Abstract
Introduction
Most of the drugs associations that have been used to treat patients with SARS-CoV-2 infection increase the risk of prolongation of the corrected QT interval (QTc).
Objective
To evaluate the effects of an association therapy of hydroxychloroquine (HY) plus ritonavir/darunavir (RD) or azithromycin (AZ) on QTc intervals.
Methods
At the beginning of COVID-19 pandemic patients admitted to our hospital were treated with the empiric association of HY/RD; one week later the therapeutic protocol was modified with the combination of HY/AZ. Patients underwent an ECG at baseline, then 3 and 7 days after starting therapy. We prospectively enrolled 113 patients (61 in the HY/RD group-52 in the HY/AZ group).
Results
A significant increase in median QTc was reported after seven days of therapy in both groups: from 438 to 452 ms in HY/RD patients; from 433 to 440 ms in HY/AZ patients (p = 0.001 for both). 23 patients (21.2%) had a QTc > 500 ms at 7 days. The risk of developing a QTc > 500 ms was greater in patients with prolonged baseline QTc values (≥ 440 ms for female and ≥ 460 ms for male patients) (OR 7.10 (95% IC 1.88–26.81); p = 0.004) and in patients with an increase in the QTc > 40 ms 3 days after onset of treatment (OR 30.15 (95% IC 6.96–130.55); p = 0.001). One patient per group suffered a malignant ventricular arrhythmia.
Conclusion
Hydroxychloroquine with both ritonavir/darunavir or azithromycin therapy significantly increased the QTc-interval at 7 days. The risk of developing malignant arrhythmias remained relatively low when these drugs were administered for a limited period of time.
Keywords: COVID-19, Hydroxychloroquine, Ritonavir, Darunavir, Azithromycin
Introduction
Starting from December 2019, an outbreak of viral respiratory illness named coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, China, and spread rapidly worldwide as a pandemic [1]. Currently, no specific therapies are available for patients with SARS-CoV-2 infection and several drugs approved for other diseases are used in this context.
Hydroxychloroquine (HY) has been demonstrated to have an anti-SARS-CoV-2 activity in vitro [2]. Moreover, a recent study reported a significant viral load reduction in COVID-19 patients treated with hydroxychloroquine and this effect seemed to be reinforced by azithromycin (AZ) [3]. Based on this study, clinicians in many countries have begun to use these medications in clinical practice. It is generally used with the dosage of 200 mg b.i.d, but has often been evaluated in higher dosage as 600 mg per day [3–5].
Some retrospective studies suggested a potentially favorable effect of HIV protease inhibitors such as Darunavir in combination with Lopinavir in COVID-19 patients [6, 7]. According to this evidence, at the beginning of the outbreak, antiretroviral off-label use in COVID-19 patients was widespread [8], the results for some secondary endpoints are interesting and many studies conducted with antiviral therapy are ongoing.
Hydroxychloroquine, azithromycin and ritonavir/darunavir (RD) are generally well-tolerated but all three are associated with an increased risk of corrected QT (QTc) prolongation and cardiac arrhythmias [9–11] which may further increase when these drugs are administered together. However, safety data for these treatments, alone or in combination, are still lacking in COVID-19 patients. We evaluated the effects of hydroxychloroquine in combination with ritonavir/darunavir or azithromycin on QTc length and the risk of malignant arrhythmias in patients with COVID-19 pneumonia.
Methods
At the beginning of COVID-19 pandemic (March 2nd–8th, 2020) all of the patients with a confirmed diagnosis of SARS-CoV-2 pneumonia admitted to our hospital were treated with the empiric association of HY/RD, while the week later (March 9th–15th, 2020) the therapeutic protocol was modified by our internal ad hoc committee, and patients received the combination of HY/AZ.
Patients were included in the study if they had the following criteria: a confirmed clinical and radiological diagnosis of SARS-CoV-2 pneumonia, according to the criteria proposed by Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition, published online on March 4, 2020); positive Reverse-Transcriptase-Polymerase-Chain-Reaction (RT-PCR) assay for SARS-CoV-2 in a respiratory tract sample; electrocardiogram (ECG) recordings at baseline and then three and seven days after starting therapy; full treatment with the prescribed drugs for 7 days, unless onset of malignant ventricular arrhythmias.
