Abstract
Objective: Stimulant medication and behavior therapy are efficacious for youth with attention-deficit/hyperactivity disorder (ADHD). However, research suggests that stimulants may start and/or worsen sleep problems for youth. Further, the impact of behavior therapy for ADHD on sleep is unknown. This study examined the frequency of sleep problems and effects of stimulant medication, behavior therapy, and their combination on sleep problems in youth with ADHD. This study also explored the influence of dimensional baseline ratings of ADHD symptom subtype and psychiatric comorbidity on sleep outcomes.
Methods: Participants were 576 children (aged 7–9 years) with ADHD-Combined type from the Multimodal Treatment of ADHD study that compared methylphenidate, behavior therapy, and their combination to community care. Before treatment, parents completed the Child Behavior Checklist used to derive a total sleep problems score. Parents also completed ratings of oppositionality and ADHD symptom severity, whereas youth completed ratings of depression and anxiety. These ratings were readministered after treatment.
Results: General linear mixed-effects models were used to assess change in total sleep problems across treatment. The combined group exhibited a statistically significant reduction in total sleep problems (z = −5.81, p < 0.001). Reductions in total sleep problems in methylphenidate (z = −3.11, p = 0.05), behavior therapy (z = −2.99, p = 0.08), or community care (z = −1.59, p > 0.99) did not reach statistical significance. Change in psychiatric symptoms did not significantly moderate change in total sleep problems by treatment assignment. Greater baseline oppositional defiant disorder severity predicted less reduction in total sleep problems, χ2(1) = 3.86, p < 0.05.
Conclusions: Findings suggest that combination of methylphenidate and behavior therapy is efficacious for reducing parent-reported sleep problems in young children with ADHD-Combined type relative to community care. However, potential ameliorative effects of monotherapy treatments (i.e., methylphenidate, behavior therapy) should be examined. Future replication is needed to confirm findings.
Keywords: attention-deficit/hyperactivity disorder, oppositional defiance, stimulant medication, behavior therapy, sleep
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by persistent inattentive and/or hyperactive behavior and manifests in numerous presentations across multiple contexts (American Psychiatric Association 2013). Symptoms emerge in childhood and affect ∼5%–10% of youth (Willcutt 2012; Polanczyk et al. 2014; Thomas et al. 2015). In addition to co-occurring psychiatric conditions (major depression, anxiety disorders, oppositional defiant disorder [ODD], conduct disorder, etc.; Warikoo and Faraone 2013), ∼45%–73% of youth with ADHD experience significant sleep disturbance (Shur-Fen 2006; Sung et al. 2008; Craig et al. In Press). Commonly reported sleep problems in youth with ADHD include difficulty falling asleep, bedtime resistance, longer sleep duration, daytime sleepiness, nightmares, enuresis, and sleep talking (Cortese et al. 2009; Kirov and Brand 2014). Sleep problems are reported at dramatically higher rates in youth with ADHD than found in unaffected youth (Hvolby 2015), and exact a significant burden on youth and families across multiple domains (e.g., academic, psychosocial, family, financial) of functioning (Sung et al. 2008; Langberg et al. 2013; Lycett et al. 2016). Thus, the effective treatment/management of sleep problems and ADHD symptoms is essential to reduce burden on affected youth and their families.
There are several evidence-based treatments targeting ADHD, including stimulant medication, behavior therapy (e.g., parent training, behavior modification), and their combination (American Academy of Pediatrics 2011; Hodgson et al. 2014). However, there is some controversy surrounding the effects of stimulant medication on sleep. Several studies have shown stimulants are associated with sleep worsening (i.e., reduced sleep efficiency, delays in sleep onset, delays in rising, reduced sleep duration, etc; Galland et al. 2010; Lee et al. 2012; Kidwell et al. 2015). However, on the contrary, clinical observation and subjective report in the context of open-label treatment suggest stimulant medication may also show positive effects on sleep by reducing ADHD-linked inner restlessness and bedtime resistance (Jerome 2001; Tomás-Vila et al. 2010; Hvolby 2015). In contrast to stimulant medication for ADHD, the effect of behavioral interventions for ADHD on sleep has not yet been studied (Stein et al. 2012). Behavior therapy is an efficacious nonpharmacological alternative for addressing ADHD symptoms, often reducing child conduct problems and improving parenting skills (Daley et al. 2014). Therefore, it is possible that behavior therapy may effectively target disruptive behaviors (e.g., bedtime resistance) that impede sleep onset and maintenance. Nevertheless, despite a growing literature showing positive effects of behavioral sleep interventions on sleep functioning in youth with ADHD, at present no known studies have assessed the impact of behavioral ADHD interventions on sleep problems (Stein et al. 2012).
Further complicating these issues, sleep problems in youth with ADHD may be exacerbated by psychiatric comorbidity (Sadeh et al. 2006). For example, comorbid anxiety is commonly associated with night wakings, bedtime resistance, sleep anxiety, and difficulty falling asleep (Mick et al. 2000; Hansen et al. 2011; Accardo et al. 2012; Becker et al. 2018). The limited available research on comorbid depression suggests it is linked to delayed sleep onset, daytime sleepiness, and decreased sleep duration (Accardo et al. 2012; Becker et al. 2018). Comorbid ODD is predominantly linked to bedtime resistance (Corkum et al. 1999; Mick et al. 2000), and associated dyssomnias (i.e., difficulty rising, delayed sleep onset; Corkum et al. 1999). Interestingly, the association between bedtime resistance and ODD may be driven, in part, by comorbid anxiety (Mick et al. 2000; Becker et al. 2018), and it is therefore important that the influence of both disorders be explored when examining effects of comorbidity on sleep.
In addition, ADHD subtype may impact the presentation of sleep problems. Specifically, differential associations have been shown between subtype and sleep disturbance. ADHD-Combined type has been associated with difficulty falling asleep, lowered sleep duration, enuresis, restless sleep, night waking, nightmares, sleep talking, and sleep-related involuntary movements broadly (Corkum et al. 1999; Mayes et al. 2009; Chiang et al. 2010). In contrast, ADHD-Inattentive subtype has been linked to daytime sleepiness and napping (Mayes et al. 2009; Chiang et al. 2010). Less is known about ADHD-Hyperactive/Impulsive subtype; however, it has been linked to increased parasomnias, night wakings, nightmares, and bruxism (Chiang et al. 2010; Becker et al. 2018). In sum, research suggests differential associations between psychiatric comorbidity and ADHD subtype in relation to sleep problems. However, few studies have examined dimensional influences of these factors on sleep, and it is not yet known how these factors might predict or moderate sleep problems in response to ADHD intervention.
