Key Points
Question
Compared with usual care, does the use of a long-acting formulation of antipsychotic medication reduce the risk of hospitalization in early-phase schizophrenia?
Findings
In this cluster randomized trial of 489 participants, use of long-acting injectable aripiprazole monohydrate was associated with a significant delay in time to first hospitalization, with the number needed to treat to prevent 1 hospitalization of 7.
Meaning
Long-acting formulations are infrequently used in early-phase treatment, and the association between their use and decreased hospitalization risk can have implications for individual treatment decisions and public health efforts.
Abstract
Importance
Long-acting injectable antipsychotics (LAIs) can potentially reduce hospitalization risk by enhancing medication adherence but are rarely considered for early-phase schizophrenia treatment.
Objective
To determine whether encouraging use of a LAI compared with usual care delays the time to first hospitalization with patients with early-phase illness.
Design, Setting, and Participants
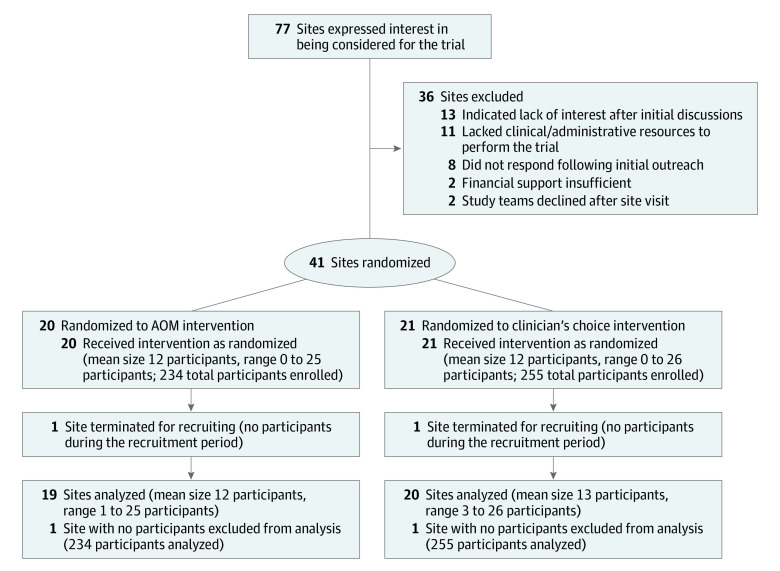
The Prevention of Relapse in Schizophrenia (PRELAPSE) trial was cluster randomized with a follow-up duration of 2 years. The study began in December 2014, was completed in March 2019, and was conducted in 39 mental health centers in 19 US states. Site randomization assigned 19 clinics to encourage treatment with long-acting aripiprazole monohydrate (aripiprazole once monthly [AOM] condition) and 20 to provide treatment as usual (clinician’s choice [CC] condition). Participant eligibility criteria included (1) schizophrenia diagnosis confirmed by a structured clinical interview, (2) fewer than 5 years of lifetime antipsychotic use, and (3) age 18 to 35 years. The AOM sites identified 576 potentially eligible participants, of whom 234 (40.6%) enrolled; CC sites identified 685 potentially eligible participants, of whom 255 (37.2%) enrolled.
Interventions
There were no restrictions on treatment at CC sites (including using LAIs) or at AOM sites with the exception that aripiprazole monohydrate had to be prescribed within US Food and Drug Administration–approved guidelines.
Main Outcomes and Measures
The primary outcome was time to first psychiatric hospitalization based on participant interviews every 2 months, the service use resource form administered every 4 months, and other sources (eg, health records) as available. Potential events were adjudicated by an independent committee masked to treatment assignment.
