Abstract
Exosomes can reach distant tissues through blood circulation to communicate directly with target cells and rapidly regulate intracellular signals. Exosomes play an important role in cardiovascular pathophysiology. Different exosomes derived from different sources, and their cargos have different mechanisms of action. In addition to being biomarkers, exosomes also have a certain significance in the diagnosis, treatment, and even prevention of cardiovascular diseases. Here, we provide a review of the up-to-date applications of exosomes, derived from various sources, in the prognosis and diagnosis of cardiovascular diseases.
1. Introduction
Exosomes are endosomal-derived vesicles that play a critical role in cell-to-cell communication and are secreted in several biological fluids including serum, saliva, urine, ascites, and cerebrospinal fluid among others [1]. Exosomes are small (30~150 nm diameter) with a distinctive bilipid protein structure. They can carry and exchange various cargos between cells and are used as a noninvasive biomarker for several diseases. Moreover, exosomes are considered the best biomarkers for disease diagnosis, owing to their unique characteristics [2].
Recent studies have shown that the number of exosomes, exosomal encoded and noncoding RNA, and exosomal proteins may act as biomarkers for the diagnosis and prognosis of cardiovascular diseases [3], whose death brings a great threat to global health [4, 5]. Some heart protection strategies have been shown to increase the number of exosomes in the blood [6–8]. The release of exosomes in the circulation of rats with the ischemia-reperfusion model increased significantly [9], in which the main exosomes containing HSP-60 were found [10]. A group of exosomes rich extracellular vesicles purified from the blood and proved to protect rat heart and myocardial cells from acute ischemia and reperfusion injury when administered in vivo or in vitro [9]. The exosomes containing HSP70 exist in the outer membrane of normal cardiac myocytes, which can activate the MAPK/ERK1/2 signal pathway through interaction with the TLR4 receptor and thus play a role in myocardial protection [9]. Exosomes rich in miR-143 and miR-145 have atherosclerotic protective effects in mouse models [11]. In addition, the exosomes containing miR-208a are related to the level of cardiac troponin I after coronary artery bypass grafting, while miR-208a in the blood circulation does not find this relationship, which indicates that the exosomal miRNAs may be helpful for the diagnosis of cardiovascular diseases [12]. Moreover, miR-192 and miR-194 in exosomes are also considered as prognostic biomarkers of cardiovascular diseases [13]. It is worth noting that miR-34a is highly expressed in myocardial infarction and preferentially integrated into the exosomes derived from cardiomyocytes and fibroblasts [14], making it possible for early diagnosis of myocardial infarction.
Here, we critically review the advances in exosomes derived from different cell sources and cardiovascular diseases (Figure 1), and we mainly highlight the exosomes derived from cardiomyocytes, cardiac progenitor cells, fibroblasts, and mesenchymal stem cells, which communicate intensively to facilitate proper cardiac function through direct cell-cell contact and paracrine interactions [15, 16]. This knowledge may be helpful to promote and develop diagnostic markers and therapeutic approaches, which may be beneficial to the management of cardiovascular patients.
Figure 1.
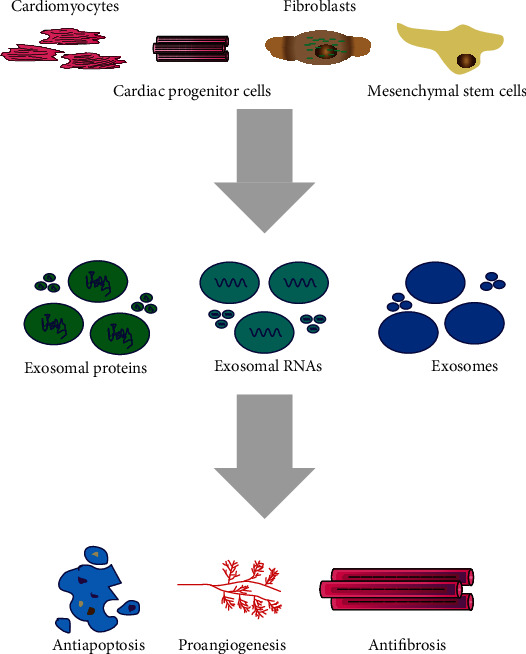
Exosomes derived from different cell sources and cardiovascular diseases.
2. Exosomes Derived from Different Cell Sources
2.1. Exosomes Derived from Cardiomyocytes
Exosomes derived from cardiomyocytes were initially found under hypoxia and reoxygenation conditions and may contain biological molecules [17, 18]. HSPs, which play essential roles in cellular survival and adaptation under numerous stresses [19], are found to be enriched in cardiac exosomes. HSP20 contained in cardiomyocyte-derived circulating exosomes is considered to be a new type of cardiac motility factor, which can promote the formation of myocardial neovascularization by activating VEGFR2 [20] and AKT signaling pathway, repressing TNF-α and IL-1β factors to alleviate myocardial infarction [21]. HSP60, which is thought to be a danger signal to the immune system and is also highly immunogenic [22], is released via exosomes, and that within the exosome, HSP60 is tightly attached to the exosome membrane [23]. The cardiomyocyte-derived exosomes are enriched for HSP70 involved in regulating cardiomyocyte growth and survival under stress [16]. The myocyte-derived HSP90 orchestrates not only p65-mediated IL-6 synthesis but also its release in exosomal vesicles, and such exosomes and myocyte-secreted IL-6 are responsible in unison for the biphasic activation of STAT-3 signaling in cardiac fibroblasts that culminates in excess collagen synthesis, leading to severely compromised cardiac function during cardiac hypertrophy [24]. TNF-α can also be isolated from cardiomyocyte-derived exosomes and interact with HIF-1α to contribute to cardiac remodeling [25]. Furthermore, exosomes derived from cardiomyocytes were found to carry functional GLUT (GLUT4 and GLUT1) and glycolytic enzymes (lactate dehydrogenase) and were shown to have specialized functions in glucose transport and metabolism in endothelial cells [26].
