Abstract
This study aimed to conduct a systematic review to sum up evidence of the associations between different aspects of night shift work and female breast cancer using a dose–response meta-analysis approach.
We systematicly searched all cohort and case–control studies published in English on MEDLINE, Embase, PSYCInfo, APC Journal Club and Global Health, from January 1971 to May 2013. We extracted effect measures (relative risk, RR; odd ratio, OR; or hazard ratio, HR) from individual studies to generate pooled results using meta-analysis approaches. A log-linear dose–response regression model was used to evaluate the relationship between various indicators of exposure to night shift work and breast cancer risk. Downs and Black scale was applied to assess the methodological quality of included studies.
Ten studies were included in the meta-analysis. A pooled adjusted relative risk for the association between ‘ever exposed to night shift work’ and breast cancer was 1.19 [95% confidence interval (CI) 1.05–1.35]. Further meta-analyses on dose–response relationship showed that every 5-year increase of exposure to night shift work would correspondingly enhance the risk of breast cancer of the female by 3% (pooled RR = 1.03, 95% CI 1.01–1.05; Pheterogeneity < 0.001). Our meta-analysis also suggested that an increase in 500-night shifts would result in a 13% (RR = 1.13, 95% CI 1.07–1.21; Pheterogeneity = 0.06) increase in breast cancer risk.
This systematic review updated the evidence that a positive dose–response relationship is likely to present for breast cancer with increasing years of employment and cumulative shifts involved in the work.
Keywords: breast cancer, case–control studies, cohort studies, dose–response relationship, meta-analysis, shift work
introduction
Breast cancer is the leading cancer incidence and mortality among women in both developed and developing countries [1, 2]. The incidence rate increased rapidly in Asian countries during the past two decades, although, it decline in North America [3]. This rising trend among Asian women was considered to be in relation with ‘westernization’ of lifestyle, in which endogenous and exogenous female sex hormones exposure, alcohol drinking, physical activities reduction, and adiposity were the primary causes [4]. These well-known risk factors, together with genetic susceptibilities (such as family history of breast and ovarian cancer, BRCA1 and BRCA2 mutations), contributed to only 41% of the breast cancer risk in the female population [5, 6], and the majority fractions of breast cancer etiology remains poorly understood. A recent research showed that the high mammographic density might contribute 16% of breast cancers risk [7]; however, the mammographic density has been documented to be altered by many risk factors (e.g. age, body mass index, parity, menopause, and sex hormones) and its independent role on the breast cancer etiology has yet been well established.
Meanwhile, shift work as a popular job nature in the general population has attracted growing attentions from the public regarding its carcinogenesis. In 2007, the International Agency for Research on Cancer (IARC) classified ‘shift work that involves circadian disruption’ as probably carcinogenic to human (Group 2A) based on sufficient evidence from experimental study but limited evidence on epidemiological studies [8]. Shift work, essential to be part of some occupation, such as healthcare, transportations, and services, is becoming a more common practice among different sectors. It is reported that in 2004–2005, the prevalence of shift work among the US female working population was 12.4% and it was 17.4% in European countries, while the trend is increasing.
Epidemiological studies published after 2007 reported positive association between night shift work and female breast cancer, but statistically insignificant associations were frequently seen in many individual studies owing to limited power, and some studies even presented with conflicting findings [9, 10]. Two meta-analyses were published in the past 10 years and the most recent one was published in 2013, with pooled adjusted risk estimates of 1.48 [95% confidence interval (CI) 1.36–1.61, 1960–2005] [11] and 1.20 (95% CI 1.08–1.33, 1980–2012) [12], for the association between ‘ever worked in night shifts’ and breast cancer. None of the previous meta-analyses of breast cancer studies attempted to summarize evidence according to the frequency, duration, or cumulative amount of night shift work. This study aimed to conduct a systematic review to sum up evidence of the associations between different aspects of night shift work and female breast cancer using a dose–response meta-analysis approach.
methods
search strategy and eligible studies
We systematically sought literatures in Medline, Embase, Global Health, ACP Journal Club, and PsycINFO to identify all cohort and case–control studies published during January 1971 to May 2013 according to the search strategies as described in supplementary file 1, available at Annals of Oncology online.
