Abstract
The treatment of chronic hepatitis C (CHC) has been revolutionized in an era of all-oral direct-acting antivirals (DAAs) since 2014. Satisfactory treatment efficacy and tolerability can be provided by novel DAAs. Nevertheless, there are still some unmet needs and emerging issues in the treatment of CHC in the DAA era. Certain hard-to-cure populations are prone to have inferior treatment responses, including patients with severe liver decompensation, active hepatocellular carcinoma (HCC), and hepatitis C virus (HCV) genotype 3 (HCV-3) infection and those who experience multiple DAA treatment failures. Hepatitis B virus (HBV) reactivation during and after DAA treatment has raised concern regarding the use of prophylactic antivirals against HBV throughout DAA treatment. However, the standard strategy for the use of prophylactic antivirals is not uniform across regional guidelines. In the post-sustained virological response (SVR) period, HCC still occurs in a substantial proportion of patients. Due to the relatively short follow-up period, the net benefit of the achievement of an SVR by DAAs in the reduction of extrahepatic manifestations has not yet been determined. Attention must also be paid to HCV reinfection, particularly in high-risk populations. The most critical and unmet need for HCV elimination is the large gap in the HCV care cascade at the population level. To accomplish the World Health Organization (WHO)’s goal for HCV elimination by 2030, the expansion of access to HCV care requires a continuous effort to overcome practical and political challenges.
Keywords: Hepatitis C virus, Direct-acting antivirals, Treatment
INTRODUCTION
The treatment of chronic hepatitis C (CHC) has been revolutionized in an era of all-oral direct-acting antivirals (DAAs) since 2014. With the use of current novel DAAs, a sustained virological response (SVR) rate >95% can be attained in addition to satisfactory tolerability. Nevertheless, there are still some unmet needs and emerging issues in the treatment of CHC in the DAA era. Inferior treatment efficacies are observed in some hard-to-cure populations, including patients with severe liver decompensation, active hepatocellular carcinoma, hepatitis C virus (HCV) genotype 3 (HCV-3) infection and those who experience multiple DAA failures. Hepatitis B virus (HBV) reactivation during and after DAA treatment has raised concern for the use of prophylactic antivirals against HBV throughout the course of DAA treatment. However, there is no definite recommendation to guide which patients should be prescribed these prophylactic antivirals and for how long. In the post-SVR period, hepatocellular carcinoma (HCC) still occurs in a substantial proportion of patients, especially among those with advanced fibrosis and subjects who possess ongoing risk factors (e.g., diabetes, HBV dual infection, alcoholism). Due to the relatively short follow-up period, the net benefit of the achievement of an SVR by DAAs in the reduction of extrahepatic manifestations has not yet been settled. Attention must also be paid to HCV reinfection, particularly in high-risk populations. Last but not least, the most critical issue for HCV elimination is the large gap in the HCV care cascade at the population level. The abovementioned issues will be highlighted and discussed in the current review (Table 1).
Table 1.
Unmet needs of HCV care in the DAA era
| Gap to HCV elimination, from diagnosis to linking-to-care | |
| Difficult-to-cure populations | |
| Active HCC, including treatment timing | |
| Severe decompensation, including treatment timing | |
| HCV genotype three patients with cirrhosis | |
| Multiple DAA treatment failures, including the necessity of RAS testing | |
| Prophylactic anti-HBV treatment: Which patients to start and when to stop? | |
| Post-SVR period | |
| HCV reinfection in high-risk populations | |
| HCC risk may not be reduced in decompensated patients | |
| Residual HCC risk remains in low-risk patients: which patients and when to discharge? | |
| Net benefit of extrahepatic outcomes not justified | |
| Survival benefits of an SVR in patients with active HCC are uncertain | |
HCV, hepatitis C virus; DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; RAS, resistance-associated substitution; HBV, hepatitis B virus; SVR, sustained virological response.