In response to limited research regarding the impact of stimulant and behavioral treatment approaches on sleep among youth with ADHD, this study examined the frequency and change in sleep problems across youth receiving intervention in the Multimodal Treatment of ADHD (MTA). The present study aimed (1) to examine rates of parent-reported sleep problems in youth with ADHD, (2) to assess the degree to which sleep problems changed as a function of ADHD treatment in the MTA, and (3) explored whether dimensional ratings of psychiatric comorbidity (i.e., anxiety, depression, and ODD) and ADHD symptom presentation predicted change in sleep problems over time and moderated change in sleep problems by treatment group. Given equivocal findings regarding the effect of stimulant medication on sleep problems in youth with ADHD, and the effectiveness of parent training on sleep problems in youth (Daley et al. 2014), we hypothesized that youth randomized to behavioral treatment alone would exhibit a significant decrease in sleep problems relative to community care, and youth randomized to medication conditions (i.e., medication, combined treatment) would not.
Methods
Participants
Participants included 576 children aged 7–9 years (mean = 7.77, standard deviation [SD] = 0.81) diagnosed with ADHD-Combined type, who participated in the MTA Study. The sample was predominantly boys (80.2%), with almost one-third on a stimulant medication before study entry (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics
| Full sample (N = 576) | Behavioral treatment (n = 144) | Medication management (n = 143) | Combined treatment (n = 144) | Community care (n = 145) | Test statistic | P | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, mean (SD) | 7.77 (0.81) | 7.64 (0.77) | 7.87 (0.81) | 7.74 (0.79) | 7.85 (0.84) | 2.38 | 0.07 |
| Male, n (%) | 462 (80.2) | 114 (79.2) | 117 (81.8) | 113 (78.5) | 118 (81.4) | 0.73 | 0.87 |
| Minority, n (%) | 218 (38.7) | 61 (42.7) | 52 (36.9) | 54 (38.6) | 51 (36.4) | 1.45 | 0.70 |
| School grade level, mean (SD) | 2.38 (0.90) | 2.46 (0.95) | 2.50 (0.87) | 2.36 (0.90) | 2.46 (0.95) | 2.47 | 0.06 |
| Highest parent education: college graduate or above,an (%) | 263 (46.7) | 67 (46.9) | 53 (37.6) | 65 (46.8) | 78 (55.7) | 9.27 | 0.03* |
| Highest parent employment: full time, n (%) | 478 (84.8) | 120 (83.9) | 117 (83.0) | 119 (85.0) | 122 (87.1) | 1.05 | 0.79 |
| Annual income $50,000 or above,an (%) | 212 (38.4) | 54 (38.0) | 48 (35.0) | 60 (43.5) | 50 (37.0) | 2.27 | 0.52 |
| On stimulant medication at study outset, n (%) | 175 (31.0) | 38 (21.7) | 38 (26.6) | 43 (24.6) | 49 (28.0) | 2.42 | 0.49 |
| Baseline clinical measures, mean (SD) | |||||||
| SNAP-IV hyperactivity/impulsivity score | 1.91 (0.65) | 1.89 (0.64) | 1.92 (0.62) | 1.88 (0.68) | 1.91 (0.65) | 0.96 | 0.96 |
| SNAP-IV inattention score | 2.05 (0.64) | 2.00 (0.63) | 2.08 (0.64) | 2.09 (0.62) | 2.05 (0.66) | 0.51 | 0.67 |
| SNAP-IV ODD score | 1.44 (0.75) | 1.38 (0.71) | 1.51 (0.82) | 1.41 (0.75) | 1.49 (0.69) | 1.00 | 0.39 |
| MASC total | 106.98 (35.55) | 104.97 (35.41) | 104.26 (35.99) | 110.78 (33.27) | 107.95 (33.39) | 1.05 | 0.37 |
| CDI total | 10.75 (8.48) | 10.13 (8.85) | 10.04 (7.04) | 11.81 (9.02) | 11.02 (8.80) | 1.36 | 0.25 |
| Total sleep problems score | 1.98 (1.88) | 1.88 (1.87) | 1.96 (1.90) | 2.04 (1.89) | 2.04 (1.91) | 0.24 | 0.87 |
p < 0.05.
Drawn from a categorical variable.
CDI, Children's Depression Inventory; MASC, Multidimensional Anxiety Scale for Children; ODD, oppositional defiant disorder; SD, standard deviation; SNAP-IV, Swanson, Nolan and Pelham-IV.
Measures
Swanson, Nolan and Pelham Rating Scale–Parent
The MTA version of the Swanson, Nolan and Pelham-IV (SNAP-IV) (Swanson et al. 2001) is a 26-item measure used to collect parent and teacher ratings of children's ADHD and ODD symptoms. In the present analysis, only the parent version was used. The 26 items are the sum of 18 ADHD symptoms (9 for inattentive, 9 for hyperactive/impulsive) and 8 ODD symptoms specified in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association 1994). The items are scored on a scale from 0 (not at all) to 3 (very much). Average rating-per-item subscale scores are calculated for the inattention, hyperactivity/impulsivity, and opposition/defiance domains, generating three SNAP-IV subscale scores each ranging from 0 to 3 (Swanson et al. 2001). The SNAP-IV displays acceptable reliability and acceptable to excellent internal consistency (Stevens and Quittne 1998; Bussing et al. 2008), and good to excellent internal consistency (Swanson et al. 2001).