Exclusion criteria were: QTc > 500 ms on baseline ECG, history of severe systolic dysfunction (left ventricular ejection fraction < 35%), history of arrhythmias, bradycardia < 50 bpm, concomitant drugs that could cause QTc prolongation or early interruption of the medical therapy due to side effects.
The drugs were given at the following doses: HY 200 mg b.i.d; RD 100/800 mg q.d.; azithromycin 500 mg q.d. Standard 12-lead ECG was recorded at screening time before the beginning of the therapy (day 0) and after three (day 3) and 7 days (day 7). Triplicate ECG collection was obtained at three time points to reduce noise of individual QT interval measurements. The QTc interval was calculated according to Bazett's formula (QTc = QT ÷ √RR) in the II lead or V5 lead. ECGs were independently evaluated and a manual adjudication of automated QTc interval measurements was done by two senior cardiologists (M.L. and L.M.). Significant changes in QTc (ΔQTc) and development of prolonged QTc interval > 500 ms were reported in the whole cohort. Clinical and laboratory data were recorded as far as in-hospital outcomes. The study was approved by our Ethical Committee.
Statistical analysis
Continuous variables were summarized as median with interquartile range (IQR) and compared with the Mann–Whitney U test and Wilcoxon matched pairs signed rank. Categorical variables were presented as number (%), and proportions for categorical variables were compared using the χ2 or Fisher exact test as appropriate. Odds ratio (OR) was calculated using logistic regression. A two-sided p < 0.05 was considered statistically significant. Statistical analyses were done using the SPSS version 21.0 software (IBM, New York, USA).
Results
During the study period, a total of 137 patients were considered for treatment with the combinations of drugs. Twenty-four patients were excluded from the analysis: two had a severe systolic dysfunction; six history of arrhythmias and/or bradycardia < 50 bpm; five were chronically taken drugs that could cause QTc prolongation; 11 subjects were excluded for the appearance of drug-related side effects: ten complained of nausea and diarrhea and one referred dizziness. Finally, the study population consisted of 113 patients: 61 subjects in the HY/RD group, and 52 patients in the HY/AZ group.
Baseline characteristics and clinical outcomes
Clinical characteristics and laboratory tests of the two groups are reported in Tables 1 and 2. The most common comorbidities were hypertension (28%) and diabetes mellitus (14%); 61 patients (54%) needed non-invasive ventilation (34 in the HY/RD group and 27 in the HY/AZ group). Only one patient required mechanical ventilation.
Table 1.
Clinical characteristics and in-hospital outcomes
| Parameters | Total (n = 113) | HY/RD (n = 61) | HY/AZ (n = 52) |
|---|---|---|---|
| Age (years) | 68 (61–74) | 67 (61–74) | 68 (60–74) |
| Gender (male) | 85 (75%) | 49 (80%) | 36 (69%) |
| Hypertension | 32 (28%) | 18 (29%) | 14 (27%) |
| Diabetes | 16 (14%) | 10 (16%) | 6 (12%) |
| Smoking | 10 (9%) | 7 (11%) | 3 (6%) |
| COPD | 4 (4%) | 2 (3%) | 2 (4%) |
| Chronic kidney disease | 6 (5%) | 4 (7%) | 2 (4%) |
| Atrial Fibrillation | 8 (7%) | 2 (3%) | 6 (11%) |
| CAD | 12 (11%) | 7 (11%) | 5 (10%) |
| NIV | 42 (37%) | 26 (43%) | 16 (31%) |
| Intubation | 1 (0.8%) | 1 (1.6%) | 0 |
| Death | 9 (8%) | 7 (11%) | 2 (4%) |
Data are presented as median (IQR) or n (%); HY hydroxychloroquine, msec milliseconds, COPD chronic obstructive pulmonary disease CAD coronary artery disease, NIV non-invasive ventilation; QTc corrected QT interval, ΔQTc change in corrected QT interval
Table 2.
