Abstract
Objective
Baloxavir marboxil is a novel anti-influenza drug reported to have an early antiviral effect, although it also causes the appearance of variant viruses with a reduced susceptibility to baloxavir. In Japan, four neuraminidase inhibitors (NAIs) have been commonly used to treat patients with influenza. In clinical practice, the differences in the effects of baloxavir and NAIs have not been sufficiently examined. Our objective was to clarify the clinical differences in efficacy between baloxavir and NAIs.
Methods
A multicenter, observational study was conducted using postcard questionnaires during the 2018-19 influenza season. Patients who were prescribed anti-influenza drugs were provided postcard questionnaires asking about their background characteristics and their body temperatures. The factors associated with the early alleviation of the fever were analyzed, and the duration of the fever was compared between the baloxavir group and the NAI group.
Results
A total of 295 patients with influenza A, ranging in age from 0-91 years old, were enrolled in this study. A multivariate analysis showed that treatment with baloxavir and a duration from the onset to the start of treatment ≥2.5 days were factors contributing to the early alleviation of the fever from the start of treatment. The duration of the fever was significantly shorter in the baloxavir group than in the NAI group (p=0.002).
Conclusion
The present survey showed that baloxavir was significantly more effective than NAIs for treating patients with influenza A in clinical practice.
Keywords: influenza, baloxavir marboxil, neuraminidase inhibitor
Introduction
Influenza A and B viruses cause seasonal epidemics every year, and if novel strains of influenza A virus spread worldwide, a global pandemic can ensue. Elderly patients, pregnant women, and immunocompromised patients are at a particularly high risk of hospitalization and mortality due to influenza virus infection; the control of the influenza virus is therefore an important issue (1). Although neuraminidase inhibitors (NAIs) are widely used for the treatment of influenza, new anti-influenza drugs are being developed in the face of resistance to NAIs and future pandemic threats.
Baloxavir marboxil is a novel anti-influenza drug that suppresses viral growth by inhibition of Cap-dependent endonuclease (2). In Japan, baloxavir marboxil (baloxavir) was released in March 2018, before the rest of the world. Until then, four NAIs (oseltamivir, zanamivir, peramivir, and laninamivir) had been used for the treatment of influenza virus A and B infections. Outside Japan, the prescription of NAIs is commonly recommended for groups at a high risk of developing severe or complicated illness (3). However, in Japan, 80-95% of influenza patients visit a medical facility within 48 hours from the onset of influenza infection, and anti-influenza drugs are prescribed to most patients diagnosed based on a positive influenza antigen detection kit rather than being limited to high-risk patients (4).
Oseltamivir requires oral administration twice daily for 5 days. It is the most commonly prescribed NAI, and its efficacy and safety have been reported (5). However, an oseltamivir-resistant influenza virus emerged in 2007 and entered global circulation in the 2008-2009 influenza season (6). Zanamivir is an inhaled drug used twice a day for 5 days. Zanamivir is effective against oseltamivir-resistant virus (7). Peramivir is intravenously delivered once a day on the first treatment day only or several days as needed. The efficacy of peramivir has been reported in patients with severe influenza who cannot take other NAIs (8). Laninamivir is a relatively new NAI approved only in Japan; it is a single-dose inhalation drug that has the advantage of good adherence with medication (9-11). Therefore, laninamivir is prescribed as often as oseltamivir in Japan. Aside from efficacy against oseltamivir-resistant influenza viruses, it has been reported that there is no significant difference in efficacy among the NAIs (7, 12-14).
Baloxavir is a single oral medication that reduces influenza virus titers significantly more rapidly than oseltamivir (2). However, after the administration of baloxavir, amino acid substitutions at position 38 of polymerase acidic protein in the influenza virus (PA/I38X) have occasionally emerged in pediatric and adult patients (23% and 10%, respectively) (2). The susceptibility of the PA/I38T mutant virus to baloxavir is reportedly reduced by about 50-fold. However, influenza viruses with this mutation have a reduced proliferation ability. Whether or not baloxavir has less of an effect on the I38X mutant virus than virus of non-mutant has not been proved (15).
There are few reports comparing the clinical effects of baloxavir and NAIs in clinical practice. A postcard questionnaire survey of patients diagnosed with influenza virus infection and prescribed anti-influenza drugs in the winter of 2018-2019 was conducted, and the efficacies of baloxavir and NAIs were compared to clarify the clinical differences between them.
