Abstract
Mild traumatic brain injury (mTBI) is a risk for military personnel due to blast overpressures, which may result from a variety of sources, including artillery and improvised explosive devices. Much research has gone into the search for a biomarker to identify patients with a TBI. The FDA recently identified two proteins, glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase-L1 (UCH-L1), as biomarkers to evaluate suspected brain injury. Our group previously observed changes in UCH-L1 in a military population exposed to repeated blast. In our current study we assessed GFAP protein levels in a military population exposed to repeated blast during a 2-week training protocol. We observed GFAP levels were reduced in the moderate blast cases on days 6 and 7 during the training. Specifically, moderate blast cases showed a 24.07% reduction from baseline on day 6 and a 29.61% reduction on day 7. Further, GFAP levels were negatively correlated with cumulative blast experienced during training and with duration of military service. We observed that repeated blast exposure at low levels may impact acute changes in GFAP. Additionally subacute cumulative blast exposure or duration of service was also a factor in influencing GFAP levels.
Keywords: blast, biomarker, GFAP, military, overpressure, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a common injury seen both in the civilian and military populations. Although the mechanisms of injury may differ in these two populations, such as a sports concussion versus a combat-related blast exposure, the search for a biomarker able to identify people with TBI provides promise for improved diagnoses of head injuries in both populations. There are various severities of TBI ranging from severe to mild TBI (mTBI). We know that exposure to an overpressure wave can result in injury to the brain and body.1–3 In military combat settings, overpressures can occur due to a variety of sources, including artillery and improvised explosive devices. As many as 22% of military personnel injured in combat operations during the Operation Iraqi/Enduring Freedom campaigns were diagnosed with an, TBI as a result of blast exposure.4 Over the course of a career, these mild blast exposures may have cumulative effects such as headache, or psychological or cognitive changes, some of which may be longlasting.5,6
Accurate and acute diagnosis of TBI is a critical capability for improving management and interventions in patients with these injuries. A single biomarker that could identify a range of TBI severity is yet to be identified, although in February of 2018 the U.S. Food and Drug Administration (FDA) authorized marketing of both glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase-L1 (UCH-L1) as biomarkers to evaluate concussion.7 The use of these proteins has been met with both interest and scrutiny as to their reliability as biomarkers, especially for TBI associated with blast exposures.8,9 In some studies, these proteins were correlated with mTBI. For example, in a study examining adult patients with mTBI and a Glasgow Coma Scale (GCS) score of 9–15, elevations in both GFAP and UCH-L1 were detectable within hours following injury.10 In another study in children with a GCS score of 13–15, UCH-L1 strongly correlated with findings of traumatic intracranial lesions.10 However, there has also been evidence to indicate the lack of usefulness for these proteins as biomarkers of mTBI. In a recent study, proteins such as S100B, tau, GFAP, and UCH-L1 were examined to determine their efficacy as biomarkers; it was found that only S100B was helpful in making an informed decision in the emergency department following mTBI.11 Previous studies have highlighted the time course of GFAP as 6 h to 3 days following impact injury.12,13 One study in particular showed that GFAP was highest in rodents in the serum at 6 h following a fluid percussion injury, whereas some of the human studies saw a peak at 24 h.12,13 To date, no studies have followed a time course of GFAP levels following blast exposure.
Our group has previously published changes in UCH-L1, amyloid beta (Aβ), tau, and neurofilament light chain (NFL) in an experienced breacher population, that is, military personnel exposed to repeated blast as part of their regular training.14,15 Specifically, altered levels of UCH-L1, Aβ, tau, and NFL proteins were observed in the days following blast exposure during a breacher training course.14,15
The objectives of this study were to identify molecular changes following chronic exposure to low-level blast overpressure in a professional community of “breachers.” Further, we sought to assess the relationship of molecular changes with pressure levels in those exposed to higher blast pressure. Ultimately, one of the ultimate goals in studying protein changes in operational populations is to identify a biomarker that can provide an indication of when an individual has been exposed to excessive blast overpressure and may not be ready to return to full duty, or in the sports community, when an athlete is not ready to return to play. These biomarkers need to be reliable in all environments, or, if warranted, clarified when confounding factors such as history of exposure apply. Such confounding factors may need to be taken into account when utilizing biomarkers such as GFAP as an indicator of TBI. In this study we assessed GFAP levels in service members participating in a 2-week breacher training course.