Many studies have demonstrated that exosomes derived from cardiomyocytes can also carry DNA/RNA [19]. Exosomes secreted by cells derived from cardiomyocytes can improve myocardial function, inhibit cell apoptosis, and promote the proliferation of cardiomyocytes when expressed in a mouse myocardial infarction model, which may be related to the enhancement of the expression of vascular gene SIS [27]. These effects can also be achieved by increasing the expression of miR-146a, which is significantly enriched in exosomes [27]. In addition, in an adriamycin-induced dilated cardiomyopathy mouse model, systemic administration of these exosomes can reduce apoptosis and fibrosis [28]. Cardiomyocytes exert an antiangiogenic function in type 2 diabetic rats through an exosomal transfer of miR-320 into endothelial cells [29]. MiR-30a, which is derived from hypoxic cardiomyocytes, is efficiently transferred between cardiomyocytes via exosomes and regulates autophagy by affecting the expression of Beclin-1, ATG12, and the ratio of LC3II/LC3I, which are important regulators of autophagy [30]. Exosomes from cardiomyocytes were enriched for certain miRNAs (particularly miR-29b, miR-323-5p, miR-455, and miR-466) and mediated the regulation of MMP9, which is involved in matrix degradation and leads to fibrosis and myocyte uncoupling [31]. Additionally, miR-208a was upregulated in cardiomyocyte-derived exosomes and contributed to increased fibroblast proliferation and differentiation into myofibroblasts via targeting Dyrk2 [32]. In a different way, exosomes from the cardiac spheroid are used to activate fibroblasts, which can increase the secretion of angiogenic factor, SDF1 and VEGF by fibroblasts [33, 34]. When injected into the heart of chronic myocardial perfusion model rats, activated fibroblasts were found to significantly promote angiogenesis and cardiac protection [34]. Recent evidence established that exosomes secreted from cardiomyocytes can deliver a wide variety of biomolecules into other types of cells and regulate gene expression in these cells [19].
2.2. Exosomes Derived from Cardiac Precursor Cells
Exosomes of cardiac precursor cells can be obtained from auricular implants of patients undergoing valve surgery [35]. When these exosomes were used in myocardial infarction model rats, it was found that myocardial apoptosis decreased, angiogenesis increased, and left ventricular ejection fraction improved significantly [36]. Saphenous vein-derived pericyte progenitor cells transplantation produces a long-term improvement of cardiac function through a novel paracrine mechanism involving the secretion of miR-132 and inhibition of its target genes [37]. Microarray analysis of exosomes secreted by hypoxic cardiac precursor cells identified 11 miRNAs that were upregulated compared with exosomes secreted by cardiac precursor cells grown under normoxic conditions, and those miRNAs improved cardiac function and reduced fibrosis [38]. Cardiac precursor cell-derived exosomes have a high-level expression of GATA4-responsive-miR-451 and can protect H9C2 from oxidative stress by inhibiting caspase 3/7 activation in vitro and inhibit cardiomyocyte apoptosis in vivo [39]. Regardless of the type of stem cells used, most cells die or lose shortly after implantation. In order to solve this problem, some researchers found that cotransfection of cardiac progenitor cells and nonviral small ring plasmids carrying HIF1 can improve their resistance to hypoxia [40]; this cotransfection resulted in high expression of HIF1 in endothelial cells, production of exosomes with a high content of miR-126 and miR-210, active internalization by receptor cardiac progenitor cells, activation of kinase, and induction of glycolysis [40]. Cardiac precursor cell-derived exosomal miR-21 had an inhibiting role in the apoptosis pathway through downregulating PDCD4 [41]. Moreover, miR-21 bonded with PTEN and suppressed PTEN expressions to downregulate both infarction size and injury marker expressions in vivo and promote endothelial cell proliferation, inhibit apoptosis, and stimulate angiogenesis in vitro [42]. Human cardiac precursor cell-derived exosomes were highly enriched in miR-146a-5p and attenuated doxorubicin/trastuzumab-induced oxidative stress in cardiomyocytes through suppressing miR-146a-5p target genes Traf6, Smad4, Irak1, Nox4, and Mpo [43]. Additionally, miR-133a-cardiac progenitor cells clearly improved cardiac function in a rat myocardial infarction model by reducing fibrosis and hypertrophy and increasing vascularization and cardiomyocyte proliferation [44]. The cardiac precursor cell-secreted exosomes promote the infarct healing through the improvement of cardiomyocyte survival and angiogenesis, and the cardioprotective effects can be enhanced by hypoxia conditioning of cardiac precursor cells and are partially contributed by MALAT1 via targeting the miRNA [45]. In contrast, exosomes from cardiac progenitor cells have also been shown to stimulate cell migration [46].
2.3. Exosomes Derived from Fibroblasts
The results showed that the exosomes derived from fibroblasts rich in miR-21-3p could induce cardiomyocyte hypertrophy by targeting SORBS2 and PDLIM5. Inhibition of miR-21-3p reduced cardiac hypertrophy in animals treated with Ang II [47]. Besides, circulatory miR-29 and miR-30 are considered as biomarkers of left ventricular hypertrophy, the correlation of circulatory miR-21 in the diagnosis and prognosis of cardiac hypertrophy deserves further study [48]. In addition, exosomes extracted from endothelial cells expressing KLF2 can attenuate the formation of atherosclerosis [49]. It is also important that exosomes from atherosclerotic plaques and macrophages from peripheral blood participate in the development of atherosclerosis [50]. Patients with atherosclerosis have a higher level of extracellular vesicles derived from leukocyte compared with healthy participants [51].