Only English articles were included into this review. Articles were considered to be ineligible when the reported risk estimates for female was not separated from that for the male. Studies in which breast cancer was not separated from other types of cancer were also excluded. For the purpose of the current meta-analysis, we also excluded studies with less than three levels of night shift exposure to ensure an implementation of dose–response analysis using meta-regression [13, 14].
types of outcome measurements
We extracted three indicators (employment years of night shift work, cumulative work nights, and frequency of night shift work per month) to describe the exposure of night shift. We also extracted the number of incident cases of breast cancer, the effect measures and the 95% CI from each eligible study. Effect measures for this review included hazard ratio (HR), relative risk (RR), and odds ratio (OR). We recorded all types of confounding factors for each adjusted HR, RR, or OR.
quality assessment
The quality of included articles was assessed according to the checklist developed by Downs and Black [15]. There were totally 27 items in the checklist distributed among five subscales: reporting (10 items), external validity (3 items), bias (7 items), confounding (6 items), and power (1 item). Most of the questions scored 0 or 1, while only one item regarding Reporting had a 0–2 score and another item on Power which scored 0–5. The maximum possible score of each article is 32 based on this checklist. In this review, the quality of a study was determined by comparing its score with the median score calculated for all included studies. A ‘better quality’ study referred to a score greater than or equal to the median; otherwise, the study was deemed ‘low quality’. All studies were reviewed by the two reviewers (KY and FW), independently. There was no obvious difference between the judgments of these two reviewers concerning the quality of individual studies.
statistical analysis
We used the generalized least-squares trend (GLST) model proposed by Greenland and Longnecker [13] and Orsini et al. [14] to compute the trend of effect measure (HR, RR, or OR) across ordinal level of night shift work. This method required the effect measure with its variance estimate for at least three known categories of exposure. RR from individual studies and corresponding s.e. (derived from the CIs) were transformed to their natural logarithms to stabilize the variance and normalize the distributions. For each study, we assigned the midpoint of the upper and lower boundaries in each category as the average of night shift work exposure. If the upper boundary of the highest category was not provided, it was then assigned by 125% of the lower boundary. We used two-stage random-effect model to estimate the pooled dose–response effect of night shift work and breast cancer risk and explored potential heterogeneity using meta-regression. Random effect model was applied to the pooled effect if I2 index for heterogeneity test was larger than 50%; otherwise, fixed effect model was carried out. Publication bias was evaluated with Egger's regression asymmetry test [16]. Statistical analyses were conducted with Stata 11.0 (StataCorp, College Station, TX).
results
description of studies
We identified 10 eligible articles from the database (Figure 1), including three cohort studies [10, 17, 18], three nested case–control studies [19., 20., 21.] and four case–control studies [9, 22., 23., 24.]. The main characteristics of these 10 eligible studies are summarized in Table 1, five of them focused on nursing occupation [17, 18, 20, 21, 23], one study concerned about the female military employees [19], while the occupations for the remaining four studies were mixed [9, 10, 22, 24].
Figure 1.

Flow chart of articles' selection.
Table 1.