Methods
Standard protocol approvals, registrations, and patient consents
Prior to enrollment, the research protocol was approved by the Institutional Review Boards (IRBs) at the Walter Reed Army Institute of Research, National Institutes of Health, and the Naval Medical Research Center in compliance with all applicable Federal regulations governing the protection of human subjects. All participants provided written, informed consent prior to participation in the study.
Study design and setting
All participants were recruited from service members (SMs) engaged in a 10-day breacher training program for an all-male cohort (n = 50) as previously described.14 Baseline serum samples were collected on day 1 of a 10-day training program, with daily follow-up samples. Pressure sensors were used to measure blast exposure. On day 7, some individuals (n = 29) experienced a moderate blast, defined as a peak overpressure ≥5 psi. Moderate blast cases were matched on age, blast exposure history, and previous TBI to individuals in a no/low blast control group (n = 21), who never experienced blast exposure ≥3 psi at any point during the 10-day training program. All participants were recruited from a well-characterized cohort engaged in a 10-day military blast training program as previously described.16 Training occurred during the hours of 0600 to 1600 daily.
At baseline, self-report data were collected from participants including: age, education, marital status, duration of military service, exercise routine, tobacco use, history of TBI, prior blast exposure (yes/no), and number of lifetime blast exposures. Blood draws were collected on day 1 of training and at the end of each training day between 1600 and 1800; changes in protein levels were assessed using blood collected on days 1 (baseline), 6 (day before moderate blast), 7 (day of moderate blast), and post-blast days 8, 9, and 10. On each day of training, all participants wore a helmet equipped with bilateral sensors mounted above the ear cups (micro Data Acquisition System [μDAS]; Applied Research Associates, Inc., Albuquerque, NM). This sensor-equipped helmet enabled continuous detection of changes in ambient pressure from each side of the head. Data were recorded when a threshold of 0.4 psi was crossed, based on the technical specifications of the sensors as well as considerations for signal-to-noise ratios as they relate to data interpretation. For each recorded event, the highest peak pressure was recorded (i.e., maximal overpressure during the blast exposure). The peak impulse was also recorded (i.e., integral of the overpressure across time throughout the blast exposure); peak impulse represents the amount of force per unit area applied during the positive pressure phase. Readings from the left and right sensor were averaged to produce the data used in analysis. Cumulative pressure was calculated as the summed pressure exposure over the 2-week training course.
Laboratory methods
Peripheral blood samples were drawn between 1600 and 1800 h and processed for serum, then aliquoted and stored at −80°C until processing. An ultra-sensitive immunoassay analyzer capable of single molecule array (Simoa™; Quanterix, Lexington, MA) was used to run an enzyme-linked immunosorbent assay (ELISA) measuring GFAP protein levels. This method results in greater sensitivity in the detection of biomarkers compared with a standard ELISA. The GFAP assays have low limits of detection (LLOD) at 0.276 pg/mL, and all intra- and inter-plate coefficient of variation (CV) values were less than 20%. If a sample was measured as 0.276 pg/mL it was used, and if a sample measured below the LLOD the sample was not included in data analysis.
Statistical analyses
The Statistical Package for the Social Sciences version 24 (SPSS; IBM Corporation, Armonk, NY) was used for database management and statistical analysis. GraphPad Prism version 6.02 (GraphPad Software, San Diego, CA) was used to produce the graphs. Concentrations of GFAP were considered as continuous data. Demographic variables including age, duration of service, education, previous blast exposure, tobacco and alcohol use, hours of exercise, and hours of sleep were analyzed using either t test (age and duration of service) or chi-square (χ2). For GFAP distribution we assessed the normality of the concentration data, and we are within the parameters of the assumptions required to perform parametric statistics. Analysis of variance (ANOVA) was used to compare the moderate group with the no/low blast group based on protein levels on days 1, 6, 7, 8, 9, and 10 of training; an outlier check was performed on all samples and any values above or below two standard deviations (SDs) from the average were removed prior to analysis. Levene's test was used to check the homogeneity of variance and the Welch test was used if needed. To assess changes in protein levels across groups and across training days, a repeated measures (RM)-ANOVA was used; if the test of sphericity showed significance, the Greenhouse-Geisser correction was applied. Multi-variate analysis was performed to determine the effects of age, duration of service, cumulative pressure exposure over the training period, and any interactions of these on the GFAP levels on days 6, 7, and 8, and on the percent change from baseline of GFAP on days 6, 7, and 8. For all categorical variables, chi-square analysis was used to compare the moderate blast cases with no/low blast controls. Self-reported history of exposure was categorized as follows: 1 = 0–10 events, 2 = 11–50 events, 3 = 51–150 events, 4 = 151–350 events, and 5 = 351+ events.