2.4. Exosomes Derived from Mesenchymal Stem Cells
The exosomal proteins derived from mesenchymal stem cells can reduce the infarct area by half [52], inhibit the proliferation and migration of vascular smooth muscle [53], reduce cardiomyocyte apoptosis, promote angiogenesis, reduce ventricular remodeling, and protect cardiac function [54, 55]. Exosomes isolated from macrophage migration inhibitory factor-pretreated mesenchymal stem cells protected cardiomyocytes from apoptosis [56] through the lncRNA-NEAT1/miR-142-3p/FOXO1 signaling pathway [57]. At the same time, the exosomes derived from mesenchymal stem cells can also reduce the levels of inflammatory factors, such as IL-6 and MCP-1 [53], through activating the signal pathways involved in IGF-1/PI3K/Akt and GSK-3p [52, 54, 58, 59]. In the hypoxia-induced pulmonary hypertension mouse model, intravenous infusion of the exosomes derived from mesenchymal stem cells can also inhibit vascular remodeling and hypertension, which may be achieved by inhibiting the STAT3 signaling pathway [60]. Another interesting study confirmed the potential of mesenchymal stem cell-derived exosomes in reversing pulmonary hypertension in mice and further showed that exosomes from drug-induced pulmonary hypertension mice can induce pulmonary hypertension when injected into nondiseased animals [61]. These differences may be related to different miRNA expression patterns of exosomes.
Stem cells can be injected into the heart muscle to prevent myocardial ischemia and reperfusion injury and improve myocardial function through repair and gradual regeneration [62]. Transplantation of human CD34+ve hematopoietic stem cells into ischemic tissue can induce neovascularization in preclinical models, which has been proved to be related to the treatment of angina pectoris and the improvement of exercise time in phase II clinical trials. However, the benefits in these experiments seem to depend more on the effect of paracrine signals than on stem cell transplantation of cardiomyocytes [63]. At present, exosomes have been used to regulate paracrine benefits [16, 64]. In fact, the exosomes of CD34+ve have angiogenic activity in vitro and in vivo. Interestingly, although the benefits of CD34+ve exosomes on angiogenesis have been observed, CD34+ve hematopoietic stem cells do not have cardioprotective effects in the expression of the SHH gene after acute myocardial infarction [65]. Injection of exosomes from embryonic stem cells into the heart of mice with myocardial infarction increased neovascularization and cardiomyocyte survival and reduced fibrosis, which is related to the transmission of miR-294 and C-kit+ve cardiac progenitor cells in the myocardium, thus increasing their regeneration activity [66]. In the peripheral blood of patients with chronic heart failure, CD34+ stem cell-derived exosomes rich in angiogenesis-related miR-126 and miR-130a were significantly reduced [67].
3. Future Perspectives
Exosomes act as messengers of intercellular communication among cardiomyocytes, fibroblasts, smooth muscle cells, and endothelial cells and participate in the regulation of cardiac regeneration, ventricular remodeling, and angiogenesis in cardiovascular diseases [68]. Because of its perfect properties as a carrier of signal molecules, circulating exosomes transmit both protective and harmful information [69, 70]. Since it is difficult to obtain the heart tissue samples of patients, it may be a useful strategy to detect the changes of exosomes in peripheral blood circulation to obtain the pathophysiological process information of cardiovascular diseases [8, 71] and guide the treatment of patients [61, 72]. In this new and exciting field of exosome research, there are still many problems. In contrast, there are relatively few studies on exosomal protein content and its significance in diagnosis, treatment, and prevention, although they have been proved to be accurate prognostic tools for predicting negative cardiovascular events, including CD31+/Annexin V+ and CD144-positive exosomes [73, 74]. In addition, the preparation and purification of exosomes have some limitations. Biologically, the exact mechanism of exosomes remains to be verified. The ability to study the role of exosomes in vivo will be greatly enhanced by the discovery of a specific production or absorption inhibitor. In terms of treatment, a better understanding of the pharmacokinetics and pharmacodynamics of exosomes is essential. All available preclinical and clinical data strongly support the hypothesis that exosomes have therapeutic value or play an important role in the treatment of many diseases [75].
The release of exosomes can also be regulated by different exogenous stimuli. Inflammatory response following myocardial infarction dramatically increased the number of circulating exosomes carrying alarmins such as IL-1α, IL-1β, TNF-α, and Rantes [76, 77]. In fact, recent studies demonstrated that exosome numbers may be increased by some specific chemical compounds. Monensin induces the formation of exosome and stimulates exosome secretion in K562 cells [78], which are human myeloid leukemia cells that secrete exosomes [79], in a Ca2+-dependent manner through an increase in transferrin [78]. Quantitative analysis demonstrates activation of histamine H1 receptor in HeLa cells which increases Ser110 phosphorylation of SNAP23, promoting multivesicular body-plasma membrane fusion and the release of CD63-enriched exosomes [80]. In addition to compounds, some drugs can also affect the secretion of exosomes. Atorvastatin enhances the numbers of exosomes derived from mesenchymal stem cells, which improve cardiac function and promote blood vessel formation, thus enhancing the therapeutic efficacy for myocardial infarction [81]. Ticagrelor, another commonly prescribed for cardiovascular diseases, can be leveraged to modulate the release of antihypoxic exosomes from resident human cardiac-derived mesenchymal progenitor cells through acute phosphorylation of ERK42/44 [82]. Besides, recent studies have shown that exosomes are also a safe alternative to drug delivery systems [83]. Ideally, a method of targeting the heart or target cells is needed to avoid intramyocardial or nonspecific systemic administration. Despite these barriers, the potential of exosomes as a therapeutic drug for cardiovascular diseases is still exciting, and there has been a blueprint for treatment based on extracellular vesicles in clinical trials [84]. However, it is important to do a lot of research on exosomes.
Exosomes can transfer proteins, RNAs, and other bioactive molecules to recipient cells to influence their biological properties [85], suggesting the potential functional diversity of exosomes [86]. The exosomes from different cardiac cells deliver a specific protein or RNA. The cardiac precursor cell-secreted exosomes rich in PAPP-A had a cardioprotection profile through releasing IGF-1 via proteolytic cleavage of IGFBP-4, resulting in IGF-1R activation and intracellular Akt and ERK1/2 phosphorylation [87]. It was found that exosomes secreted by endothelial progenitor cells derived from patients with coronary atherosclerotic heart disease could inhibit the migration and angiogenesis through expressing more miR-146a-5p and miR-146b-5p [88]. Another study indicated that normoxia endothelial progenitor cell-derived exosomes rich in miR-10b-5p could significantly ameliorate cardiac fibroblast activation in vitro [89].
