Abstract
A 53-year-old man presented with fulminant hepatitis due to de novo hepatitis B. He had been diagnosed previously with adult T-cell leukemia (ATL) and previously resolved hepatitis B virus infection. The ATL had been treated with cord blood transplantation (CBT). He developed fulminant hepatitis 18 months after CBT, 15 months after the withdrawal of immunosuppressants, and 10 months after vitreous injections of methotrexate for ATL-related retinal infiltration. The aggressive medical protocol included entecavir, prednisolone, plasma exchange, hemodialysis, and bilirubin adsorption. We herein report successful medical treatment for fulminant de novo hepatitis B in a patient considered unsuitable for liver transplantation.
Keywords: acute liver failure, artificial liver support, de novo hepatitis B, fulminant hepatitis, hepatitis B virus, hepatitis B virus reactivation
Introduction
Hepatitis B virus (HBV) infection is an important clinical condition in Japan; the number of Japanese HBV carriers is estimated to exceed 1 million (1), and more than 20% of Japanese people >50 years old has a history of transient HBV infection (2), defined as serum anti-HBs and/or anti-HBc positivity despite a negative serum HBs-antigen (Ag) status. These data suggest that more than 10 million Japanese people have either persistent or previously resolved HBV (prHBV) infection. Such individuals are at a high risk of developing liver injury after HBV reactivation during and after immunosuppressive therapies and antineoplastic chemotherapies.
HBV reactivation in patients with prHBV infection was originally documented in 1975 by Wands et al. (3). The development of liver injury after HBV reactivation in patients with prHBV infection, so-called ‘‘de novo hepatitis B,’’ is a serious clinical problem, especially in those receiving chemotherapy for hematological malignancies (3, 4). With regard to the incidence of fulminant hepatitis in Japan, de novo cases are more common than acute hepatitis B. Furthermore, fulminant de novo hepatitis B is associated with a grim prognosis, and the survival is difficult to achieve with medications alone (5). The prognosis is also poor in patients with HBV reactivation associated with chemotherapy and/or immunosuppressive therapy, as confirmed in the nationwide surveys of acute liver failure (ALF) and late-onset hepatic failure (LOHF) conducted in Japan from 2010 to 2015 (6).
To our knowledge, there are no reports of rescue following medical treatment of fulminant de novo hepatitis B. We herein report a case of fulminant hepatitis due to de novo hepatitis B after cord blood transplantation (CBT, representing stem cell therapy) and treatment with immunosuppressants for adult T-cell leukemia (ATL). This patient is a rare case rescued by medical therapy, which was the only available option due to the inappropriateness of liver transplantation (LT) for this patient.
Case Report
A 53-year-old Japanese man had a history that included the diagnosis of ATL and prHBV infection (positive for anti-HBc, anti-HBs, negative for HBs Ag, HBV-DNA). He received CBT in November, 2017, as part of the therapeutic management of ATL. The mother of the donor via the cord blood bank was confirmed to be HBV negative (negative for anti-HBc, HBs Ag and HBV-DNA). Subsequently, the patient received immunosuppressants [oral tacrolimus (TAC) daily at 1.1 mg/day, plus intravenous methotrexate (MTX) at 6.2 mg] at 1, 3, and 6 days after CBT to protect against possible graft-versus-host disease. TAC was withdrawn in February, 2018. Afterwards, the patient developed loss of vision in the right eye. This was suspected to be ATL retinal infiltration and treated with a total of five vitreous injections of MTX between May to July, 2018. Subsequently, ATL was considered to be in remission, and no treatment with immunosuppressants was provided during the monthly follow up. He was also not given HBV vaccine after transplantation. The last evaluation for HBV markers conducted in August 2018 showed negativity for HBs Ag and HBV-DNA. In addition, the liver function tests were within the normal ranges in April 2019.
