Abstract
Background: Lung transplant recipients who experience serious illness could benefit from specialty palliative care (SPC), but evidence suggests that referral has been rare.
Objective: Examine the characteristics of post-transplant SPC encounters, utilization trends, and patient characteristics associated with SPC at a center with established SPC services.
Design: Retrospective cohort study of SPC utilization by 597 lung transplant recipients transplanted between 2010 and 2015. We collected data on pretransplant demographics and post-transplant SPC encounters, including timing, location, and referral reasons. Cumulative incidence of SPC and patient characteristics associated with SPC were examined by competing risks methods. Utilization in the first two post-transplant years was compared between subcohorts defined by year of transplantation.
Results: SPC cumulative incidence was 27% and 43% at one and five years. More than 60% of encounters occurred in the first post-transplant year including 34% during the index transplant hospitalization. Over 90% of encounters occurred in the inpatient setting. The majority of consults were for symptom management. From 2010 to 2015 inpatient utilization in the first two post-transplant years increased from 23% to 42%, and outpatient utilization increased from 2% to 16%. Accounting for increasing utilization, pretransplant SPC and double-lung transplantation were associated with greater incidence of post-transplant SPC.
Conclusions: Lung transplant recipients may have palliative care needs early after transplantation. Increasing utilization suggests greater awareness of or changing attitudes about the utility of SPC for lung transplant recipients. Understanding transplant recipients' palliative care needs and transplant physicians' views of SPC is critical to improving the provision of SPC in lung transplantation.
Keywords: competing risks, lung transplantation, lung transplant recipients, solid organ transplantation, specialty palliative care
Introduction
Lung transplantation is an increasingly common therapy for patients with end-stage lung disease.1 Lung transplant recipients face foreshortened survival and are at risk of experiencing declines in quality of life due to physical and psychological symptoms.1–3 Lung transplant recipients who experience serious illness could potentially benefit from specialty palliative care (SPC), but post-transplantation utilization is not well characterized.
Limited prior evidence suggests that lung transplant recipients rarely receive SPC; and if they do, this likely occurs near end of life in the hospital.4,5 No studies to date have described the longitudinal use of SPC services by lung transplant recipients beginning at transplantation. Understanding longitudinal SPC utilization is important because post-transplant outcomes are unpredictable and transplant recipients can experience serious illness at multiple time points. Furthermore, prior studies have not examined temporal trends, and SPC use may be increasing as the availability of SPC services and awareness of its potential benefits grow.6–9 Characterizing current practices and identifying trends in utilization is an important step to improving the use of SPC in lung transplantation.
To address this gap in knowledge, we explored SPC utilization in a cohort of lung transplant recipients at a large tertiary medical center with well-established transplantation and SPC services. The aims of this study were to describe the location, timing, and reasons for SPC encounters after lung transplantation; examine temporal changes in SPC utilization; and identify pretransplant patient characteristics associated with the receipt of post-transplant SPC.
Materials and Methods
Study design
We conducted a single-center, retrospective cohort study on patients who underwent lung transplantation at the University of Pittsburgh Medical Center (UPMC). This study was approved by the University of Pittsburgh Institutional Review Board.
Setting
The UPMC Lung Transplant Program has a multidisciplinary practice with initial co-management by the surgical and pulmonary transplant teams. Following index transplant hospitalization, the pulmonary team, which includes social workers, transplant psychologists, and behavioral health clinicians, cares for transplant recipients for the remainder of their lives. The inpatient SPC service, established in 1997, is a multidisciplinary consult team of physicians, advanced practice nurses, pharmacists, psychologists, and social workers that sees patients across 10 academic, community, and specialty hospitals. Among the eight outpatient SPC clinics is included the Cardiopulmonary Program Clinic for patients with advanced heart and lung disease, which was established in 2005. During the study period there were no programmatic guidelines for referral to SPC.
Participants
All patients who received a lung transplant between January 1, 2010 and December 31, 2015 at the UPMC were included and followed until transfer of care to another transplant center, death, or through December 31, 2017, at the conclusion of the study period.