Increasing studies also have shown that exosomes play an active role in patients with cardiovascular crisis and patients with complications. Acute myocardial infarction is a common critical disease of cardiovascular diseases. Exosomes released by chronically hypoxic cardiac precursor cells deliver a pool of miRNAs (miR-15b, miR-17, miR-20a, miR-103, miR-199a, miR-210, and miR-292) that enhance angiogenesis, reduce profibrotic gene expression, preserve myocardial contractile function, and improve cardiac function in the early hours after the onset of acute myocardial infarction [90]. Heart failure is the end-stage state of various cardiovascular diseases [91, 92], and heart failure pathological condition altered the miRNA cargos of cardiac-derived exosomes and impaired their regenerative activities. It may be related to miR-21-5p, the exosome released from explant-derived cardiac stromal cells, contributing to heart repair by enhancing angiogenesis and cardiomyocyte survival through the phosphatase and tensin homolog/Akt pathway [93]. Both heart failure and diabetes independently increase the morbidity of another disease and associated with considerable mortality [94, 95]. Diabetic cardiomyocytes exhibited increased secretion of detrimental exosomes containing decreased HSP20 levels, which contributed to diabetes-induced organ damage [96]. In a transgenic mouse model with cardiac-specific overexpression of HSP20, overexpression of HSP20 significantly attenuated cardiac dysfunction, hypertrophy, apoptosis, fibrosis, and microvascular rarefaction, in other words, protected against in vivo cardiac adverse remodeling by increasing generation/secretion of exosomes, indicating that elevation of HSP20 in cardiomyocytes can offer protection in diabetic hearts through the release of instrumental exosomes [96]. Poststroke cardiac complications are common, and diabetes exacerbates poststroke cardiac injury. In type 2 diabetes mellitus-stroke mice, exosomes harvested from human umbilical cord blood-derived CD133+ cell treatment significantly increased miR-126 expression in the heart and decreased its target gene expression and decreased myocardial cross-sectional area, interstitial fibrosis, TGFβ, numbers of M1 macrophages, and oxidative stress markers 4-HNE and NOX2 in heart tissue, meaning that exosome treatment significantly improves cardiac function [97]. Additionally, uremic cardiomyopathy contributes to chronic kidney disease-induced morbidity and mortality. Overexpression of exosome-encapsulated miR-26a in muscle attenuated cardiomyopathy via exosome-mediated miR-26a transfer, which may be related to decreased the upregulation of FBXO32/atrogin-1 and TRIM63/MuRF1 and depressed cardiac fibrosis lesions [98]. Although exosomes show exciting and gratifying cardioprotection effects, it should be noted that the evidences of this cardioprotection are mostly from in vivo or in vitro, which still needs to be verified in the clinic.
4. Conclusion
In this review, ample preclinical and biomedical data were summarized (Table 1), which can provide a reference for the study of exosomes and their application in the diagnosis and prognosis of cardiovascular diseases. Although the current evidences show that exosomes derived from different cell sources are helpful for the diagnosis and prognosis of cardiovascular diseases, most of the evidences come from preclinical studies; solid clinical data are urgently needed. Moreover, there are some limitations in the preparation and purification of exosomes, and their exact mechanisms of action still need to be validated. These challenges need to be addressed before exosomes can proceed to clinical application.
Table 1.
Summary of exosomes derived from different cell sources in cardiovascular diseases.
| Source | Cargos | Biological effects | Evidences | References |
|---|---|---|---|---|
| Cardiomyocytes | HSP20 | Promote angiogenesis by activating VEGFR2, and activate AKT signaling pathway and repress TNF-α and IL-1β factors to alleviate myocardial infarction | Preclinical evidences (in vivo and in vitro) | [20, 21] |
| HSP60 | Promote immune responses | Preclinical evidences (in vitro) | [23] | |
| HSP70 | Activate monocytes alone, resulting in monocyte adhesion to endothelial cells; improve cardiac function | Preclinical evidences (in vitro) | [16, 17] | |
| HSP90 and IL-6 | Active STAT-3 signaling in cardiac fibroblasts that culminates in excess collagen synthesis, leading to severely compromised cardiac function during cardiac hypertrophy | Preclinical evidences (in vivo and in vitro) | [24] | |
| TNF-α | Interact with HIF-1α to contribute to cardiac remodeling | Preclinical evidences (in vitro) | [25] | |
| GLUT | Increase glucose transport | Preclinical evidences (in vitro) | [26] | |
| miR-15b, miR-17, miR-20a, miR-103, miR-199a, miR-210, and miR-292 | Enhance angiogenesis, reduce profibrotic gene expression, preserve myocardial contractile function, and improve cardiac function | Preclinical evidences (in vivo and in vitro) | [90] | |
| miR-29b, miR-323-5p, miR-455, and miR-466 | Mediate the regulation of MMP9, which is involved in matrix degradation and leads to fibrosis and myocyte uncoupling | Preclinical evidences (in vitro) | [31] | |
| miR-30a | Regulate autophagy by affecting the expression of Beclin-1, ATG12, and the ratio of LC3II/LC3I | Preclinical evidences (in vitro) | [30] | |
| miR-34a | Biomarkers of myocardial infarction | Preclinical evidences (in vitro) | [14] | |
| miR-146a | Inhibit apoptosis and promote proliferation of cardiomyocytes, while enhancing angiogenesis | Preclinical evidences (in vivo and in vitro) | [27] | |
| miR-208a | Increase fibroblast proliferation and differentiation into myofibroblasts via targeting Dyrk2 | Preclinical evidences (in vivo and in vitro) | [32] | |
| miR-320 | Inhibit proliferation, migration, and tube-like formation | Preclinical evidences (in vivo and in vitro) | [29] | |
| miR-451 | Protect H9C2 from oxidative stress by inhibiting caspase 3/7 activation and inhibit cardiomyocyte apoptosis | Preclinical evidences (in vivo and in vitro) | [39] | |
| NA | Reduce apoptosis and fibrosis | Preclinical evidences (in vivo and in vitro) | [28] | |
| NA | Activate fibroblasts, which can increase the secretion of angiogenic factor, SDF1 and VEGF by fibroblasts | Preclinical evidences (in vivo and in vitro) | [33, 34] | |
| NA | Promote angiogenesis and cardiac protection | Preclinical evidences (in vivo and in vitro) | [34] | |
|
| ||||