In middle of May, 2019, the patient complained of general fatigue and poor appetite (this was subsequently considered the date of the onset of hepatitis). He visited our hospital 10 days later and was found to have severe liver injury upon admission. Based on the results of the laboratory tests at admission (Table 1), he was diagnosed with ALF associated with HBV reactivation (both HBs Ag and HBV-DNA were positive) after exclusion of other causes of hepatic injury, including hepatitis A, hepatitis C, autoimmune hepatitis, cytomegalovirus, and Epstein-Barr virus infections. He was also not taking any drugs that affect the prothrombin time-international normalized ratio (PT-INR) (anticoagulants or antibacterial agents).
Table 1.
Laboratory Data on Admission.
| Peripheral blood | Biochemistry | Viral markers | ||||||
| Leukocyte count | 12,200 | /μL | TP | 7.2 | g/dL | HBsAg | 120,000 | IU/mL, (+) |
| Neutrophil | 73.5 | % | Albumin | 4.1 | g/dL | Anti-HBs | <2.5 | mIU/mL, (-) |
| Lymphocyte | 18 | % | T-Bil | 8.9 | mg/dL | HBeAg | 687.5 | S/CO, (+) |
| Monocyte | 6.5 | % | D-Bil | 6.6 | mg/dL | Anti-HBe | 0.1 | %, (-) |
| Eosinophil | 2 | % | AST | 4,886 | U/L | Anti-HBc | 4.34 | S/CO, (+) |
| Basophil | 0 | % | ALT | 6,839 | U/L | HBV-DNA | 7.5 | LogIU/mL |
| Atypical lymphocyte | 0 | % | LDH | 1,960 | U/L | HBV genotype | C | |
| Erythrocyte count | 4.52×106 | /μL | ALP | 541 | U/L | HBV pre-core | Wild 0%, Mutant 100% | |
| Hemoglobin | 14.0 | g/dL | γ-GTP | 202 | U/L | HBV core-promoter | Wild | |
| Hematocrit | 41.4 | % | BUN | 32 | mg/dL | IgM anti-HAV | (-) | |
| Platelet count | 16.0×104 | /μL | CRE | 1.17 | mg/dL | Anti-HCV | (-) | |
| Coagulation | Na | 136 | mEq/L | IgA anti-HEV | (-) | |||
| PT% | 24.2 | % | K | 4.6 | mEq/L | IgM/IgG anti-CMV | (-)/(+) | |
| PT-INR | 2.22 | Cl | 101 | mEq/L | IgM/IgG anti-EB-VCA | (-)/(+) | ||
| APTT | 51.9 | s | NH3 | 55 | μg/dL | IgG Anti EBNA | (+) | |
| Serology | CRP | 2.18 | mg/dL | |||||
| ANA | (-) | |||||||
| AMA | (-) | |||||||
T: prothrombin time, INR: international normalized ratio, ANA: antinuclear antibody, AMA: anti-mitochondria antibody, TP: total protein, T-Bil: total bilirubin, D-Bil: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, CRE: creatinine, Ag: antigen, HAV: hepatitis A virus, HBV: hepatitis B virus, HCV: hepatitis C virus, HEV: hepatitis E virus, CMV: cytomegalovirus, EB: Epstein-Barr, VCA: viral capsid antigen, EBNA: Epstein-Barr nuclear antigen, S/CO: signal/cut-off
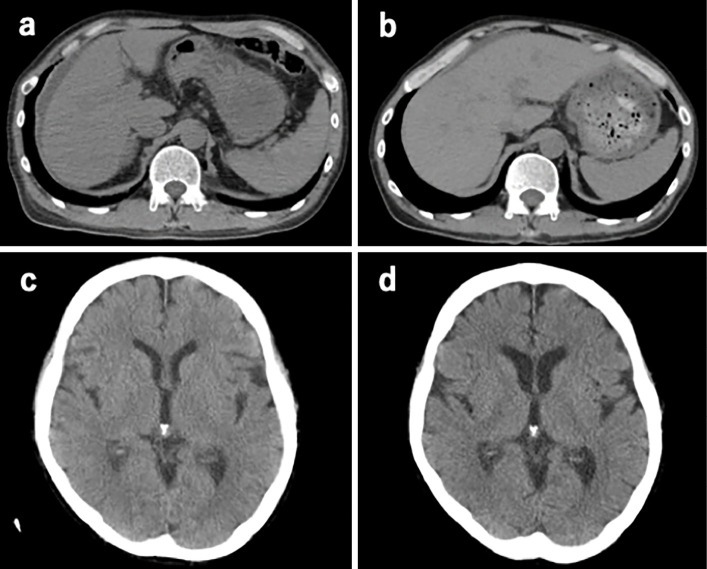
A physical examination on admission showed neither ascites nor signs of hepatic coma (as proposed by the Inuyama Symposium) (7). The clinical course is summarized in Fig. 1 (date of hospitalization labeled as day 1). Table 2 shows the serial changes in the viral markers, α-fetoprotein (AFP), and hepatocyte growth factor (HGF), which were measured as prognostic factors, throughout the clinical course. HGF was ≥1 ng/mL from the day of admission, which was considered to reflect a poor prognosis. The initial treatment included oral prednisolone (30 mg/day), entecavir (ETV) (0.5 mg/day), lactulose, and rifaximin along with plasma exchange (PE). Each PE session included the administration of 30 units of fresh-frozen plasma (FFP). On the PE-free days, 4 units of FFP were transfused. However, the patient developed grade II hepatic coma on day 6 (15 days after onset), consistent