Achenbach Child Behavior Checklist 6-18
The Child Behavior Checklist (CBCL; Achenbach et al. 2001) is a widely used 113-item parent-completed questionnaire evaluating child behavioral and emotional functioning. Items are scored on a 3-point Likert scale (0 = not true; 1 = somewhat or sometimes true; 2 = very true or often true) based on the child's behavior over the previous 6 months. The measure includes eight syndrome scales (anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior). To assess sleep when dedicated measures are not available, researchers have generated sleep scores using some or all of the following seven items of the CBCL: (47) “Nightmares,” (57) “Overtired without good reason,” (76) “Sleeps less than most kids,” (77) “Sleeps more than most kids during day and/or night,” (92) “Talks or walks in sleep,” (100) “Trouble sleeping,” (108) “Wets the bed” (Gregory et al. 2011; Becker et al. 2015). In this study, total sleep problems were calculated as the sum of all seven items. Cronbach's alpha for the CBCL sleep problems score in the MTA sample ranged from 0.46 to 0.52 across time points. Although lower, this range is consistent with that reported in other studies using this scale (Gregory and O'Connor 2002; Becker et al. 2015; Wang et al. 2016).
Children's Depression Inventory
The Children's Depression Inventory (CDI; Kovacs 1985) is a 27-item self-report measure of depressive symptom severity over the past 2 weeks in youth aged 7–17 years. Items are rated on a 0–2 scale, and summed to produce a total score (range: 0–54) with higher scores indicative of greater depressive symptoms (Kovacs 1985). The CDI demonstrates acceptable to excellent internal consistency and adequate to good test–retest reliability (Saylor et al. 1984; Smucker et al. 1986).
Multidimensional Anxiety Scale for Children
The Multidimensional Anxiety Scale for Children (MASC; March et al. 1997) is a 39-item self-report measure of anxiety symptom severity in children and adolescents aged 8–18 years (March et al. 1997). Items are rated on a 4-point Likert scale with greater scores indicative of higher anxiety. The MASC demonstrates satisfactory-to-excellent test–retest reliability (March et al. 1997; Baldwin and Dadds 2007; Osman et al. 2009), poor-to-good internal consistency (Rynn et al. 2006; Baldwin and Dadds 2007; Osman et al. 2009; Wei et al. 2014), adequate convergent and divergent validity (March et al. 1997; Rynn et al. 2006; Baldwin and Dadds 2007), and good concurrent validity (Osman et al. 2009).
Procedure
MTA study procedures were approved by the local institutional review boards at the recruitment sites where the data were collected. Parents provided informed consent for participation and children provided assent. Full details regarding the study protocol and procedures can be found elsewhere (The MTA Cooperative Group 1999). Participants completed a pretreatment assessment to determine eligibility that included parent-reported ratings of ADHD symptom severity (SNAP-IV) and general functioning (CBCL), and youth ratings of anxiety (MASC) and depression (CDI). Afterward, participants were randomly assigned to one of the four treatment groups: behavior therapy, medication management, combined behavior therapy and medication management, and community care. After a treatment course of 14 months, youth and parents were readministered the same pretreatment scales to ascertain change in symptoms after intervention.
Behavior therapy
Participants randomized to the behavior therapy condition received treatment involving parent training, child-focused treatment, and school-based intervention. Parent training consisted of 27 group family sessions and 8 individual family sessions provided by a clinician. Treatment began weekly, with sessions decreasing in frequency over the 14-month period. Child-focused treatment involved an 8-week summer program that spanned 5 days per week and 9 hours per day. Child-focused treatment was administered in group format in a recreational environment and featured several components such as behavioral rewards, time out, social reinforcement, modeling of appropriate behavior, problem solving, motor (i.e., sports) skills, social skills training, classroom-based academic skills learning, and positive reinforcement. Finally, school-based intervention included biweekly teacher consultation to implement classroom behavior management strategies over 10 to 16 weeks. In addition, children received one-on-one child-focused treatment from part-time behaviorally trained aides over ∼12 weeks. Throughout the intervention children received daily report cards to foster parental reinforcement of achievements.
Medication management
Participants randomized to medication management received methylphenidate. After undergoing dose optimization (see The MTA Cooperative Group 1999 for a complete description of optimization procedure), participants received monthly 30-minute visits with clinicians, who provided guidance and support in addition to medication management. However, clinicians were not permitted to provide any behavioral treatment recommendations or guidance. Adjustments to methylphenidate dosage of ±10 mg/day could be made at these visits based on a review of all relevant information. For youth only receiving medication management, the average daily dose of methylphenidate was 37.7 mg (The MTA Cooperative Group 1999; Jensen et al. 2001).
Combined treatment
Participants randomized to combined treatment received a combination of behavior therapy and medication management interventions described previously. For participants in this combined group, the average daily dose of methylphenidate was 31.2 mg (Jensen et al. 2001).
Community care
Participants randomized to community care received reports of their initial study evaluation and community mental health referrals for treatment. Participants were asked to return for follow-up assessments that paralleled the other three treatment conditions. Most (67.4%) youth randomized to this group received stimulant medication from a healthcare provider during the study period (e.g., primary care provider; The MTA Cooperative Group 1999). Of note, medication visits with the community provider were less frequent relative to medication management and combined treatment condition (Jensen et al. 2001). With respect to psychosocial treatment, a very low proportion of the sample used behavior therapy services (Pelham 1999). For participants in this community care group, the average daily stimulant dose was 23.0 mg/day with 2.3 times daily dosing on average (The MTA Cooperative Group 1999). Sixteen youth who were receiving stimulant medication in this group also received a concurrent antidepressant (excluding tricyclics or bupropion; The MTA Cooperative Group 1999).
Analytic plan
Although the initial MTA study included 579 participants, three participants were missing outcome measures of interest at baseline and were excluded from the current analyses. Descriptive statistics were computed for basic demographic characteristics and clinical measures of interest (Table 1). To assess pre- to posttreatment changes in total sleep problems by treatment assignment, general linear mixed-effects models (GLMM) were estimated using STATA 14.2. Linear mixed-effects modeling is advantageous over other statistical approaches as it allows for the analysis of incomplete data. This allows for the use of all available data despite missing values (Tasca and Gallop 2009). Predictors in the GLMM included time (i.e., baseline, posttreatment), treatment assignment (i.e., referral, medication, behavioral, combined medication, and behavioral), and the interaction between time and treatment assignment. A significant interaction between time and treatment assignment indicated differences in the effect of treatment assignment on sleep over time. Time and treatment assignment were analyzed as categorical predictors, where the reference groups were baseline time point and community care treatment assignment, respectively. To account for individual differences in baseline sleep, the models each included a random intercept term.