DIFFICULT-TO-CURE POPULATIONS
HCV-3
HCV-1 is considered a difficult-to-cure genotype in the interferon era. In the landscape of the DAA era, the treatment response of HCV-1 infection is no longer suboptimal. Rather, a relatively lower SVR rate could be observed in the treatment of HCV-3 infection, particularly in patients with liver cirrhosis who failed prior antivirals. Ironically, patients with HCV-3 infection are prone to have advanced liver disease at the time of presentation [1]. In the ASTRAL-3 study, a high SVR12, defined as undetectable HCV RNA throughout 12 weeks of the posttreatment follow-up period, of 95% could be attained in HCV-3 infected patients treated with sofosbuvir (SOF) plus velpatasvir (VEL). However, the SVR rate was only 89% in the subgroup of treatment-experienced patients with cirrhosis [2]. While the pooling analysis of phase III studies of SOF/VEL revealed an SVR rate of 93% in patients with nonstructural protein 5A (NS5A) resistance-associated substitutions (RASs) [3], a lower SVR rate of 84% was denoted in patients with the Y93H mutation in the ASTRAL-3 study [2]. This finding may prompt RAS testing or the addition of ribavirin to the regional guidelines for the treatment of patients with cirrhosis with HCV-3 infection receiving SOF/VEL [4-6]. Beyond the impact of RASs, HCV-3 subtyping may also account for treatment inferiority. In a phase III study using SOF/VEL for 12 weeks in Asia, an SVR rate of only 76% was noted in HCV-3b-infected patients. Among them, an SVR rate of 89% in patients without cirrhosis but only 50% in patients with cirrhosis patients was depicted [7]. For the other pangenotypic DAA regimen, glecaprevir (GLE)/pibrentasvir (PIB), an SVR rate of 94.9% could be attained in HCV-3 treatment-naïve patients without cirrhosis who received 8 weeks of treatment in the ENDURANCE-3 study. Among them, the SVR rate of patients with the A30K mutation in NS5A was 75% (12/16) compared to 99% (135/137) in those without the mutation [8]. As HCV-3 treatment-naïve compensated patients with cirrhosis could be allocated to abbreviated 8-week GLE/PIB regimen with an SVR rate of 98.4% (60/61) in the per-protocol analysis of the EXPEDITION-8 study [9], interferon/ribavirin- or SOF (PRS)-experienced patients should remain on a 12- to 16-week regimen of GLE/PIB to ensure treatment efficacy [4,5].
Liver decompensation
The portal-systemic-shunting-related poor first-pass effect and bioavailability of DAAs may result in the suboptimal antiviral response in patients with liver decompensation [10]. SOF plus NS5A inhibitor-based therapy is recommended since protease inhibitors are contraindicated. In the SOLAR-1 and SOLAR-2 studies, the SVR12 rate was 87% and 85–86% in Child-Pugh class B and Child-Pugh class C patients after 12 weeks of SOF/ledipasvir (LDV) treatment, respectively [11,12]. In the ASTRAL-4 study, although the treatment efficacy could be improved up to 94% in Child-Pugh class B patients who were allocated to 12 weeks of SOF/VEL plus ribavirin treatment, the SVR12 rate was as low as 85% in patients with HCV-3-infection [13]. Adding ribavirin to DAAs in the population is warranted to ascertain treatment efficacy. The role of ribavirin in the improvement in DAA efficacy is not fully understood [14], and whether it improves early kinetics and promotes immune modulations to diminish the chance of relapse following viral mutation awaits further identification [15]. It should be noted that the failure to attain an SVR in a significant proportion of decompensated patients was due to adverse events or mortality-related treatment discontinuation rather than to virological failure in the trials [11-13], which reflected the difficulty in the management of the fragile subjects in the clinical setting. For example, a recent study using SOF/VEL plus ribavirin in the treatment of 23 patients with Child-Pugh class C resulted in an SVR12 of only 70% (16/23) in an intention-to-treat analysis. The lack of assessment in six of the seven patients during the study period was due to mortality not related to the study drugs [16].
Apart from the issue of treatment efficacy, ongoing deterioration of the Model for End-Stage Liver Disease (MELD) score was noted in 17–43% of Child-Pugh class B and 11–18% of Child-Pugh class C patients, even after the eradication of HCV [17]. The timing of DAA initiation before or after liver transplantation in decompensated patients on the waiting list increases the complexity when donor feasibility is taken into account. It is recommended that patients whose MELD score is >18–20 should be considered for liver transplantation first if the local situation allows and if the donor is available within 6 months [4,18]. On the other hand, although patients could be delisted due to an improvement in liver function reserve by DAAs before liver transplantation, this may occur in only a limited subset of patients [19]. Furthermore, re-decompensation [20], a loss of transplantation priority with the occurrence of HCC, and a poor quality of life (so-called MELD purgatory) [21] become challenging tasks clinicians must face in the postSVR period.