with the diagnosis of fulminant hepatitis (subacute type) (8). Computed tomography (CT) showed mild liver atrophy, ascites on the liver surface, and mild brain edema (Fig. 2). At this stage, LT was considered inappropriate because he had been treated with ATL for 18 months. From day 8, we started combined hemodialysis (HD) as treatment enhancement. This was followed by the resolution of his hepatic coma on day 10. By day 17, the patient was able to remain alert, and the PT increased to 50% (PT-INR 1.35). The blood purification treatment was withdrawn, as was prednisolone, but the bilirubin level continued to rise. In addition to the oral administration of ursodeoxycholic acid, bilirubin adsorption therapy was started from day 29. After 4 courses of bilirubin adsorption therapy (until day 37), the serum bilirubin level decreased gradually, allowing the patient to be discharged on day 63. CT before discharge confirmed improvements in his liver atrophy, ascites, and brain edema (Fig. 2). The total number of PE sessions was 11, HD 4, and bilirubin adsorption therapy 4.
Figure 1.
Clinical course. Days indicates days from admission. FFP: fresh-frozen plasma, HD: hemodialysis, PE: plasma exchange therapy, PSL: prednisolone
Table 2.
Serial Changes in Viral Markers, AFP, and HGF during the Clinical Course.
| Diagnosis of ATL | CBT treatment | ATL retinal infiltration | Follow up at the previous facility | Admission | Onset of coma | After discharge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| date | 2017/8 | 2017/11 | 2018/5 | 2018/8 | 2019/5 | ||||||||||
| date from admission | 21 months ago | 18 months ago | 12 months ago | 9 months ago | Day 1 | 6 | 13 | 27 | 34 | 41 | 56 | 71 | 107 | 133 | 198 |
| MTX 6.2 mg | MTX | ETV 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | ETV 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | 0.5 mg/day | |||
| Treatment contents | - | (1, 3 and 6 days after CBT) | (vitreous injections) | - | PSL 30 mg/day | 30 mg/day | 20 mg/day | 10 mg/day | 5 mg/day | - | - | - | - | - | - |
| TAC 1.1 mg/day (~2018/2) | (~2018/7) | PE | PE | PE+HD | - | Bilirubin adsorption | - | - | - | - | - | - | |||
| HBs Ag (IU/mL) | 0.01 | N.E. | N.E. | 0.01 | 120,000 | 4,600 | 82.31 | 45.55 | 32.03 | 16.03 | 4.77 | 0.3 | 0.01 | 0.01 | 0.01 |
| Anti-HBs (mIU/mL) | 62.3 | N.E. | N.E. | 5.9 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 | N.E. | N.E. | 3.1 | 9.9 |
| Anti-HBc (S/CO) | 43.6 | N.E. | N.E. | 5 | 4.34 | N.E. | N.E. | N.E. | N.E. | N.E. | N.E. | N.E. | N.E. | N.E. | N.E. |
| IgM anti-HBc (S/CO) | N.E. | N.E. | N.E. | N.E. | N.E. | 15.6 | 3.2 | 5 | 4.9 | 3.4 | 2.7 | N.E. | N.E. | N.E. | N.E. |
| HBe Ag (S/CO) | N.E. | N.E. | N.E. | N.E. | 687.5 | 19 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 |
| Anti-HBe (S/CO) | N.E. | N.E. | N.E. | N.E. | 0.1 | 15.6 | 24.7 | 32.6 | 33.7 | 15.5 | 29.2 | 33.7 | 40.5 | 40 | 44 |
| HBV-DNA (LogIU/mL) | negative | N.E. | N.E. | negative | 7.5 | 4.6 | 3.4 | 2.7 | 2.6 | 2.3 | 2.2 | 1.6 | <1.0 | negative | negative |
| AFP (ng/mL) | N.E. | N.E. | N.E. | N.E. | 5.6 | 89.7 | 45.2 | 65 | N.E. | 33.5 | 27.8 | 15.6 | 7.1 | 6 | 5.6 |
| HGF (ng/mL) | N.E. | N.E. | N.E. | N.E. | 1.62 | 1.61 | 3.06 | 2.06 | N.E. | 2.47 | 1.29 | N.E. | N.E. | N.E. | N.E. |