Materials and Methods
This multicenter, observational study was conducted in Osaka Prefecture, Japan. Physicians, pediatricians, and otorhinolaryngologists at 50 clinics or general hospitals participated in this study. Patients who were diagnosed using an influenza antigen detection kit and treated with baloxavir or NAIs from December 1, 2018, through April 30, 2019, were enrolled. After obtaining informed consent, clinicians completed the sections on age, sex, type of influenza (A or B), and the anti-influenza drug prescribed, and then they handed the postcard questionnaires to the patients. Patients recorded their highest body temperatures twice a day (in the morning and evening) and completed the questionnaire forms on their influenza vaccination statuses, underlying diseases, the date (AM or PM) of onset, and the start date (AM or PM) of the anti-influenza drug. After filling out the postcards, patients mailed them to the Department of Respiratory Medicine, Osaka City University, Graduate School of Medicine. Cases with any missing data for age, type of flu, or prescribed NAIs were excluded.
The duration of the fever was defined as the period of time from the administration of the anti-influenza drugs until the fever was alleviated for more than one day with no relapse thereafter. A decrease in the fever was defined as temperatures under 37.5℃ in patients under 10 years of age or under 37.0℃ in patients ≥10 years of age, as previously described (9, 10).
The study protocol was approved by the Institutional Ethics Committee (Osaka City University, Graduate School of Medicine).
Statistical analyses
The statistical analyses were performed with the JMP software program, ver. 10 (SAS Institute, Cary, USA). A p value less than 0.05 was considered significant. Pearson's chi-squared test was used to assess group differences in the percentages of patients. Mann-Whitney's U test was used to compare averages. A multivariate analysis was conducted and followed by a univariate analysis with logistic regression models to examine the factors that were related to the alleviation of the fever two days after the treatment with anti-influenza drugs started. Wilcoxon's rank-sum test was used to compare the median times until the alleviation of the fever.
Results
Patients' characteristics
A total of 900 postcards were handed to patients with positive rapid diagnostic kit results. The response rate was 34.6% (311 of 900). Fifteen patients were excluded from the analyses because of incomplete questionnaire data for age, type of influenza, body temperature, or prescribed NAI. One patient had influenza B, and the remaining 295 had influenza A infection, so the data of patients with influenza A were analyzed.
Table 1 lists the clinical characteristics of 111 patients who received baloxavir and 184 patients who received NAIs. The NAIs prescribed for the patients were oseltamivir for 74, zanamivir for 24, peramivir for 4, and laninamivir for 77. With the exception of the mean age and the rate of chills as an initial symptom, the baseline characteristics did not differ markedly between the two groups. The mean age and range were 26.32±20.40 and 2-72 years old for the baloxavir group and 21.21±22.99 and 0-91 years old for the NAI group.
Table 1.
Clinical Characteristics of Patients with Influenza A.
| Baloxavir | NAIs | p | |||
|---|---|---|---|---|---|
| No. of patients | 111 | 184 | |||
| Oseltamivir | 74 | ||||
| Zanamivir | 29 | ||||
| Peramivir | 4 | ||||
| Laninamivir | 77 | ||||
| Gender F/M/unknown | 50/60/1 | 73/108/3 | 0.60 | ||
| Age | Average (Range) | 26.32±20.40 (2-72) | 21.21±22.99 (0-91) | 0.002 | |
| 0-4 | 4 | 32 | |||
| 5-9 | 34 | 64 | |||
| 10-19 | 18 | 30 | |||
| 20- | 55 | 58 | |||
| Vaccinated this season yes/no/unknown | 44/65/2 | 77/96/11 | 0.08 | ||
| Duration from onset to drug administration | 0.99±0.84 | 0.96±0.75 | 0.95 | ||
| First symptoms (Multiple answers allowed) |
Number of Total answer | 242 | 157 | ||
| Fever | 103 (39.9%) | 60 (36.1%) | 0.43 | ||
| Cough | 51 (19.7%) | 22 (13.3%) | 0.08 | ||
| Sore throat | 33 (12.8%) | 26 (15.7%) | 0.40 | ||
| Chills | 7 (2.7%) | 12 (7.2%) | 0.03 | ||
| General malaise | 12 (4.7%) | 9 (5.4%) | 0.72 | ||
| Head ache | 15 (5.8%) | 9 (5.4%) | 0.86 | ||
| Myalgia or joint pain | 7 (2.7%) | 11 (6.6%) | 0.05 | ||
| Nasal discharge | 14 (5.4%) | 8 (4.8%) | 0.78 | ||
NAIs: neuraminidase inhibitors
Factors related to the early alleviation of the fever
A univariate analysis showed that the type of anti-influenza drug and duration between onset of symptoms and the start of anti-influenza drugs were associated with the alleviation of the fever within two days. In a multivariate analysis, the type of anti-influenza drug and the time from the onset of symptoms to the start of anti-influenza drugs were the factors significantly correlated with the alleviation of the fever in 2 days (odds ratio 1.72, 95% confidence interval 1.05-2.84, p=0.03; and odds ratio 2.78, 95% confidence interval 1.08-8.65, p=0.03, respectively.) This means that treatment with baloxavir and a time from the onset to the start of treatment ≥2.5 days were the factors contributing to the early alleviation of the fever after the start of treatment (Table 2).
