Abstract
Vascular calcification is a pathophysiological process that is associated with coronary atherosclerosis, and is a prognostic marker of cardiovascular morbidity and mortality. The process of arterial wall calcification is triggered and accompanied by pro-osteogenic phenotypical modifications of resident smooth muscle cells (SMC). Vitamin C (ascorbic acid) is an essential nutrient required to support the production of extracellular matrix components and maintain healthy connective tissue. In this study we investigated the effects of ascorbic acid on cultured human aortic SMC calcification process in vitro. Our results demonstrate that supplementation of SMC cultures with ascorbic acid significantly decreases calcium accumulation in SMC-produced and -deposited extracellular matrix. These effects were accompanied by a reduction in cell-associated alkaline phosphatase activity. Significantly, treatment of cultured SMC with HMG-CoA reductase inhibitors, simvastatin and mevastatin, resulted in increased calcium accumulation in cultured SMC. These effects were blocked by ascorbic acid. The effects of ascorbic acid supplementation on pro-osteogenic modification were compared in different cell types. Analysis of the expression of osteogenic markers in cultured human aortic SMC, human dermal fibroblasts and immortalized human osteoblasts (hFOB) revealed cell type-specific responses to ascorbate supplementation. We conclude that ascorbic acid supplementation can actively and beneficially interfere with the process of arterial wall calcification, with potential implications for human health.
Keywords: Smooth muscle cells, fibroblasts, osteoblasts, calcification, extracellular matrix, alkaline phosphatase, osteogenic markers
Introduction
Vascular calcification is a relevant pathophysiological process that is associated with coronary atherosclerosis, and is a prognostic marker of cardiovascular morbidity and mortality [1-3].
Vascular smooth muscle cells (SMC) have an extraordinary capacity to undergo osteoblastic phenotypical differentiation. Whether it is a smooth muscle cell, a mesenchymal cell, or vascular pericyte, calcification of the intimal and/or medial vascular cell layer leads to differentiation of osteoblasts, and is characterized by increased alkaline phosphatase activity, osteocalcin production and bone matrix secretion [4]. Biochemical mechanisms associated with the conversion of SMC into osteoblastic cells have been elaborated; however the decisive mechanisms of what triggers and/or regulates this process have remained largely elusive.
Recent studies showed that plaque calcification is a dynamic process and relates to the degree of vascular inflammation. Several inflammatory factors produced during the different phases of atherosclerosis can induce the expression and activation of osteoblastic cells located within the arterial wall, which, in turn, promote the deposition of calcium.
The presence of regulatory proteins along with differentiated osteoblast-like cells was demonstrated to originate from vascular smooth muscle cells that were designated calcifying vascular cells [5]. These cells are implicated in the synthesis/reabsorption of bone in atherosclerotic plaques, especially in the process of calcification [6]. Thus, it has been proposed that bone cell function in the vascular wall is in some respects similar to that found in bones [7]. However, in vitro studies provide evidence that the regulation of bone synthesis in the vascular wall is different from that which takes place in the skeleton. When stimulated by oxidative stress or with oxidized LDL, osteoblasts of the skeleton and CVCs (a population of vascular cells with osteoblastic characteristics) show opposing responses: a decrease and increase in bone formation, respectively [8,9].
Vitamin C is a very powerful antioxidant and is essential for the formation of collagen and optimum extracellular matrix (ECM) [10,11]. It has been demonstrated to prevent lipoprotein deposition and development of atherosclerosis by protecting the integrity and strength of the vascular wall [12].
Our previous studies have shown that ascorbate can inhibit excessive proliferation and migration of SMC in vitro [13]. Also, dietary vitamin C is essential in preventing the deposition of lipoproteins in the vascular wall and atherosclerosis in genetically engineered mice, mimicking human metabolism in relation to their inability to produce vitamin C and their expression of human lipoprotein(a) [14]. In a clinical study, a daily micronutrient supplement, including about 4 grams of vitamin C, was able to halt the progression of coronary calcifications in patients diagnosed with early coronary artery disease [15].