| Cardiac progenitor cells | PAPP-A | Cardioprotection profile through releasing IGF-1 via proteolytic cleavage of IGFBP-4, resulting in IGF-1R activation, intracellular Akt and ERK1/2 phosphorylation | Preclinical evidences (in vivo and in vitro) | [87] |
| miR-15b and miR-20a | Stimulate angiogenesis | Preclinical evidences (in vivo and in vitro) | [38] | |
| miR-17 and miR-103 | Promote angiogenesis, inhibit myocardial fibrosis | Preclinical evidences (in vivo and in vitro) | [38] | |
| miR-21 | Inhibit cardiomyocyte apoptosis through downregulating PDCD4; downregulate both infarction size and injury marker expressions in vivo and promote endothelial cell proliferation, inhibit the apoptosis, and stimulate angiogenesis in vitro by targeting PTEN | Preclinical evidences (in vivo and in vitro) | [41, 42] | |
| miR-126 and miR-210 |
Active kinase and induce glycolysis | Preclinical evidences (in vivo and in vitro) | [40] | |
| miR-132, miR-210, and miR-146a-3p | Decrease myocardial apoptosis, increase angiogenesis, and improve left ventricular ejection fraction | Preclinical evidences (in vivo and in vitro) | [27, 36, 37] | |
| miR-133a | Improve cardiac function by reducing fibrosis and hypertrophy and increasing vascularization and cardiomyocyte proliferation | Preclinical evidences (in vivo and in vitro) | [44] | |
| miR-146a-5p | Attenuate doxorubicin/trastuzumab-induced oxidative stress in cardiomyocytes through suppressing target genes Traf6, Smad4, Irak1, Nox4, and Mpo | Clinical evidences | [43] | |
| miR-181a and miR-323-5p | Promote angiogenesis | Preclinical evidences (in vivo and in vitro) | [27, 36] | |
| miR-210 | Promote angiogenesis, inhibit cardiomyocyte apoptosis, improve heart function | Preclinical evidences (in vivo and in vitro) | [38] | |
| lnc RNA MALAT1 | Promote the infarct healing through improvement of cardiomyocyte survival and angiogenesis by targeting the miRNA | Preclinical evidences (in vivo and in vitro) | [45] | |
| NA | Stimulate cell migration | Preclinical evidences (in vitro) | [46] | |
|
| ||||
| Fibroblasts | miR-21-3p | Induce cardiomyocyte hypertrophy by targeting SORBS2 and PDLIM5 | Preclinical evidences (in vivo and in vitro) | [47] |
| miR-21, miR-29, and miR-30 | Biomarkers of left ventricular hypertrophy | Preclinical evidences (in vivo and in vitro) | [48] | |
| miR-34a | Biomarkers of myocardial infarction | Preclinical evidences (in vitro) | [14] | |
|
| ||||
| Mesenchymal stem cells | NA | Reduce the infarct area, inhibit the proliferation and migration of vascular smooth muscle, reduce cardiomyocyte apoptosis, promote angiogenesis, reduce ventricular remodeling, and protect cardiac function | Preclinical evidences (in vivo and in vitro) | [52–55] |
| NA | Protect cardiomyocytes from apoptosis through lncRNA-NEAT1/miR-142-3p/FOXO1 signaling pathway | Preclinical evidences (in vitro) | [56, 57] | |
| NA | Reduce the levels of inflammatory factors, such as IL-6 and MCP-1, through activating the signal pathways involved in IGF-1/PI3K/Akt and GSK-3p | Preclinical evidences (in vivo and in vitro) | [52–54, 58, 59] | |
| NA | Inhibit vascular remodeling and hypertension by inhibiting STAT3 signaling pathway | Preclinical evidences (in vivo and in vitro) | [60] | |
| NA | Reverse pulmonary hypertension | Preclinical evidences (in vivo) | [61] | |
| miR-126 and miR-130a | Biomarkers of chronic heart failure | Clinical evidences | [67] | |
| miR-294 | Increase neovascularization, cardiomyocyte survival, and reduce fibrosis | Preclinical evidences (in vivo and in vitro) | [66] | |
|
| ||||
| Endothelial cells | KLF2 | Attenuate the formation of atherosclerosis | Preclinical evidences (in vivo and in vitro) | [49] |
| miR-10b-5p | Ameliorate cardiac fibroblast activation | Preclinical evidences (in vitro) | [89] | |
| miR-146a-5p and miR-146b-5p | Inhibit the migration and angiogenesis | Preclinical evidences (in vitro) | [88] | |
|
| ||||
| Macrophages and leukocyte | NA | Promote vascular smooth muscle cells migration and adhesion, which may be mediated by the integration of extracellular vesicles into vascular smooth muscle cells and the subsequent downstream activation of ERK and Akt | Preclinical evidences (in vitro) | [51] |
| Biomarkers of atherosclerosis | Clinical evidences | [50] | ||
| Cardiac stromal cells | miR-21-5p | Contribute to heart repair by enhancing angiogenesis and cardiomyocyte survival through the phosphatase and tensin homolog/Akt pathway | Pre-clinical evidences (in vivo and in vitro) | [93] |
Acknowledgments
We thank all the scientists involved in exosomes and cardiovascular diseases. This work was supported by the National Natural Science Foundation of China (81774229), the Jiangsu Leading Talent Project of Traditional Chinese Medicine (Jiangsu TCM 2018 No. 4), the Major Project of Nanjing Medical Science and Technology Development During 13th Five-year Plan (ZDX16013), the Jiangsu Universities Nursing Advantage Discipline Project (2019YSHL095), and the Jiangsu Science and Technology Program (BK20161115).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Chung I.-M., Rajakumar G., Venkidasamy B., Subramanian U., Thiruvengadam M. Exosomes: current use and future applications. Clinica Chimica Acta. 2020;500:226–232. doi: 10.1016/j.cca.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Yuan D., Zhao Y., Banks W. A., et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu R., Gao W., Yao K., Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Frontiers in Immunology. 2019;10:p. 648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang B., Qu Y., Zhao Q.-F., Gu N. Guanxin V for coronary artery disease: a retrospective study. Biomedicine and Pharmacotherapy. 2020;128, article 110280 doi: 10.1016/j.biopha.2020.110280. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–1788. doi: 10.1016/s0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sluijter J. P. G., Condorelli G., Davidson S. M., et al. Novel therapeutic strategies for cardioprotection. Pharmacology and Therapeutics. 2014;144(1):60–70. doi: 10.1016/j.pharmthera.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Yellon D. M., Davidson S. M. Exosomes: nanoparticles involved in cardioprotection? Circulation Research. 2014;114(2):325–332. doi: 10.1161/circresaha.113.300636. [DOI] [PubMed] [Google Scholar]
- 8.Lawson C., Vicencio J. M., Yellon D. M., Davidson S. M. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. Journal of Endocrinology. 2016;228(2):R57–R71. doi: 10.1530/joe-15-0201. [DOI] [PubMed] [Google Scholar]