Laboratory data
| Parameters | Normal range | HY/RD (n = 61) | HY/AZ (n = 52) |
|---|---|---|---|
| D-dimer (μg/ml) | 0–0.5 | 2.4 (0.9–3.1) | 2.3 (0.98–2.7) |
| Hs-TnI(ng/L) | 0–34 | 12.7 (8.15–52.6) | 3.6 (0.6–118) |
| CRP (mg/L) | 0–5 | 51 (51–174) | 56 (24–159) |
| WBC (*103/mm3) | 3.9–10.6 | 5.3 (3.8–8.1) | 6.2 (4.8–8.3) |
| Potassium (mEq/L) | 3.5–5 | 4 (3.8–4.1) | 3.8 (3.7–4) |
| Sodium (mEq/L) | 135–145 | 134 (134–138) | 139 (134–140) |
| ALT (U/L) | 0–41 | 25 (24–48) | 25 (17–32) |
| AST (U/L | 10–40 | 37 (37–50) | 37 (33–60) |
| LDH (U/L) | < 248 | 345 (178–453) | 232 (182–297) |
| Creatinine (mg/dL) | 0.7–1.18 | 1 (0.99–1.3) | 0.9 (0.75–1) |
| eGFR (ml/min/1.73mq) | > 60 | 59 (53–60) | 63 (59–89) |
| Pa02 (mmHg) | 80–100 | 64 (54–65) | 64 (57–64) |
| Pa02/Fi02 ratio (mmHg/%) | > 300 | 305 (257–309) | 304 (271–419) |
| S02 (%) | 95–100% | 94 (90–94) | 94 (90–97) |
Data are presented as median (IQR) or n (%). hs-TnI high sensitive troponin I, CRP C-reactive protein; WBC white blood cell count, ALT alanine aminotransferase, AST aspartate transaminase, INR international normalized ratio, LDH lactate dehydrogenase, eGFR estimated glomerular filtration rate, Pa02 arterial oxygen partial pressure, S02 oxygen saturation, Fi02 fraction of inspired oxygen
In-hospital death occurred in 9 (8%) patients (7 in the HY/RD group and 2 in the HY/AZ group).
All of them died of acute respiratory distress syndrome or multi-organ failure. Ventricular arrhythmias were recorded in two cases (1.8%): one patient in the HY/RD group developed torsade de point (TdP) and ventricular fibrillation successfully treated with 200 J Direct-Current shock after 6 days of therapy and one patient in the HY/AZ group developed non-sustained ventricular tachycardia after 4 days. Both patients presented a QTc value > 500 ms and stopped the therapy.
ECG evaluation
In the 111 patients who had taken the drugs for the entire length of the study, a statistically significant increase of QTc interval was reported at 7 days. A QTc interval > 500 ms was observed in 15 (13%) patients on day 3 and in 23 (20%) on day 7. In 18 (16%) patients, an increase in the QTc > 40 ms three days after onset of treatment was documented. At the univariate regression analysis, the risk of developing a QTc > 500 ms was greater in patients with prolonged baseline QTc values (≥ 440 ms for female and ≥ 460 ms for male) (OR 3.9 (95% CI, 1.47–10.17); p = 0.006) and in patients with an increase in the QTc > 40 ms three days after onset of treatment (OR 19.9 (95% CI, 5.93–66.44); p = 0.001) (Table 3). Impaired renal function in terms of estimated glomerular filtration rate < 60 ml/min/1.73mq was related to the risk of developing a QTc interval > 500 ms (OR 3.5 (95% CI 1.33–9.44), p = 0.012).
Table 3.
Predisposing risk factors for QTc interval prolongation (≥ 500 ms)
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| QTc ≥ 500 ms OR (95% CI) |
p value | QTc ≥ 500 ms OR (95% CI) |
p value | |
| Baseline QTc prolongation (≥ 440 ms female, ≥ 460 ms male) | 3.86 (1.47–10.17) | 0.006 | 7.10 (1.88–26.81) | 0.004 |
| Delta QTc > 40 ms (0–3 days) | 19.86 (5.93- 66.44) | 0.001 | 30.15 (6.96- 130.55) | 0.001 |
| eGFR < 60 ml/min/1.73mq | 3.53 (1.32–9.44) | 0.012 | 1.38 (0.41–4.67) | 0.600 |
| Non-invasive ventilation | 0.43 (0.17–1.08) | 0.072 | ||
| CRP > 50 mg/L | 1.40 (0.47–4.18) | 0.543 | ||
| Baseline serum K < 3.8 mg/dL | 0.68 (0.22–1.89) | 0.417 | ||
| Baseline serum Na < 138 mg/dL | 1.71 ( 0.68–4.30) | 0.259 | ||
QTc corrected QT interval, eGFR estimated glomerular filtration, CRP C-reactive protein
The multivariate regression analysis indicated that prolonged baseline QTc values (OR 7.10 (95% IC 1.88–26.81); p = 0.004) and the increase in the QTc > 40 ms three days after onset of treatment (OR 30.15 (95% IC 6.96–130.55); p = 0.001) were independent predictors of QTc prolongation (> 500 ms); estimated glomerular filtration rate < 60 ml/min/1.73mq was not significant (p = 0.6) (Table 3).