To investigate potential predictors and moderators of change in, and treatment of, sleep problems, separate GLMMs were estimated. Based on the extant literature, theoretical candidate predictors/moderators included inattention (SNAP-IV inattention total score), hyperactivity/impulsivity (SNAP-IV hyperactivity/impulsivity total score), oppositional defiance (SNAP-IV ODD total score), anxiety symptom severity (MASC total score), and depressive symptom severity (CDI total score). Each predictor/moderator was entered into the model as a time-varying covariate, with unique pre- and posttreatment observations. Each GLMM included time (i.e., baseline and treatment endpoint; baseline as referent), treatment assignment (referral as referent), candidate predictors/moderators, and all two-way and three-way interaction terms. A candidate moderator was considered a moderator of treatment if the multivariate three-way interaction term (time by treatment by moderator) was significant. A candidate predictor was considered only a predictor of treatment if the multivariate two-way interaction term (time by moderator) was significant, but the three-way interaction (i.e., moderator) was not. Each model included a random intercept term to account for individual differences in the respective baseline sleep subtotal.
Results
Rates of parent-reported sleep problems in youth with ADHD
Descriptive statistics revealed 72.3% of youth had one or more parent-reported sleep problems, as defined by a score of 1 (somewhat or sometimes true) or 2 (very true or often true) on the CBCL. Nightmares were endorsed at the highest rate (39.8%), followed by sleeps less than most kids (29.4%), trouble sleeping (27.0%), wets the bed (20.2%), talks or walks in sleep (16.5%), overtired without reason (15.8%), and sleeps more than most kids (8.2%). See Table 2 for frequency of baseline sleep problems for the total sample and by treatment group.
Table 2.
Frequency of Baseline Child Behavior Checklist Sleep Problems
| Full sample (N = 576) | Behavioral treatment (n = 144) | Medication management (n = 143) | Combined treatment (n = 144) | Community care (n = 145) | χ2 | p | |
|---|---|---|---|---|---|---|---|
| CBCL sleep item endorsement, n (%) | |||||||
| 47. Nightmares | 224 (39.8) | 48 (33.6) | 51 (36.2) | 69 (49.6) | 56 (40.0) | 8.72 | 0.03* |
| 54. Overtired without Reason | 89 (15.8) | 19 (13.3) | 24 (17.0) | 21 (14.9) | 25 (17.9) | 1.37 | 0.71 |
| 76. Sleeps less than most kids | 166 (29.4) | 40 (28.0) | 39 (27.7) | 40 (28.4) | 47 (33.6) | 1.59 | 0.66 |
| 77. Sleeps more than most kids | 46 (8.2) | 11 (7.7) | 15 (10.6) | 9 (6.4) | 11 (7.9) | 1.77 | 0.62 |
| 92. Talks or walks in sleep | 93 (16.5) | 26 (18.2) | 20 (14.2) | 25 (17.7) | 22 (15.7) | 1.06 | 0.79 |
| 100. Trouble sleeping | 152 (27.0) | 41 (28.7) | 32 (22.7) | 45 (32.1) | 34 (24.5) | 3.86 | 0.28 |
| 108. Wets the bed | 114 (20.2) | 35 (24.5) | 31 (22.0) | 26 (18.4) | 22 (15.7) | 3.92 | 0.27 |
| One or more sleep problems | 404 (72.3) | 101 (70.6) | 100 (70.9) | 37 (73.0) | 103 (74.6) | 0.74 | 0.86 |
For CBCL sleep item endorsement, scores of 1 and 2 were collapsed into 1 to obtain frequency of endorsement of sleep problems broadly.
p < 0.05.
CBCL, Child Behavior Checklist.
Change in sleep problems by treatment assignment
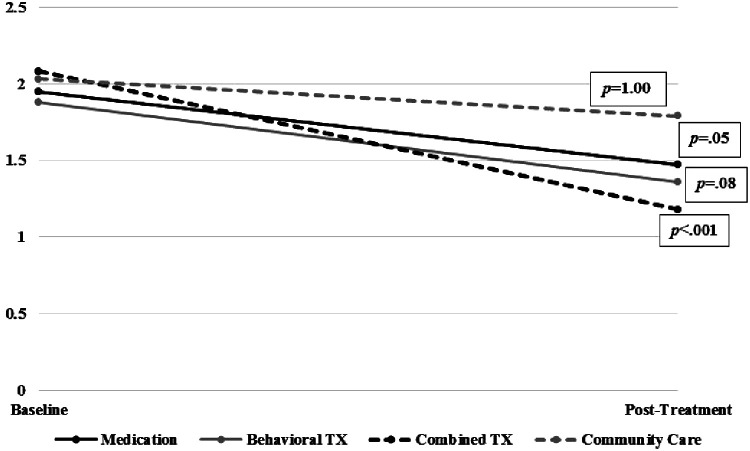
The GLMM model revealed no significant simple effects of treatment assignment on baseline sleep problems score for medication (z = −0.37, p = 0.71), behavioral treatment (z = −0.74, p = 0.46), or combined treatment (z = 0.44, p = 0.85) relative to community care (Table 3). There were no significant simple effects of treatment assignment on posttreatment sleep problems score for medication (z = −1.11, p = 0.27) or behavioral treatment (z = −1.17, p = 0.24) relative to community care. However, there was a significant simple effect of combined treatment on sleep problems relative to community care (z = −3.02, p = 0.003). Results yielded a significant time × treatment assignment interaction (χ2 = 9.30, p = 0.03). Follow-up contrasts with Bonferroni correction (Fig. 1) showed significant pre-to-posttreatment reductions in sleep problems for combined treatment (z = −5.81, p < 0.001). The contrast score revealed a 0.90 SD decrease for combined treatment. There were no significant pre-to-posttreatment reductions in sleep problems for the medication group (z = −3.11, p = 0.05; contrast = −0.48), behavioral therapy (z = −2.99, p = 0.08; contrast = −0.51), or community care (z = −1.59, p > 0.99; contrast = −0.24).
Table 3.