Multiple DAA treatment failures
For patients with previous DAA treatment failures, 12-week SOF/VEL/voxilaprevir (VOX) and 24-week SOF/VEL plus ribavirin treatment for patients with HCV-1-6 infection have been approved [22-24]. For HCV-1 infected patients, 12-week GLE/PIB treatment for prior NS3/4A protease inhibitor-experienced patients and 16-week GLE/PIB treatment for prior NS5A inhibitor-experienced patients have also been approved [25]. Recently, 179 DAA-experienced patients were treated with SOF/VEL/VOX with or without RBV in the real-world setting. Of them, 82% of patients carried a RAS in the NS3, NS5A or NS5B regions before retreatment. The overall SVR12 rate was 96% in a per-protocol analysis. Nevertheless, patients with liver cirrhosis (91%, 71/78) and HCC (71%, 5/7) had a significantly lower SVR rate. Due to overlapping antiviral classes, a relatively low SVR rate of 88.4% (23/26) was noted in SOF/VEL-experienced patients who received SOF/VEL/VOX [26], indicating that the retreatment of prior-DAA-failed patients should be managed sophisticatedly and on an individual basis.
Certain unapproved strategies with multitarget regions have also been adopted with satisfactory efficacy [27,28]. The timing and necessity of RAS testing remain elusive [4,5]. Currently, the treatment strategy for “very difficult-to-cure” patients, who have been defined as patients with NS5A RASs who failed twice to achieve an SVR after a combination regimen including a protease inhibitor and/or an NS5A inhibitor, remains unclear. The European Association for the Study of the Liver (EASL) guideline has advocated the use of SOF/VEL/VOX or SOF/GLE/PIB in addition to ribavirin and has extended the treatment duration to 16–24 weeks. Nevertheless, the recommendation awaits validation by prospective studies (Table 2).
Table 2.
Clinical trials of DAA rescue therapy in patients with prior DAA treatment failure*
| Trial | Study design | Prior DAAs | GT | Salvage DAA | Duration (weeks) | SVR12 (%), n/N |
|---|---|---|---|---|---|---|
| POLARIS-1 [22] (2017) | Phase III, double-blind, multicenter, randomized controlled trial | NS5AI+NS3I±NS5BI (32%); NS5AI+NS5BI (61%); NS5AI (7%) | 1–6 | SOF/VEL/VOX | 12 | 96 (253/263) |
| POLARIS-4 [22] (2017) | Phase III, multicenter, randomized, open-label study | NS5BI+NS3I (25%); NS5BI (74%) | 1–4 | SOF/VEL/VOX | 12 | 98 (178/182) |
| POLARIS-4 [22] (2017) | Phase III, multicenter, randomized, open-label study | NS5BI+NS3I (25%); NS5BI (72%) | 1–3 | SOF/VEL | 12 | 90 (136/151) |
| GS-US-342-1553 [23] (2017) | Phase II, open-label study | SOF/VEL (39%); SOF/VEL+RBV (20%); SOF/VEL/VOX (41%) | 1–3 | SOF/VEL+RBV | 24 | 91 (63/69) |
| C-SWIFT [70] (2017) | Phase II, open-label, single-center, multiple-arm study | EBR/GZR+SOF (100%) | 1 | EBR/GZR+SOF+RBV | 12 | 100 (23/23) |
| CERTAIN-1 [71] (2018) | Phase III, open-label, multicenter study | DCV+ASV (91%); PegIFN/RBV+SMV (6%); SOF+RBV (3%) | 1, 2 | GLE/PIB | 12 | 94 (31/33) |
| ANRS HC34 REVENGE [28] (2018) | Phase II, open-label study | SOF+DCV (29%); SOF/LDV (64%); SOF+SMV (7%) | 1, 4 | EBR/GZR+SOF+RBV | 16/24 | 96 (25/26) |
| Izumi et al. [24] (2018) | Phase III, multicenter, open-label study | NS5AI+NS3I±NS5BI (79%); NS5BI±NS3I (16%); NS5AI±NS5BI (5%) | 1, 2 | SOF/VEL+RBV | 12 | 82 (47/57) |
| Izumi et al. [24] (2018) | Phase III, multicenter, open-label study | NS5AI+NS3I±NS5BI (72%); NS5BI±NS3I (15%); NS5AI±NS5BI (13%) | 1, 2 | SOF/VEL+RBV | 24 | 97 (58/60) |