Thus, it is conceivable that vitamin C plays a decisive role in regulating the cellular and extracellular architecture and function inside the vascular wall. With an optimum availability of ascorbate, the integrity and stability of the vascular wall would be provided, above all, with an optimum synthesis of collagen and other ECM molecules. In chronic ascorbate deficiency or subclinical scurvy, the demand for compensatory mechanisms may arise to add compensatory stability to a structurally impaired vascular wall-including by means of calcification.
In this study we investigated the effects of vitamin C on vascular SMC, human dermal fibroblasts (DF) as well as on immortalized human fetal osteoblasts (FOB), as well as the potential of these cells to contribute to vascular calcification. Moreover, we evaluated the role of statins in connection with this regulatory process, in light of the fact that these drugs are currently taken by millions of patients in the expectation that they curb vascular calcification.
Materials and methods
Reagents
All reagents were from Sigma-Aldrich (St. Louis, MO) except when indicated differently.
Cell cultures
Normal human dermal fibroblasts (hDF) and immortalized human fetal osteoblasts (hFOB) were supplied by ATCC (Manassas, VA). Human aortic smooth muscle cells (AoSMC) were purchased from Cambrix (East Rutherford, NJ) and used in experiments at 5-7 passages. Cell cultures were maintained in DMEM medium (ATCC) containing antibiotics and 5% fetal bovine serum (FBS, ATCC). In some experiments, cells were incubated in pro-osteogenic medium, defined as 5% FBS/DMEM fortified with 5 mM beta-glycerophosphate with or without 25 mcM forskolin. All cell cultures were maintained at 37°C and 5% CO2 atmosphere. Cell viability was monitored with MTT assay.
Alkaline phosphatase activity assay in AoSMC
AoSMC were plated in 96-well plates and grown to confluent layer. Cells were incubated with ascorbic acid in growth medium for 3 days. Cells were washed with phosphate buffered saline (PBS) and supplemented with 25 mcg/ml 4-MUP (fluorescent ALP substrate, Sigma) in alkaline buffer (Sigma)/1% Triton X100 for 1 hour at room temperature. Enzyme activity was evaluated by the level of fluorescence measured at 360 nm excitation/450 nm emission with Cytofluor 4000 multiwell plate reader (Perseptive Biosystems).
Calcium accumulation in extracellular matrix
AoSMC were seeded on fibronectin-covered plastic plates at a density of 25,000 per square cm and grown to confluence for 5-7 days. Ascorbic acid was added to cells at indicated concentrations for 72 hours in DMEM supplemented with 2% FBS and cell-produced extracellular matrix was exposed by sequential treatment with 0.5% Triton X100 and 20 mM ammonium sulfate in phosphate buffered saline (PBS, Life Technologies) for 3 min each at room temperature as described previously [16]. After 4 washes with PBS, ECM layers were solubilized by incubation in 0.6 N HCl for 48 hours at 37°C. Calcium content in solubilized samples was measured with calcium diagnostic kit (TECO Diagnostics) according to manufacturer’s protocol.
Expression of osteoblast markers in human cultured cells
For experiments, AoSMC, DF and hFOB cells were seeded in separate 96-well plastic plates at density 25,000 per square cm and grown to confluence. Cells were cultured in plain 2% FBS/DMEM or in pro-osteogenic medium (2% FBS/DMEM supplemented with 5 mM beta-glycerophosphate and 25 mcM forskolin) for 4 weeks in either the presence or absence of 200 mcM ascorbic acid. Cell layers were washed 3 times with PBS and fixed with 3% formaldehyde in PBS at 4°C for 1 hour. Fixed cell layers were washed 4 times with PBS and treated with 1% BSA/PBS for 1 hour at room temperature. Immunoasssay for osteogenic markers was done by sequential incubation with primary monoclonal antibodies (R&D Systems) in 1% BSA/PBS for 2 hours followed by 1 hour incubation with secondary goat anti-mouse IgG antibodies labeled with horse radish peroxidase (HRP). Retained peroxidase activity was measured after the last washing cycle (3 times with 0.1% BSA/PBS) using TMB peroxidase substrate reagent (Rockland). Optical density was read with plate reader (Molecular Devices) at 450 nm and expressed as a percentage of control cell samples incubated in unsupplemented 2% FBS/DMEM. To ensure a direct comparison of osteogenic markers’ expression on different cell types, all cell-covered plates were treated identically and simultaneously during immunoassay.