Characteristics of three cohort and seven case–control studies included in meta-analysis
| Studies, location | Source of participants | Definition of night shift | Adjusted confounders | Night shift work |
Score | ||
|---|---|---|---|---|---|---|---|
| Exposure years | Number of cases | Relative risk (95% CI) | |||||
| Cohort study | |||||||
| Schernhammer et al. [18], USA | Nurses' Health Study I | Rotating night shifts with at least three nights per month | Age, age at menarche, parity, age at first birth, weight change between age 18 years and menopause, body mass index, family history of breast cancer, benign breast disease, oral contraceptive use, current alcohol consumption, age at menopause, use of postmenopausal hormones, height | None | 925 | 1.00 | 17 |
| 1–14 | 1324 | 1.08 (0.99–1.18) | |||||
| 15–29 | 134 | 1.08 (0.90–1.30) | |||||
| ≥30 | 58 | 1.35 (1.04–1.78) | |||||
| Schernhammer et al. [17], USA | Nurses' Health Study II | Rotating night shifts with at least three nights per month | Age, age at menarche, menopausal status, age at menopause, age at first birth combined, parity, body mass index, current alcohol consumption, oral contraceptive use, use of postmenopausal hormones, smoking status, benign breast disease, family history of breast cancer, physical activity | None | 441 | 1.00 | 17 |
| 1–9 | 816 | 0.98 (0.87–1.10) | |||||
| 10–19 | 80 | 0.91 (0.72–1.16) | |||||
| ≥20 | 15 | 1.79 (1.06–3.01) | |||||
| Pronk et al. [10], Shanghai China | Population Cohort | Starting work after 10 PM at least 3 times a month for over 1 year. | Age, education, family history of breast cancer, number of pregnancy, age at first birth combined, physical activity | None | 423 | 1.0 | 15 |
| 0–14 | 108 | 1.1 (0.9–1.3) | |||||
| 14–25 | 89 | 0.9 (0.7–1.1) | |||||
| >25 | 97 | 1.0 (0.8–1.3) | |||||
| Nested Case–control Study | |||||||
| Lie et al. [21], Norway | Nurses | All work at infirmaries was assumed to include night work. | parity, total employment time | None | 50 | 1.00 | 19 |
| 0–14 | 362 | 0.95 (0.67–1.33) | |||||
| 15–29 | 101 | 1.29 (0.82–2.02) | |||||
| ≥30 | 24 | 2.21 (1.10–4.45) | |||||
| Lie et al. [20], Norway | Nurses | A shift that lasted from at least 12 PM until 6 AM. | Age, parity, family history of breast cancer, current alcohol consumption | None | 102 | 1.0 | 19 |
| 1–14 | 390 | 1.2 (0.9–1.6) | |||||
| 15–29 | 152 | 1.2 (0.9–1.7) | |||||
| ≥30 | 27 | 0.8 (0.5–1.4) | |||||
| Hansen and Lassen [23], Denmark | Military Employees | Working for at least 1 year during hours beginning after 17:00 and ending before 9:00, not including overtime | Age, use of postmenopausal hormones, number of childbirth, age at menarche, education, smoking status | None | 89 | 1.0 | 19 |
| 1–5.9 | 13 | 0.9 (0.4–1.7) | |||||
| 6–14.9 | 18 | 0.7 (0.9–3.2) | |||||
| ≥15 | 12 | 2.1 (1.0–4.5) | |||||
| Case–control Study | |||||||
| Davis et al. [22], USA | Population-based Study | Graveyard shift which began after 7:00 PM and leaving work before 9:00 AM | Parity, family history of breast cancer, oral contraceptive use, use of postmenopausal hormones | None | 733 | 1.0 | 18 |
| <3 | 15 | 1.4 (0.6–3.2) | |||||
| 3–10 | 19 | 1.6 (0.8–3.2) | |||||
| O'Leary et al. [24], USA | Population-based Study | Overnight shift which could start as early as 7:00 p.m. and continue until the following morning | Age, parity, family history of breast cancer, education, benign breast disease | None | 469 | 1.00 | 18 |
| 1–8 | 11 | 0.74 (0.32–1.68) | |||||
| ≥8 | 6 | 0.32 (0.12–0.83) | |||||
| Pesch et al. [9], Germany | Population-based Study | Night work was defined as working the fulltime period between 24:00–05:00 h | Age, family history of breast cancer, use of postmenopausal hormones, number of mammograms | None | 698 | 1.00 | 18 |
| 1–4 | 15 | 0.64 (0.34–1.24) | |||||
| 5–9 | 11 | 0.93 (0.41–2.15) | |||||
| 10–19 | 10 | 0.91 (0.38–2.18) | |||||
| ≥20 | 12 | 2.49 (0.87–7.18) | |||||
| Hansen and Stevens [23], Denmark | Nurses Interview-based Study | Graveyard shifts which worked after midnight (about 8 h of work between 19 and 9) for at least 1 year | Age, weight, use of postmenopausal hormones, age at menarche, menopausal status, menstrual regularity, age at first birth combined | None | 37 | 1.0 | 19 |
| 1–5 | 55 | 1.5 (0.99–2.5) | |||||
| 5–10 | 70 | 2.3 (1.4–3.5) | |||||
| 10–20 | 66 | 1.9 (1.1–2.8) | |||||
| ≥20 | 39 | 2.1 (1.3–3.2) | |||||
assessing quality of studies