| MAGELLAN-1 (part 2) [25] (2018) | Phase III, multicenter, randomized, open-label study | NS3/4A PI (32%); NS5AI (36%); N3/4A PI+NS5AI (32%) | 1, 4 | GLE/PIB | 12 | 89 (39/44) |
| MAGELLAN-1 (part 2) [25] (2018) | Phase III, multicenter, randomized, open-label study | NS3/4A PI (28%); NS5AI (38%); N3/4A PI+NS5AI (34%) | 1, 4 | GLE/PIB | 16 | 91 (43/47) |
| RESOLVE [72] (2019) | Phase IIb, multicenter, open-label study | LDV/SOF (89%); PrOD (4%); DCV/ASV (4%); EBR/GZR (3%); SMV+SOF (3%); DCV+SOF (1%); SOF/VEL (1%) | 1 | SOF/VEL/VOX | 12 | 91 (70/77) |
| MAGELLAN-3 [27] (2019) | Phase IIIb, open-label, nonrandomized, multicenter study | GLE/PIB (100%) | 1–3 | GLE/PIB+SOF+RBV | 12/16 | 96 (22/23) |
| Lok et al. [73] (2019) | Phase IIIb, open-label, randomized, study | SOF/LDV (95%); SOF/VEL (5%) | 1, non-LC | GLE/PIB | 12 | 90 (70/78) |
| Lok et al. [73] (2019) | Phase IIIb, open-label, randomized, study | SOF/LDV (92%); SOF/VEL (6%); SOF+DCV (2%) | 1, non-LC | GLE/PIB | 16 | 94 (46/49) |
| Lok et al. [73] (2019) | Phase IIIb, open-label, randomized, study | SOF/LDV (100%) | 1, LC | GLE/PIB+RBV | 12 | 86 (18/21) |
| Lok et al. [73] (2019) | Phase IIIb, open-label, randomized, study | SOF/LDV (90%); SOF/VEL (10%) | 1, LC | GLE/PIB | 16 | 97 (28/29) |
DAA, direct-acting antiviral; GT, genotype; SVR, sustained virological response; NS, nonstructural; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir; RBV, ribavirin; EBR, elbasvir; GZR, grazoprevir; DCV, daclatasvir; ASV, asunaprevir; PegIFN, pegylated interferon; SMV, simeprevir; GLE, glecaprevir; PIB, pibrentasvir; LDV, ledipasvir; PI, protease inhibitor; PrOD, paritaprevir/ritonavir/ombitasvir+dasabuvir; LC, liver cirrhosis.
Adapted from 2020 Taiwan Consensus Statement on the Management of Hepatitis C.
Finally, since protease inhibitors are contraindicated for decompensated patients, the only recommended regimen is the combination of SOF/VEL+RBV for 24 weeks. However, the SVR rate might be suboptimal in patients with baseline NS5A RASs and those who could not tolerate full course ribavirin. This highlights the urgent need for early identification and treatment for CHC patients to avoid progression of liver disease to decompensated cirrhosis [29].
Patients with active HCC
Treatment efficacy in patients with HCC has been widely discussed (Fig. 1). Compared to patients without HCC or with inactive HCC, whether patients with active HCC have an inferior treatment response remains controversial [10,30]. A recent meta-analysis of 49 studies showed that the SVR rate was significantly lower in patients with active HCC (73.1%) than in those with inactive HCC (92.6%) or without HCC (93.3%) [31]. The argument exists that in these studies, there were unequal patient and viral characteristics as well as suboptimal regimens in early studies, which may end in different treatment responses between groups. To overcome this pitfall, Ogawa et al. [32] conducted a propensity-score-matched study for age, sex, cirrhosis, prior treatment, HCV genotype, treatment regimen, baseline platelet count, HCV RNA, total bilirubin, alanine aminotransferase, and albumin level to evaluate the treatment outcome in a large Asian cohort with or without HCC. Patients with active HCC (85.5%) but not those with inactive HCC (93.7%) had a lower SVR rate than those without HCC (95.0%; adjusted odds ratio, 0.28; P=0.01).
Figure 1.
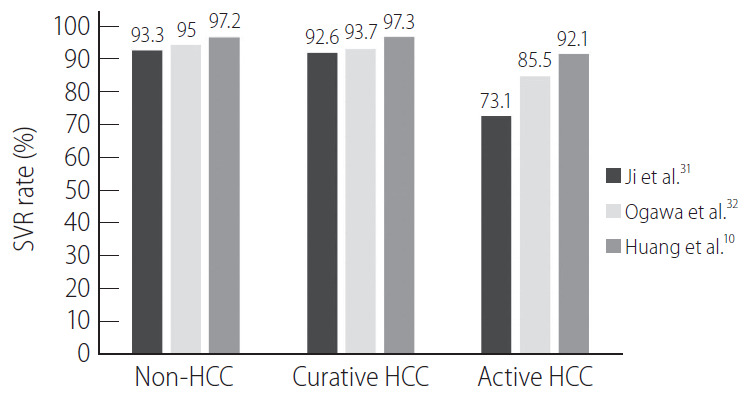
Treatment responses of chronic hepatitis C patients with different hepatocellular carcinoma status across studies. SVR, sustained virological response; HCC, hepatocellular carcinoma.