- 9.Vicencio J. M., Yellon D. M., Sivaraman V., et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. Journal of the American College of Cardiology. 2015;65(15):1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Giricz Z., Varga Z. V., Baranyai T., et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. Journal of Molecular and Cellular Cardiology. 2014;68:75–78. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Hergenreider E., Heydt S., Tréguer K., et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology. 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 12.Emanueli C., Shearn A. I. U., Laftah A., et al. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac MicroRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLOS ONE. 2016;11(4, article e0154274) doi: 10.1371/journal.pone.0154274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto S., Sakata Y., Suna S., et al. Circulating p53-Responsive MicroRNAs are predictive indicators of heart failure after acute myocardial infarction. Circulation Research. 2013;113(3):322–326. doi: 10.1161/circresaha.113.301209. [DOI] [PubMed] [Google Scholar]
- 14.Tian C., Gao L., Zimmerman M. C., Zucker I. H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 2018;314(5):H928–h939. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talman V., Kivela R. Cardiomyocyte-endothelial cell interactions in cardiac remodeling and regeneration. Frontiers in Cardiovascular Medicine. 2018;5:p. 101. doi: 10.3389/fcvm.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y., Huang W., Meng W., et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem cells. 2014;32(2):462–472. doi: 10.1002/stem.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan R., Leng X., Liu X., et al. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochemical and Biophysical Research Communications. 2009;387(2):229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 18.Waldenström A., Gennebäck N., Hellman U., Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PloS One. 2012;7(4, article e34653) doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H., Wang Z. Cardiomyocyte-derived exosomes: biological functions and potential therapeutic implications. Frontiers in Physiology. 2019;10:p. 1049. doi: 10.3389/fphys.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Wang X., Zhu H., et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PloS One. 2012;7(3, article e32765) doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D. W., Ge P. P., Liu A. L., Yu X. Y., Liu T. T. HSP20-mediated cardiomyocyte exosomes improve cardiac function in mice with myocardial infarction by activating Akt signaling pathway. European Review for Medical and Pharmacological Sciences. 2019;23(11):4873–4881. doi: 10.26355/eurrev_201906_18075. [DOI] [PubMed] [Google Scholar]
- 22.Malik Z. A., Kott K. S., Poe A. J., et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. American Journal of Physiology Heart and Circulatory Physiology. 2013;304(7):H954–H965. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S., Knowlton A. A. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. American Journal of Physiology Heart and Circulatory Physiology. 2007;292(6):H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 24.Datta R., Bansal T., Rana S., et al. Myocyte-derived Hsp 90 modulates collagen upregulation via biphasic activation of STAT-3 in fibroblasts during cardiac hypertrophy. Molecular and Cellular Biology. 2017;37(6) doi: 10.1128/mcb.00611-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X., Deng L., Wang D., et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. Journal of Molecular and Cellular Cardiology. 2012;53(6):848–857. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Garcia N. A., Moncayo-Arlandi J., Sepulveda P., Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovascular Research. 2016;109(3):397–408. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim A. G.-E., Cheng K., Marbán E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Reports. 2014;2(5):606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandergriff A. C., de Andrade J. B. M., Tang J., et al. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells International. 2015;2015:8. doi: 10.1155/2015/960926.960926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Huang W., Liu G., et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of Molecular and Cellular Cardiology. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Li Y., Chen X., Cheng X., Liao Y., Yu X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. Journal of Molecular Medicine (Berlin, Germany) 2016;94(6):711–724. doi: 10.1007/s00109-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi P., Kalani A., Medina I., Familtseva A., Tyagi S. C. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine. 2015;19(9):2153–2161. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J., Yu X., Xue F., Li Y., Liu W., Zhang S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. American Journal of Translational Research. 2018;10(12):4350–4366. [PMC free article] [PubMed] [Google Scholar]
- 33.Bromage D. I., Davidson S. M., Yellon D. M. Stromal derived factor 1α: A chemokine that delivers a two-pronged defence of the myocardium. Pharmacology and Therapeutics. 2014;143(3):305–315. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseliou E., Fouad J., Reich H., et al. Fibroblasts Rendered Antifibrotic, Antiapoptotic, and Angiogenic by Priming With Cardiosphere-Derived Extracellular Membrane Vesicles. Journal of the American College of Cardiology. 2015;66(6):599–611. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Jia Q., Xinnong C., et al. Role of cardiac progenitor cell‐derived exosome‐mediated microRNA‐210 in cardiovascular disease. Journal of Cellular and Molecular Medicine. 2019;23(11):7124–7131. doi: 10.1111/jcmm.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barile L., Lionetti V., Cervio E., et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular Research. 2014;103(4):530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 37.Katare R., Riu F., Mitchell K., et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation Research. 2011;109(8):894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray W. D., French K. M., Ghosh-Choudhary S., et al. Identification of therapeutic covariant MicroRNA Clusters in hypoxia-treated cardiac progenitor cell exosomes using systems Biology. Circulation Research. 2015;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L., Wang Y., Pan Y., et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochemical and Biophysical Research Communications. 