Statistical analysis
Results in figures are means ± SD from 3 or more repetitions from the most representative of at least 2 independent experiments. Differences between samples were estimated with a two-tailed Student’s t-test using Excel software (Microsoft) and accepted as significant at p values less than 0.05.
Results
The cellular calcification process was investigated in human AoSMC cultured in a regular cell growth medium (5% FBS/DMEM) in both the absence and presence of various amounts of ascorbic acid. The calcification process of AoSMC was evaluated by the activity of cellular alkaline phosphatase and calcium accumulation in the cell-produced extracellular matrix (Figure 1).
Figure 1.
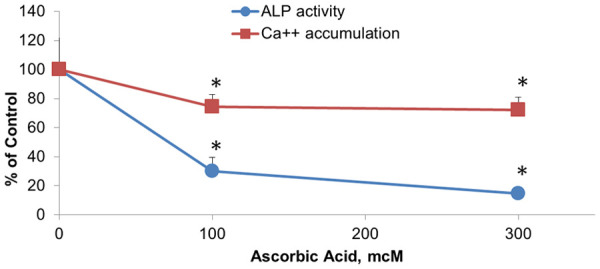
Effects of ascorbic acid on calcification of extracellular matrix in cultured human aortic smooth muscle cells (AoSMC). Human AoSMC were seeded in 96 well plates and grown to confluency in 5% FBS/DMEM. Cells were incubated with increasing concentrations of ascorbic acid for four days in cell growth medium. Cell associated alkaline phosphatase activity was evaluated by accumulation of fluorescent product (360 nm exitation/450 nm emmission) after cell incubation with 25 mcg/ml 4-MUP (fluorescent ALP substrate, Sigma) in alkaline buffer (Sigma)/1% Triton X100 for 1 h at room temperature. In separate plate cell layers were washed with PBS and extracellular matrix (ECM) was exposed by cell removal with NH4OH/Triton X100 treatment. Calcium from ECM was solubilized by incubation in 0.6 N HCl for 48 hours at 37°C. Calcium content in solubilized samples was measured with Calcium diagnostic kit (TECO Diagnostics). Results were expressed as percentage of cell samples incubated in plain unsupplemented cell growth medium. *, indicates significant differences from unsupplemented controls (P<0.05) in two-tailed t-test.
The results show that supplementation of AoSMC medium with ascorbic acid up to 300 mcM resulted in a significant decrease in the level of extracellular calcium as well as in the reduced activity of cellular alkaline phosphatase in a dose-dependent manner. In the presence of 300 mcM ascorbate the extracellular Calcium accumulation by AoSMC decreased by 20% and alkaline phosphatase activity decreased by 80%.
The effect of ascorbate on AoSMC calcification was also tested in the presence of agents known to stimulate arterial calcifications, such as statins [17,18]. The results presented in Figure 2A show that calcium accumulation in AoSMC layers was increased in the presence of simvastatin by 23%. However, the concomitant presence of 100 mcM calcium ascorbate resulted in a 54% decrease of accumulated calcium to the value of 0.2 mcg/well, which correlated with the values observed in cells not exposed to simvastatin.
Figure 2.