A significantly improved survival benefit has been observed in early-stage HCC patients who received curative cancer treatment followed by DAA therapy [33]. If liver function does not compromise cancer treatment (e.g., as in the case of the ability to undergo anesthesia for surgical resection), HCC with curative potential should be treated first before antiviral therapy to ensure a higher chance of treatment success. On the other hand, the timing of the initiation of DAAs in patients with incurable HCC becomes a challenging issue since residual HCC may compromise DAA treatment efficacy. Patients with active HCC may have more safety concerns during DAA treatment [10]. In addition, with the breakthrough innovations of systemic therapy in advanced HCC, whether the treatment with DAAs and immunotherapy/target therapy alter the clinical outcome remains unknown [34]. Furthermore, the most critical point is whether HCV eradication will prolong long-term survival in patients with incurable HCC. Dang et al. [35] conducted a propensity-matched study enrolling 1,239 untreated patients and 437 patients with an SVR. Among them, 70.6% of patients received curative HCC treatment, whereas the remaining 29.4% received palliative treatment. The results demonstrated that the attainment of an SVR significantly reduced 5-year all-cause mortality and liver relative mortality in both groups [35], indicating that treatment should not be withheld from those who are not eligible for curative HCC therapy. One of the postulated reasons is that the recovery or preservation of liver function after HCV eradication may offer chances for repeated cancer therapy. Nevertheless, patient numbers in certain subpopulations were too limited for the conclusions to be generalized. Upon closer look, patients with Child-Pugh class B did not benefit much from HCV eradication in terms of the long-term outcome, whereas the achievement of an SVR did not provide survival benefits in patients with Child-Pugh class C [35]. Unlike decompensated patients without HCC, the deteriorated liver function in HCC patients may be due to cancer burden, which also precludes them from liver transplantation. The role of HCV eradication in the terminally ill population remains an unknown domain for exploration.
HBV REACTIVATION IN PATIENTS WITH DUAL HCV/HBV INFECTION
The issue of HBV reactivation after HCV viral suppression deserves more attention, particularly in HBV hyperendemic areas [36-38]. A meta-analysis of 17 studies observed that the rate of HBV activation was 24% in chronic hepatitis B patients, which in turn led to 9% clinical hepatitis. A total of 1.4% of patients with resolved HBV experienced HBV reactivation, but none had clinical relapse [39]. Until now, no prospective controlled study has denoted which, when, or for how long HBV patients should receive prophylactic nucleoside/nucleotide analogs (NUCs) or monitoring during/post-DAA therapy. The recommendations of regional guidelines are largely based on clinical rationality, and the level of evidence is not strong enough to draw conclusions. In addition, there are somewhat different viewpoints among regional guidelines [4,5,40]. For example, the EASL suggests that patients who are hepatitis B virus surface antigen (HBsAg)-positive should receive HBV prophylaxis at least until week 12 post-DAA therapy and be monitored monthly if HBV treatment is stopped [4]. The Asian Pacific Association for the Study of the Liver (APASL) suggests pre-emptive NUC prophylaxis in HBV patients with advanced liver disease or pre-existing HCC. For patients with mild liver disease, close monitoring without anti-HBV therapy is another option. When to stop NUCs should follow APASL HBV guidelines [40]. One of the best ways to monitor these patients is to identify a surrogate marker that can positively and negatively predict HBV reactivation. Factors predictive of HBV flares have been detectable HBV DNA before DAA therapy [39], alanine aminotransferase >2 times the upper limit of normal at baseline [37], and HBsAg >10 IU/mL [41] at baseline when using DAAs or baseline HBV DNA >300 IU/mL when using interferon-based therapy [38]. However, the surrogates were just identified as statistically significant in distinguishing events between groups, and their accuracies in guiding clinical decisions need to be validated by prospective studies. HBV-related clinical hepatitis may lead to severe decompensation and even mortality [41]. From this viewpoint, patients with advanced liver fibrosis should at least receive HBV prophylaxis before and during DAA therapy. The treatment duration and follow-up strategy after the discontinuation of NUCs awaits further clarification.