SPC utilization
SPC encounters were defined as either consults or follow-up visits by a SPC physician or advanced care provider, and abstracted from the electronic health records (EHRs) by identifying SPC consult and progress notes. During each hospitalization, SPC encounters after the initial SPC consultation were considered follow-up visits. Inpatient encounters were classified by location (hospital floor or ICU) and occurring during or after index transplant hospitalization. Outpatient encounters were initial outpatient consultations and any subsequent outpatient visits. Outpatient visits following an inpatient SPC encounter or an initial outpatient consultation were considered follow-up visits. Time from transplant to SPC encounters was calculated in years. The primary reason for SPC consultation was abstracted from the initial field in the SPC consultation note, which uses four mutually exclusive categories: symptom management (specified as pain or nonpain symptoms), psychosocial support, goals of care, or hospice. Before 2012, reasons were entered in a free-text field and grouped by the authors into four categories. In cases with a lack of clarity the primary reason for consultation was determined by chart review.
Patient characteristics
All patient characteristics were abstracted from the EHRs, including age at transplantation, gender, race, pretransplant SPC, lung allocation score, transplant indication, type of surgical procedure, and pretransplant hospitalization, mechanical ventilation, and extracorporeal membrane oxygenation utilization at the time of transplantation. Transplantation procedure was defined as single-lung transplantation (SLTx) or double-lung transplantation (DLTx). Transplant indication was defined by five categories: chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis (IPF), cystic fibrosis, connective tissue-associated interstitial lung disease (CT-ILD), and other. CT-ILD was separated from IPF as patients with CT-ILD have systemic autoimmune diseases that may affect the receipt of SPC because of the potential for increased extrapulmonary symptoms compared with other lung transplant recipients. Graft survival was defined as alive, dead, or lost to follow-up through December 31, 2017. Patients who underwent a second transplantation during the study period were considered to have expired at re-transplantation because within-subject correlation from repeated observations increases the risk of type II error.10
Statistical analysis
Cohort demographics
Baseline demographics were summarized using descriptive statistics, including means, medians, ranges, and frequencies. Cohort one- and five-year survival were estimated by Kaplan-Meier analysis. Demographics of transplant recipients who did and did not receive post-transplant SPC were compared using two-tailed Student's t-test for continuous normally distributed variables or the Mann-Whitney test for continuous, nonparametric variables, and the chi-square test or Fisher's exact test for categorical variables.
Characteristics and timing of SPC encounters
The location, timing, and reasons for SPC encounters were summarized using descriptive statistics. Time of SPC from transplant was compared by Mann-Whitney or Kruskal-Wallis tests with Dunn's test for multiple comparisons. We used a competing risks model to estimate the cumulative incidence of any post-transplant SPC, inpatient SPC, and outpatient SPC with first SPC and death as competing events.11
Temporal changes in SPC use in the first two post-transplant years
We compared SPC utilization in subcohorts defined by calendar year of transplantation (i.e., January 1, 2010–December 31, 2010; January 1, 2011–December 31, 2011). We examined utilization in the first two post-transplant years because all patients were followed for a minimum of two years following transplantation. The percentage of transplant recipients receiving inpatient and outpatient SPC was summarized and compared using the p-value for trend.
Patient characteristics associated with the receipt of post-transplant SPC
To determine if pretransplant characteristics affect the probability of receiving post-transplant SPC, we used the Fine and Gray cumulative incidence function (CIF) regression model with time to first SPC encounter and death as competing events.11,12 We regressed each covariate on the CIF of post-transplant SPC, included covariates with a p-value <0.2 in the initial multivariable model, and reached the final model by stepwise backwards elimination with p-value criteria <0.05. We tested proportional hazards with Schoenfeld residuals and time interactions.13 Potential outliers were identified by Pregibon's delta beta and examined by sensitivity analysis. Model fit was assessed by Cox-Snell residual plot and Harrell's concordance using the equivalent Cox proportional hazards regression model.13 Stata 14.2 (StataCorp, College Station, TX) was used for analysis. A two-tailed p-value <0.05 was considered statistically significant.
Results
Cohort demographics
Between January 1, 2010 and December 31, 2015, 597 transplants were performed. Follow-up for all patients was through December 31, 2017, and the median follow-up time was 3.6 years (interquartile range [IQR], 1.9–5.4 years). During the follow-up period, 42% (249) of patients died, 6% (37) transitioned care to another transplant center, and 0.5% (3) underwent re-transplantation. Cohort graft survival at one and five years was 86% (95% confidence interval [CI], 82%–88%) and 57% (95% CI, 52%–61%), respectively. After transplantation, 42% (251) of transplant recipients received SPC (Table 1), of whom 44% (110) were alive at follow-up.
Table 1.