Results
When assessing demographic variables such as age, duration of service, education, previous blast exposure, tobacco and alcohol use, and hours of exercise, we observed no differences between the groups (Table 1). When we assessed hours of sleep we found that moderate cases overall slept fewer hours compared with the no/low blast cases (χ2 = 6.561, p = 0.0376) (Table 1).
Table 1.
Demographics
| |
No/low blast (n = 21) |
Moderate blast (n = 29) |
|
|---|---|---|---|
| Mean (SEM) | Mean (SEM) | P | |
| Age | 29.81 ± 0.79 | 30.52 ± 0.83 | ns (0.540) |
| Duration of service | 8.55 ± 0.78 | 10.76 ± 0.88 | ns (0.067) |
| N (%) | N (%) | (p) | |
|---|---|---|---|
| Education |
|
|
2.048 (0.359) |
| High school or below |
9 (43%) |
7 (24%) |
|
| Some college |
7 (33%) |
14 (48%) |
|
| College of more |
5 (24%) |
8 (28%) |
|
| Previous blast exposure |
|
0.511 (0.475) |
|
| No |
7 (33%) |
7 (24%) |
|
| Yes |
14 (67%) |
22 (76%) |
|
| Tobacco use |
|
|
1.394e-4 (0.991) |
| No |
13 (62%) |
18 (62%) |
|
| Yes |
8 (38%) |
11 (38%) |
|
| Alcohol use |
|
|
2.167 (0.339) |
| None |
2 (10%) |
7 (24%) |
|
| 1–9 per week |
15 (71%) |
19 (66%) |
|
| 10+ per week |
4 (19%) |
3 (10%) |
|
| Hours of exercise |
|
|
2.445 (0.294) |
| None |
0 (0%) |
3 (10%) |
|
| 1–6 per week |
7 (33%) |
10 (34%) |
|
| 7+ per week |
14 (67%) |
16 (56%) |
|
| Hours of sleep |
|
|
6.561 (0.0376) |
| None |
0 (0%) |
2 (7%) |
|
| 1–5 per night |
3 (14%) |
12 (41%) |
|
| 6+ per night | 18 (86%) | 15 (52%) | |
SEM, standard error of the mean.
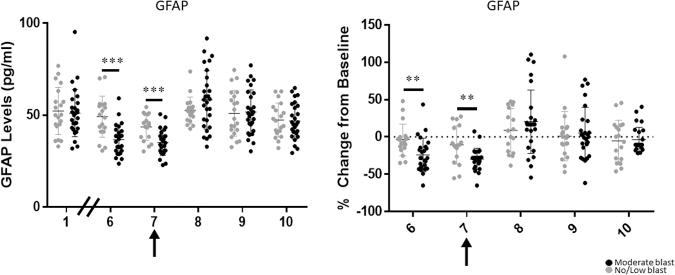
GFAP protein levels were lower on day 7 in both groups when compared across time as a combined data set (Fig. 1). GFAP protein levels were also lower in the moderate blast cases than in the control cases on days 6 (control mean = 49.207 and SD = 11.162; moderate mean = 36.595 and SD = 8.174; F1,43 = 19.189; p < 0.001; Cohen's d = 1.289) and 7 (control mean = 43.510 and SD = 7.099; moderate mean = 35.091 and SD = 6.960; F1,43 = 15.551; p < 0.001; Cohen's d = 1.154) of training (Table 2, Fig. 2). Additionally, when assessing the difference between groups in their change from baseline, moderate blast cases showed a 24.07% reduction (SD = 22.54%) from baseline on day 6, whereas the no/low blast controls showed a 3.94% reduction (SD = 21.08%) from baseline with a large effect size (F1,42 = 11.895; p = 0.001; Cohen's d = −0.922) (Table 2, Fig. 2).
FIG. 1.