Table 2.
Factors Related to Alleviation of Fever in 2 Days in the Patients with Influenza A.
| Variable | Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | O.R. | 95% CI | p value | O.R. | 95% CI | p value | ||||
| Gender | Female | 93/168 (55.0) | 1 | - | - | |||||
| Male | 75/123 (61.0) | 1.26 | 0.79-2.03 | 0.33 | ||||||
| 10> years old | 78/134 (58.2) | 1 | - | - | ||||||
| 10≤ years old | 93/161 (57.8) | 0.94 | 0.62-1.56 | 0.94 | ||||||
| Vaccinated this year | No | 88/161 (54.7) | 1 | - | - | |||||
| Yes | 72/121 (59.5) | 1.22 | 0.76-1.97 | 0.42 | ||||||
| Anti influenza drug | NAIs | 97/184 (52.7) | 1 | - | - | |||||
| Baloxavir | 74/111 (66.7) | 1.79 | 1.10-2.94 | 0.02 | 1.72 | 1.05-2.84 | 0.03 | |||
| Duration from onset to drug administration |
≤2 days | 152/270 (56.3) | 1 | - | - | |||||
| ≥2.5 days | 19/24 (79.2) | 2.95 | 1.15-9.10 | 0.02 | 2.78 | 1.08-8.65 | 0.03 | |||
We also compered the rate of alleviation of the fever within 1 day from the start of anti-influenza drugs between the groups with times from the onset to the start of anti-influenza drugs ≤2 days and ≥2.5 days; the results were 3.8% (10/270), and 20.8% (5/24), respectively (p=0.003).
The comparison of the duration of the fever
Figure shows that the duration of the fever was significantly shorter in the baloxavir group than in the NAI group (p=0.002). The mean time to the alleviation of the fever ± standard deviation was 1.94±0.09 days for the baloxavir group and 2.35±0.08 days for the NAI group.
Figure.
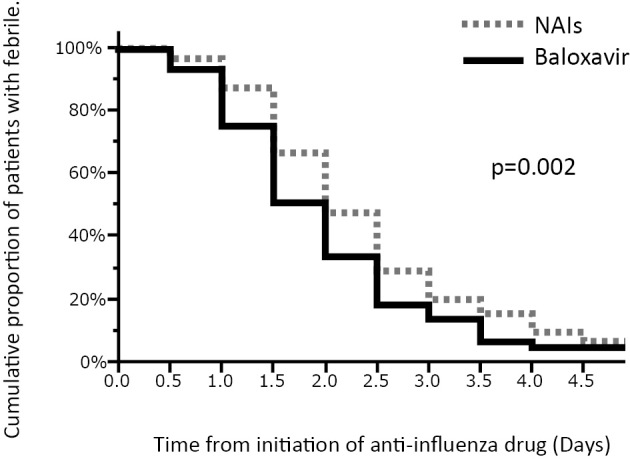
Kaplan-Meier curves of the time to the alleviation of the fever. The duration of the fever in the baloxiavir group was significantly shorter than in the NAIs group. NAIs: neuraminidase inhibitors
Discussion
In this survey, the new anti-influenza drug baloxavir and the time from onset of symptoms to administration of the anti-influenza drug ≥2.5 days were factors significantly associated with early alleviation of the fever; sex, age group (<10 or ≥10 years old), and vaccination this season were not associated with early alleviation of the fever. The duration of the fever from the initial administration of anti-influenza drug was significantly shorter in the baloxavir group than in the NAI group. We consider that the early and high antiviral activity of baloxavir contributed to the early alleviation of the fever.