Linear Mixed Model Summary of Changes in Total Sleep Problems by Attention-Deficit/Hyperactivity Disorder Treatment Assignment
| Estimated marginal mean (SE) | Coefficient (SE) | z | p | CI | |
|---|---|---|---|---|---|
| Time | −0.24 (0.15) | −1.59 | 0.11 | −0.54 to 0.06 | |
| Pretreatment | |||||
| Medication | 1.95 (0.15) | −0.08 (0.21) | −0.37 | 0.71 | −0.50 to 0.34 |
| Behavioral treatment | 1.87 (0.15) | −0.16 (0.21) | −0.74 | 0.46 | −0.38 to 0.46 |
| Combined | 2.08 (0.15) | 0.04 (0.22) | 0.20 | 0.85 | −0.38 to 0.46 |
| Community care | 2.03 (0.15) | ||||
| Posttreatment | |||||
| Medication | 1.47 (0.16) | −0.24 (0.22) | −1.11 | 0.27 | −0.67 to 0.19 |
| Behavioral treatment | 1.36 (0.18) | −0.27 (0.23) | −1.17 | 0.24 | −0.72 to 0.18 |
| Combined | 1.18 (0.16) | −0.65 (0.22) | −3.02 | 0.003 | −1.08 to −0.23 |
| Community care | 1.79 (0.15) | ||||
| Intercept | 2.03 (0.15) | 13.37 | <0.001 | 1.74 to 2.33 | |
| Variance intercept | 1.75 (0.17) | 1.46 to 2.11 | |||
| Variance residual | 1.48 (0.10) | 1.30 to 1.69 | |||
CI, confidence interval; SE, standard error.
FIG. 1.
Change in total sleep problems from baseline to posttreatment. Combined, Combined Medication and Behavior Therapy; TX, treatment.
Moderators of change in sleep problems by treatment assignment
Change in hyperactivity/impulsivity (χ2(3) = 2.27, p = 0.52), inattention (χ2(3) = 1.56, p = 0.67), ODD (χ2(3) = 1.44, p = 0.70), anxiety, (χ2(3) = 0.83, p = 0.84), and depression (χ2(3) = 2.13, p = 0.55) severity did not significantly moderate pre-to-posttreatment change in total sleep problems by treatment assignment.
Predictors of change in sleep problems across ADHD treatment
GLMM model terms (i.e., interaction between time and predictor) assessing predictors of change in sleep problems across treatment showed that baseline hyperactivity/impulsivity (χ2(1) = 1.51, p = 0.22), inattention (χ2(1) = 2.60, p = 0.11), anxiety (χ2(1) = 0.41, p = 0.52), and depression (χ2(1) = 0.01, p = 0.93) did not significantly predict pre-to-posttreatment change in CBCL sleep composite. However, ODD symptom severity did significantly predict pre-to-posttreatment change in sleep problems (χ2(1) = 3.86, p < 0.05), such that greater symptoms of ODD at baseline were associated with less change in sleep problems (β = 0.29, standard error = 0.24).
Discussion
This study evaluated the frequency and change across treatment of parent-reported sleep problems in a large sample of young children with ADHD-Combined type receiving stimulant medication, behavioral treatment, their combination, or community care as part of MTA. Moreover, this study explored whether symptoms of inattention, hyperactivity, oppositional defiance, anxiety, and depression influenced change in total sleep problems broadly or by treatment group. Findings revealed a sizeable portion (72.3%) of the sample had one or more parent-reported sleep problem, consistent with previous research showing rates of sleep problems as high as 73% in youth with ADHD (Sung et al. 2008). Nightmares, sleeping less than most youth, and trouble sleeping were the three most commonly endorsed sleep-related problems. With respect to treatment effects on sleep functioning, findings revealed that combined treatment (stimulant medication plus behavior therapy) was the only approach associated with statistically significant reductions in sleep problems. Although change in psychiatric symptom measures did not moderate the effect of treatment assignment on sleep problems, increased baseline ODD symptoms predicted less of a decline in sleep problems across treatment broadly.
With respect to treatment effects on sleep, contrary to the hypothesis, reductions in sleep problems in behavior therapy alone were not statistically significant. This may have been partially related to the fading of behavior therapy in the final 4–5 months of the 14-month MTA trial period (Pelham 1999). With respect to medication management alone, we found no worsening effect of methylphenidate on sleep. Of note, sleep problems displayed a decreasing pattern (i.e., p-value of 0.05). The lack of robustness of this finding may have been related to correction for multiple comparisons. Previous research on the effects of methylphenidate on sleep is mixed, with several studies showing sleep worsening (De Crescenzo et al. 2014; Kidwell et al. 2015), some showing sleep improvement (Kim et al. 2010; Tomás-Vila et al. 2016), and others showing minimal to no impact on sleep (Wolraich et al. 2001; Wilens et al. 2003). Mixed findings are likely because of interindividual differences, in addition to variation in study methodology, including trial duration, study design, sleep and ADHD assessment measures, length of titration, handling of attrition, and sample size (Stein et al. 2012). More favorable sleep outcomes have been observed with use of extended-release formulations, a history of stimulant use, longer duration of use, lower dose, and increased body weight (Stein et al. 2012; Hvolby 2015; Becker et al. 2016).
Despite the finding that neither monotherapy treatment (methylphenidate or behavior therapy) was associated with a statistically significant reduction in sleep problems, their combination was significantly efficacious in this regard. The mechanism by which combined treatment influenced sleep problems is unclear, as change in ADHD symptom severity did not moderate effects of treatment assignment on sleep. Theoretical and clinical accounts suggest that stimulants may improve sleep in some by targeting brain structures implicated in sleep (Kooij et al. 2001; Sobanski et al. 2008), reducing inner feelings of restlessness at bedtime (Jerome 2001), and decreasing daytime sleepiness (Kooij et al. 2001; Sobanski et al. 2008). Thus, stimulant medication may have optimized combined treatment through these means. It is also possible that aspects of behavior therapy contributed to positive effects of combined treatment on sleep. Select behavioral treatment components used in MTA, such as parent training (MTA 1999), overlap with those used in behavior therapies specifically targeting sleep in children in general (Morgenthaler et al. 2006) and in those with ADHD specifically (Keshavarzi et al. 2014; Corkum et al. 2016). For example, parent training within the context of combined treatment may have facilitated changes in sleep by addressing problematic behaviors at bedtime (i.e., bedtime resistance, difficulty winding down). Alternatively, reduced strain at bedtime because of improved behavior may have led to a reduction in parental perceptions of sleep problems, even if sleep remained a problem area for these youth. Future work using multimodal assessment (sleep diary, actigraphy, and parent- and child-report of sleep problems, etc.) is needed to parse treatment effects on sleep.