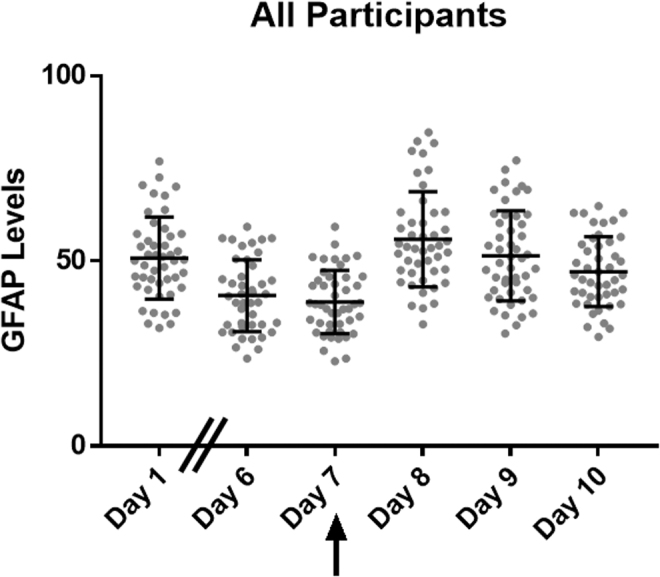
GFAP protein changes for all participants. Arrow indicates largest blast was experienced on day 7. GFAP, glial fibrillary acidic protein.
Table 2.
Mean, Standard Deviation (SD), and Statistics for Analyses Performed
| |
Mean (SD) no/low blast |
IQR for no/low blast |
Mean (SD) moderate blast |
IQR for moderate blast |
F statistic (df) |
P |
Welch statistic |
Effect size (Cohen's d) |
|---|---|---|---|---|---|---|---|---|
| GFAP protein levels | ||||||||
| Day 1 | 52.280 (12.835) | 17.460 | 49.525 (9.694) | 11.290 | 0.704 (1,45) | 0.406 | 0.242 | |
| Day 6 | 49.207 (11.162) | 14.680 | 36.595 (8.174) | 11.240 | 19.189 (1,43) | <0.001 | 1.289 | |
| Day 7 | 43.510 (7.099) | 12.980 | 35.091 (6.960) | 9.040 | 15.551 (1,43) | <0.001 | 1.154 | |
| Day 8 | 52.546 (7.343) | 1.421 | 58.278 (15.845) | 24.560 | 0.105 | 2.747 | −0.464 | |
| Day 9 | 51.008 (12.478) | 20.260 | 51.703 (12.122) | 20.600 | 0.038 (1,46) | 0.847 | −0.056 | |
| Day 10 | 47.375 (9.187) | 14.210 | 46.848 (9.838) | 1.929 | 0.033 (1,43) | 0.856 | 0.055 | |
| Percent change from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 6 |
−3.94% (21.08%) |
13.80% |
−24.07% (22.54%) |
28.34% |
11.895 (1,42) |
0.001 |
|
−0.922 |
| Day 7 |
−10.56% (25.54%) |
41.27% |
−29.61% (14.28%) |
10.71% |
|
0.008 |
8.481 |
−0.920 |
| Day 8 |
8.668% (29.08%) |
62.52% |
20.4% (42.38%) |
37.11% |
0.331 (1,45) |
0.568 |
|
−0.322 |
| Day 9 |
1.019% (32.98%) |
34.09% |
5.318% (34.32%) |
47.08% |
0.780 (1,44) |
0.382 |
|
−0.128 |
| Day 10 | −5.336% (27.83%) | 46.80% | −3.776% (16.62%) | 9.50% | 0.815 | 0.056 | 0.068 | |
| Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time x Group | 5.481 (3.4,97.3) | 0.001 | 0.870 | |||||
| Pearson correlation statistics (r, p) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative pressure by day 6 | Cumulative pressure by day 7 | Total cumulative pressure | ||||||
| GFAP day 6 |
r = 0.333, p = 0.022 |
|
|
|||||
| GFAP day 7 |
|
r = −0.473, p = 0.001 |
|
|||||
| Duration of service | r = 0.258, p = 0.045 | |||||||
df, degrees of freedom; IQR, interquartile range; GFAP, glial fibrillary acidic protein.
FIG. 2.
GFAP protein changes and percent change from baseline for moderate and no/low blast groups. Arrow indicates largest blast was experienced on day 7. ** ≤0.05; *** ≤0.001. GFAP, glial fibrillary acidic protein.