POST-SVR ERA
HCC
Similar to interferon-based therapy [42], the achievement of an SVR by DAAs decreases the risk of HCC occurrence and does not increase the risk of HCC recurrence [43,44]. Notably, the benefits of the achievement of an SVR in the reduction of HCC risk in decompensated patients are controversial [20,45]. Again, this controversy raises the importance of the discussion of the ideal timing for liver transplantation in the treatment of decompensated patients mentioned earlier. Several factors have been predictive of HCC in the post-SVR period [46-50]. The developed prediction model for HCC has also been created to guide follow-up strategies [51,52]. It is noteworthy that the risk of HCC persists even after 10 years of viral eradication [53]. It has been suggested that HCV-induced oncogenic effects are elicited before treatment and that the “epigenetic scar” may leave and persist long after viral eradication, leading to a life-long risk of HCC [54]. The EASL advocates that patients with an SVR could be discharged if they do not possess advanced fibrosis or other comorbidities. The recommendation may be based on the cost-effectiveness of surveillance. Nevertheless, HCC still occurs in so-called low-risk patients after viral eradication [49]. As the updated APASL guidelines recommended the follow-up of patients with an SVR at different intervals based on their underlying risks [40], the surveillance of HCC, particularly low-risk patients, in the post-SVR period should be judged on a case-by-case basis including local medical accessibility and feasibility [55].
Extrahepatic manifestations
HCV eradication may also improve long-term extrahepatic manifestations. Due to the short follow-up period of the post-DAA period and low incidences of index outcomes, the benefits of DAAs in the reduction in extrahepatic complications are not universally granted [56]. A retrospective cohort study has shown that the risk of non-Hodgkin lymphoma was not significantly reduced after DAA-induced SVR (hazard ratio, 0.86; 95% confidence interval, 0.52–1.43) after only 2.01 years of follow-up [56]. HCV eradication may reduce the risk of vascular events [57]. However, this was not always the case. A study comprising 160,875 subjects showed that the risk of coronary heart disease and stroke did not differ between patients with and without an SVR [58]. Hypolipidemia during viremic status can be reversed to deteriorate lipid profiles after DAA therapy [59]. An increase in the small dense low-density lipoprotein cholesterol level corresponding to increased carotid intima-media thickness 1 year after DAA therapy has been reported [60]. Attention should be paid not only to liver-related complications but also to the extrahepatic consequence of cardio-cerebrovascular disease in the post-SVR period.
Reinfection
The reinfection rate after HCV eradication should be rare in the general population. Nevertheless, reinfection has been frequently encountered in patients with high-risk behaviors. A systemic review showed that the HCV reinfection rate 5 years after HCV eradication was 0.95% in a low-risk population, 10.7% in a high-risk population (prisoners and patients who inject drugs), and up to 15.0% in subjects with HCV and human immunodeficiency virus (HIV) coinfection [61]. Due to the ease of access and the fact that more treatment candidates are allowed in the DAA era, it is postulated that more HCV reinfection may occur [62]. An annual incidence of up to 5.9% per person-year has been reported in HCV/HIV co-infected men who have sex with men after DAA therapy [63]. Multidisciplinary approaches to high-risk populations should be adopted, which include health counseling for safe sex, harm reduction services, opioid substitution therapy and so forth. Treatment as prevention and an increase in the treatment uptake rate followed by a decrease in viral reservoirs at the population level are the most critical policies to be applied [64].
GAP TO ACHIEVE HCV ELIMINATION
The WHO has set ambitious goals for the control of viral hepatitis by 2030 [65]. However, only a few countries are on track for HCV elimination. In the DAA era, where treatment efficacy and tolerability are no longer the major concern, the identification of multiple barriers that exist in patients, providers and institutions that prevent the delivery of HCV care is the most difficult task [66]. A comprehensive HCV care cascade involves blood safety and infection control, harm reduction, proper screening for unawareness, accurate and efficient diagnosis, and linking to medical care. To scale up prevention and treatment, outreach screening programs would be the key determinant for HCV elimination [67]. Several outreach screening programs and treatment strategies with the concept of microelimination in hyperendemic areas and high-risk populations have been adopted in Taiwan [68,69].