Characteristics of Transplant Recipients at Time of Transplant by Receipt of Post-Transplant Specialty Palliative Care
| Characteristic (unit) | All patients, N = 597 | Did not receive SPC, N = 346 (58%) | Received SPC, N = 251 (42%) | p |
|---|---|---|---|---|
| N (%) or median (IQR) | ||||
| Age (years) | 60 (49–66) | 60 (48–66) | 61 (50–66) | 0.804 |
| Lung allocation score | 45.1 (35.2–66.6) | 44.7 (35.0–65.6) | 46.9 (35.4–71.4) | 0.336 |
| Female sex | 252 (42) | 133 (38) | 119 (47) | 0.028 |
| Pretransplant SPC | 61 (10) | 19 (5) | 42 (17) | <0.001 |
| Caucasian race | 533 (89) | 307 (89) | 226 (90) | 0.609 |
| Transplant indication | ||||
| COPD | 157 (26) | 87 (25) | 70 (28) | 0.928 |
| IPF | 189 (32) | 112 (32) | 77 (31) | |
| CF | 67 (11) | 40 (12) | 27 (11) | |
| CT-ILD | 75 (13) | 42 (12) | 33 (13) | |
| Other | 109 (18) | 65 (19) | 44 (17) | |
| Transplantation procedure | ||||
| Double lung | 514 (86) | 289 (83) | 225 (90) | 0.033 |
| Single lung | 83 (14) | 57 (17) | 26 (10) | |
| Pretransplant course | ||||
| Hospitalization | 144 (24) | 79 (23) | 65 (26) | 0.414 |
| Mechanical ventilation | 85 (14) | 44 (13) | 41 (16) | 0.225 |
| ECMO | 46 (8) | 26 (8) | 20 (8) | 0.837 |
Other: sarcoidosis, primary pulmonary hypertension, adenocarcinoma in situ, bronchiectasis, silicosis, alpha-1 antitrypsin, re-transplantation or other indications.
CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; CT-ILD, connective tissue-associated interstitial lung disease; ECMO, extracorporeal membrane oxygenation; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; SPC, specialty palliative care.
Location of SPC encounters
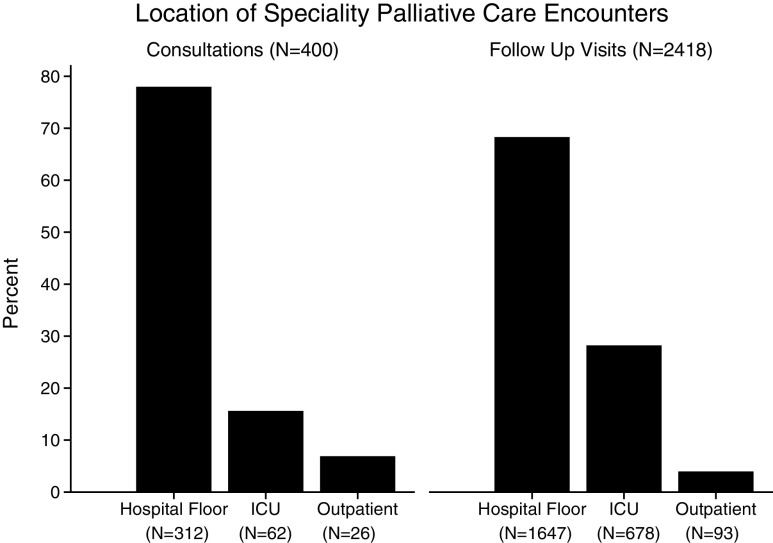
During the follow-up period there were 2818 SPC encounters. Most consults, 93% (374/400), and follow-up visits, 96% (2325/2418), occurred in the inpatient setting (Fig. 1). Only 7% (26/400) of consults and 4% (93/2418) of follow-up visits occurred in outpatient clinics. Among the 251 transplant recipients who received post-transplant SPC, a total of 18% (45) received outpatient SPC.
FIG. 1.
Location of SPC consultations and follow-up visits. SPC, specialty palliative care.
Timing of SPC encounters
Overall 64% (1795/2818) of SPC encounters occurred in the first post-transplant year, including 64% (1738/2699) of inpatient and 48% (57/119) of outpatient encounters (Fig. 2). Median time of SPC consultation was 0.53 year post-transplant (IQR, 0.14–2.32) with outpatient consultations occurring later than inpatient consultations (median [IQR], 1.59 [0.31–2.91] vs. 0.50 [0.13–2.23] years; p = 0.02). Notably, 45% (112/251) who received SPC did so during index hospitalization, accounting for 34% (966/2818) of encounters. Of the 249 decedents, 56% (141) first received SPC a median of 0.68 year from death (IQR, 0.21–1.84).