Results
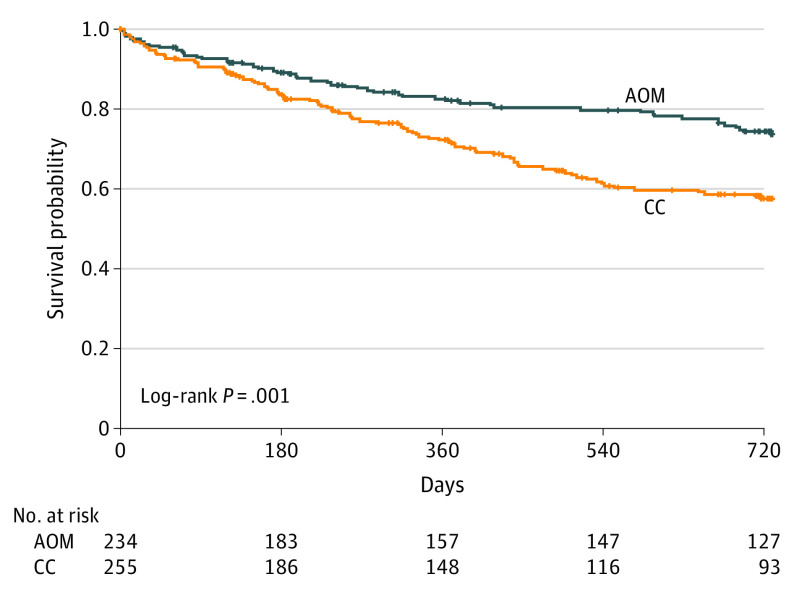
The 489 participants (368 men [75.3%]) had a mean (SD) age of 25.2 (4.2) years and 225 (46.0%) had 1 year or less lifetime antipsychotic use. Fifty-two AOM (22%) and 91 CC participants (36%) had at least 1 hospitalization. The mean survival time until first hospitalization was 613.7 days (95% CI, 582.3-645.1 days) for AOM participants and 530.6 days (95% CI, 497.3-563.9 days) for CC participants. For time to first hospitalization, the hazard ratio was 0.56 (95% CI, 0.34- 0.92; P = .02), favoring AOM. Survival probabilities were 0.73 (95% CI, 0.65-0.83) for AOM participants and 0.58 (95% CI, 0.50-0.67) for CC participants. The number needed to treat to prevent 1 additional hospitalization was 7 participants treated with AOM compared with CC.
Conclusions and Relevance
Long-acting injectable antipsychotic use by patients with early-phase schizophrenia can significantly delay time to hospitalization, a personally and economically important outcome. Clinicians should more broadly consider LAI treatment for patients with early-phase illness.
Trial Registration
ClinicalTrials.gov Identifier: NCT02360319
This randomized clinical trial examines the association of use of long-acting injectable antipsychotics for patients with early-phase schizophrenia with delayed time to hospitalization.
Introduction
The prevention of relapse and hospitalization in schizophrenia is a major public health challenge. Antipsychotic medications reduce the relapse risk compared with placebo with a number to treat (NNT) of 3,1 and epidemiological studies find substantial reductions in all-cause mortality compared with no use.2 Long-acting injectable antipsychotics (LAIs) are superior to oral counterparts in this regard.3,4,5
The consequences of relapse can be substantial for patients at all illness stages.6 For patients with early-phase illness, an additional consequence is that prospective studies7 report that second episodes of psychosis in the same individual respond less well to the same treatment than the first episode. Despite these risks, Tiihonen et al8 reported that 35.7% of patients admitted for their first hospitalization with schizophrenia stopped taking their antipsychotic within 30 days of discharge and 54.3% discontinued taking antipsychotics within 60 days of discharge. Adherence enhancement interventions are needed for patients with early-phase illness.9 Long-acting injectable antipsychotics can enhance adherence,10 but surveys of physician attitudes about LAIs show that they consider LAIs primarily for patients who have already had repeated hospitalizations and relapses.11
For patients with first-episode and early-phase schizophrenia, some randomized clinical trials (RCTs)12,13 and naturalistic/cohort studies8 have shown substantial superiority for LAIs, whereas others have not.14,15 Although RCTs can provide the highest level of evidence, this design may selectively recruit participants who are more adherent to treatment than general patient populations.16 For studies that focus on nonadherence and its consequences, this may diminish the possibility of detecting differences between alternative treatment approaches. A partial solution to this challenge is large simple trials.17 Such trials are less likely to affect adherence as they are embedded in the delivery of care, demand little extra effort from physicians and patients, and involve 1 or 2 objective outcome measures.
This study was designed with this intent, focusing on patients in the early phase of schizophrenia illness before a long-term pattern of frequent hospitalizations has been established. The primary outcome measure, time to first psychiatric hospitalization, is one that can be verified objectively. The primary objective was to determine on an individual level the time to first psychiatric hospitalization with the LAI aripiprazole monohydrate compared with antipsychotic treatment as usual over a 2-year period.
Using cluster randomization at the clinic level enabled us to train the clinical staff at clinics randomized to provide LAI treatment with long-acting aripiprazole monohydrate (aripiprazole once monthly [AOM] condition) without affecting the care at clinics providing antipsychotic medication treatment as usual (clinician’s choice [CC] condition). In addition, participants were not required to consent to individual treatment randomization, but only to consent to the treatment assigned to their clinic site.