Twenty-seven questions included in the Downs and Black checklist were available for quality assessment, however, eight items were not suitable for our review as these eight items were designed for assessing the qualities of randomized, controlled trial (RCT), while our review only included cohort or case–control studies. Therefore, only 19 items were applied to our eligible studies, and 24 is the maximum score which represents the best quality (supplement 2, available at Annals of Oncology online). Among our articles, the total scores ranged from 15 to 19. The median score was 18 while only three articles were having scores below this value. The lower scored articles were all cohort studies, of which loss of follow-up and their characteristics of exposure were less frequently reported. Articles with higher scores tended to taking more consideration on the adjustment of confounding factors. In general, the nested case–control studies had a better quality on average (supplement 3, available at Annals of Oncology online).
a summary of the definition of night shift work and combined association with duration of exposure
The cohort studies about nurses' health were published by Schernhanner et al. in 2001 [18] and 2006 [17], with a definition on night shift workers as those working on rotating night shifts for at least three nights per month on top of their usual working schedules in daytime or evenings in that month. To be eligible, the workers were required to have worked on the shifts for no <6 months. These Nurses' Health Studies (NHS I & II) indicated same positive effect of night shift work over longer exposure duration. The RR were 1.35 and 1.79 for more than 30 and 20 years correspondingly to NHS I and II. Nevertheless, the results from a prospective cohort study among general population led by Pronk et al. [10] did not provide any significant association between breast cancer and increasing years of employment on night shift. In the study of Pronk et al., night shift work was specified a time interval ‘started after 22:00’ for at least three times a month for over 1 year.
The nested case–control studies consisted of two articles by Lie et al. in 2006 [21] and 2011 [20] on Norwegian nurses. In Lie's study in 2006, night shift work was assumed to be included by all work at infirmaries, except for managerial jobs, teaching, and work at the out-patients' or physiotherapy departments, while work site other than infirmaries were also assumed to have daytime work only. In Lie's another study published in 2011, night shift was specified as the shift which lasted through at least 00:00 to 06:00, and the shift that had an earlier start and later end in additional to the required inclusive period were also counted. Lie's study in 2006 showed a highest and significant RR of 2.21 (95% CI 1.10–4.45) for an exposure of more than 30 years; however, conflicting findings were shown in the study in 2011, in which the RR was 0.8 (95% CI 0.5–1.4) for those were in the highest category of exposure (≥30 years). Results of the military employees from Denmark by Hansen and Lassen suggested a significantly increased RR of 2.1 (95% CI 1.0–4.5) for the exposure over 15 years.
Three of the four case–control studies were population-based studies conducted in the general hospital patients. The study of Hansen and Stevens focused on nurses which based on interview [23]. Davis et al. defined night shift work as the graveyard shift which began after 19:00 and left work before 09:00, while the female should worked for at least once per week on this shift [22]. Hansen and Stevens regarded night shift work as the graveyard shift which started after midnight, between 19:00 and 09:00 for around 8 h, and the worker was also required to have worked for at least 1 year. The RR in these two studies showed a generally positive association with the increasing exposure duration to night shift work. In Pesch et al.'s study [9], night shift was defined as working for the fulltime period between 24:00–05:00, which was adopted from the International Labor Organization (ILO) convention on night work [25]. It only identified a positive relationship (RR = 2.49) when exposure duration was longer than 20 years, while a negative association was observed by any other shorter exposure duration. The study by O'Leary et al. considered night shift work as the overnight shifts which started as early as 19:00 and continued until the following morning. It was the only study that concluded a negative association even over a long exposure.