FIG. 2.
Timing of all SPC encounters after lung transplantation for inpatient and outpatient SPC.
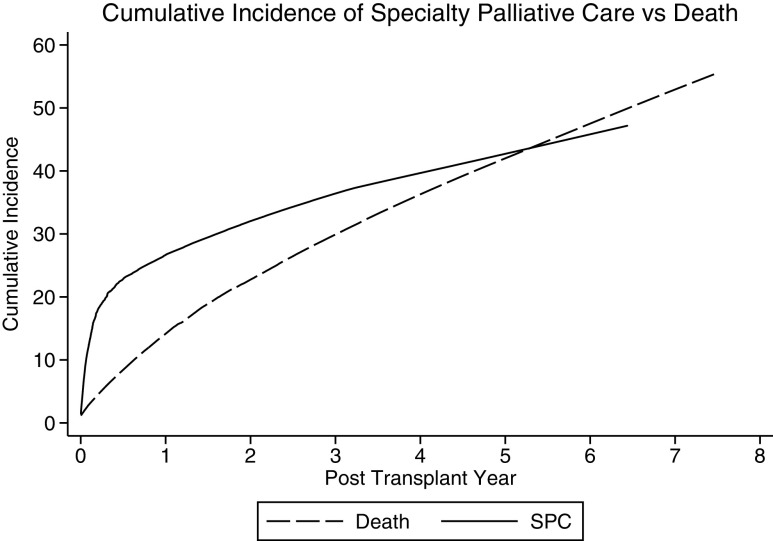
The cumulative incidence of SPC was 27% (95% CI, 23%–30%) at one year and 43% (95% CI, 39%–47%) at five years (Fig. 3). The cumulative incidence of inpatient SPC at one and five years was 25% (95% CI, 22%–28%) and 40% (95% CI, 36%–44%), and the cumulative incidence of outpatient SPC at one and five years was 4% (95% CI, 2%–6%) and 8% (95% CI, 6%–11%).
FIG. 3.
Cumulative incidence of receiving any SPC accounting for the competing risk of death compared with the cumulative incidence of death.
Reasons for SPC consultation
Of 400 consultations, 76% were for symptom management (N = 304), 13% for psychosocial support (N = 54), 9% for goals of care (N = 35), and 2% for hospice (N = 7). Of all consultations requesting symptom management (N = 304), 68% (N = 208) were for nonpain symptoms, 25% (N = 75) for pain, and 7% (N = 21) did not specify. Consultations for symptoms and support occurred earlier post-transplant than goals of care or hospice (Fig. 4). Most consultations for goals of care (25/35) and hospice (6/7) occurred after the first post-transplant year.
FIG. 4.
Time of SPC consultations by reason. Consultation for symptom management (median, 0.41 year; IQR, 0.11–1.88) or support (median, 0.64 year; IQR, 0.17–2.67) occurred earlier than goals of care (median, 1.88 years; IQR, 0.98–3.70) or hospice (median, 3.34 years; IQR, 1.60–4.86). *Symptom management versus goals of care (p ≤ 0.001) and versus hospice (p = 0.006). **Support versus goals of care (p = 0.005) and versus hospice (p = 0.038). IQR, interquartile range.
Changes in SPC utilization over time
The overall percentage of transplant recipients receiving SPC in the first two post-transplant years increased significantly over time (Fig. 5). Inpatient SPC increased from 23% in 2010 to 42% in 2015 (p-value for trend = 0.001). Outpatient SPC increased from 2% in 2010 to 16% in 2015 (p-value for trend <0.001).
FIG. 5.
Percentage of patients transplanted in each calendar year receiving inpatient or outpatient SPC in the first two post-transplant years.