Hydroxychloroquine plus ritonavir/darunavir group
The QTc interval increased progressively overtime from 438 ms (421–454) at day 0 to 448 ms (429–483) at day 3 and to 452 ms (430–490) at day 7 (baseline vs. day 3, p = 0.001; baseline vs. day 7, p = 0.001; day 3 vs. day 7, p = 0.001) (Fig. 1).
Fig. 1.

QTc interval trend (minimum, first quartile, median, third quartile and maximum) at baseline, day 3 and day 7 in hydroxychloroquine plus ritonavir/darunavir group
Hydroxychloroquine plus azithromycin group
The QTc interval increased progressively overtime from 433 ms (412–447) at baseline to 430 ms (420–447) at 3 days and to 440 ms (428–464) at 7 days (baseline vs. day 7, p = 0.001) (Fig. 2).
Fig. 2.
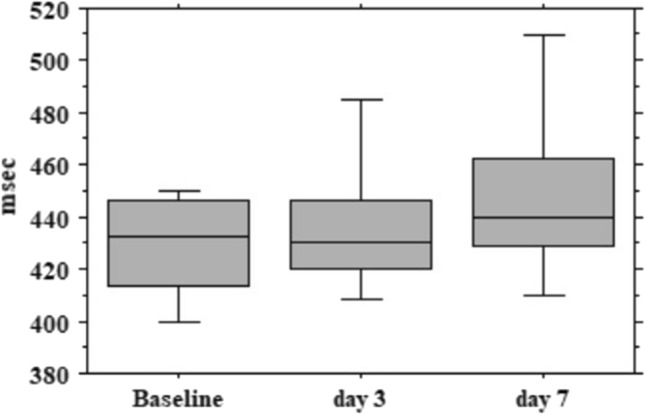
QTc interval trend (minimum, first quartile, median, third quartile and maximum) at baseline, day 3 and day 7 in hydroxychloroquine plus azithromycin group
Discussion
Drug-induced QT prolongation is considered a surrogate indicator for increased risk of drug-associated TdP [12]. Although only a small proportion of patients with QTc prolongation suffer from TdP, drug-associated QT prolongation is related to increased arrhythmic and non-arrhythmic mortality [13, 14]. Hydroxychloroquine and azithromycin are known to induce QTc prolongation directly by blocking inward cellular potassium current and indirectly by inhibiting CYP2D6 and therefore increasing the circulating levels of eventual concomitant drugs that prolong QTc, which can promote life-threatening ventricular arrhythmias [15, 16]. Ritonavir is a protease inhibitor developed for the treatment of HIV infection used in combination with other antiretroviral drugs such as lopinavir or darunavir. A recent trial reported a QT prolongation in COVID-19 patients treated with ritonavir and lopinavir but no pro-arrhythmic adverse events were observed [9]. However, ritonavir is a potent CYP3A4 inhibitor and hence its potential pro-arrhythmic risk. These drugs are generally well tolerated when used in chronic diseases, but data about their safety came mainly from in vitro studies and small non-randomized clinical trials. Evidence about their use alone or in combination in an acute setting such as COVID-19 pneumonia is still limited. COVID-19 patients are likely to have longer baseline QTc as a result of the metabolic and physiologic sequelae of their illness and particularly they are exposed to a systemic inflammatory response, electrolyte imbalance and concomitant drugs leading to an increased risk of QTc prolongation [17]. All these factors could increase the risk of adverse drug effects related to the use of hydroxychloroquine, especially in combination with ritonavir/darunavir or azithromycin [18, 19]. Our study reported a significant prolongation of QTc interval at 7 days in patients with severe COVID-19 pneumonia treated with hydroxychloroquine with both combinations. This prolongation of QTc was already present at 3 days after the onset of treatment in the HY/RD group.