To estimate the association between ever exposure of night shift work and breast cancer risk, a two-stage meta-analysis was carried out and result showed that the adjusted RR was 1.19 (95% CI 1.05–1.35; random effect model) (supplement 4, available at Annals of Oncology online). Further dose–response meta-analyses were conducted separately to analyze the pooled effect of night shift work on the risk of breast cancer regarding duration of exposure in cohort studies, nested case–control studies and case–control studies, and an overall analysis. Figure 2 shows the pooled results from overall meta-analysis, there is a 3% increasing in the risk of breast cancer for each increment of 5-year night shift under random effect model (combined RR = 1.03; 95% CI 1.01–1.05; Pheterogeneity < 0.001). The corresponding pooled RR for case–control studies and cohort studies was 1.06 (95% CI 1.02–1.09; Pheterogeneity = 0.001; seven studies) and 1.02 (95% CI 1.00–1.04; Pheterogeneity = 0.218; three studies). Results from Egger's regression did not suggest significant publication bias (P = 0.365).
Figure 2.
Forest plot of pooled studies for the duration of night shift work. Duration of night shift work means the total years of working schedule with night shift. Unit: 5-years incremental risk.
combined effect of cumulative shift of night work
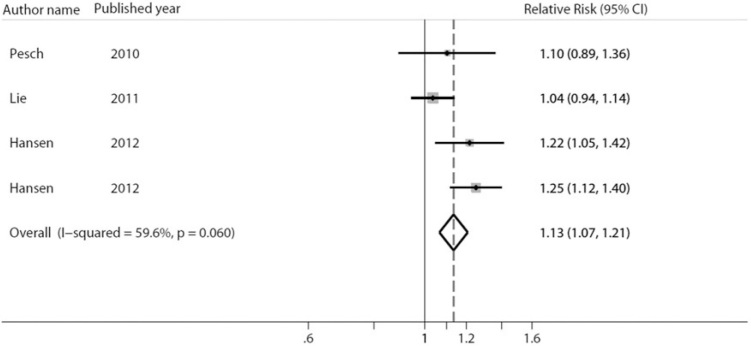
Four included studies provided the effect measures of cumulative night shift work on the risk of breast cancer [9, 19, 20, 23] (Table 2). In general, a positive gradient of breast cancer risk was suggested with the increasing amount of cumulated night shift work, with the exception of the study of Lie et al. A meta-analysis regarding the association with cumulative night shifts revealed a 13% increased risk of breast cancer (RR = 1.13; 95% CI 1.07–1.21; Pheterogeneity = 0.06) by every 500 nights increase of shift work (Figure 3). No evidence of publication bias was presented (P = 0.688).
Table 2.
Relative risks of breast cancer for the frequency and cumulative number of night shift work
| Studies | Frequency of night shift |
Cumulative night shift work |
||||
|---|---|---|---|---|---|---|
| Freq. of shifts | Number of cases | Relative risk (95% CI) | Number of shift | Number of cases | Relative risk (95% CI) | |
| Pesch et al. [9] | NA | NA | NA | None | 698 | 1.00 |
| <1056 | 25 | 0.80 (0.47–1.36) | ||||
| ≥1056 | 20 | 1.66 (0.80–3.46) | ||||
| Hansen and Stevens [23] | NA | NA | NA | None | 37 | 1.00 |
| <468 | 63 | 1.6 (1.0–2.6) | ||||
| 468–1095 | 80 | 2.0 (1.3–3.0) | ||||
| ≥1096 | 87 | 2.2 (1.5–3.2) | ||||
| Pronk et al. [10] | Per month | NA | NA | NA | ||
| None | 276 | 1.0 | ||||
| <8 | 8 | 0.6 (0.3–1.2) | ||||
| =8 | 45 | 0.9 (0.7–1.3) | ||||
| >8 | 20 | 0.9 (0.5–1.3) | ||||
| Lie et al. [20] | Per month | |||||
| None | 102 | 1.0 | None | 102 | 1.00 | |
| <4 | 415 | 1.2 (0.9–1.6) | <1,007 | 396 | 1.2 (0.9–1.6) | |
| ≥4 | 182 | 1.2 (0.8–1.6) | ≥1,007 | 201 | 1.2 (0.9–1.7) | |
| Hansen and Lassen [23] | Per week | |||||
| None | 82 | 1.0 | None | 82 | 1.00 | |
| 1–2 | 15 | 1.0001 (0.5–1.9) | <416 | 9 | 0.8 (0.4–1.9) | |
| ≥3 (1–5.9 years) | 9 | 1.1 (0.5–2.3) | 416–1560 | 14 | 1.4 (0.7–2.9) | |
| ≥3 (6–14.9 years) | 11 | 2.1 (1.0–4.8) | >1560 | 17 | 2.3 (1.2–4.6) | |
| ≥3 (≥15 years) | 9 | 2.5 (1.0–6.6) | ||||
Figure 3.