Patient characteristics associated with the receipt of post-transplant SPC
Transplant recipients who received SPC were more likely to be female (47% vs. 38%; p = 0.028), receive pretransplant SPC (17% vs. 6%; p < 0.001), and undergone DLTx (90% vs. 83%; p = 0.033). In univariable CIF regressions (Table 2), pretransplant SPC (subdistribution hazard ratio [SDH], 2.88; 95% CI, 1.98–4.18), female sex (SDH, 1.38; 95% CI, 1.08–1.77), year of transplant (SDH, 1.12 per year increase; 95% CI, 1.04–1.21), and DLTx (SDH, 1.49; 95% CI, 1.00–2.22) were associated with greater relative incidence of post-transplant SPC. After multivariable regression (Table 2), pretransplant SPC (SDH, 2.70; 95% CI, 1.86–3.29), year of transplant (SDH, 1.10 per year increase; 95% CI, 1.02–1.19), and DLTx (SDH, 1.54; 95% CI, 1.01–2.32) were associated with post-transplant SPC. No covariates violated the proportional hazards assumption and no outliers were identified. Predictive accuracy was acceptable in the analogous Cox model (Harrell's C = 0.610).
Table 2.
Factors Associated with the Receipt of Post-Transplant Specialty Palliative Care in Univariable and Multivariable Cumulative Incidence Function Models
| Characteristic (unit or comparison group) | Univariate SDH (95% CI) | p | Multivariate SDH (95% CI) | p |
|---|---|---|---|---|
| Age (years) | 0.99 (0.99–1.00) | 0.263 | ||
| LAS (per one unit increase) | 1.00 (1.00–1.01) | 0.109 | ||
| Female sex | 1.38 (1.08–1.77) | 0.010 | ||
| Pretransplant SPC | 2.88 (1.98–4.18) | <0.001 | 2.70 (1.86–3.29) | <0.001 |
| Non-Caucasian race | 0.91 (0.60–1.40) | 0.683 | ||
| Double- vs. single-lung transplantation | 1.49 (1.00–2.22) | 0.048 | 1.54 (1.01–2.32) | 0.043 |
| Transplant indication (reference COPD) | ||||
| IPF | 0.91 (0.67–1.24) | 0.551 | ||
| CF | 0.96 (0.61–1.50) | 0.848 | ||
| CT-ILD | 1.13 (0.74–1.73) | 0.577 | ||
| Other | 0.98 (0.68–1.44) | 0.935 | ||
| Pretransplant course | ||||
| Hospitalization | 1.22 (0.92–1.65) | 0.170 | ||
| Mechanical ventilation | 1.35 (0.94–1.93) | 0.100 | ||
| Extracorporeal membrane oxygenation | 1.00 (0.64–1.56) | 0.992 | ||
| Year of transplant (per one year increase) | 1.12 (1.04–1.21) | 0.002 | 1.10 (1.02–1.19) | 0.014 |
95% CI, 95% confidence interval; LAS, lung allocation score; SDH, subdistribution hazard ratio.
Discussion
Both the American Thoracic Society and the American College of Chest Physicians recommend that SPC be used concurrently with curative or life-extending care.14,15 In spite of this, limited evidence suggests that SPC has been rarely received by lung transplant recipients, but most commonly provided near end of life. In this large retrospective cohort study, we examined the patterns of post-transplant SPC utilization at a center with well-established SPC services. We found frequent SPC utilization during the first post-transplant year, including during index hospitalization, for symptom management and psychosocial support. Utilization increased over time, although outpatient SPC was received relatively less frequently. Accounting for increasing utilization, pretransplant SPC and DLTx were associated with post-transplant SPC. Our findings have several implications regarding the use of SPC after lung transplantation.
First, our cohort received SPC more frequently and earlier after transplantation than previously reported. A survey about SPC utilization conducted at 18 academic lung transplant centers performing >15 transplants a year found that most centers estimated referring <5 transplant recipients to SPC per year.16 More recently, a cohort study at a center with established SPC services found that only 24 of ∼600 lung transplant recipients were referred to SPC over 16 months.4 In both these studies, most referrals occurred in the last month of life. In contrast, nearly half of our cohort received SPC most commonly in the first post-transplant year, and decedents receiving SPC did so more than half a year before death.