Hayden et al. reported that the virus titer was significantly lower from the second day of administration in the baloxavir group than in the oseltamivir group (2). However, they also reported that the median time to alleviation of symptoms (TTAS) was similar in the baloxavir group and the oseltamivir group. In the present study, only the alleviation of the fever, but not the resolution of other symptoms, was assessed. The effect of the antiviral activity of baloxavir may have been more reflected in the length of the febrile period than in other symptoms. Uehara et al. reported that 3-9 days after baloxavir treatment, viruses with reduced susceptibility due to PA/I38X occasionally emerged (16). They also reported that, in the baloxavir group with substituted PA/I38X, the virus titer increased again from day 4 to day 5 after receiving baloxavir. The median TTAS was 12.0 hours longer in the group with PA/I38X-substituted viruses than in the group without PA/I38X-substituted viruses. The emergence rate of viruses with PA/I38X-substituted has been reported to be 9.7% in patients 12 to 65 years old and 23% in those younger than 12 years old (2, 17). A certain number of these mutations might have emerged in the present subjects treated with baloxavir. However, on the whole, the duration of the fever was significantly shorter in the baloxavir group than in the NAI group. Based on previous reports (2, 17), the number of patients without PA/I38-substituted would have been more than that of patients with PA/I38-substituted in this study. Therefore, even if the febrile period had been prolonged in patients with the mutation, the overall data of the duration of the fever might not have been markedly affected. Further clinical studies are needed to determine the impact of influenza infection with PA/I38X-substituted viruses in terms of the clinical efficacy.
In the present multivariate analysis, a time from the onset to the start of anti-influenza drug ≥2.5 days also contributed to the early alleviation of the fever from the start of treatment. It is recommended that both baloxavir and NAIs be started within 48 hours from the onset of symptoms (18). Most patients assessed in this survey had started treatment with anti-influenza drugs within 2 days of the onset, and only 24 patients had taken more than 2.5 days to start treatment. We compared the rate of fever alleviation within 1 day from the start of anti-influenza drugs between the groups with a time from the onset to the start of treatment ≤2 days and ≥2.5 days, and the rate was significantly higher in the group with ≥2.5 days than in the group with ≤2 days. This result suggested that the patients in the group with ≥2.5 days might have been recovering at the start of treatment. Influenza is a self-limiting disease in most cases, and the median time to the resolution of the fever, even in placebo groups, has been shown to be about 2 days (2, 3). This is considered why taking ≥2.5 days from the onset of symptoms to the initial treatment was significantly associated with the early alleviation of the fever from the start of treatment. It has been reported that initiating baloxavir within 24 hours after the onset contributes more profoundly to the earlier recovery of symptoms than initiating baloxavir between 24 and 48 hours after the onset (2).
The present study had three limitations. First, the return rate of the postcard questionnaires was not high. Second, the answers including the temperatures were self-reported data, thus limiting the reliability. In addition, parents would have filled in the questionnaire on behalf of their young children with influenza, and some of the elderly patients might not have answered this survey sufficiently compared to younger patients. There may therefore be a difference in the data reliability depending on the age group. Third, the subtypes of influenza A virus that infected the present subjects and the emergence of PA/T38X-substituted viruses could not be evaluated. However, this postcard survey provides important information about background characteristics and the clinical course of the outpatients every year. In Japan, the Infectious Disease Surveillance Center (IDSC) of the National Institute of Infectious Diseases reported the proportion of subtypes of circulating influenza in the 2018-2019 influenza season, and the rates of A(H1N1)pdm09, A(H3N2), and Type B were 38%, 56%, and 6%, respectively. The IDSC also reported that the proportions of A(H1N1)pdm09 strains resistant to oseltamivir and baloxavir were 0.8% and 1.5%, respectively, and the proportions of A(H3N2) strains resistant to oseltamivir and baloxavir were 0% and 9.4%, respectively (19). However, no regional spread of resistant mutant viruses was reported. These data will help predict the viral characteristics of the patient groups analyzed.