2013;431(3):566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong S. G., Lee W. H., Huang M., et al. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal MicroRNA Transfer. Circulation. 2014;130(11_suppl_1):S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao J., Pan Y., Li X. H., et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death & Disease. 2016;7(6, article e2277) doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F., Liu W., Yan X., et al. Effects of mir-21 on cardiac microvascular endothelial cells after acute myocardial infarction in rats: role of phosphatase and tensin homolog (PTEN)/vascular endothelial growth factor (VEGF) signal pathway. Medical Science Monitor. 2016;22:3562–3575. doi: 10.12659/MSM.897773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milano G., Biemmi V., Lazzarini E., et al. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovascular Research. 2020;116(2):383–392. doi: 10.1093/cvr/cvz108. [DOI] [PubMed] [Google Scholar]
- 44.Izarra A., Moscoso I., Levent E., et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports. 2014;3(6):1029–1042. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Q., Wang J., Tan W. L. W., et al. Extracellular vesicles from human embryonic stem cell-derived cardiovascular progenitor cells promote cardiac infarct healing through reducing cardiomyocyte death and promoting angiogenesis. Cell Death & Disease. 2020;11(5):p. 354. doi: 10.1038/s41419-020-2508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrijsen K. R., Sluijter J. P., Schuchardt M. W., et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. Journal of Cellular and Molecular Medicine. 2010;14(5):1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bang C., Batkai S., Dangwal S., et al. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. Journal of Clinical Investigation. 2014;124(5):2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan W., Zhong Y., Cheng C., et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. Plo S One. 2013;8(1, article e53950) doi: 10.1371/journal.pone.0053950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordes K. R., Sheehy N. T., White M. P., et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leroyer A. S., Isobe H., Lesèche G., et al. Cellular Origins and Thrombogenic Activity of Microparticles Isolated From Human Atherosclerotic Plaques. Journal of the American College of Cardiology. 2007;49(7):772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 51.Niu C., Wang X., Zhao M., et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. Journal of the American Heart Association. 2016;5(10) doi: 10.1161/jaha.116.004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai R. C., Arslan F., Lee M. M., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Liu R., Shen H., Ma J., Sun L., Wei M. Extracellular vesicles derived from adipose mesenchymal stem cells regulate the phenotype of smooth muscle cells to limit intimal hyperplasia. Cardiovascular Drugs and Therapy. 2016;30(2):111–118. doi: 10.1007/s10557-015-6630-5. [DOI] [PubMed] [Google Scholar]
- 54.Kang K., Ma R., Cai W., et al. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells International. 2015;2015:14. doi: 10.1155/2015/659890.659890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma J., Zhao Y., Sun L., et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Translational Medicine. 2017;6(1):51–59. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X., Li X., Zhu W., et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. Journal of Cellular Physiology. 2020 doi: 10.1002/jcp.29456. [DOI] [PubMed] [Google Scholar]
- 57.Chen H., Xia W., Hou M. LncRNA-NEAT1 from the competing endogenous RNA network promotes cardioprotective efficacy of mesenchymal stem cell-derived exosomes induced by macrophage migration inhibitory factor via the miR-142-3p/FOXO1 signaling pathway. Stem Cell Research & Therapy. 2020;11(1):p. 31. doi: 10.1186/s13287-020-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arslan F., Lai R. C., Smeets M. B., et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research. 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Lai R. C., Arslan F., Tan S. S., et al. Derivation and characterization of human fetal MSCs: An alternative cell source for large-scale production of cardioprotective microparticles. Journal of Molecular and Cellular Cardiology. 2010;48(6):1215–1224. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Lee C., Mitsialis S. A., Aslam M., et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aliotta J. M., Pereira M., Wen S., et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovascular Research. 2016;110(3):319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maghin E., Garbati P., Quarto R., Piccoli M., Bollini S. Young at heart: combining strategies to rejuvenate endogenous mechanisms of cardiac repair. Frontiers in Bioengineering and Biotechnology. 2020;8:p. 447. doi: 10.3389/fbioe.2020.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madonna R., Van Laake L. W., Davidson S. M., et al. Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. European Heart Journal. 2016;37(23):1789–1798. doi: 10.1093/eurheartj/ehw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kishore R., Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circulation Research. 2016;118(2):330–343. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackie A. R., Klyachko E., Thorne T., et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circulation Research. 2012;111(3):312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan M., Nickoloff E., Abramova T., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation Research. 2015;117(1):52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jakob P., Doerries C., Briand S., et al. Loss of AngiomiR-126 and 130a in angiogenic early outgrowth cells from patients with chronic heart Failure. Circulation. 2012;126(25):2962–2975. doi: 10.1161/CIRCULATIONAHA.112.093906. [DOI] [PubMed] [Google Scholar]
- 68.Cosme J., Liu P. P., Gramolini A. O. The cardiovascular exosome: current perspectives and potential. Proteomics. 2013;13(10-11):1654–1659. doi: 10.1002/pmic.201200441. [DOI] [PubMed] [Google Scholar]
- 69.Pant S., Hilton H., Burczynski M. E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochemical Pharmacology. 2012;83(11):1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita T., Kamada H., Kanasaki S., et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Die Pharmazie. 2013;68(12):969–973. doi: 10.1691/ph.2013.3599. [DOI] [PubMed] [Google Scholar]