With respect to the influence of psychiatric symptoms on outcomes, increased baseline ODD symptoms predicted less decline in sleep problems across treatment broadly. ODD symptoms are primarily linked to bedtime resistance (Corkum et al. 1999; Mick et al. 2000), thus this may be one mechanism by which ODD symptoms disrupted reduction in sleep problems across treatment. Of note, the CBCL does not include an item assessing bedtime resistance, and instead includes a question regarding “trouble sleeping.” There is research to suggest parents are able to discriminate between these two sleep difficulties when presented with both items and more detailed wording (Blader et al. 1997). However, because of the brevity of the phrasing of the CBCL item, it is possible that this item may be capturing both difficulty falling and staying asleep (i.e., insomnia) in addition to reluctance to go to bed. Previous literature has hypothesized that anxiety drives the effect of ODD on sleep problems (i.e., bedtime resistance; Mick et al. 2000; Becker et al. 2018). Of note, anxiety did not predict or moderate change in sleep problems over time in this sample.
Findings are preliminary and should be interpreted cautiously. The sleep-related items of the CBCL do not compose a well-validated sleep measure. Internal consistency found in this study was in the lower range as shown in previous research (Gregory and O'Connor 2002; Becker et al. 2015) and is likely attributed to conflicting item content (e.g., sleeps more vs. sleeps less) and variation in the type of sleep problems assessed (Becker et al. 2015). This limits conclusions which may be drawn from the present analyses. Of importance, findings require replication with well-validated parent- and child-reported sleep rating scales in addition to objective sleep measures. In addition, the sample was homogenous with respect to ADHD subtype as all participants were diagnosed with ADHD-Combined type. Although this categorical diagnostic classification does not preclude dimensional examination of ADHD subtypes, it may have restricted the variation in expression of these symptom clusters across the sample. Furthermore, the participant age range is restricted to young children limiting extension of conclusions to older children and adolescents. In addition, study attrition because of lack of tolerance of medication side effects may have skewed outcomes for combined and medication-alone treatment groups. Furthermore, the lower average daily methylphenidate dose in the community care group may have confounded group effects. Moreover, we lack information on concurrent psychiatric or hypnotic medication, which may have influenced outcomes.
The present findings confirm the prevalence of sleep problems in youth with ADHD. This study is the first to compare stimulant medication, behavioral treatment, and combined treatment effects on sleep problems in ADHD. Although findings are preliminary, they suggest that in young children with ADHD-Combined type who have sleep problems, the combination of stimulant medication and behavior therapy may provide some therapeutic benefit. However, the potential ameliorative effects of the monotherapy treatments—especially methylphenidate—should be further examined. Findings also suggest increased ODD symptoms may dampen positive effects of ADHD treatment broadly on sleep problems, which has clinical implications for treatment sequencing. Of note, despite statistically significant positive effects of combined treatment on parent-reported sleep problems, effects were modest. It is likely that targeted sleep interventions (e.g., behavior therapy, melatonin) would provide greater therapeutic gains. Future replication using well-validated multimodal (i.e., subjective, objective) sleep measures assessing a range of sleep problems is needed to fully understand the effects of ADHD treatment on sleep functioning. Future research should also explore potential biological markers (i.e., genes, hormones, neural functioning) associated with stimulant effects on sleep.
Acknowledgments
The research reported in this publication was supported by NIMH T32MH073517 fellowships to Drs. McGuire and Ricketts, and K23MH113884 grant support to Dr. Ricketts. Data and/or research tools used in the preparation of this article were obtained and analyzed from the controlled access data sets distributed from the National Institutes of Health (NIH)-supported National Database for Clinical Trials (NCDT). NDCT is a collaborative informatics system created by the National Institute of Mental Health to provide a national resource to support and accelerate discovery related to clinical trial research in mental health. Data set identifier(s): NCT00000388 and U01MH050453. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or the submitters of the original data to NDCT.
Disclosures
Dr. Ricketts has received research support from the National Institute of Mental Health (NIMH), the Tourette Association of America (TAA), and the TLC Foundation for Body-Focused Repetitive Behaviors. Dr. Sturm has nothing to disclose. Dr. McMakin has received research support from the NIMH. Dr. McGuire has received research support from the NIMH, the TAA, American Academy of Neurology, and the American Brain Foundation. He is a consultant for Bracket, and also receives royalties from Elsevier. Dr. Tan has received research support from the NIMH. Miss Smalberg has nothing to disclose. Dr. McCracken has received grant or research support from the National Institutes of Health, Seaside Therapeutics, Roche, and Otsuka. He has served as a consultant to BioMarin and PharmaNet. Dr. Colwell has received grant support from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Piacentini has received grant or research support from the NIMH, Pfizer Pharmaceuticals through the Duke University Clinical Research Institute CAPTN Network, Psyadon Pharmaceuticals, and the TAA. He has received financial support from the Petit Family Foundation and the Tourette Syndrome Association Center of Excellence Gift Fund. He has received royalties from Guilford Press and Oxford University Press. He has served on the speakers' bureau of the TAA, the International Obsessive Compulsive Disorder Foundation, and the TLC Foundation for Body-Focused Repetitive Behaviors.