Methods
The Prevention of Relapse in Schizophrenia (PRELAPSE) study was an investigator-initiated, multicenter, cluster-randomized clinical trial. The trial design, education strategies for informing potential participants about the trial, and baseline participant characteristics have been published previously.18 The trial protocol (Supplement 1) was approved by the Feinstein Institute for Medical Research Northwell Health institutional review board and local institutional review boards as required by individual sites. The study used an independent data and safety monitoring board to evaluate participant safety, the adequacy and integrity of accumulating data, and the study’s ability to test the hypotheses. Written informed participant consent was obtained before initiating any participant study procedures. Enrollment began in December 2014 and the last assessment occurred in March 2019.
Sites and Randomization
Clinics were eligible to participate if they had adequate populations of patients with early-phase illness, the capacity to offer LAI medication, and the willingness to be randomized. Clinics were paired based on having similar site and patient population factors (eg, urban vs rural, academic vs community, and presence of first-episode treatment vs no specialized treatment) and randomly assigned to treatment condition based on a predetermined computer-generated randomization list. No clinic withdrew after learning of their randomization. Forty-one clinics were randomized; 1 in each condition did not recruit any participants and were terminated, leaving 39 active clinical sites in 19 states. Nineteen (48.7%) of these were randomized to provide LAI treatment with long-acting aripiprazole monohydrate (AOM condition) and 20 (51.3%) to provide antipsychotic medication treatment as usual (CC condition).
Participants
Patients receiving treatment at participating clinics were approached about their willingness to engage in the study and screened to determine eligibility. Inclusion and exclusion criteria were kept to the minimum needed to address the trial questions. Participant inclusion criteria included: (1) schizophrenia diagnosis confirmed by the Structured Clinical Interview for DSM-5, Research Version (SCID-5),19 (2) less than 5 years of lifetime antipsychotic use, (3) age of 18 to 35 years, and (4) ability to provide informed consent. Exclusion criteria included (1) primary DSM-5 diagnosis other than schizophrenia, (2) pregnant or lactating women, (3) unstable medical condition making trial participation unwise, (4) prior clozapine use, and (5) history of intolerance to aripiprazole (AOM sites only). Consent was obtained from individual participants after the cluster randomization of the sites took place (AOM staff training about presenting the AOM condition is described in the eAppendix in Supplement 2).
Treatment
The CC sites offered CC (including possible LAI) medication and other available services to their participants. The AOM sites offered AOM without charge to their participants in addition to other available services. The AOM prescribers were required to prescribe AOM within its US Food and Drug Administration–approved guidelines, which included oral aripiprazole use before LAI AOM initiation and adherence to the approved injection interval, but were not otherwise restricted in their treatment. Participants at AOM sites who did not tolerate aripiprazole, had inadequate response to aripiprazole, or wished to stop AOM for other reasons received alternative treatment as clinically determined at their site and continued with the study outcome assessment.
Outcomes
Outcomes were assessed at the individual participant level only. Participants were assessed for 2 years regardless of the treatment being received.
Primary Outcome
Participants were interviewed via telephone every other month to obtain data on hospitalizations and emergency department and crisis unit use and every 4 months via completion of the service use resource form.20,21 Hospitalizations were verified from other sources (eg, medical records) as available. Potential events were adjudicated by an independent committee masked to treatment assignment. Included in the definition of psychiatric hospitalization were 2 related events: overnight stays in crisis stabilization units and overnight stays in psychiatric emergency departments for treatment of psychiatric symptoms. Inpatient admissions solely for substance detoxification were not counted as psychiatric hospitalizations (additional information is available in the eAppendix in Supplement 2).
Other Outcomes
Assessments conducted via live, 2-way video by central, masked assessors included a SCID-5 interview19 (baseline only), the Brief Psychiatric Rating Scale (BPRS),22 the Clinical Global Impressions Scale,23 the Columbia Suicide Severity Rating Scale,24 and the Heinrichs-Carpenter Quality of Life Scale (QLS)25 at baseline, 12 months, and 24 months. The Repeatable Battery for Assessment of Neuropsychological Status 26 was obtained by site personnel at baseline, 12 months, and 24 months. Adverse event assessments using unstructured inquiries and laboratory tests were obtained at baseline 6, 12, 18, and 24 months. Compared with most RCTs, this limited assessment schedule was designed to minimize contact with participants outside of routine clinical care.
Sample Size
Sample size calculations were performed using the formula developed by Latouche et al27 for proportional hazards modeling of Cox regression. The proportion of failures of interest (ie, first hospitalization) was set at 60% for the treatment as usual (CC) arm. For the sample size calculation, we hypothesized the corresponding proportion in the LAI arm to be 30%. The minimum detectable subdistribution hazard ratio (HR) at 80% power and a 5% significance level with 398 participants equally allocated in the 2 treatment arms was 1.48. Accounting for a 20% dropout rate from the hospitalization analyses, the corresponding sample was 498, or 249 per treatment group. With 400 participants who completed the study (ie, 200 per group), the cause-specific HR was estimated at 80% power and 5% significance level was 1.38.