AFP: α-fetoprotein, Ag: antigen, ATL: adult T-cell leukemia, CBT: cord blood transplantation, ETV: entecavir, HBV: hepatitis B virus, HD: hemodialysis, HGF: hepatocyte growth factor , MTX: methotrexate, N.E.: not examined, PE: plasma exchange therapy, PSL: prednisolone, S/CO: signal/cut-off, TAC: tacrolimus
Figure 2.
CT scans for the abdomen (a, b) and brain (c, d) obtained on Days 6 and 62 from the onset, respectively. Note the liver atrophy, ascites, and mild brain edema on day 6 and the improvement in these parameters on day 62.
Discussion
We reported a case of fulminant de novo hepatitis B successfully managed with aggressive treatment without the need for LT.
We considered the HBV pre-core (PC) mutation important as a viral factor that made this case fulminant. The PC and core-promoter (CP) mutations are very frequent in patients with fulminant hepatitis from Japan (9). The HBV subgenotype and PC and CP mutations have been linked to high replication rates in acute HBV infection and in turn to a fulminant outcome (10). The evaluation of the presence of a PC mutation is thus considered an important factor in the course of hepatitis B, including the prediction of the likelihood of a fulminant outcome.
Thus far, the only successful treatment for ALF has been urgent LT. Artificial liver support (ALS) systems maintain stable condition until recovery of the liver function or performance of LT and are beneficial in improving the prognosis of ALF. However, ALS do not promote liver regeneration and are not known to improve the survival without LT (6, 8, 11-13). Clinically speaking, however, treatment of hematological malignancies, as in this case, cannot be followed by LT in all cases, so ALS should be used in such patients. The purpose of ALS is to support the minimal liver function required to sustain the life of ALF patients through (i) removal of toxic compounds causing hepatic coma; (ii) supply of useful substances, such as clotting factors, which are often deficient due to an impaired liver production; (iii) correction of water, electrolyte, and acid-base balance; and (iv) removal of various pro-inflammatory cytokines believed to be associated with the progression of ALF. In this regard, PE is the first-line ALS. It removes several compounds of various molecular weights from the blood stream non-selectively and replenishes plasma components, including coagulation factors and opsonins. The substances involved in the induction and maintenance of hepatic coma with known central nervous system toxicity are considered to have a low-to-medium molecular weight. However, the efficacy of PE is limited to the removal of low- to medium-molecular-weight substances. Furthermore, PE consumes large amounts of FFP, and the number of PE sessions per patient is strictly limited by Japanese National Health Insurance. It has also possible side effects, such as hypernatremia, metabolic alkalosis, and rapid change in colloid osmotic pressure. In contrast, HD efficiently removes low-molecular-weight substances, such as ammonia, while hemofiltration removes medium-molecular-weight substances, such as pro-inflammatory cytokines. Accordingly, it is desirable to apply hemodiafiltration (HDF) for the treatment of patients with ALF. Thus, HDF is typically administered in conjunction with PE to remove low- to medium-weight molecules and reduce the potential side effects of PE (14). In our case, the resolution of hepatic coma occurred relatively rapidly after the combined use of HD and PE.