CONCLUSIONS
By using multitarget DAA therapy with high genetic barriers, adding ribavirin and extending treatment duration, difficult-to-cure patients may no longer be difficult to cure. The long-term benefit of HCV eradication in decompensated and/or active HCC patients remains elusive. Since it is impractical to conduct a prospective untreated-comparator study, larger observational studies comprising diverse patient characteristics with a longer follow-up period are warranted to judge the impact of SVR. For subjects with HBV/HCV dual infection who do not fulfill the indications for anti-HBV therapy before DAA treatment, the addition of prophylactic NUCs is practical and essential. Further studies that explore the pathophysiological interaction of the two viruses may help to identify the candidates for and strategy of NUC prophylaxis. Much work remains to be done with many barriers standing in the way of achieving the WHO goal of HCV elimination. Each step may require the support and engagement of the local healthcare system, infrastructural commitment and nongovernment organizations. The expansion of access to HCV care requires a continuous effort to overcome the practical and political challenges.
Acknowledgments
The study was supported by grants from Kaohsiung Medical University (MOST 107-2314-B-037 -025 -MY2, MOST 108-2314-B-037-003) and Kaohsiung Medical University Hospital (MOHW108-TDU-B-212-133006).
Abbreviations
- APASL
Asian Pacific Association for the Study of the Liver
- CHC
chronic hepatitis C
- DAAs
direct-acting antivirals
- EASL
European Association for the Study of the Liver
- GLE
glecaprevir
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LDV
ledipasvir
- MELD
Model for End-Stage Liver Disease
- NS5A
nonstructural protein 5A
- NUC
nucleotide analog
- NUCs
nucleos(t)ide analogs
- PIB
pibrentasvir
- RAS
resistance-associated substitutions
- SOF
sofosbuvir
- SVR
sustained virological response
- VEL
velpatasvir
- VOX
voxilaprevir
- WHO
World Health Organization
Footnotes
Authors’ contributions
CFH and MLY contributed to the design and writing of the manuscript.
Conflicts of Interest
Ming-Lung Yu: Research support (grant) from AbbVie, Abbott, BMS, Gilead, and Merck. Consultant for AbbVie, Abbott, Ascletis, BMS, Gilead, Merck and PharmaEssentia. Speaker for AbbVie, Abbott, Ascletis, BMS, Gilead, and Merck.
Chung-Feng Huang: Speaker for AbbVie, Abbott, BMS, Gilead, and Merck.
REFERENCES
- 1.Huang JF, Huang CF, Yeh ML, Dai CY, Yu ML, Chuang WL. Updates in the management and treatment of HCV genotype 3, what are the remaining challenges? Expert Rev Anti Infect Ther. 2018;16:907–912. doi: 10.1080/14787210.2018.1544492. [DOI] [PubMed] [Google Scholar]
- 2.Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 3.Hezode C, Reau N, Svarovskaia ES, Doehle BP, Shanmugam R, Dvory-Sobol H, et al. Resistance analysis in patients with genotype 1-6 HCV infection treated with sofosbuvir/velpatasvir in the phase III studies. J Hepatol. 2018;68:895–903. doi: 10.1016/j.jhep.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 5.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD) HCV guidance: recommendations for testing, managing, and treating hepatitis C. doi: 10.1002/hep.31060. AASLD web site, < http://www.hcvguidelines.org/>. Accessed 15 Dec 2019. [DOI] [PMC free article] [PubMed]
- 6.Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, et al. 2019 Taiwan Consensus Statement on the Management of Hepatitis C. J Formos Med Assoc. 2020 doi: 10.1016/j.jfma.2020.04.003. accepted. [DOI] [PubMed] [Google Scholar]
- 7.Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127–134. doi: 10.1016/S2468-1253(18)30343-1. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 9.Brown RS, Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72:441–449. doi: 10.1016/j.jhep.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Huang CF, Yeh ML, Huang CI, Liang PC, Lin YH, Hsieh MY, et al. Equal treatment efficacy of direct-acting antivirals in patients with chronic hepatitis C and hepatocellular carcinoma? A prospective cohort study. BMJ Open. 2019;9:e026703. doi: 10.1136/bmjopen-2018-026703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 13.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 14.Yu ML, Hung CH, Huang YH, Peng CY, Lin CY, Cheng PN, et al. Efficacy and safety of 12 weeks of daclatasvir, asunaprevir plus ribavirin for HCV genotype-1b infection without NS5A resistance-associated substitutions. J Formos Med Assoc. 2019;118:556–564. doi: 10.1016/j.jfma.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Huang CF, Yeh ML, Huang CI, Liang PC, Lin YH, Hsieh MY, et al. Ribavirin facilitates early viral kinetics in chronic hepatitis C patients receiving daclatasvir/asunaprevir. J Gastroenterol Hepatol. 2020;35:151–156. doi: 10.1111/jgh.14815. [DOI] [PubMed] [Google Scholar]
