.S. Intra-articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model. Arthroscopy. 2020 doi: 10.1016/j.arthro.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H.P., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Chen S., Tang Y., Liu Y., Zhang P., Lv L., Zhang X., Jia L., Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52 doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N., Miyado K., Higashi Y., Ochi M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016;5:1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo S.C., Tao S.C., Yin W.J., Qi X., Sheng J.G., Zhang C.Q. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int. J. Biol. Sci. 2016;12:1262–1272. doi: 10.7150/ijbs.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia Y., Qiu S., Xu J., Kang Q., Chai Y. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcif. Tissue Int. 2020;106:509–517. doi: 10.1007/s00223-019-00656-4. [DOI] [PubMed] [Google Scholar]
- 45.Kuang M.J., Huang Y., Zhao X.G., Zhang R., Ma J.X., Wang D.C., Ma X.L. Exosomes derived from Wharton's jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int. J. Biol. Sci. 2019;15:1861–1871. doi: 10.7150/ijbs.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Liu D., Li C., Zhou S., Tian D., Xiao D., Zhang H., Gao F., Huang J. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017;41:1379–1390. doi: 10.1002/cbin.10869. [DOI] [PubMed] [Google Scholar]
- 47.Li W., Liu Y., Zhang P., Tang Y., Zhou M., Jiang W., Zhang X., Wu G., Zhou Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018;10:5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- 48.Li R., Chen C., Zheng R.Q., Zou L., Hao G.L., Zhang G.C. Influences of hucMSC-exosomes on VEGF and BMP-2 expression in SNFH rats. Eur. Rev. Med. Pharmacol. Sci. 2019;23:2935–2943. doi: 10.26355/eurrev_201904_17573. [DOI] [PubMed] [Google Scholar]
- 49.Li X., Zheng Y., Hou L., Zhou Z., Huang Y., Zhang Y., Jia L., Li W. Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs. Oral Dis. 2020;26:131–144. doi: 10.1111/odi.13202. [DOI] [PubMed] [Google Scholar]
- 50.Liang B., Liang J.M., Ding J.N., Xu J., Xu J.G., Chai Y.M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019;10:335. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao W., Ning Y., Xu H.J., Zou W.Z., Hu J., Liu X.Z., Yang Y., Li Z.H. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin. Sci. 2019;133:1955–1975. doi: 10.1042/CS20181064. [DOI] [PubMed] [Google Scholar]
- 52.Liu X., Li Q., Niu X., Hu B., Chen S., Song W., Ding J., Zhang C., Wang Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017;13:232–244. doi: 10.7150/ijbs.16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W., Li L., Rong Y., Qian D., Chen J., Zhou Z., Luo Y., Jiang D., Cheng L., Zhao S., Kong F., Wang J., Zhou Z., Xu T., Gong F., Huang Y., Gu C., Zhao X., Bai J., Wang F., Zhao W., Zhang L., Li X., Yin G., Fan J., Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., Hu B., Wang Y., Li X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016;12:836–849. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeuchi R., Katagiri W., Endo S., Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H.J., Liao W., Liu X.Z., Hu J., Zou W.Z., Ning Y., Yang Y., Li Z.H. Down-regulation of exosomal microRNA-224-3p derived from bone marrow-derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. Faseb. J. 2019;33:8055–8068. doi: 10.1096/fj.201801618RRR. [DOI] [PubMed] [Google Scholar]