Statistical Analyses
The intention-to-treat analyses included all participants who provided informed consent and met eligibility criteria that were comprehensively assessed at the first postconsent visit. The primary analysis involved a Cox regression model with a robust sandwich covariance matrix estimate to account for intracluster dependence.28 As a sensitivity analysis, we conducted a discrete-time mixed-effects survival analysis29,30 for the primary end point. In terms of secondary end points, a mixed-effects Poisson regression model31 was used to examine overall rates of hospitalizations. Three-level linear mixed-effects regression models31 were used to study differences between treated and control participants in terms of QLS and BPRS changes from baseline at months 12 and 24 adjusted for clustering of participants nested within study sites and repeated observations nested within participants. Results of secondary analyses were reported as point estimates of treatment-related effects and 95% confidence intervals. A Kaplan-Meier survival plot was used to illustrate the overall marginal effect for time to first hospitalization. Analyses were conducted using SAS, version 9.4 (SAS Institute).
Results
Figure 1 presents the site participant flow. There were 489 participants overall, with 234 AOM (47.9%) and 255 CC patients (52.1%). A detailed individual participant flow chart has been published previously.18 At AOM sites, 576 potential participants were identified who met inclusion/exclusion criteria based on a screening interview. Of these, as previously reported,18 83 (14.4%) declined participation because they would not consider LAI treatment and 165 (28.6%) declined for other reasons.
Figure 1. Site Participant Flow.
AOM indicates aripiprazole once monthly.
Patient Characteristics
Patients were mostly men (368 [75.3%]) (Table 1). The mean (SD) age of CC participants was 24.7 (4.1) years and for AOM 25.7 (4.3) years. The mean (SD) number of prior hospitalizations was 3 (2.5) and 3.4 (2.9) for AOM and CC, respectively; 97 AOM (41.5%) and 94 CC patients (36.9%) had 0 or only 1 prior hospitalizations. The mean (SD) duration of antipsychotic treatment was 612.1 (547.4) days for CC and 646.6 (554.4) for AOM; importantly, 102 participants in AOM (43.6%) and 123 in CC (48.2%) had 1 year or less of lifetime antipsychotic treatment.
Table 1. Participant Characteristics at Study Entry.
| Characteristic | No. (%) | |
|---|---|---|
| AOM (n = 234) | CC (n = 255) | |
| Age, mean (SD), y | 25.7 (4.3) | 24.7 (4.1) |
| Age at first antipsychotic treatment, mean (SD), y | 21.9 (4.4) | 21.3 (4.2) |
| Men | 172 (73.5) | 196 (76.9) |
| Race | ||
| White | 80 (34.2) | 91 (35.7) |
| Black | 109 (46.6) | 104 (40.8) |
| Other | 45 (19.2) | 58 (22.7) |
| Unknown | 0 (0.0) | 2 (0.8) |
| Marital status | ||
| Married | 13 (5.6) | 9 (3.5) |
| Divorced | 7 (3.0) | 8 (3.1) |
| Single | 212 (90.6) | 238 (93.3) |
| Unknown | 2 (0.9) | 0 (0.0) |
| Current residence | ||
| Living alone | 32 (13.7) | 33 (12.9) |
| Supported living environment | 10 (4.3) | 5 (2.0) |
| Structured environment | 8 (3.4) | 12 (4.7) |
| Family of origin | 154 (65.8) | 174 (68.2) |
| Family of orientation | 17 (7.3) | 13 (5.1) |
| Other | 13 (5.6) | 18 (7.1) |
| Duration of antipsychotic treatment, mean (SD), d | 646.6 (554.4) | 612.1 (547.4) |
| Log (duration of antipsychotic treatment +1) (SD) | 5.86 (1.41) | 5.83 (1.32) |
| Had <1 y or less of antipsychotic treatment | 102 (43.6) | 123 (48.2) |
| No. of hospitalizations for psychiatric illness | ||
| 0 | 29 (12.4) | 32 (12.5) |
| 1 | 68 (29.1) | 62 (24.3) |
| 2 | 38 (16.2) | 41 (16.1) |
| 3 | 31 (13.2) | 37 (14.5) |
| 4 | 26 (11.1) | 24 (9.4) |
| ≥5 | 40 (17.1) | 54 (21.2) |
| No. of hospitalizations for psychiatric illness (treated as continuous variable), mean (SD) | 3 (2.5) | 3.4 (2.9) |
Abbreviations: AOM, aripiprazole once monthly; CC, clinician’s choice.