Forest plot of pooled studies for the cumulative night shifts. Cumulative night shifts means the total days of night work. Unit: 500-night shifts incremental risk.
combined effect for the frequency of night shift work
Three of the included articles provided information on the effect of the frequency of night shift on the risk of breast cancer [10, 19, 20] (Table 2), but the results were inconsistent. Pronk et al. did not observe an association between the frequency of night shift and breast cancer risk, while the gradient tendency was not obvious in Lie's study and only a slightly increased risk was observed in the high-exposure categories (RR = 1.2). A clear exposure–response relationship was indicated by Hansen and Lassen who they observed an increased risk breast cancer was associated with the increasing frequency of night shift, with the highest effect observed in the most intensive group (RR = 2.5, frequency ≥3/week for 15 years or more).
Figure 4 shows the meta-analysis regarding frequency of night shift work, we found a pooled RR of 1.02 (95% CI 0.97–1.09; Pheterogeneity = 0.072) which suggests that an increase of three nights per month would result in a 2% breast cancer increase; nevertheless, the combined effect was only borderline. No publication bias was detected (P = 0.271).
Figure 4.
Forest plot of pooled studies for the frequency of night shift. Frequency of night shift means the days of night work in one month. Unit: 3-night shifts/month incremental risk.
discussion
This meta-analysis regarding the association between night shift work and the risk of breast cancer among female population included 10 observational studies. The dose–response analysis showed a positive gradient of breast cancer risk with the year of night shift work and cumulative night work. Our study renewed the evidence that was not covered by the previous meta-analyses and summarized more up-to-date evidence by introducing the meta-regression on dose–response analysis.
There were three systematic reviews published in 2013 [12], 2008 [6], and 2005 [11] to summarize the evidence about night shift work and breast cancer. Jia et al. (2013) showed an overall positive association of 1.20 (95% CI 1.08–1.33; 13 studies) between the risk of breast cancer and night work (never versus ever) [12]. Kolstad (2008) indicated that evidence to support a causal association between night shift work and breast cancer was limited [6], and no meta-analysis was conducted to evaluate the dose–response relationship with exposure to night shift work. The earliest meta-analysis about night shift and breast cancer was carried out by Megdal et al. (2005) [11], who identified a 48% (RR = 1.48, 95% CI 1.36–1.61) increased risk for those ever working for night shift by including studies published during 1995–2005.