Additionally, moderate blast cases showed a 29.61% reduction (SD = 14.28%) on day 7, whereas the no/low blast cases showed a 10.56% reduction (SD = 25.54%) from baseline with a large effect size (Welch statistic = 8.481; p = 0.008; Cohen's d = −0.920) (Table 2, Fig. 2). Moreover, when RM-ANOVA was used to explore changes in GFAP levels over time, there was no significant main effect of time (p = 0.078), age (p = 0.423), or group (p = 0.819), nor was there a time by age interaction (p = 0.100). However, there was a significant time by group interaction with a large effect size (F3.4, 97.3 = 5.481; p = 0.001; Cohen's d = 0.870), indicating that GFAP levels differed between the no/low and moderate blast groups over time (Table 2, Fig. 2).
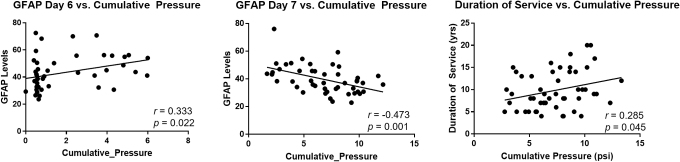
GFAP levels were positively correlated with cumulative pressure on day 6 (r = 0.333; p = 0.022) and negatively on day 7 (r = −0.473; p = 0.001) of training, with higher cumulative pressure exposure co-occurring with higher or lower GFAP levels on the given day (Table 2, Fig. 3). Cumulative pressure overall was also correlated with duration of service (r = 0.258; p = 0.045) (Table 2, Fig. 3).
FIG. 3.
Correlations of cumulative pressure, GFAP levels, and duration of service. GFAP, glial fibrillary acidic protein.
The multi-variate analyses indicate that cumulative pressure had an effect on GFAP levels on days 6 (t = −2.72, p = 0.010) and 7 (t = −3.31, p = 0.002), as well as on the change from baseline levels on days 6 (t = −2.47, p = 0.018) and 7 (t = −2.27, p = 0.029). There were no effects of age and duration of service on the GFAP levels, nor the percent change, and there were no interaction effects between age, duration of service, and cumulative pressure.
Discussion
Our findings show that GFAP protein levels are suppressed in moderate blast cases on days 6 and 7 of training compared with controls, and these differences revealed a large effect size. This indicates protein levels for GFAP were elevated on day 6 prior to moderate blast exposure in those SMs who were exposed to larger blast pressure (11 psi) on day 7 of training. However, on day 7 following blast exposure, the GFAP levels were reduced. When we examined cumulative pressure exposure and GFAP levels on day 7, we observed a negative correlation with GFAP protein levels. This indicates the reduction in GFAP levels may be due to the service member's cumulative exposure during the entire training period and not just the large blast exposure experienced on day 7. Further, duration of service and cumulative pressure for the training period were positively correlated. These findings indicate that those personnel who have a longer duration of service may be in positions of leadership and thus put in a position to be exposed to more cumulative pressure throughout the training period. We postulate that changes in GFAP protein levels may in fact be a reflection of the participants' previous exposures, and not just blast exposure experienced during the training course observed in this study. These findings confirm that patient history is important, perhaps more important than acute exposure to a relatively large overpressure event, and needs to be taken into account when utilizing GFAP as a biomarker of blast exposure. Further, in a previously published study the moderate blast exposure described here was associated with changes in the instructors' California Verbal Learning Test (CVLT) scores and therefore contribute to the ambivalence around the use of GFAP as a biomarker for subclinical neurotrauma in specific populations.