Early referral is notable because many lung transplant providers equate SPC with end of life. In a survey of transplant providers' views of post-transplant SPC, 65% identified perception of SPC as end-of-life care as a barrier to referral.16 Perception that SPC precludes aggressive treatment was also a barrier for 40%. Earlier referral in our cohort suggests that these views are not universally shared in the lung transplant community. Increasing use of SPC in the absence of programmatic guidelines also suggests that views may be changing, perhaps informed by recent publications highlighting the potential benefits of co-managing transplant patients with SPC or increasing availability of SPC services.4,6,8,9,17 Referral for goals of care or hospice were uncommon, especially in the first post-transplant year, potentially reflecting physicians' preference in addressing goals of care themselves, regulatory emphasis on early outcomes, or other factors.18
The majority of SPC encounters occurring in the first post-transplant year suggests that many lung transplant recipients experience palliative care needs early after transplantation. Transplant recipients can experience distress from new extrapulmonary symptoms, including side effects related to immunosuppressant medications, pain, postoperative cognitive dysfunction, and psychologic symptoms such as depression and post-traumatic stress disorder.19–28 Other sources of distress may include adjusting to the challenges of post-transplant life, such as the intensity of post-transplant care, changes in social relationships, caregiver burdens, worry about allograft function, and uncertainty about long-term outcomes.3,29–32 Consistent with this, most consultations in this study were for symptom management and psychosocial support.
The frequency of inpatient SPC suggests that transplant recipients experience heightened palliative care needs while hospitalized. One study suggests that many transplant recipients experience psychological distress and lower quality of life compared with population norms during index hospitalization, with the latter potentially associated with persistently lower quality of life and greater psychological distress six months post-transplant.33 Beyond index hospitalization, rehospitalization has been associated with lower perception of general health, lower physical and social functioning, and greater bodily pain, which is notable as the majority of transplant recipients are re-hospitalized during the first post-transplant year.34–39
Lastly, the cumulative incidence of SPC increased with time following transplantation. Transplant recipients' palliative care needs may change over time as they develop potentially life-limiting complications later. Chronic rejection is associated with dyspnea, depression, and lower overall quality of life.5,40–42 Symptoms of dyspnea and physical limitations are particularly distressful because transplant recipients may feel they are returning to a pretransplant state.43 In addition to chronic rejection, malignancy and end-stage renal disease elevate the need for advance care planning.
Accounting for increasing SPC use, pretransplant SPC and DLTx were associated with the receipt of post-transplant SPC. Patients or their caregivers already familiar with SPC services could request SPC consultation for symptom management or support following transplantation. A pre-existing relationship may also ameliorate physicians' concerns that transplant recipients will feel abandoned by their physicians after referral.16,44 The association of DLTx with post-transplant SPC is interesting, as it may be associated with a greater improvement in quality of life compared with SLTx.2,45 DLTx, however, may also result in a more complicated post-transplant course (i.e., longer ICU and hospital stays in some studies) with greater palliative care needs.33,46–50
Our study has limitations. First, this study was conducted at a center with well-established SPC services, and our results may not apply to centers with less established SPC. Second, our results may reflect institutional culture and not broader trends. The symptom and support needs prompting SPC referral at our institution could potentially be managed by other specialty services or the primary transplant team at other institutions. Future studies addressing specific reasons for SPC referral and the services provided by SPC will be paramount to understanding SPC's role in lung transplantation. Finally, the primary reason for consultation may not reflect or may underestimate transplant recipients' actual palliative care needs. Despite these limitations, our findings are an important step toward understanding post-transplant SPC utilization.
In conclusion, lung transplant recipients in our cohort received SPC predominantly early after transplantation in the inpatient setting. Our findings suggest that many transplant recipients have palliative care needs soon after transplant, including during index hospitalization. Increasing SPC utilization over time suggests that the views of SPC in the transplant community may be changing beyond the view that SPC is solely for end of life. Characterizing the palliative care needs of transplant recipients and their caregivers will be essential to identifying the indications for SPC consultation and understanding the potential benefits of SPC in lung transplantation.
Acknowledgment
This work was supported by 5T32HL007563-30.
Author Disclosure Statement
None of the authors has a financial relationship with commercial entities with an interest in the subject matter or other conflicts of interest to disclose.