References
- Accardo JA, Marcus CL, Leonard MB, Shults J, Meltzer LJ, Elia J: Associations between psychiatric comorbidities and sleep disturbances in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 33:97–105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Dumenci L, Rescorla LA: Ratings of Relations Between DSM-IV Diagnostic Categories and Items of the CBCL/6-18, TRF, and YSR. Burlington (Vermont), University of Vermont, 2001 [Google Scholar]
- American Academy of Pediatrics: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- Baldwin JS, Dadds MR: Reliability and validity of parent and child versions of the Multidimensional Anxiety Scale for Children in community samples. J Am Acad Child Adolesc Psychiatry 46:252–260, 2007 [DOI] [PubMed] [Google Scholar]
- Becker SP, Cusick CN, Sidol CA, Epstein JN, Tamm L: The impact of comorbid mental health symptoms and sex on sleep functioning in children with ADHD. Eur Child Adolesc Psychiatry 27:353–365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Froehlich TE, Epstein JN: Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 37:395–404, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Ramsey RR, Byars KC: Convergent validity of the Child Behavior Checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center. Sleep Med 16:79–86, 2015 [DOI] [PubMed] [Google Scholar]
- Blader JC, Koplewicz HS, Abikoff H, Foley C: Sleep problems of elementary school children: A community survey. Arch Pediatr Adolesc Med 151:473–480, 1997 [DOI] [PubMed] [Google Scholar]
- Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, Swanson JM: Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms: Psychometric properties and normative ratings from a school district sample. Assessment 15:317–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HL, Shur-Fen Gau SS, Ni HC, Chiu YN, Shang CY, Wu YY, Lin LY, Tai YM, Soong WT: Association between symptoms and subtypes of attention-deficit hyperactivity disorder and sleep problems/disorders. J Sleep Res 19:535–545, 2010 [DOI] [PubMed] [Google Scholar]
- Corkum P, Lingley-Pottie P, Davidson F, McGrath P, Chambers CT, Mullane J, Laredo S, Woodford K, Weiss SK: Better nights/better days-distance intervention for insomnia in school-aged children with/without ADHD: A randomized controlled trial. J Pediatr Psychol 41:701–713, 2016 [DOI] [PubMed] [Google Scholar]
- Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R: Sleep problems in children with attention-deficit/hyperactivity disorder: Impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry 38:1285–1293, 1999 [DOI] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, Lecendreux M: Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry 48:894–908, 2009 [DOI] [PubMed] [Google Scholar]
- Craig SG, Weiss MD, Hudec KL, Gibbins C: The functional impact of sleep disorders in children with ADHD. J Atten Disord 2017. [Epub ahead of print]; DOI: 10.1177/1087054716685840 [DOI] [PubMed]
- Daley D, van der Oord S, Ferrin M, Danckaerts M, Doepfner M, Cortese S, Sonuga-Barke EJ, European ADHD Guidelines Group: Behavioral interventions in attention-deficit/hyperactivity disorder: A meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 53:835–847, 2014 [DOI] [PubMed] [Google Scholar]
- De Crescenzo F, Armando M, Mazzone L, Ciliberto M, Sciannamea M, Figueroa C, Janiri L, Quested D, Stefano V: The use of actigraphy in the monitoring of methylphenidate versus placebo in ADHD: A meta-analysis. Atten Defic Hyperact Disord 6:49–58, 2014 [DOI] [PubMed] [Google Scholar]
- Galland BC, Tripp E, Taylor BJ: The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: A matched case–control study. J Sleep Res 19:366–373, 2010 [DOI] [PubMed] [Google Scholar]
- Gregory AM, Cousins JC, Forbes EE, Trubnick L, Ryan ND, Axelson DA, Birmaher B, Sadeh A, Dahl RE: Sleep items in the Child Behavior Checklist: A comparison with sleep diaries, actigraphy, and polysomnography. J Am Acad Child Adolesc Psychiatry 50:499–507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, O'Connor TG: Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry 41:964–971, 2002 [DOI] [PubMed] [Google Scholar]
- Hansen BH, Skirbekk B, Oerbeck B, Richter J, Kristensen H: Comparison of sleep problems in children with anxiety and attention deficit/hyperactivity disorders. Eur Child Adolesc Psychiatry 20:321–330, 2011 [DOI] [PubMed] [Google Scholar]
- Hodgson K, Hutchinson AD, Denson L: Nonpharmacological treatments for ADHD: A meta-analytic review. J Atten Disord 18:275–282, 2014 [DOI] [PubMed] [Google Scholar]
- Hvolby A: Associations of sleep disturbance with ADHD: Implications for treatment. Atten Def Hyp Disord 7:1–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conner CK, Arnold LE, Abikoff HB, Elliott G, Hechtman L, Hoza B, March JS, Newcorn JH, Severe JB, Vitiello B, Wells K, Wigal T: Findings from the NIMH multimodal treatment study of ADHD (MTA): Implications and applications for primary care providers. J Dev Behav Pediatr 22:60–73, 2001 [DOI] [PubMed] [Google Scholar]
- Jerome L: Can methylphenidate facilitate sleep in children with Attention Deficit Hyperactivity Disorder? J Child Adolesc Psychopharmacol 11:109, 2001 [DOI] [PubMed] [Google Scholar]
- Keshavarzi Z, Bajoghli H, Mohamadi MR, Salmanian M, Kirov R, Gerber M, Holsboer-Trachsler E, Brand S: In a randomized case-control trial with 10-year olds suffering from attention deficit/hyperactivity disorder (ADHD) sleep and psychological functioning improved during a 12-week sleep-training program. World J Biol Psychiatry 15:609–619, 2014 [DOI] [PubMed] [Google Scholar]
- Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD: Stimulant medications and sleep for youth with ADHD: A meta-analysis. Pediatrics 136:1144–1153, 2015 [DOI] [PubMed] [Google Scholar]
- Kim H, Yoon I, Cho S, Kim B, Chung S, Lee H, Kim C, Park S, Yoo H. The effect of OROS methylphenidate on the sleep of children with attention-deficit/hyperactivity disorder. Int J Clin Pharm 25:107–115, 2010 [DOI] [PubMed] [Google Scholar]
- Kirov R, Brand S: Sleep problems and their effect in ADHD. Expert Rev Neurother 14:287–299, 2014 [DOI] [PubMed] [Google Scholar]
- Kooij JJS, Middlekoop HAM, van Gils K, Buitelaar JK: The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: An open-label case-control study. J Clin Psychiatry 62:952–956, 2001 [DOI] [PubMed] [Google Scholar]
- Kovacs M: The Children's Depression Inventory (CDI). Psychopharmacol Bull 2:995–998, 1985 [PubMed] [Google Scholar]