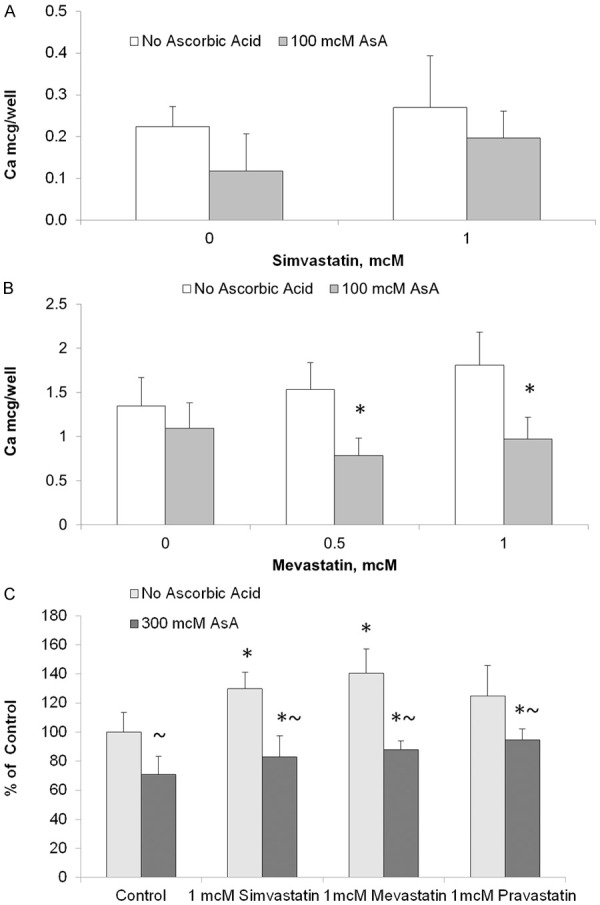
Effects of statins and ascorbic acid on AoSMC culture calcification. Human AoSMC were seeded in plastic plates in 5% FBS/DMEM. Additions of statins and ascorbic acid (AsA) started after cells grew to confluent layer in 5 mM b-glycerophoshate/5% FBS/DMEM supplemented (B, C) or nonsupplemented (A) with 25 mcM forskolin. Medium was changed every two or three days. After 10 day incubation cell culture Calcium content (A, B) was measured in 0.6 N HCl whole cell layer extracts (48 hours incubation at 37°C). Cell-associated alkaline phosphatase activity (C) was analyzed by fluorescent assay after incubation with 25 mcg/ml 4-MUP (fluorescent ALP substrate, Sigma). Experimental procedures are described in more details in Materials and Methods section and in the Figure 1 legend. * and ~, indicate significant differences from unsupplemented controls and from ascorbiate-free samples, respectively (P<0.05) in two-tailed t-test.
The effect of ascorbate on calcium accumulation in AoSMC under enhanced pro-calcification conditions (with forskolin) and in the presence of a statin (mevastatin) is presented in Figure 2B. The results show that in the presence of 1 mM mevastatin, calcium accumulation increased from 1.35 mcg/well in control to 1.8 mcg/well with mevastatin. However, when 100 mcM ascorbate was added, calcium accumulation decreased by 19% to below control (unsupplemented) values. Alkaline phosphatase activity in AoSMC increased significantly in the presence of 1 mcM statin (Figure 2C). These pro-calcification effects of statins were diminished in the presence of 300 mcM ascorbic acid.
In addition to AoSMC, we studied the effect of ascorbate on the cellular calcification process in human dermal fibroblasts (hDF) and immortalized human fetal osteoblasts (hFOB) by evaluating changes in the expression of different pro-osteogenic markers in these cells. The results show that the expression of all tested osteogenic markers was significantly reduced by 200 mcM ascorbic acid supplementation in both AoSMC and hDF cultures (Figure 3).
Figure 3.
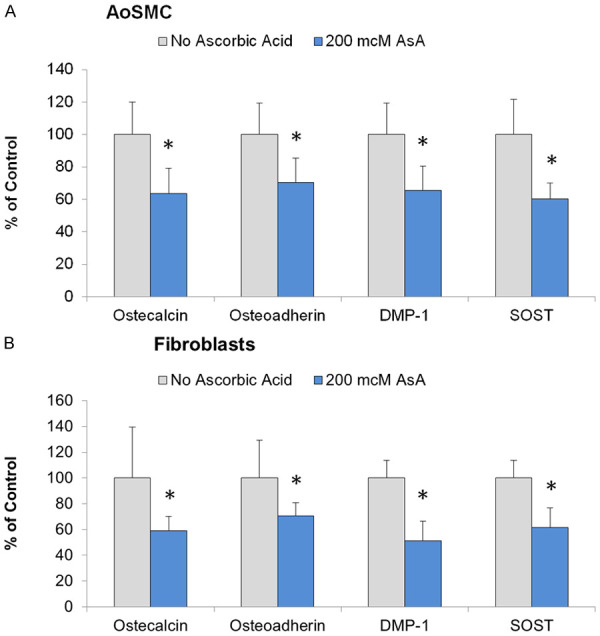
Effects of ascorbic acid supplementation on osteogenic markers expression in human cells cultured in pro-osteogenic cell culture media. Human AoSMC (A) and human dermal fibroblasts (B) were cultured in 5% FBS/DMEM supplemented with 5 mM beta-Glycerophosphate and 25 mcM Forskolin for four weeks. Osteogenic markers expression was measured by immunoassay as described in Materials and Methods. AoSMC (A) and human dermal fibroblasts (B) were seeded in separate 96 well plastic plates and grown to confluence. Cells were cultured in 2% FBS/DMEM supplemented or not with 200 mcM ascorbic acid for four weeks. Cell layers were washed with PBS and fixed with 3% formaldehyde. Immunoasssay for osteogenic markers on fixed cells was done by 2 hours incubation with primary monoclonal antibodies (R&D Systems) followed by 1 hour incubation with secondary goat anti-mouse IgG antibodies labeled with horse radish peroxidase (HRP). Retained peroxidase activity was measured as optical density at 450 nm and expressed as percentage of control cell samples incubated in unsupplemented 2% FBS/DMEM. For more details refer to Materials and Methods section. *, indicate significant differences from ascorbate-free samples (P<0.05) in two-tailed t-test.