LAI Usage
Seventy-two of the 234 AOM patients (30.8%) patients were receiving either AOM (36 [15.4%]) or another LAI (36 [15.4%]) at the time of consent. In comparison, 70 of the 255 CC patients (27.4%) were receiving either AOM (10 [3.9%]) or another LAI (60 [23.5%]).
Two hundred fourteen of the 234 participants at AOM sites (91.0%) received at least 1 AOM study injection during the study. Of the 20 participants who did not receive an AOM injection, only 1 refused the injection. Other reasons for not receiving an injection were because 2 were not eligible for an injection because of not tolerating an oral aripiprazole challenge, 16 dropped out before receiving an injection, and 1 relocated out of the area. Of the 214 participants who received an AOM injection during the study, 142 (66.4%) were not receiving an LAI at the time of consent. In the CC group, 130 participants (51%) were prescribed an LAI at some point during the study; of these, 23 were prescribed an LAI only after a first hospitalization.
Outcomes and Hospitalizations
The mean (SD) number of total days followed up was 594.3 (250.7) for the AOM group and 573.5 (253.9) for the CC group. The total number of psychiatric hospitalizations was 341 (133 [39.0%] among the 234 AOM participants and 208 [61.0%] among the CC participants). The primary causes of hospitalizations were psychotic symptoms (264 hospitalizations [77.4%]), other than psychotic symptoms (72 hospitalizations [21.1%]), and unclear (5 hospitalizations [1.5%]).
Primary Outcome
Fifty-two AOM participants (22.2%) met criteria for a first hospitalization and 91 of the CC group (36.0%). Of these, 40 of the AOM group were receiving aripiprazole monohydrate at the time of first hospitalization and 22 of the CC group any LAI. The mean survival time until first hospitalization was 613.7 days (95% CI, 582.3-645.1 days) for AOM participants and 530.6 days (95% CI, 497.3-563.9 days) for CC participants.
Under the proportional hazards assumption, the HR for time to first hospitalization was 0.56 (95% CI, 0.34-0.92; P = .02), favoring AOM. A sensitivity analysis using a discrete-time survival model with random site-effect produced identical results (odds ratio [OR], 0.56; 95% CI, 0.34-0.91; P = .02). The Kaplan-Meier curves are displayed in Figure 2. The mean survival time until first hospitalization was 613.7 days (95% CI, 582.3-645.1 days) for AOM participants and 530.6 days (95% CI, 497.3-563.9 days) for CC participants. The estimated survival probabilities from the Cox model were 0.73 (95% CI, 0.65-0.83) for AOM participants and 0.58 (95% CI, 0.50-0.67) for CC participants. This translates to an NNT for prevention of 1 additional hospitalization of 6.67 participants treated with AOM relative to CC. The mean (SD) time to first hospitalization for those hospitalized was 275.1 (234.6) days with AOM and 261.1 (188.9) days for CC.
Figure 2. Time Remaining Without Having a First Hospitalization.
Log rank test χ2 = 11.373; df = 1; P < .001. AOM indicates aripiprazole once monthly; CC, clinician’s choice.
Secondary Outcomes
The AOM option was associated with a 36% decrease in rate for total hospitalizations but the difference was not statistically significant (relative risk, 0.64; 95% CI, 0.33-1.26; P = .20). The estimated change from baseline to month 12 or month 24 did not differ between the conditions for either QLS or BPRS total scores or the respective subfactor scores (Table 2 and eFigures 1-3 in Supplement 2).
Table 2. Change in Heinrich Carpenter Quality of Life and Brief Psychiatric Rating Scale Total Scores Between AOM and CC Conditions From Baseline to 12 Months and 24 Monthsa.
| Score | Estimate (95% CI) | |
|---|---|---|
| Change to mo 12 | Change to mo 24 | |
| Heinrich Carpenter quality of life | ||
| Total score | 1.068 (−2.911 to 5.047) | −2.014 (−6.252 to 2.225) |
| Subscale scores | ||
| Interpersonal relationsb | 0.166 (−1.703 to 2.034) | 0.247 (−1.737 to 2.231) |
| Instrumental rolec | 1.079 (−0.575 to 2.732) | −1.095 (−2.856 to 0.666) |
| Intrapsychic foundationsd | 0.137 (−1.285 to 1.559) | −1.015 (−2.535 to 0.504) |
| Common objects and activitiese | −0.055 (−0.459 to 0.349) | −0.104 (−0.535 to 0.328) |
| BPRS scores | ||
| Total score | 0.960 (−1.105 to 3.025) | 0.334 (−1.993 to 2.661) |
| Factor scores | ||
| Anxiety/depressionf | 0.080 (−0.126 to 0.286) | 0.016 (−0.204 to 0.23)7 |
| Anergiag | 0.150 (−0.049 to 0.350) | 0.136 (−0.090 to 0.362) |
| Thought disturbanceh | 0.035 (−0.185 to 0.254) | −0.141 (−0.376 to 0.095) |
| Activationi | −0.021 (−0.129 to 0.087) | −0.017 (−0.138 to 0.105) |
| Hostile suspiciousnessj | 0.062 (−0.128 to 0.252) | −0.014 (−0.219 to 0.191) |
Abbreviations: AOM, aripiprazole once monthly; BPRS, Brief Psychiatric Rating Scale; CC, clinician’s choice.