- Langberg JM, Dvorsky MR, Marshall S, Evans SW: Clinical implications of daytime sleepiness for the academic performance of middle school-aged adolescents with attention deficit hyperactivity disorder. J Sleep Res 22:542–548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Seo WS, Sung HM, Choi TY, Kim SY, Choi S, Koo BH, Lee JH: Effect of methylphenidate on sleep parameters in children with ADHD. Psychiatry Investig 9:384–390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett K, Sciberras E, Hiscock H, Mensah FK: Sleep problem trajectories and well-being in children with attention-deficit hyperactivity disorder: A prospective cohort study. J Dev Beh Pediatr 37:405–414, 2016 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK: The multidimensional anxiety scale for children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36:554–565, 1997 [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Bixler EO, Vgontza AN, Mahr F, Hillwig-Garcia JE, Elamir B, Edhere-Ekezie L, Parvin M: ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: Differences in sleep problems. J Pediatr Psychol 34:328–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Jetton J, Faraone SV. Sleep disturbances associated with attention deficit hyperactivity disorder: The impact of psychiatric comorbidity and pharmacotherapy. J Child Adolesc Psychopharmacol 10:223–231, 2000 [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Owens J, Alessi C, Boehlecke B, Brown TM, Coleman J, Friedman L, Kapur VK, Lee-Chion T, Pancer J, Swick TJ: Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children: An American Academy of Sleep Medicine Report. Sleep 29:1277–1281, 2006 [PubMed] [Google Scholar]
- Osman A, Williams JE, Espenschade K, Gutierrez PM, Bailey JR, Chowdhry O: Further evidence of the reliability and validity of the multidimensional anxiety. J Psychopathol Behav Assess 31:202–214, 2009 [Google Scholar]
- Pelham WE: The NIMH multimodal treatment study for Attention-Deficit Hyperactivity Disorder: Just say yes to drugs alone? Can J Psychiatry 44:981–990, 1999 [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA: ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol 43:434–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynn MA, Barber JP, Khalid-Khan S, Siqueland L, Dembiski M, McCarthy KS, Gallop R. The psychometric properties of the MASC in a pediatric psychiatric sample. J Anxiety Disorders 20:139–157, 2006 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Pergamin L, Bar-Haim Y: Sleep in children with attention-deficit hyperactivity disorder: A meta-analysis of polysomnographic studies. Sleep Med Rev 10: 381–398, 2006 [DOI] [PubMed] [Google Scholar]
- Saylor CF, Finch AI, Spirito A, Bennett B: The Children's Depression Inventory: A systematic evaluation of psychometric properties. J Consult Clin Psychol 52:955–967, 1984 [DOI] [PubMed] [Google Scholar]
- Shur-Fen GS: Prevalence of sleep problems and their association with inattention/hyperactivity among children aged 6–15 in Taiwan. J Sleep Res 15:403–414, 2006 [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ: Normative and reliability data for the Children's Depression Inventory. J Abnom Child Psychol 14:25–39, 1986 [DOI] [PubMed] [Google Scholar]
- Sobanski E, Schredl M, Kettler N, Alm B: Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: A controlled polysomnographic study. Sleep 31:375–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Weiss M, Hlavaty L: ADHD treatments, sleep, and sleep problems: Complex associations. Neurotherapeutics 9:509–517, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Quittner AL: Factors influencing elementary school teachers' ratings of ADHD and ODD behaviors. J Clin Child Psychol 27:406–414, 1998 [DOI] [PubMed] [Google Scholar]
- Sung V, Hiscock H, Sciberras E, Efron D: Sleep problems in children with attention-deficit/hyperactivity disorder: Prevalence and the effect on the child and family. Arch Pediatr Adolesc Med 162:336–342, 2008 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold E, Conners K, Abikoff HB, Clevenger W, Davies M, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Newcorn JH, Owens EB, Pelham WE, Schiller E, Severe JB, Simpson S, Vitiello B, Wells K, Wigal T, Wu M: Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry 40:168–179, 2001 [DOI] [PubMed] [Google Scholar]
- Tasca GA, Gallop R: Multilevel modeling of longitudinal data for psychotherapy researchers: I. The basics. Psychother Res 19:429–437, 2009 [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 56:1073–1086, 1999 [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P: Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 135:e994–e1001, 2015 [DOI] [PubMed] [Google Scholar]
- Tomás Vila M, Aleu Pérez-Gramunt M, Beseler Soto B, Benac Prefasi M, Pantoja Martínez J, Pitarch Castellano I: Methylphenidate and sleep: Results of a multicenter study on a population of children with attention deficit hyperactivity disorder. An Pediatr 73:78–83, 2010 [DOI] [PubMed] [Google Scholar]
- Wang B, Isensee C, Becker A, Wong J, Eastwood PR, Huang R RU.nions KC, Stewart RM, Meyer T, Brüni LG, Zepf FD, Rothenberger A: Developmental trajectories of sleep problems from childhood to adolescence both predict and are predicted by emotional and behavioral problems. Front Psychol 7:1874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warikoo N, Faraone SV: Background, clinical features and treatment of attention deficit hyperactivity disorder in children. Expert Opin Pharmacother 14:1885–1906, 2013 [DOI] [PubMed] [Google Scholar]
- Wei C, Hoff A, Villabø MA, Peterman J, Kendall PC, Piacentini J, McCracken J, Walkup JT, Albano AM, Rynn M, Sherrill J, Sakolsky D, Birmaher B, Ginsburg G, Keaton C, Gosch E, Compton SN, March J: Assessing anxiety in youth with the multidimensional anxiety scale for children. J Clin Child Adolesc Psychol 43:566–578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens T, Pelham W, Stein M, Conners CK, Abikoff H, Atkins M, August G, Greenhill L, McBurnett K, Palumbo D, Swanson J, Wolraich M: ADHD treatment with once-daily OROS methylphenidate: Interim 12-month results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry 42:424–433, 2003 [DOI] [PubMed] [Google Scholar]
- Willcutt EG: The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 9:490–499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich MI, Greenhill LI, Pelham W, Swanson J, Wilens T, Palumbo D, Atkins M, McBurnett K, Bukstein O, August G, on behalf of the Concerta Study Group. Randomized, controlled trial of oros methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Am Acad Pediatr 108:833–892, 2001 [DOI] [PubMed] [Google Scholar]