We compared the levels of osteogenic markers in tested human cell types as presented in Table 1. The results indicate that in a regular growth medium, the expression of osteocalcin, osteoadherin, dentin matrix protein 1 (DMP-1) and sclerostin (SOST) were most prominent in osteoblast cells (hFOB) closely followed by fibroblasts (hDF); however, in relation to the expression of DMP-1, fibroblasts slightly surpassed FOB cultures. Cellular expression of these 4 osteogenic markers in AoSMC cultured in regular growth medium was significantly (2-4)-fold less prominent than in hFOB and hDF cultures. Different cell types responded differently to a challenge with a pro-osteogenic medium. Thus the levels of all 4 tested osteogenic markers were increased in AoSMC cultures (from 23% increase for osteoadherin to 48% increase for osteocalcin). Whereas cell expression of all markers was decreased in hDF and hFOB cultures. In hDF cultures osteoadherin expression was reduced by 55%, followed by osteocalcin and sclerostin (44% reduction each) and by DMP-1 with 25% reduction. In hFOB cultures the most pronounced reduction was observed for the expression of sclerostin (34% decrease), followed by osteoadherin and osteocalcin (29% and 17% reduction, respectively). DMP-1 levels decreased only slightly (4% reduction).
Table 1.
Direct comparison of the levels of osteogenic markers expression in human aortic smooth muscle cells (hAoSMC), human dermal fibroblasts (hDF) and human fetal osteoblast (hFOB)
| Cell Type | Cell Culture Medium | Osteogenic Markers | |||
|---|---|---|---|---|---|
|
| |||||
| Osteocalcin | Osteoadherin | Dentin Matrix Protein 1 | Sclerostin | ||
| AoSMC | Plain | 0.29 +/- 0.05a | 0.26 +/- 0.03a | 0.41 +/- 0.06a | 0.21 +/- 0.03a |
| pro-Osteogenic | 0.43 +/- 0.09 (148%)* | 0.32 +/- 0.06 (123%)* | 0.57 +/- 0.11 (139%)* | 0.29 +/- 0.06 (138%)* | |
| hDF | Plain | 1.09 +/- 0.05b | 0.89 +/- 0.09b | 1.14 +/- 0.09b | 0.66 +/- 0.06b |
| pro-Osteogenic | 0.61 +/- 0.24 (56%)* | 0.40 +/- 0.12 (45%)* | 0.85 +/- 0.12 (75%)* | 0.37 +/- 0.05 (56%)* | |
| hFOB | Plain | 1.21 +/- 0.29b | 1.49 +/- 0.15c | 0.82 +/- 0.31c | 0.96 +/- 0.20c |
| pro-Osteogenic | 1.00 +/- 0.21 (83%) | 1.05 +/- 0.21 (71%)* | 0.79 +/- 0.08 (96%) | 0.63 +/- 0.13 (66%)* | |
Cells were cultured in plain (2% FBS/DMEM) or in pro-osteogenic (medium supplemented with 5 mM b-glycerophosphate and 25 mcM forscolin) cell culture media for four weeks. Immunoassay for osteogenic markers was performed simultaneously in plates with all cell types at identical conditions. Data presented as averages +/- SD (n=8) of optical density reading at 450 nm expressed in OD arbitrary units. Numbers in parenthesis represent percentage changes in osteogenic markers expression in cell cultured in pro-osteogenic medium as compared to plain medium.
indicate significant differences from plain medium samples (P<0.05) in two-tailed t-test.