Moreover, during the study period, 20% of our patients developed a QTc > 500 ms. The risk of developing a QTc interval > 500 ms was greater in patients presenting with a QTc prolongation at baseline and in those with an increase in the QTc interval > 40 ms compared with the pre-drug baseline values. This value is a more stringent criterion compared to the one reported by the current guidelines on the prevention of TdP that used to consider significant interval of least 60 ms [15]. In our population, we observed two cases (1.8%) of ventricular arrhythmias (one of TdP and another of recurrent episodes of non-sustained ventricular tachycardia) both in patients with a QTc > 500 ms. While the risk of developing malignant arrhythmias remains relatively low in a short period of administration, our observations confirm the safety concerns raised by two recent studies about the use of hydroxychloroquine in COVID-19 patients [18, 19]. Because of this evidence, every patient hospitalized for COVID-19 should be subjected to a careful assessment of baseline risk of QT prolongation, including baseline ECG, laboratory exams, and collection of medical and pharmacological history to identify and to correct potentially arrhythmogenic risk factors such as electrolyte disturbances or concomitant use of QTc prolonging agents [20]. In our experience, impaired renal function (estimated glomerular filtration rate < 60 ml/min/1.73 mq) and the need for non-invasive ventilation were not associated with the risk of developing a QTc > 500 ms. These findings seem to suggest that the prolongation of the QTc was not related to the characteristics associated with the severity of the underlying disease and the clinical course.
The design of the study does not allow a comparison between groups: however, in HY/RD group the QTc seemed to increase to higher values than in HY/AZ group. Further studies are needed to understand if the combination HY/RD should be regarded as a less convenient option for the higher risk of QTc prolongation.
In conclusion, in patients with COVID-19 pneumonia treated with hydroxychloroquine in association with antiviral drugs ritonavir/darunavir or azithromycin, a close QTc surveillance should be performed especially in high-risk patients such as those who present a QTc prolongation at baseline or those who experienced an increase in the QTc > 40 ms compared with the pre-drug value. This close monitoring would allow the prompt identification of subjects with increased pro-arrhythmic risk in which discontinuation of the treatment should be considered after a careful evaluation of the risk/benefit of the ongoing therapy.
Limitations
This study has several limitations. First, the sample size is relatively small. Second, the study population consisted of two consecutive series of patients with different characteristics that do not allow a comparison between groups. Moreover, we do not have a control group of treatment with only hydroxychloroquine. Finally, higher risk subjects may not have been represented in our population because we excluded individuals with prolonged QTc intervals at baseline as recommended by international guidelines.
Compliance with ethical standards
Conflict of interest
The author(s) declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Van Ierssel S, Dauby N, Bottieau E, Huits R (2020) Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. https://epidemio.wiv-sp.be/ID/Documents/Covid19/COVID19_InterimGuidelines_Treatment_ENG.pdf?fbclid=IwAR2P. Accessed 3 July 2020.
- 5.Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: Recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 6.Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H, Zuo Y, Li H, Wang J, Xv Q, Yu W, Liu J, Shao C, Hao J, Wang C, Ma Y, Wang Z, Yanagihara R, Wang J, Deng Y. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. MedRxiv. 2020 doi: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei L, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. The cardiotoxicity of antimalarials: Malaria Policy Advisory Committee Meeting. https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2. Accessed 21 Apr 2020.
- 10.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chorin E, Dai M, Shulman E, Wadhwani L, Cohen RB, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Soinelli M, Park DS, Chinitz L, Jankelosn L. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. MedRxiv. 2020 doi: 10.1101/2020.04.02.20047050. [DOI] [PubMed] [Google Scholar]
- 12.Simpson TF, Kovacs RJ, Stecker EC (2020) Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19. Accessed 22 Apr 2020.
- 13.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Ju J, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson T, Salazar J, Vittinghoff E, Probert J, Iwahashi A, Olginet JE, Ursell P, Hart A, Moffatt E, Tseng ZH. Association of QT prolonging medications with risk of autopsy causes of sudden death. JAMA Intern Med. 2020;180(5):1–9. doi: 10.1001/jamainternmed.2020.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W. American heart association acute cardiac care committee of the council on clinical cardiology, the council on cardiovascular nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdany J, Kim AHJ. Use of Hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020 doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etchegoyen CV, Keller GA, Mrad S, Cheng S, Di Girolamo G. Drug-induced QT interval prolongation in the intensive care unit. Curr Clin Pharmacol. 2017;12(4):210–222. doi: 10.2174/1574884713666180223123947. [DOI] [PubMed] [Google Scholar]
- 18.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, Cour M. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Chi-Chung Cheng V, Edwards KM, Gandhi R, Muller WJ, O’Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y (2020) Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 infection. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management. Accessed 21 Apr 2020. [DOI] [PMC free article] [PubMed]