References
- 1. Chambers DC, Yusen RD, Cherikh WS, et al. : The registry of the international society for heart and lung transplantation: Thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047–1059 [DOI] [PubMed] [Google Scholar]
- 2. Singer JP, Singer LG: Quality of life in lung transplantation. Semin Respir Crit Care Med 2013;34:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singer JP, Chen J, Katz PP, et al. : Defining novel health-related quality of life domains in lung transplantation: A qualitative analysis. Qual Life Res 2015;24:1521–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colman R, Singer LG, Barua R, Downar J: Characteristics, interventions, and outcomes of lung transplant recipients co-managed with palliative care. J Palliat Med 2015;18:266–269 [DOI] [PubMed] [Google Scholar]
- 5. Song M-K, De Vito Dabbs A, Studer SM, Zangle SE: Course of illness after the onset of chronic rejection in lung transplant recipients. Am J Crit Care 2008;17:246–253 [PubMed] [Google Scholar]
- 6. Wentlandt K, Dall'Osto A, Freeman N, et al. : The transplant palliative care clinic: An early palliative care model for patients in a transplant program. Clin Transplant 2016;30:1591–1596 [DOI] [PubMed] [Google Scholar]
- 7. Wentlandt K, Weiss A, O'Connor E, Kaya E: Palliative and end of life care in solid organ transplantation. Am J Transplant 2017;17:3008–3019 [DOI] [PubMed] [Google Scholar]
- 8. Colman R, Singer LG, Barua R, Downar J: Outcomes of lung transplant candidates referred for co-management by palliative care: A retrospective case series. Palliat Med 2015;29:429–435 [DOI] [PubMed] [Google Scholar]
- 9. Freeman N, Le LW, Singer LG, et al. : Impact of a transplant palliative care clinic on symptoms for patients awaiting lung transplantation. J Heart Lung Transplant 2016;35:1037–1039 [DOI] [PubMed] [Google Scholar]
- 10. Sainani K: The importance of accounting for correlated observations. PM R 2010;2:858–861 [DOI] [PubMed] [Google Scholar]
- 11. Austin PC, Fine JP: Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 13. An Introduction to Survival Analysis Using Stata 3rd ed. www.stata.com/news/saus3.html (Last accessed June13, 2018)
- 14. Selecky PA, Eliasson CAH, Hall RI, et al. : Palliative and end-of-life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest 2005;128:3599–3610 [DOI] [PubMed] [Google Scholar]
- 15. Lanken PN, Terry PB, Delisser HM, et al. : An official American Thoracic Society clinical policy statement: Palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008;177:912–927 [DOI] [PubMed] [Google Scholar]
- 16. Song M-K, De Vito Dabbs A, Studer SM, Arnold RM: Palliative care referrals after lung transplantation in major transplant centers in the United States. Crit Care Med 2009;37:1288–1292 [DOI] [PubMed] [Google Scholar]
- 17. Dumanovsky T, Augustin R, Rogers M, et al. : The growth of palliative care in U.S. hospitals: A status report. J Palliat Med 2016;19:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maxwell BG, Levitt JE, Goldstein BA, et al. : Impact of the lung allocation score on survival beyond 1 year. Am J Transplant 2014;14:2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dew MA, DiMartini AF, DeVito Dabbs AJ, et al. : Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psychiatry 2012;34:127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dew MA, Myaskovsky L, Switzer GE, et al. : Profiles and predictors of the course of psychological distress across four years after heart transplantation. Psychol Med 2005;35:1215–1227 [DOI] [PubMed] [Google Scholar]
- 21. Gries CJ, Dew MA, Curtis JR, et al. : Nature and correlates of post-traumatic stress symptomatology in lung transplant recipients. J Heart Lung Transplant 2013;32:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen DG, Christie JD, Anderson BJ, et al. : Cognitive function, mental health, and health-related quality of life after lung transplantation. Ann Am Thor Soc 2014;11:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diamond JM, Mikkelsen ME: From research to clinical practice. Cognitive trajectory after lung transplantation. Ann Am Thor Soc 2014;11:1604–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith PJ, Rivelli S, Waters A, et al. : Neurocognitive changes after lung transplantation. Ann Am Thor Soc 2014;11:1520–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith PJ, Blumenthal JA, Carney RM, et al. : Neurobehavioral functioning and survival following lung transplantation. Chest 2014;145:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffman BM, Blumenthal JA, Carney RC, et al. : Changes in neurocognitive functioning following lung transplantation. Am J Transplant 2012;12:2519–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith PJ, Blumenthal JA, Hoffman BM, et al. : Postoperative cognitive dysfunction and mortality following lung transplantation. Am J Transplant 2018;18:696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanuza DM, Lefaiver CA, Brown R, et al. : A longitudinal study of patients' symptoms before and during the first year after lung transplantation. Clin Transplant 2012;26:E576–E589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haines AJ: Tales of roller coaster rides and resilience: Lung transplant caregivers in their own words. J Heart Lung Transplant 2016;35:S346 [Google Scholar]