Different low case letters (a, b, c) next to plain medium samples indicate significant differences from other cell type samples for individual markers (P<0.05). Experimental details described in Materials and Methods.
Discussion
Due to their implication in the origins of cardiovascular disease and related mortality, vascular calcifications represent a major clinical problem [19]. The degree of calcification correlates with the advancement of atherosclerosis, age, and hypertension as dominant risk factors for systemic calcified atherosclerosis [17].
In a recent statement by the Cardiac Society of Australia and New Zealand, coronary artery calcium (CAC) scoring was analyzed in its connection with the diagnosis and prognosis of cardiovascular disease [20]. CAC scoring is a non-invasive method for quantifying coronary artery calcification using computed tomography. The statement indicates that CAC scoring is a marker of atherosclerotic plaque burden and the strongest independent predictor of future myocardial infarction and mortality. CAC scoring provides incremental risk information beyond traditional risk calculators such as the Framingham Risk Score. Its use for risk stratification is confined to the primary prevention of cardiovascular events, and can be considered as an individualized coronary risk scoring for intermediate risk patients, allowing patients’ risk to be reclassified as low or high based on the score.
The process of vascular calcification requires a phenotypic transformation of vascular smooth muscle cells into osteogenic cells [21]. In this study we demonstrated that ascorbic acid tested up to a concentration of 300 mcM can reduce calcium accumulation produced by AoSMC in the ECM. This effect was accompanied by the blockage of osteogenic transformation in the SMC as indicated by changes in specific metabolic parameters, such as reduction in cellular alkaline phosphatase activity, and cellular expression of osteoblast marker proteins. A high level of serum alkaline phosphatase (ALP) is associated with an increased risk of mortality and myocardial infarction [22]. ALP hydrolyses inorganic pyrophosphate, which is a strong inhibitor of calcium phosphate deposition.
Special interest was ascribed to the evaluation of ascorbate’s effects on vascular calcification in the presence of cholesterol-lowering drugs, namely statins. It has been known that statins increase vascular calcifications, which is a recognized risk factor for heart disease [23]. In the recent analysis of 8 prospective randomized trials using serial coronary intravascular ultrasound, Puri et al. [24] concluded that independent of their plaque-regressive effects, statins promote coronary atheroma calcification. Still there is a controversy between arterial calcification being a well-established marker and prognostic indicator for cardiovascular disease development as well as statins stimulating effects on arterial calcification and ostensible beneficial effects of statin supplementation on clinical events in CVD patients. Some researchers are providing a plausible explanation of these conflicting facts as a “special” mechanism of arterial calcification under statin treatment which results in greater lesion stability, defined as fewer VH-thin-cap fibroatheromas and plaque ruptures and more calcified thick-cap fibroatheromas [25].
Our literature searches did not reveal any literature data elaborating the effects of statins and ascorbate on vascular SMC calcification process in vitro. Despite the fact that pro-calcification effects of statins have been reported in various clinical studies previously, apparently there was no attempt to investigate the molecular mechanisms involved. Here we demonstrate novel findings that statins in both the absence and presence of pro-calcification agent (forskolin), significantly increase extracellular calcium deposition in cultured human SMC, and that ascorbic acid can alleviate the adverse effect of these drugs by inhibiting statin-induced calcification of SMC. Statin-driven calcification of AoSMC culture was associated with the stimulation of cell-associated alkaline phosphatase, a key enzyme in the calcification process [4], as presented in Figure 2C. Ascorbic acid decreased alkaline phosphatase activity in statin-free AoSMC cultures and counteracted a statin-dependent increase of the enzyme activity (Figure 2C). These findings indicate that regulation of the cell-associated activity of alkaline phosphatase could be a possible cellular mechanism involved. Elucidation of the particular molecular pathways involved in these effects was outside the scope of this study and should be addressed in further research.