In addition to the above ALS endeavors, bilirubin adsorption therapy was also used. To our knowledge, there is little or no information on the benefits of such therapy and whether or not it leads to an improvement in the prognosis of ALF. Since LT was considered inappropriate, and the overall condition was considered to be severe de novo hepatitis B with a poor prognosis, it was necessary to administer multidisciplinary treatment centering on the frequent application of ALS. Although various factors may have played a role in the positive outcome seen in our patient, ALS was one of the main factors and was considered effective.
Other factors that contributed to the good outcome of this case were the lack of any complications, such as infections and DIC. In addition, the scoring criteria for the LT indications for fulminant hepatitis in Japan (15) was 4 points (the interval between hepatitis onset and the development of hepatic coma was 15 days, which was 2 points; the ratio of direct to total bilirubin concentrations was 0.52, which was 1 point; liver atrophy present, which was 1 point) at the development of hepatic coma, and this corresponded to a mortality of 50%. This scoring system predicts death if a total of ≥5 points is noted at the onset of a grade II coma. Although the use of FFP had an effect on PT in this case, this system may be useful for predicting the prognosis of cases of fulminant de novo hepatitis B.
In Japan, severe hepatitis due to viral reactivation is a serious health problem. In our case, 18 months had passed since the CBT, 15 months since the withdrawal of oral immunosuppressants, and 10 months since the end of intraocular MTX injection. The Guidelines of the Japan Society of Hepatology (JSH Guideline) advise monthly HBV-DNA monitoring in patients undergoing hematopoietic stem cell transplantation (HSCT) or chemotherapy including rituximab, corticosteroids, or fludarabine during the treatment and for at least 12 months after its completion (16). In addition, the Guidelines of the Japan Society of Hematopoietic Cell Transplantation indicate that HBV-DNA monitoring post-HSCT should be continued during immunosuppressant administration and for at least 12 months after termination. The results of the present case indicate that prHBV patients who undergo HSCT require monthly monitoring, including for HBV-DNA, for as long as possible, even beyond 12 months later.
The effect of antiviral treatment was shown to be attenuated in patients with HBV reactivation who received HSCT, which appears to be involved in the reduction of the immune response associated with the decrease in CD8+ T cells against HBV (17). The administration of ETV in this case was thought to have been useful for suppressing the progression of liver failure, but caution must be exercised to avoid reducing the effectiveness of antiviral treatment after reactivation.
One strategy for preventing reactivation is to administer HBV vaccines. For prHBV recipients after HSCT, HBV vaccination is expected to prevent HBV reactivation by immunizing engrafted donor cells and maintaining the anti-HBs titer (18). Indeed, the anti-HBs titer in this case decreased and disappeared. Since anti-HBs disappears before HBV reactivation (19), monitoring the anti-HBs titer after transplantation may lead to early vaccine administration and prediction of reactivation risk. Clinical research (UMIN000034113) is currently being carried out, which we hope will supply meaningful results. In addition, when HSCT donors are vaccinated or prHBV, the hazard ratio is 0.12, which reduces the risk of reactivation. Therefore, donor-derived anti-HBs may act to suppress reactivation (20).
The risk of HBV reactivation must be explained to patients, and advice should be given regarding the benefits of prompt medical therapy on noting the appearance of new clinical symptoms, such as general fatigue, jaundice, and poor eating habits. To prevent ALF in the future, new alternative treatment modalities and lateral thinking continue to be important for both healthcare professionals and patients.
Author's disclosure of potential Conflicts of Interest (COI).