The merit of our review is that we systematically conducted dose–response meta-analysis for various exposure indicators (i.e. duration, frequency, cumulative shifts) that had never been emphasized by previous meta-analyses. However, several limitations in our review should be mentioned. (i) Some of the articles required night shift workers to work on night shift for more than three nights per months [10, 17, 18], some defined more strictly to only include those working more than once per week [22], while others may not have such requirement but only on the period of time the shift should fall into. But even the period of time regarded as night shift did had several variations across the definitions from the studies. This lack of consistent definition of night shift in different individual studies may lead in certain degree of misclassification, and consequently may cause a dilution of pooled effects when doing syntheses; (ii) The effect measures retrieved from individual articles were adjusted by different confounding factors, though many of the included studies had adjusted for the common confounders including age, menopausal status, age of first birth, smoking, and alcohol drinking. Residual confounding effect in some studies with less adjustment cannot be ruled out. Overall, there is no evidence indicating obvious publication bias in our systematic review; (iii) Many of the included individual studies have adjusted their results for several risk factors (such as menopausal status, age, social-economic status, and family history), but the results of this meta-analysis itself has not adjusted the results for further factors. In addition, given the majority of night shift workers belonging to the lower social classes who tend to have a lower incidence of breast cancer and a lower uptake of screening and therefore at a reduced risk of over-diagnosis, this might suggest that if there is an effect then the RR of 1.03 might be an underestimate; (iv) The 95% confidence interval of the pooled risk estimate in terms of the frequency and cumulative number of night shift work tends to be broad due to the relatively low case load of each included studies (Tables 1 and 2), so the results from this meta-analysis would have to be updated and confirmed when evidence gets accumulating in the future.
The theoretical biological plausibility for the positive association between night work and breast cancer risk are (i) long-term exposure to night shift work and/or light-at-night can disturb the human day-night rhythm and result in circadian disruption, causing the resetting of the internal pacemaker of the synchronization nuclei and the suppression of the hormone melatonin output [26]. Melatonin appears to protect against cancer development through several pathways, including anti-oxidation, anti-mitosis, anti-angiogenesis, and the regulation of the immune system [27]. Decreased excretion of melatonin may induce continuous production of estrogen and alter the function of estrogen receptor (ER) to increase the risk of breast cancer [28]. The long-term impact of oral melatonin supplements is unknown, although animal study showed protective effect of breast cancer [29]; (ii) Evidence from experimental studies suggested that vitamin D intake and higher circulating vitamin D levels may be protective against breast cancer [30]. As a substantial proportion of time in adult life, night work may decrease the exposure of sun light and subsequently reduce vitamin D levels. Evidence from epidemiological studies revealed that the circulating concentration of 25-hydroxyvitamin D [25(OH)D] was significantly lower in female night workers [31], and there was a inverse association between plasma 25(OH)D and the breast cancer risk in postmenopausal women [32].
We recommend that more specific exploration could be done based on various ethnicities. Most of the articles included were showing certain degree of positive association depending on the exposure duration. In contrast, the study by Pronk et al. was focusing on Shanghai, within the Asia region, and the association was found to be rather neutral, even under long exposure duration category. The difference may be influenced by the ethnicity in a way yet to be discovered. Researches could also be done to further look into the mechanisms of how night shift work affects the risk of breast cancer. Knowing how the exposure to shift work biologically served as a risk factor for breast cancer may help the establishment or modification of current policies and regulation on how shift work should be executed so as to provide a better protection to female or even investigate possibilities such as supplements or change of environment which lessen the carcinogenic effect of night shift work.
In conclusion, this systematic review updated the evidence from current literature that a positive dose–response relationship is likely to present for breast cancer with increasing years of night shift and cumulative shifts involved in the work. Given the increasing prevalence of shift work worldwide and heavy economic burden of breast cancer, improved administrative measures should be implemented into the company's management scheme to effectively protect women from breast cancer.
Supplementary Material
acknowledgements
We acknowledge Wilson Wai-san Tam for his input on statistical analysis.
funding
This work was supported by Research Grants Council of Hong Kong [Grant number 474811].
disclosure
The authors have declared no conflicts of interest.