Ascorbic acid is a versatile multifunctional biological agent essential for several crucial physiological pathways. The most notable role of ascorbic acid is in the prevention of scurvy by providing vitamin-like effects (vitamin C). The stability of connective tissue is dependent on a proper supply of vitamin C. Scorbutic symptoms are characterized by a weakened stability of connective tissue, manifesting in the inability of blood vessels to contain blood. It takes 3-4 months of acute Vitamin C deficiency to develop clinical scurvy. It has been proposed that chronic subclinical deficiency in vitamin C intake, as has been widely reported as occurring in modern societies, could result in the gradual development of weakened arterial wall syndrome: an underlying cause of vascular plaque formation and atherosclerosis [26]. In this regard, the atherosclerotic process-characterized by thickening of the arterial wall with abnormally increasing number of resident smooth muscle cells that produce increased amounts of structurally modified extracellular matrix and that triggers lipid accumulation/lipoprotein retention-is a compensatory mechanism in the body’s attempt to maintain sufficient mechanical stability of blood vessels.
As such, arterial calcification could also be regarded as a compensatory process to strengthen mechanical stability of the arterial wall when it has been compromised by a chronic subclinical deficiency of Vitamin C [26].
In addition to serving as a cofactor in enzymatic reactions in collagen synthesis and fibril tri-dimensional organization, ascorbic acid has been demonstrated to be a powerful inhibitor of inflammation and oxidative stress [27-29]: pathological processes contributing to arterial wall calcification.
Other cell types present in the vascular wall also can contribute to the process of vascular calcification. We demonstrated that vascular fibroblasts undergo myogenic and osteogenic phenotype modifications, especially in the media and adventitia layers of the arterial wall. In this study we observed that under ascorbic acid supplementation there was a reduction in the osteogenic modification of fibroblasts, as measured by a reduced expression of osteogenic markers. The effects of ascorbic acid on the expression of osteogenic markers in osteoblast cells in comparison with vascular SMC showed that its effects on pro-osteoblastic differentiation were not parallel when SMC were directly compared to osteoblasts. This suggests the possible existence of generalized effects of ascorbic acid supplementation on calcium metabolism in the entire organism in addition to its cell-specific effects. As such, a sufficient level of ascorbic acid supplementation seems to be essential for the proper maintenance of calcium homeostasis.
Our study shows that different cell types respond quite differently to pro-osteogenic treatment. When cells were challenged with pro-osteogenic conditions, the expression of all 4 osteogenic markers in AoSMC cultures were significantly elevated as compared to regular growth medium, whereas cell expression of all 4 osteogenic markers was diminished in osteoblasts and fibroblasts.
In light of these results, the increased calcification observed under long-term statin treatment needs to be evaluated. The hypothetical interpretation that such increased calcification under statin therapy could be beneficial [24] or that there could be a beneficial macro calcification, as opposed to a detrimental micro calcification [5] are little more than speculation.
Our data suggests that ascorbate is a key regulator in vascular cells and plays a decisive role in preventing the formation of calcium deposits by smooth muscle cells, the most abundant cell type in larger, muscular arteries. Thus, ascorbate supplementation should be part of any effective approach to cardiovascular disease prevention and treatment.
Acknowledgements
This research study was funded by the Dr. Rath Health Foundation, San Jose, CA: a nonprofit organization. We would like to thank Ms. Elizabeth Wells for her proofreading assistance.
Disclosure of conflict of interest
None.