Norio Akuta: Honoraria, AbbVie. Masahiro Kobayashi: Honoraria, Eisai. Yasuji Arase: Honoraria, AbbVie. Hiromitsu Kumada: Honoraria, MSD, Gilead Sciences, AbbVie, Eisai and Dainippon Sumitomo Pharma.
Acknowledgement
The authors thank Drs. T. Fukuda, J. Hoshino, A. Sekine, Y. Tohyama, K. Koda and M. Matsugaki, for their cooperation in the management of the reported case.
References
- 1. Tanaka J, Kumagai J, Katayama K, et al. Sex- and age-specific carriers of hepatitis B and C virus in Japan estimated by the prevalence in the 3,485,648 first-time blood donors during 1995-2000. Intervirology 47: 32-40, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Kusumoto S, Tanaka Y, Mizokami M, et al. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 90: 13-23, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Wands JR, Chura CM, Roll FJ, et al. Serial studies of hepatitis associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology 68: 105-112, 1975. [PubMed] [Google Scholar]
- 4. Lok AS, Liang RH, Chiu EK, et al. Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 100: 182-188, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Umemura T, Kiyosawa K. Fatal HBV reactivation in a subject with anti-HBs and anti-HBc. Intern Med 45: 747-748, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Nakao M, Nakayama N, Uchida Y, et al. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol 53: 752-769, 2018. [DOI] [PubMed] [Google Scholar]
- 7.In: The Proceedings of the 12th Inuyama Symposium. Hepatitis type A and fulminant hepatitis. Chugai Igaku-sha, Tokyo, 1982: (in Japanese). [Google Scholar]
- 8. Fujiwara K, Mochida S, Matsui A, Nakayama N, Nagoshi S, Toda G. Fulminant hepatitis and late onset hepatic failure in Japan. Hepatol Res 38: 646-657, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Omata M, Ehata T, Yokosuka O, et al. Mutations in the precore region of hepatitis B virus DNA in patients with fulminant and severe hepatitis. N Engl J Med 324: 1699-1704, 1991. [DOI] [PubMed] [Google Scholar]
- 10. Ozasa A, Tanaka Y, Orito E, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology 44: 326-334, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Oketani M, Ido A, Nakayama N, et al. Etiology and prognosis of fulminant hepatitis and late-onset hepatic failure in Japan: summary of the annual nationwide survey between 2004 and 2009. Hepatol Res 43: 97-105, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Fujiwara K, Yasui S, Tawada A, et al. Autoimmune fulminant liver failure in adults: experience in a Japanese center. Hepatol Res 41: 133-141, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Yasui S, Fujiwara K, Haga Y, et al. Infectious complications, steroid use and timing for emergency liver transplantation in acute liver failure: Analysis in a Japanese center. J Hepatobiliary Pancreat Sci 23: 756-762, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Fujiwara K, Abe R, Yasui S, Yokosuka O, Kato N, Oda S. High recovery rate of consciousness by high-volume filtrate hemodiafiltration for fulminant hepatitis. Hepatol Res 49: 224-231, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Naiki T, Nakayama N, Mochida S, et al. ; Intractable Hepato-Biliary Disease Study Group supported by the Ministry of Health, Labor and Welfare of Japan. Novel scoring system as a useful model to predict the outcome of patients with acute liver failure: Application to indication criteria for liver transplantation. Hepatol Res 42: 68-75, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res 44 (Suppl): 1-58, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Kimura M, Nishikawa K, Sakamaki H, Mizokami M, Kimura K. Reduced therapeutic effect of antiviral drugs in patients with hepatitis B virus reactivation after hematopoietic stem cell transplantation. Hepatol Res 48: 469-478, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Onozawa M, Hashino S, Darmanin S, et al. HB vaccination in the prevention of viral reactivation in allogeneic hematopoietic stem cell transplantation recipients with previous HBV infection. Biol Blood Marrow Transplant 14: 1226-1230, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Onozawa M, Hashino S, Izumiyama K, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 79: 616-619, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Mikulska M, Nicolini L, Signori A, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect 20: 694-701, 2014. [DOI] [PubMed] [Google Scholar]