We hypothesize that history of exposure may be an associated factor and therefore has the potential to be a confounding factor when assessing serum GFAP levels. When used as a biomarker of mild neurotrauma, GFAP may be best for those who do not have an extensive history of TBI or blast exposure, because previous injuries may shape GFAP changes more than changes that may occur following a blast. It is important to point out that in this unique population, no participants were diagnosed with an mTBI nor had any post-traumatic amnesia (PTA) or loss of consciousness (LOC). Additionally, blast exposure in this training cohort does not reach the concussion threshold, and the repetitive low-level exposure seen in this cohort results in a different, lower severity event. Also, we are reporting on blast exposure, as compared with blunt impact, which may have different physiological effects to be considered. The activity of GFAP in subconcussive blast events may be different compared with the activity of GFAP in patients with moderate or severe TBI. GFAP as a biomarker in patients with focal mass lesions compared with diffuse injury has previously been shown to be less sensitive, and peripheral levels of GFAP have been correlated with worse health condition as reflected in their GCS score.9
GFAP's status as a neurotrauma biomarker has been debated; some studies indicate GFAP may be best used as a biomarker for moderate/severe TBI.9 Several other studies have examined the benefit of combining multiple proteins into a panel to improve classification of TBI. Specifically, a recent study assessing interleukin-10 (IL-10) and heart fatty acid binding protein (H-FABP) showed differences between computed tomography (CT)-negative and CT-positive patients, and, when combined with GFAP, improved specificity and sensitivity of identifying patients at risk.8 Also important is the acuteness of GFAP, which appears to be elevated at acute time-points and less sensitive at 3 months post-injury; thus it may be most useful in acute mTBI, but not chronic.17–19 Ultimately, a panel of biomarkers that combine proteins changed in both acute and chronic phases of TBI would be ideal to identify TBI in patients in the days or weeks following injury.
Finally, the role of glymphatic clearance is also of importance regarding serum biomarkers and blast exposure that result in possible neuronal changes.20 The glymphatic system is essentially the waste clearance system of the brain, moving waste products through cerebrospinal fluid (CSF) and out to the periphery.21 The vasculature that support the glymphatic system have been shown to be impacted by blast exposure; specifically, blood–brain barrier permeability is disrupted following blast exposure up to 72 h post-blast.22 In a mouse model of TBI, suppression of glymphatic clearance reduced serum concentrations of GFAP in the TBI-only animals, indicating the important role of glymphatic flow in moving proteins from the central nervous system (CNS) into the periphery.23 Further, in a study assessing sleep the induction of sleep improved glymphatic clearance of amyloid-β40 in a mouse model.24 Given that we found the moderate cases to have reported less sleep, the sleep and glymphatic relationship may be an important avenue for further study. Future studies should assess the impact of repeated blast and sleep on serum biomarkers and the relationship of glymphatic flow.
In summary, GFAP is one of many potential biomarkers that has some utility, albeit limited by specific factors such as severity and acuteness of injury. Further, reported large effects suggest history of previous blast exposure contributes to altered protein levels and thus GFAP alone may not be an ideal diagnostic for clinical use in evaluating acute blast exposure effect unless a patient's head injury history is well known. These findings support the importance of patient history when interpreting GFAP findings. Because lower GFAP levels were also found to correlate with longer duration of service, this finding may indicate a long-term or chronic injury response and possibly suppression of protein levels over time.
Institution Disclaimers
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The study protocol was approved by the Walter Reed Army Institute of Research, National Institutes of Health, and the Naval Medical Research Center Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. Some of the authors are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. The opinions and assertions contained herein are the private ones of the authors/speakers and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of the Health Sciences, or any other agency of the U.S. Government.
Funding Information
This work was supported/funded by Defense Health Program (DHP) work unit number 603115HP.3730.001.A1118.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Risling M., and Davidsson J. (2012). Experimental animal models for studies on the mechanisms of blast-induced neurotrauma. Front. Neurol. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stein M.B., and McAllister T.W. (2009). Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatry 166, 768–776 [DOI] [PubMed] [Google Scholar]
- 3. Sajja V.S.S.S., Galloway M., Ghoddoussi F., Kepsel A., and VandeVord P. (2013). Effects of blast-induced neurotrauma on the nucleus accumbens. J. Neurosci. Res. 91, 593–601 [DOI] [PubMed] [Google Scholar]