References
- 1.Raggi P, Callister TQ, Cooil B, He ZX, Lippolis NJ, Russo DJ, Zelinger A, Mahmarian JJ. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 3.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–230. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 4.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 5.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. Beta glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–2009. doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 6.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boström K, Watson KE, Stanford WP, Demer LL. Atherosclerotic calcification: relation to developmental osteogenesis. Am J Cardiol. 1995;75:88B–91B. doi: 10.1016/0002-9149(95)80020-s. [DOI] [PubMed] [Google Scholar]
- 8.Demer LL. Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int J Epidemiol. 2002;31:737–741. doi: 10.1093/ije/31.4.737. [DOI] [PubMed] [Google Scholar]
- 9.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 10.Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–1140S. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- 11.Ronchetti IP, Quaglino D Jr, Bergamini G. Ascorbic acid and connective tissue. Subcell Biochem. 1996;25:249–264. doi: 10.1007/978-1-4613-0325-1_13. [DOI] [PubMed] [Google Scholar]
- 12.Rath M, Pauling L. Hypothesis: lipoprotein(a) is a surrogate for ascorbate. Proc Natl Acad Sci U S A. 1990;87:6204–6207. doi: 10.1073/pnas.87.16.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov V, Ivanova S, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Extracellular matrix-mediated control of aortic smooth muscle cell growth and migration by a combination of ascorbic acid, lysine, proline, and catechins. J Cardiovasc Pharmacol. 2007;50:541–547. doi: 10.1097/FJC.0b013e318145148e. [DOI] [PubMed] [Google Scholar]
- 14.Cha J, Niedzwiecki A, Rath M. Hypoascorbemia induces atherosclerosis and vascular deposition of lipoprotein(a) in transgenic mice. Am J Cardiovasc Dis. 2015;5:53–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Rath M, Niedzwiecki A. Nutritional supplement program halts progression of early coronary atherosclerosis documented by ultrafast computed tomography. J Appl Nutr. 1996;48:67–78. [Google Scholar]
- 16.Ivanov V, Ivanova S, Kalinovsky T, Niedzwiecki A, Rath M. Plant-derived micronutrients suppress monocyte adhesion to cultured human aortic endothelial cell layer by modulating its extracellular matrix composition. J Cardiovasc Pharmacol. 2008;52:55–65. doi: 10.1097/FJC.0b013e31817e692f. [DOI] [PubMed] [Google Scholar]
- 17.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 18.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis heart study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton-Craig CR, Chow CK, Younger JF, Jelinek VM, Chan J, Liew GY. Cardiac Society of Australia and New Zealand position statement executive summary: coronary artery calcium scoring. Med J Aust. 2017;207:357–361. doi: 10.5694/mja16.01134. [DOI] [PubMed] [Google Scholar]
- 21.Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25:267–274. doi: 10.1016/j.tcm.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panh L, Ruidavets JB, Rousseau H, Petermann A, Bongard V, Bérard E, Taraszkiewicz D, Lairez O, Galinier M, Carrié D, Ferrières J. Association between serum alkaline phosphatase and coronary artery calcification in a sample of primary cardiovascular prevention patients. Atherosclerosis. 2017;260:81–86. doi: 10.1016/j.atherosclerosis.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Ikegami Y, Inoue I, Inoue K, Shinoda Y, Iida S, Goto S, Nakano T, Shimada A, Noda M. The annual rate of coronary artery calcification with combination therapy with a PCSK9 inhibitor and a statin is lower than that with statin monotherapy. NPJ Aging Mech Dis. 2018;4:7. doi: 10.1038/s41514-018-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Kadohira T, Mintz GS, Souza CF, Witzenbichler B, Metzger DC, Rinaldi MJ, Mazzaferri EL Jr, Duffy PL, Weisz G, Stuckey TD, Brodie BR, Crowley A, Kirtane AJ, Stone GW, Maehara A. Impact of chronic statin therapy on clinical presentation and underlying lesion morphology in patients undergoing percutaneous intervention: an ADAPT-DES IVUS substudy. Coron Artery Dis. 2017;28:218–224. doi: 10.1097/MCA.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 26.Rath M, Pauling L. Unified theory of human cardiovascular disease leading the way to the abolition of this disease as a cause for human mortality. J Orthomol Med. 1992;7:5–15. [Google Scholar]
- 27.Ellulu MS. Obesity, cardiovascular disease, and role of vitamin C on inflammation: a review of facts and underlying mechanisms. Inflammopharmacology. 2017;25:313–328. doi: 10.1007/s10787-017-0314-7. [DOI] [PubMed] [Google Scholar]
- 28.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov V, Cha J, Ivanova S, Kalinovsky T, Roomi MW, Rath M, Niedzwiecki A. Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int J Mol Med. 2008;22:731–741. [PubMed] [Google Scholar]