The 3-level model of outcome included treatment, time, treatment by time, and age.
Sum of quality of life items 1 to 8.
Sum of quality of life items 9 to 12.
Sum of quality of life items 13 to 17, 20, and 21.
Sum of quality of life items 18 to 19.
Mean of BPRS items 1, 2, 5, and 9.
Mean of BPRS items 3, 13, and 16.
Mean of BPRS items 4, 8, 11, 12. and 15.
Mean of BPRS items 6, 7, and 17.
Mean of BPRS items 10, 11, and 14.
Harms
Two deaths occurred; 1 CC participant died by suicide and 1 CC participant died of unknown causes. Four participants in each condition made suicide attempts (completed suicide excluded). Table 3 presents adverse events occurring after the baseline visit in 5% or more of either AOM or CC participants.
Table 3. Adverse Events Occurring After Baseline Visit in 5% or More of Either AOM or CC Participants.
| Adverse event | Participants experiencing adverse event, No. (%) | |
|---|---|---|
| AOMa | CCb | |
| Worsening of psychotic symptoms | 51 (23) | 97 (40.2) |
| Weight gain | 32 (14.4) | 47 (19.5) |
| Suicidal ideation without suicide attemptc | 19 (8.6) | 23 (9.5) |
| Depression | 23 (10.4) | 20 (8.3) |
| Anxiety | 27 (12.2) | 15 (6.2) |
| Hyperprolactemia | 9 (4.1) | 31 (12.9) |
| Elevated cholesterol levels | 15 (6.8) | 24 (10.0) |
| Hepatic enzyme abnormalities | 15 (6.8) | 22 (9.1) |
| Insomnia | 19 (8.6) | 17 (7.1) |
| Hypertension | 12 (5.4) | 18 (7.5) |
| Somnolence | 16 (7.2) | 11 (4.6) |
| Hostility/aggression | 16 (7.2) | 7 (2.9) |
| Tachycardia | 7 (3.2) | 12 (5.0) |
| Restlessness | 14 (6.3) | 2 (0.8) |
Abbreviations: AOM, aripiprazole once monthly; CC, clinician’s choice.
Two hundred twenty-two AOM participants had a visit after the baseline visit.
Two hundred forty-one CC participants had a visit after the baseline visit.
Does not include the one CC participant who died by suicide. Participants who made suicide attempts concurrent with recorded suicidal ideation are not included; participants who made suicide attempts but also had suicidal ideation not concurrent with an attempt are included.
Discussion
To our knowledge, this is the first large-scale, multisite LAI study for patients with early-phase schizophrenia conducted in the US. The use of an LAI in this population produced a significant 44% reduction in the incidence rate of first hospitalization for the AOM group compared with the CC group and an NNT of 7 for the prevention of hospitalization. In the secondary measure of total number of hospitalizations, a 36% decrease was found, but that difference was not statistically significant. These reductions are notable given that 2 aspects of the design may have decreased the ability to find differences. First, the usual care condition allowed the use of LAIs. Clinics had to have clinical and administrative support to participate in an LAI study; therefore, not surprisingly, the study’s CC clinics had higher rates of LAI use at baseline and during the trial than US statistics would suggest. Second, psychiatric hospitalizations included those for all psychiatric symptoms. Although most of the study hospitalizations were because of psychotic symptoms, some were because of other psychiatric symptoms.
The QLS and BPRS scores did not differ. One possible factor is that the long duration between assessments limited the ability to capture the trajectories of these domains.