references
- 1.World Health Organization (WHO) Breast cancer: prevention and control. 2012 http://www.who.int/cancer/detection/breastcancer/en/ Available at:(4 June 2013, date last accessed) [Google Scholar]
- 2.Jemal A., Bray F., Center M.M. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Cancer Research (IARC) IARC; Lyon: 2008. World cancer report 2008. [Google Scholar]
- 4.Leung G.M., Thach T.Q., Lam T.H. Trends in breast cancer incidence in hong kong between 1973 and 1999: an age-period-cohort analysis. Br J Cancer. 2002;87:982–988. doi: 10.1038/sj.bjc.6600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madigan M.P., Ziegler R.G., Benichou J. Proportion of breast cancer cases in the united states explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–1685. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 6.Kolstad H.A. Nightshift work and risk of breast cancer and other cancers–a critical review of the epidemiologic evidence. Scand J Work Environ Health. 2008;34:5–22. doi: 10.5271/sjweh.1194. [DOI] [PubMed] [Google Scholar]
- 7.Assi V., Warwick J., Cuzick J. Clinical and epidemiological issues in mammographic density. Nat Rev Clin Oncol. 2012;9:33–40. doi: 10.1038/nrclinonc.2011.173. [DOI] [PubMed] [Google Scholar]
- 8.Straif K., Baan R., Grosse Y. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 9.Pesch B., Harth V., Rabstein S. Night work and breast cancer - results from the german GENICA study. Scand J Work Environ Health. 2010;36:134–141. doi: 10.5271/sjweh.2890. [DOI] [PubMed] [Google Scholar]
- 10.Pronk A., Ji B.T., Shu X.O. Night-shift work and breast cancer risk in a cohort of chinese women. Am J Epidemiol. 2010;171:953–959. doi: 10.1093/aje/kwq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megdal S.P., Kroenke C.H., Laden F. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Jia Y., Lu Y., Wu K. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37:197–206. doi: 10.1016/j.canep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 14.Orsini N., Li R., Wolk A. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M., Davey Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schernhammer E.S., Kroenke C.H., Laden F. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 18.Schernhammer E.S., Laden F., Speizer F.E. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J., Lassen C.F. Nested case-control study of night shift work and breast cancer risk among women in the danish military. Occup Environ Med. 2012;69:551–556. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- 20.Lie J.A., Kjuus H., Zienolddiny S. Night work and breast cancer risk among norwegian nurses: assessment by different exposure metrics. Am J Epidemiol. 2011;173:1272–1279. doi: 10.1093/aje/kwr014. [DOI] [PubMed] [Google Scholar]
- 21.Lie J.A., Roessink J., Kjaerheim K. Breast cancer and night work among norwegian nurses. Cancer Causes Control. 2006;17:39–44. doi: 10.1007/s10552-005-3639-2. [DOI] [PubMed] [Google Scholar]
- 22.Davis S., Mirick D.K., Stevens R.G. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 23.Hansen J., Stevens R.G. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012;48:1722–1729. doi: 10.1016/j.ejca.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 24.O'Leary E.S., Schoenfeld E.R., Stevens R.G. Shift work, light at night, and breast cancer on long island, New york. Am J Epidemiol. 2006;164:358–366. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 25.International Labour Organization (ILO) C171 - Night Work Convention, 1990 (No. 171) 1995 http://www.ilo.org/dyn/normlex/en/f?p=1000:12100:0::NO::P12100_INSTRUMENT_ID:312316 Available at:(4 June 2013, date last accessed) [Google Scholar]
- 26.Costa G., Haus E., Stevens R. Shift work and cancer - considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health. 2010;36:163–179. doi: 10.5271/sjweh.2899. [DOI] [PubMed] [Google Scholar]
- 27.Blask D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Stevens R.G., Blask D.E., Brainard G.C. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.el-Aziz M.A., Hassan H.A., Mohamed M.H. The biochemical and morphological alterations following administration of melatonin, retinoic acid and nigella sativa in mammary carcinoma: an animal model. Int J Exp Pathol. 2005;86:383–396. doi: 10.1111/j.0959-9673.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80:1721S–1724S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 31.Ward M., Berry D.J., Power C. Working patterns and vitamin D status in mid-life: a cross-sectional study of the 1958 british birth cohort. Occup Environ Med. 2011;68:902–907. doi: 10.1136/oem.2010.063479. [DOI] [PubMed] [Google Scholar]
- 32.Bauer S.R., Hankinson S.E., Bertone-Johnson E.R. Plasma vitamin d levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore) 2013;92:123–131. doi: 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.