- 57.Xu T., Luo Y., Wang J., Zhang N., Gu C., Li L., Qian D., Cai W., Fan J., Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J. Nanobiotechnol. 2020;18:47. doi: 10.1186/s12951-020-00601-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang B.C., Kuang M.J., Kang J.Y., Zhao J., Ma J.X., Ma X.L. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 2020;524:883–889. doi: 10.1016/j.bbrc.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016;7:136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Hao Z., Wang P., Xia Y., Wu J., Xia D., Fang S., Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52 doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L., Jiao G., Ren S., Zhang X., Li C., Wu W., Wang H., Liu H., Zhou H., Chen Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020;11:38. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou J., Liu H.X., Li S.H., Gong Y.S., Zhou M.W., Zhang J.H., Zhu G.Y. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4954–4960. doi: 10.26355/eurrev_201906_18086. [DOI] [PubMed] [Google Scholar]
- 63.Zuo R., Liu M., Wang Y., Li J., Wang W., Wu J., Sun C., Li B., Wang Z., Lan W., Zhang C., Shi C., Zhou Y. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res. Ther. 2019;10:30. doi: 10.1186/s13287-018-1121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N.N., Baxter A.A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A.C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borràs F.E., Bosch S., Boulanger C.M., Breakefield X., Breglio A.M., Brennan M.Á., Brigstock D.R., Brisson A., Broekman M.L., Bromberg J.F., Bryl-Górecka P., Buch S., Buck A.H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzás E.I., Byrd J.B., Camussi G., Carter D.R., Caruso S., Chamley L.W., Chang Y.T., Chen C., Chen S., Cheng L., Chin A.R., Clayton A., Clerici S.P., Cocks A., Cocucci E., Coffey R.J., Cordeiro-da-Silva A., Couch Y., Coumans F.A., Coyle B., Crescitelli R., Criado M.F., D'Souza-Schorey C., Das S., Datta Chaudhuri A., de Candia P., De Santana E.F., De Wever O., Del Portillo H.A., Demaret T., Deville S., Devitt A., Dhondt B., Di Vizio D., Dieterich L.C., Dolo V., Dominguez Rubio A.P., Dominici M., Dourado M.R., Driedonks T.A., Duarte F.V., Duncan H.M., Eichenberger R.M., Ekström K., El Andaloussi S., Elie-Caille C., Erdbrügger U., Falcón-Pérez J.M., Fatima F., Fish J.E., Flores-Bellver M., Försönits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gámez-Valero A., Gardiner C., Gärtner K., Gaudin R., Gho Y.S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D.C., Görgens A., Gorski S.M., Greening D.W., Gross J.C., Gualerzi A., Gupta G.N., Gustafson D., Handberg A., Haraszti R.A., Harrison P., Hegyesi H., Hendrix A., Hill A.F., Hochberg F.H., Hoffmann K.F., Holder B., Holthofer H., Hosseinkhani B., Hu G., Huang Y., Huber V., Hunt S., Ibrahim A.G.E., Ikezu T., Inal J.M., Isin M., Ivanova A., Jackson H.K., Jacobsen S., Jay S.M., Jayachandran M., Jenster G., Jiang L., Johnson S.M., Jones J.C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S.I., Kaur S., Kawamura Y., Keller E.T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K.P., Kislinger T., Klingeborn M., Klinke D.J., 2nd, Kornek M., Kosanović M.M., Kovács Á.F., Krämer-Albers E.M., Krasemann S., Krause M., Kurochkin I.V., Kusuma G.D., Kuypers S., Laitinen S., Langevin S.M., Languino L.R., Lannigan J., Lässer C., Laurent L.C., Lavieu G., Lázaro-Ibáñez E., Le Lay S., Lee M.S., Lee Y.X.F., Lemos D.S., Lenassi M., Leszczynska A., Li I.T., Liao K., Libregts S.F., Ligeti E., Lim R., Lim S.K., Linē A., Linnemannstöns K., Llorente A., Lombard C.A., Lorenowicz M.J., Lörincz Á.M., Lötvall J., Lovett J., Lowry M.C., Loyer X., Lu Q., Lukomska B., Lunavat T.R., Maas S.L., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E.S., Martin-Jaular L., Martinez M.C., Martins V.R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L.K., McVey M.J., Meckes D.G., Jr., Meehan K.L., Mertens I., Minciacchi V.R., Möller A., Møller Jørgensen M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D.C., Myburgh