Two previous RCTs have reported the superiority of LAIs over oral antipsychotics in first-episode or patients with early-phase schizophrenia. Schreiner et al12 reported on 715 participants within 1 to 5 years of a schizophrenia diagnosis who were randomly assigned to receive LAI paliperidone vs an array of oral medications at 141 centers in 26 countries. The time to relapse was substantially longer in the LAI group. Schreiner et al12 used the Csernansky criteria for relapse,32 which allow for a broad interpretation of worsening. Our study required hospitalization, which generally assures a more severe level of exacerbation and provides a strong health economic rationale. The Schreiner et al12 study sample was considerably older (age 33 years) than our group (age 25 years). Their inclusion criteria required 2 inpatient admissions within the past 2 years, whereas approximately 40% of our study’s sample had a lifetime history of fewer than 2 hospitalizations. Thus, our study’s sample was earlier in the illness course and at a stage when clinicians traditionally are less likely to consider LAIs. In addition, our study was conducted exclusively in the US as compared with 26 different countries. Subotnick et al,13 in a small study conducted at a single academic site, randomly assigned 86 patients with first-episode illness to receive oral or LAI risperidone and found substantially different respective relapse rates of 33% vs 5%. Our study extends these findings. To our knowledge, it is the only large-scale trial conducted in the US with first-episode and patients with early-phase schizophrenia conducted primarily in community mental health centers in addition to academic settings with a more pragmatic than explanatory design.33
Many attribute the low rate of LAI use in clinical practice to patient refusal. However, PRELAPSE demonstrates that, with proper training, practitioners are able to communicate potential advantages of LAIs, even in early illness stages, and engage patients in shared decision-making resulting in high acceptance rates. Antipsychotics should be reserved for those for whom there is a clear indication for ongoing treatment.34 Once that decision is made, the question becomes with which formulation is the patient most likely to derive the benefits intended. The use of LAIs does not deprive patients of autonomy or choice. The choice is made each time an injection is administered. As part of any such discussion with patients, the phenomenon of nonadherence needs to be normalized and destigmatized. It is human nature to have difficulty taking medication on a long-term basis, regardless of the illness and medication type.35,36,37 The recommendation of LAIs should not be construed as a pejorative or punitive stance, which can undermine the therapeutic alliance. Additionally, it should be recognized/acknowledged that even with guaranteed antipsychotic medication, some patients with schizophrenia will require hospitalization because of symptoms other than psychosis or from a relapse of psychotic symptoms, a phenomenon that requires additional research.38
Limitations
Our study’s 2 participant groups had very similar baseline characteristics (eg, baseline use of LAIs, hospitalization, and antipsychotic treatment history). Nevertheless, selection effects may have occurred with our cluster randomization design. Our results might have underestimated the effects of LAIs on hospitalization because of the relatively high LAI use rates for patients with first-episode/early-phase illness in the CC condition. The nature of the patients and clinical sites in the overall trial suggests good generalizability, but training of the AOM sites was an important element in the success of patient enrollment and strategies should be implemented to ensure that clinics generally have access to such training. This study involved only 1 long-acting injectable formulation; however, to our knowledge, there are no data suggesting differences in efficacy among the second-generation LAIs regarding prevention of hospitalization.
Conclusions
The use of LAIs for individuals with early-phase schizophrenia produced a significant and clinically meaningful 44% reduction in the incidence rate of first hospitalization and an NNT of 7 for the prevention of hospitalization.
Trial protocol
eAppendix. Staff training and determination of the primary outcome
eFigure 1. Heinricks Carpenter quality of life (QLS) scale total score over time
eFigure 2. Brief Psychiatric Rating Scale (BPRS) score total score over time
eFigure 3. Brief Psychiatric Rating Scale (BPRS) score thought disturbance factor score over time
Data sharing statement
References
- 1.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. doi: 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- 2.Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61-68. doi: 10.1002/wps.20699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen J, van Rooijen G, Doedens P, Numminen E, van Tricht M, de Haan L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol Med. 2017;47(13):2217-2228. doi: 10.1017/S0033291717000873 [DOI] [PubMed] [Google Scholar]
- 4.Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663. doi: 10.1093/schbul/sbu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiihonen J, Tanskanen A, Taipale H. 20-Year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773. doi: 10.1176/appi.ajp.2018.17091001 [DOI] [PubMed] [Google Scholar]
- 6.Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149-160. doi: 10.1002/wps.20516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042. doi: 10.1038/s41386-018-0278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. doi: 10.1176/appi.ajp.2011.10081224 [DOI] [PubMed] [Google Scholar]