- 71.Davis M. E. Exosomes: what do we love so much about them? Circulation Research. 2016;119(12):1280–1282. doi: 10.1161/CIRCRESAHA.116.309942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans S., Mann D. L. Circulating p53-Responsive MicroRNAs as predictive biomarkers in heart failure after acute myocardial Infarction. Circulation Research. 2013;113(3):242–244. doi: 10.1161/CIRCRESAHA.113.301951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinning J. M., Losch J., Walenta K., Bohm M., Nickenig G., Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. European Heart Journal. 2011;32(16):2034–2041. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 74.Nozaki T., Sugiyama S., Koga H., et al. Significance of a Multiple Biomarkers Strategy Including Endothelial Dysfunction to Improve Risk Stratification for Cardiovascular Events in Patients at High Risk for Coronary Heart Disease. Journal of the American College of Cardiology. 2009;54(7):601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 75.Jiang W., Wang M. New insights into the immunomodulatory role of exosomes in cardiovascular disease. Reviews in Cardiovascular Medicine. 2019;20(3):153–160. doi: 10.31083/j.rcm.2019.03.528. [DOI] [PubMed] [Google Scholar]
- 76.Biemmi V., Milano G., Ciullo A., et al. Inflammatory extracellular vesicles prompt heart dysfunction via TRL4-dependent NF-κB activation. Theranostics. 2020;10(6):2773–2790. doi: 10.7150/thno.39072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Correa B. L., El Harane N., Gomez I., et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovascular Research. 2020 doi: 10.1093/cvr/cvaa028. [DOI] [PubMed] [Google Scholar]
- 78.Savina A., Furlán M., Vidal M., Colombo M. I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. The Journal of Biological Chemistry. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 79.Dong Y., Lin Y., Gao X., et al. Targeted blocking of miR328 lysosomal degradation with alkalized exosomes sensitizes the chronic leukemia cells to imatinib. Applied Microbiology and Biotechnology. 2019;103(23-24):9569–9582. doi: 10.1007/s00253-019-10127-3. [DOI] [PubMed] [Google Scholar]
- 80.Verweij F. J., Bebelman M. P., Jimenez C. R., et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. Journal of Cell Biology. 2018;217(3):1129–1142. doi: 10.1083/jcb.201703206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang P., Wang L., Li Q., et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research. 2019;116(2):353–367. doi: 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casieri V., Matteucci M., Pasanisi E. M., et al. Ticagrelor Enhances Release of Anti-Hypoxic Cardiac Progenitor Cell-Derived Exosomes Through Increasing Cell Proliferation _In Vitro_. Scientific Reports. 2020;10(1):p. 2494. doi: 10.1038/s41598-020-59225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao J., Feng S., Wang X., et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peer J. 2018;6, article e5186 doi: 10.7717/peerj.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lener T., Gimona M., Aigner L., et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. Journal of Extracellular Vesicles. 2015;4(1, article 30087) doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Brien K., Breyne K., Ughetto S., Laurent L. C., Breakefield X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nature Reviews Molecular Cell Biology. 2020 doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng L., Zhao W., Hill A. F. Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Molecular Aspects of Medicine. 2018;60:62–68. doi: 10.1016/j.mam.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 87.Barile L., Cervio E., Lionetti V., et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovascular Research. 2018;114(7):992–1005. doi: 10.1093/cvr/cvy055. [DOI] [PubMed] [Google Scholar]
- 88.Xing Z., Zhao C., Liu H., Fan Y. Endothelial progenitor cell-derived extracellular vesicles: a novel candidate for regenerative medicine and disease treatment. Advanced Healthcare Materials. 2020;9(12, article 2000255) doi: 10.1002/adhm.202000255. [DOI] [PubMed] [Google Scholar]
- 89.Liu W. H., Zhang H. F., Mai J. T., et al. Distinct anti-fibrotic effects of exosomes derived from endothelial colony-forming cells cultured under normoxia and hypoxia. Medical Science Monitor. 2018;24:6187–6199. doi: 10.12659/msm.911306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terrasini N., Lionetti V. Exosomes in critical illness. Critical Care Medicine. 2017;45(6):1054–1060. doi: 10.1097/CCM.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 91.Liang B., Zhao Y.-X., Ning G. Empagliflozin improves cardiac function in heart failure with reduced ejection fraction independent of loading conditions. Cardiovascular Diabetology. 2020;19(1):p. 29. doi: 10.1186/s12933-020-01004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang B., Zhao Y.-X., Zhang X.-X., Liao H.-L., Gu N. Reappraisal on pharmacological and mechanical treatments of heart failure. Cardiovascular Diabetology. 2020;19(1):p. 55. doi: 10.1186/s12933-020-01024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiao L., Hu S., Liu S., et al. Evaluating blood–brain barrier permeability in a rat model of type 2 diabetes. Journal of Clinical Investigation. 2020;18(1):256–2250. doi: 10.1186/s12967-020-02428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seferović P. M., Coats A. J. S., Ponikowski P., et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. European Journal of Heart Failure. 2020;22(2):196–213. doi: 10.1002/ejhf.1673. [DOI] [PubMed] [Google Scholar]
- 95.McHugh K., DeVore A. D., Wu J., et al. Heart Failure With Preserved Ejection Fraction and Diabetes: Journal of the American College of Cardiology. 2019;73(5):602–611. doi: 10.1016/j.jacc.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 96.Wang X., Gu H., Huang W., et al. Hsp20-Mediated Activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic Mice. Diabetes. 2016;65(10):3111–3128. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkat P., Cui C., Chen Z., et al. CD133+exosome treatment improves cardiac function after stroke in type 2 diabetic mice. Translational Stroke Research. 2020 doi: 10.1007/s12975-020-00807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang B., Zhang A., Wang H., et al. Consequences of Confronting Patronizing Help for People with Disabilities: Do Target Gender and Disability Type Matter? Theranostics. 2019;75(3):904–923. doi: 10.1111/josi.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]