In conclusion, the present survey showed that baloxavir prescribed for patients with influenza A was significantly more effective than NAIs in clinical practice.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We gratefully acknowledge the cooperation of following clinics and hospitals for this postcard questionnaire survey in 2018-2019 influenza season; Ikuno Clinic for Internal Medicine, Ikuwakai Memorial Hospital, Ishikiriseiki Hospital, Ishitani Clinic, Izumiotsu Municipal Hospital, Izumi Municipal Hospital, Imamura Clinic, Osaka Ekisaikai Hospital, Osaka Minato Central Hospital, Oshima Clinic for Internal Medicine, Kayou Internal Medicine and Pediatric Clinic, Kourigaoka Yukeikai Hospital, Kyowa Hospital, Gotoh ENT Clinic, Sakazaki Child Clinic, Shirai Hospital, Sunami Internal Medicine Clinic, Sumiyoshi Democratic Clinic, Takamatu Internal Medicine Clinic, Takeda Children's Clinic, Takechi Internal Medicine and Pediatric Clinic, Tane General Hospital, Tooyama ENT Clinic, Tooyama Internal Medicine Clinic, Tochino ENT Clinic, Nakajima Pediatric Clinic, Nagayoshi General Hospital, Nishiyodo Hospital, Nippon Life Hospital, Hata Internal Medicine Clinic, Hananomachi Family Clinic, Hara Clinic, Higashisano Hospital, Higashisumiyosi Morimoto Hospital, Hirai Clinic, PL Hospital, Fujii Internal Medicine and Pediatric Clinic, Fujioka Pediatric Clinic, Fujitani Clinic, Fujito Clinic, Fuchu Hospital, Bel-lifecare Clinic, Masaki Clinic, Minamiura Pediatric Clinic, Moriguchi Ikuno Memorial Hospital, Yamagami Clinic, Yamada Internal Medicine Clinic, Yamamoto Internal Medicine Clinic, Yamamoto Third Hospital, and Yodogawa Christian Hospital.
References
- 1. Paules C, Subbarao K. Influenza. Lancet 390: 697-708, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 379: 913-923, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis 68: e1-e47, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Opinions Infect Dis 8: 311-328, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicholson KG, Aoki FY, Osterhaus AD, et al. ; Neuraminidase Inhibitor Flu Treatment Investigator Group. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355: 1845-1850, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Sheu TG, Deyde VM, Okomo-Adhiambo M, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 52: 3284-3292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawai N, Ikematsu H, Hirotsu N, et al. Clinical effectiveness of oseltamivir and zanamivir for treatment of influenza A virus subtype H1N1 with the H274Y mutation: a Japanese, multicenter study of the 2007-2008 and 2008-2009 influenza seasons. Clin Infect Dis 49: 1828-1835, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez JE, Adiga R, Armstrong R, et al. Clinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an Emergency IND program in the United States. Clin Infect Dis 52: 695-706, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother 54: 2575-2582, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 51: 1167-1175, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Ikematsu H, Kawai N, Iwaki N, Kashiwagi S. Duration of the fever and other symptoms after the inhalation of laninamivir octanoate hydrate; comparison of the 2011/12 to 2015/16 Japanese influenza seasons. J Infect Chemother 23: 627-633, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Shobugawa Y, Saito R, Sato I, et al. Clinical effectiveness of neuraminidase inhibitors--oseltamivir, zanamivir, laninamivir, and peramivir--for treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: an observational study in the 2010-2011 influenza season in Japan. J Infect Chemother 18: 858-864, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura S, Miyazaki T, Izumikawa K, et al. Efficacy and safety of intravenous peramivir compared with oseltamivir in high-risk patients infected with influenza A and B viruses: a multicenter randomized controlled study. Open Forum Infect Dis 4: ofx129, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koseki N, Kaiho M, Kikuta H, et al. Comparison of the clinical effectiveness of zanamivir and laninamivir octanoate for children with influenza A(H3N2) and B in the 2011-2012 season. Influenza Other Respir Viruses 8: 151-158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omoto S, Speranzini V, Hashimoto T, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep 8: 9633, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uehara T, Hayden FG, Kawaguchi K, et al. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 221: 346-355, 2020. [DOI] [PubMed] [Google Scholar]
- 17. Hirotsu N, Sakaguchi H, Sato C, et al. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 55: 1198-1204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Institute of Infectious Diseases, Ministry of Health, Labour and Welfare, Japan. [Yearly reports of influenza virus isolation/detection in 2018/19 influenza season.] [Internet]. [cited 2019 Oct 10]. Available from: https://www.niid.go.jp/niid/images/idsc/disease/influ/fludoco1819.pdf (In Japanese)