- 4. Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: Preliminary findings in a US Army brigade combat team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 5. Mac Donald C.L., Adam O.R., Johnson A.M., Nelson E.C., Werner N.J., Rivet D.J., and Brody D.L. (2015) Acute post-traumatic stress symptoms and age predict outcome in military blast concussion. Brain 138, 1314–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mac Donald C.L., Barber J., Andre J., Evans N., Panks C., Sun S., Zalewski K., Sanders R.E., and Temkin N. (2017) 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. NeuroImage Clin. 14, 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samson K. (2018). In the clinic-traumatic brain injury: FDA approves first blood test for brain bleeds after mild TBI/concussion. Neurology Today 18, 12–18 [Google Scholar]
- 8. Lagerstedt L., Egea-Guerrero J.J., Bustamante A., Rodriguez-Rodriguez. A., Rahal A.E., Quintana-Diaz M., Garcia-Armengol R., Prica C.M., Andereggen E., Rinaldi L., Sarrafzadeh A., Schaller K., Montaner J., and Sanchez J.C. (2018). Combining H-FABP and GFAP increases the capacity to differentiate between CT-positive and CT-negative patients with mild traumatic brain injury. PLoS One 13, e0200394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mondello S., Papa L., Buki A., Bullock M.R., Czeiter E., Tortella F.C., Wang K.K., and Hayes R.L. (2011). Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care 15, R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papa L., Mittal M.K., Ramirez J., Silvestri S., Giordano P., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Giordano D.M., and Zonfrillo M.R. (2017). Neuronal biomarker ubiquitin c-terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J. Neurotrauma 34, 2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mondello S., Sorinola A., Czeiter E., Vamos Z., Amrein K, Synnot A., Donoghue E., Sandor J., Wang K.K.W., Diaz-Arrastia R., Steyerberg E.W., Menon D.K., Maas A.I.R., and Buki A. (2017). Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild injury: a living systematic review and meta-analysis. J. Neurotrauma 34, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thelin E.P., Zeiler F.A., Ercole A., Mondello S., Buki A., Bellander B.M, Helmy A., Menon D.K., and Nelson D.W. (2017). Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front. Neurol. 8, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang X.J., Glushakova O., Mondello S., Van K., Hayes R.L., and Lyeth B.G. (2015). Acute temporal profiles of serum levels of UCH-L1 and GFAP and relationships to neuronal and astroglial pathology following traumatic brain injury in rats. J. Neurotrauma 32, 1179–1189 [DOI] [PubMed] [Google Scholar]
- 14. Carr W., Yarnell A.M., Ong R., Walilko T., Kamimori G.H., da Silva U., McCarron R.M., and LoPresti M.L. (2015). Ubiquitin carboxy-terminal hydrolase-L1 as a serum neurotrauma biomarker for exposure to occupational low-level blast. Front. Neurol. 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill J., Cashion A., Osier N., Arcurio L., Motamedi V., Dell K.C., Carr W., Kim H.S., Yun S., Walker P., Ahlers S., LoPresti M., and Yarnell A. (2017). Moderate blast exposure alters gene expression and levels of amyloid precursor protein. Neurology 3, e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carr W., Stone J.R., Walilko T., Young L.A., Snook T.L., Paggi M.E., Tsao J.W., Jankosky C.J., Parish R.V., and Ahlers S.T. (2016). repeated low-level blast exposure: a descriptive human subjects study. Mil. Med. 181, 28–39 [DOI] [PubMed] [Google Scholar]
- 17. Wang K.K.W., Moghieb A., Yang Z., Zhang Z. (2013). Systems biomarkers as acute diagnostics and chronic monitoring tools for traumatic brain injury. Sensing Tech Global Health, Mil. Med. and Enviro. Monitoring. Proceedings of SPIE, 8723 [Google Scholar]
- 18. Adrian H., Marten K., Salla N., and Lasse V. (2016). Biomarkers of traumatic brain injury: temporal changes in body fluids. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaz-Arrastia R., Wang K.K.W., Papa L., Sorani M., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I.R., Valadka A.B., Okonkwo D.O., and Manley G.T., and the TRACK-TBI Investigators including Casey S.S., Cheong M., Cooper S.R., Dams-O'Connor K., Gordon W.A., Hricik A.L., Menon D.K., Mukherjee P., Schnyer D.M., Sinha T.K., and Vassar M.J. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin c-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elder G.A., Gama Sosa M.A., DeGasperi R., Stone J.R., Dickstein D.L., Haghighi F., Hof P.R., and Ahlers S.T. (2015). Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front. Neurol. 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iliff J.J., Wang M., Liao Y., Plogg B.A., Pend W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., and Nedergaard M. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logsdon A.F., Meabon J.S., Cline M.M., Bullock K.M., Raskind M.A., Peskind E.R., Banks W.A., and Cook D.G. (2018). Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci. Reports 8, 11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plog B.A., Dashnaw M.L., Hitomi E., Peng W., Liao Y., Lou N., Deane R., and Nedergaard M. (2015). Biomarkers of traumatic injury are transported from brain to blood via the glympahticsystem. Neurobiol. Dis. 35, 518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O'Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., and Nedergaard M. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]