K.H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J.P., Nolte-’t Hoen E.N., Noren Hooten N., O'Driscoll L., O'Grady T., O'Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L.A., Osteikoetxea X., Østergaard O., Ostrowski M., Park J., Pegtel D.M., Peinado H., Perut F., Pfaffl M.W., Phinney D.G., Pieters B.C., Pink R.C., Pisetsky D.S., Pogge von Strandmann E., Polakovicova I., Poon I.K., Powell B.H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R.L., Raimondo S., Rak J., Ramirez M.I., Raposo G., Rayyan M.S., Regev-Rudzki N., Ricklefs F.L., Robbins P.D., Roberts D.D., Rodrigues S.C., Rohde E., Rome S., Rouschop K.M., Rughetti A., Russell A.E., Saá P., Sahoo S., Salas-Huenuleo E., Sánchez C., Saugstad J.A., Saul M.J., Schiffelers R.M., Schneider R., Schøyen T.H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G.V., Shetty A.K., Shiba K., Siljander P.R.M., Silva A.M., Skowronek A., Snyder O.L., 2nd, Soares R.P., Sódar B.W., Soekmadji C., Sotillo J., Stahl P.D., Stoorvogel W., Stott S.L., Strasser E.F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W.S., Tomasini R., Torrecilhas A.C., Tosar J.P., Toxavidis V., Urbanelli L., Vader P., van Balkom B.W., van der Grein S.G., Van Deun J., van Herwijnen M.J., Van Keuren-Jensen K., van Niel G., van Royen M.E., van Wijnen A.J., Vasconcelos M.H., Vechetti I.J., Jr., Veit T.D., Vella L.J., Velot É., Verweij F.J., Vestad B., Viñas J.L., Visnovitz T., Vukman K.V., Wahlgren J., Watson D.C., Wauben M.H., Weaver A., Webber J.P., Weber V., Wehman A.M., Weiss D.J., Welsh J.A., Wendt S., Wheelock A.M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X., Yáñez-Mó M., Yin H., Yuana Y., Zappulli V., Zarubova J., Žėkas V., Zhang J.Y., Zhao Z., Zheng L., Zheutlin A.R., Zickler A.M., Zimmermann P., Zivkovic A.M., Zocco D., Zuba-Surma E.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witwer K.W., Van Balkom B.W.M., Bruno S., Choo A., Dominici M., Gimona M., Hill A.F., De Kleijn D., Koh M., Lai R.C., Mitsialis S.A., Ortiz L.A., Rohde E., Asada T., Toh W.S., Weiss D.J., Zheng L., Giebel B., Lim S.K. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai R.C., Tan S.S., Teh B.J., Sze S.K., Arslan F., de Kleijn D.P., Choo A., Lim S.K. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Chen T.S., Lai R.C., Lee M.M., Choo A.B., Lee C.N., Lim S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silvestro S., Chiricosta L., Gugliandolo A., Pizzicannella J., Diomede F., Bramanti P., Trubiani O., Mazzon E. Extracellular vesicles derived from human gingival mesenchymal stem cells: a transcriptomic analysis. Genes. 2020;11:118. doi: 10.3390/genes11020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angulski A.B., Capriglione L.G., Batista M., Marcon B.H., Senegaglia A.C., Stimamiglio M.A., Correa A. The protein content of extracellular vesicles derived from expanded human umbilical cord blood-derived CD133+ and human bone marrow-derived mesenchymal stem cells partially explains why both sources are advantageous for regenerative medicine. Stem Cell Rev. Rep. 2017;13:244–257. doi: 10.1007/s12015-016-9715-z. [DOI] [PubMed] [Google Scholar]
- 72.Eirin A., Zhu X.Y., Puranik A.S., Woollard J.R., Tang H., Dasari S., Lerman A., van Wijnen A.J., Lerman L.O. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toh W.S., Zhang B., Lai R.C., Lim S.K. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20:1419–1426. doi: 10.1016/j.jcyt.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. National centre for the replacement, refinement and reduction of animals in research, animal research: reporting in vivo experiments--the ARRIVE guidelines. J. Cerebr. Blood Flow Metabol. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]