- 30. Gries CJ, Engelberg RA, Curtis JR, et al. : 217: Caregivers of lung transplant recipients exhibit high risk for psychological symptoms. J Heart Lung Transplant 2008;27:S139 [Google Scholar]
- 31. Myaskovsky L, Posluszny DM, Schulz R, et al. : Predictors and outcomes of health-related quality of life in caregivers of cardiothoracic transplant recipients. Am J Transplant 2012;12:3387–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lefaiver CA, Keough VA, Letizia M, Lanuza DM: Quality of life in caregivers providing care for lung transplant candidates. Prog Transplant 2009;19:142–152 [DOI] [PubMed] [Google Scholar]
- 33. Seiler A, Jenewein J, Martin-Soelch C, et al. : Post-transplant outcome-clusters of psychological distress and health-related quality of life in lung transplant recipients. Swiss Med Wkly 2015;145:w14236 [DOI] [PubMed] [Google Scholar]
- 34. Alrawashdeh M, Zomak R, Dew MA, et al. : Pattern and predictors of hospital readmission during the first year after lung transplantation. Am J Transplant 2017;17:1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osho AA, Castleberry AW, Yerokun BA, et al. : Clinical predictors and outcome implications of early readmission in lung transplant recipients. J Heart Lung Transplant 2017;36:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Courtwright AM, Zaleski D, Gardo L, et al. : Causes, preventability, and cost of unplanned rehospitalizations within 30 days of discharge after lung transplantation. Transplantation 2018;102:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Courtwright AM, Salomon S, Fuhlbrigge A, et al. : Predictors and outcomes of unplanned early rehospitalization in the first year following lung transplantation. Clin Transplant 2016;30:1053–1058 [DOI] [PubMed] [Google Scholar]
- 38. Lushaj E, Julliard W, Akhter S, et al. : Timing and frequency of unplanned readmissions after lung transplantation impact long-term survival. Ann Thorac Surg 2016;102:378–384 [DOI] [PubMed] [Google Scholar]
- 39. Vasiliadis H-M, Collet J-P, Poirier C: Health-related quality-of-life determinants in lung transplantation. J Heart Lung Transplant 2006;25:226–233 [DOI] [PubMed] [Google Scholar]
- 40. Kugler C, Fischer S, Gottlieb J, et al. : Health-related quality of life in two hundred-eighty lung transplant recipients. J Heart Lung Transplant 2005;24:2262–2268 [DOI] [PubMed] [Google Scholar]
- 41. Künsebeck HW, Kugler C, Fischer S, et al. : Quality of life and bronchiolitis obliterans syndrome in patients after lung transplantation. Prog Transplant 2007;17:136–141 [DOI] [PubMed] [Google Scholar]
- 42. Vermeulen KM, Groen H, van der Bij W, et al. : The effect of bronchiolitis obliterans syndrome on health related quality of life. Clin Transplant 2004;18:377–383 [DOI] [PubMed] [Google Scholar]
- 43. Song M-K, Devito Dabbs AJ, Studer SM, et al. : Exploring the meaning of chronic rejection after lung transplantation and its impact on clinical management and caregiving. J Pain Symptom Manage 2010;40:246–255 [DOI] [PubMed] [Google Scholar]
- 44. Colman RE, Curtis JR, Nelson JE, et al. : Barriers to optimal palliative care of lung transplant candidates. Chest 2013;143:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singer JP, Chen J, Blanc PD, et al. : A thematic analysis of quality of life in lung transplant: The existing evidence and implications for future directions. Am J Transplant 2013;13:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puri V, Patterson GA, Meyers BF: Single versus bilateral lung transplantation: Do guidelines exist? Thorac Surg Clin 2015;25:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miñambres E, Llorca J, Suberviola B, et al. : Early outcome after single vs. bilateral lung transplantation in older recipients. Transplant Proc 2008;40:3088–3089 [DOI] [PubMed] [Google Scholar]
- 48. Gammie JS, Keenan RJ, Pham SM, et al. : Single- versus double-lung transplantation for pulmonary hypertension. J Thorac Cardiovasc Surg 1998;115:397–402 [DOI] [PubMed] [Google Scholar]
- 49. Fischer S, Meyer K, Tessmann R, et al. : Outcome following single vs. bilateral lung transplantation in recipients 60 years of age and older. Transplant Proc 2005;37:1369–1370 [DOI] [PubMed] [Google Scholar]
- 50. Padia SA, Borja MC, Orens JB, et al. : Tracheostomy following lung transplantation predictors and outcomes. Am J Transplant 2003;3:891–895 [DOI] [PubMed] [Google Scholar]