- 9.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. doi: 10.1002/wps.20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. doi: 10.4088/JCP.15032su1 [DOI] [PubMed] [Google Scholar]
- 11.Iyer S, Banks N, Roy M-A, et al. A qualitative study of experiences with and perceptions regarding long-acting injectable antipsychotics: part II-physician perspectives. Can J Psychiatry. 2013;58(5)(suppl 1):23S-29S. doi: 10.1177/088740341305805s04 [DOI] [PubMed] [Google Scholar]
- 12.Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1-3):393-399. doi: 10.1016/j.schres.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 13.Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829. doi: 10.1001/jamapsychiatry.2015.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malla A, Chue P, Jordan G, et al. An exploratory, open-label, randomized trial comparing risperidone long-acting injectable with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses. 2016;9(4):198-208. doi: 10.3371/CSRP.MACH.061213 [DOI] [PubMed] [Google Scholar]
- 15.Medrano S, Abdel-Baki A, Stip E, Potvin S. Three-year naturalistic study on early use of long-acting injectable antipsychotics in first episode psychosis. Psychopharmacol Bull. 2018;48(4):25-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66(8)(suppl):S37-S41. doi: 10.1016/j.jclinepi.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eapen ZJ, Lauer MS, Temple RJ. The imperative of overcoming barriers to the conduct of large, simple trials. JAMA. 2014;311(14):1397-1398. doi: 10.1001/jama.2014.1030 [DOI] [PubMed] [Google Scholar]
- 18.Kane JM, Schooler NR, Marcy P, Achtyes ED, Correll CU, Robinson DG. Patients with early-phase schizophrenia will accept treatment with sustained-release medication (long-acting injectable antipsychotics): results from the recruitment phase of the PRELAPSE Trial. J Clin Psychiatry. 2019;80(3):18m12546. doi: 10.4088/JCP.18m12546 [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I). Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- 20.Rosenheck R, Kasprow W, Frisman L, Liu-Mares W. Cost-effectiveness of supported housing for homeless persons with mental illness. Arch Gen Psychiatry. 2003;60(9):940-951. doi: 10.1001/archpsyc.60.9.940 [DOI] [PubMed] [Google Scholar]
- 21.Rosenheck RA, Leslie DL, Sindelar J, et al. ; CATIE Study Investigators . Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry. 2006;163(12):2080-2089. doi: 10.1176/ajp.2006.163.12.2080 [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799-812. doi: 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- 23.Guy W. Clinical global impressions In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology—Revised. US Department of Health, Education, and Welfare; Public Health Service, Alcohol; Drug Abuse, and Mental Health Administration; National Institute of Mental Health; Psychopharmacology Research Branch; 1976:218-222. [Google Scholar]
- 24.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398. doi: 10.1093/schbul/10.3.388 [DOI] [PubMed] [Google Scholar]
- 26.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 27.Latouche A, Porcher R, Chevret S. Sample size formula for proportional hazards modelling of competing risks. Stat Med. 2004;23(21):3263-3274. doi: 10.1002/sim.1915 [DOI] [PubMed] [Google Scholar]
- 28.Lee EW, Wei LJ, Amato DA. (Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein P, Goel PK, eds. Survival Analysis: State of the Art. Kluwer Academic Publishers; 1992:237-247. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 29.Gibbons RD, Duan N, Meltzer D, et al. ; Institute of Medicine Committee . Waiting for organ transplantation: results of an analysis by an Institute of Medicine Committee. Biostatistics. 2003;4(2):207-222. doi: 10.1093/biostatistics/4.2.207 [DOI] [PubMed] [Google Scholar]
- 30.Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc. 1988;83:414-425. doi: 10.1080/01621459.1988.10478612 [DOI] [Google Scholar]
- 31.Hedeker D, Gibbons R. Longitudinal Data Analysis. John Wiley & Sons, Inc; 2006. [Google Scholar]
- 32.Csernansky JG, Mahmoud R, Brenner R; Risperidone-USA-79 Study Group . A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16-22. doi: 10.1056/NEJMoa002028 [DOI] [PubMed] [Google Scholar]
- 33.Bossie CA, Alphs LD, Correll CU. Long-acting injectable versus daily oral antipsychotic treatment trials in schizophrenia: pragmatic versus explanatory study designs. Int Clin Psychopharmacol. 2015;30(5):272-281. doi: 10.1097/YIC.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675-684. doi: 10.1001/jamapsychiatry.2017.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutler DM, Everett W. Thinking outside the pillbox—medication adherence as a priority for health care reform. N Engl J Med. 2010;362(17):1553-1555. doi: 10.1056/NEJMp1002305 [DOI] [PubMed] [Google Scholar]
- 36.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794-811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 38.Rubio JM, Kane JM. Psychosis breakthrough on antipsychotic maintenance medication (BAMM): what can we learn? NPJ Schizophr. 2017;3(1):36. Published online October 11, 2017. doi: 10.1038/s41537-017-0039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix. Staff training and determination of the primary outcome
eFigure 1. Heinricks Carpenter quality of life (QLS) scale total score over time
eFigure 2. Brief Psychiatric Rating Scale (BPRS) score total score over time
eFigure 3. Brief Psychiatric Rating Scale (BPRS) score thought disturbance factor score over time
Data sharing statement