- 16.Flamm S, Lawitz E, Borg B, Charlton M, Landis C, Reddy R, et al. High efficacy and improvement in CPT class with sofosbuvir/velpatasvir plus ribavirin for 12 weeks in patients with CPT C decompensated cirrhosis. Poster session presented at: EASL. The International Liver Congress; 2019 Apr 10-14; Vienna, Austria. [Google Scholar]
- 17.van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016;65(1 Suppl):S95–S108. doi: 10.1016/j.jhep.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Chhatwal J, Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65:777–788. doi: 10.1002/hep.28926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlton MR, Cheung MC, Manns MP, Sajed N, Troke P, Spellman JG, et al. Ledipasvir/sofosbuvir + ribavirin (LDV/SOF + RBV) for 12 weeks in decompensated HCV genotype 1 patients: SOLAR-1 and-2 studies compared to a real-world dataset. Hepatology. 2016;64:489A–490A. [Google Scholar]
- 20.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Barsa JE, Branch AD, Schiano TD. A pleasant dilemma to have: to treat the HCV patient on the waiting list or to treat post-liver transplantation? Clin Transplant. 2015;29:859–865. doi: 10.1111/ctr.12596. [DOI] [PubMed] [Google Scholar]
- 22.Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 23.Gane EJ, Shiffman ML, Etzkorn K, Morelli G, Stedman CAM, Davis MN, et al. Sofosbuvir-velpatasvir with ribavirin for 24 weeks in hepatitis C virus patients previously treated with a direct-acting antiviral regimen. Hepatology. 2017;66:1083–1089. doi: 10.1002/hep.29256. [DOI] [PubMed] [Google Scholar]
- 24.Izumi N, Takehara T, Chayama K, Yatsuhashi H, Takaguchi K, Ide T, et al. Sofosbuvir-velpatasvir plus ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol Int. 2018;12:356–367. doi: 10.1007/s12072-018-9878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poordad F, Pol S, Asatryan A, Buti M, Shaw D, Hézode C, et al. Glecaprevir/pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure. Hepatology. 2018;67:1253–1260. doi: 10.1002/hep.29671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onofrio FDQ, Cooper C, Borgia SM, Vachon MLC, Ramji A, Lilly L, et al. Prior therapy with sofosbuvir/velpatasvir associated with reduced response to sofosbuvir/velpatasvir/voxilaprevir: results from a Canadian prospective registry. AASLD The Liver Meeting; 2019 November 9-13; Boston, USA. [Google Scholar]
- 27.Wyles D, Weiland O, Yao B, Weilert F, Dufour JF, Gordon SC, et al. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection. J Hepatol. 2019;70:1019–1023. doi: 10.1016/j.jhep.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 28.de Lédinghen V, Laforest C, Hézode C, Pol S, Renault A, Alric L, et al. Retreatment with sofosbuvir plus grazoprevir/elbasvir plus ribavirin of patients with hepatitis C virus genotype 1 or 4 who previously failed an NS5A- or NS3-containing regimen: the ANRS HC34 REVENGE study. Clin Infect Dis. 2018;66:1013–1018. doi: 10.1093/cid/cix916. [DOI] [PubMed] [Google Scholar]
- 29.Parigi TL, Torres MCP, Aghemo A. Upcoming direct acting antivirals for hepatitis C patients with a prior treatment failure. Clin Mol Hepatol. 2019;25:360–365. doi: 10.3350/cmh.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CF, Yu ML. Direct-acting antivirals response in hepatocellular carcinoma: does the presence of hepatocellular carcinoma matter? Clin Mol Hepatol. 2019;25:168–171. doi: 10.3350/cmh.2018.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatol. 2019;71:473–485. doi: 10.1016/j.jhep.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa E, Toyoda H, Iio E, Jun DW, Huang CF, Enomoto M, et al. HCV cure rates are reduced in patients with active but not inactive hepatocellular carcinoma- a practice implication. Clin Infect Dis. 2019 Nov 28; doi: 10.1093/cid/ciz1160. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–273. doi: 10.1016/j.jhep.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Harrod E, Moctezuma-Velazquez C, Gurakar A, Ala A, Dieterich D, Saberi B. Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review. Hepatoma Res. 2019;5:28. [Google Scholar]
- 35.Dang H, Yeo YH, Yasuda S, Huang CF, Iio E, Landis C, et al. Cure with interferon free DAA is associated with increased survival in patients with HCV related HCC from both East and West. Hepatoology. 2019 Oct 14; doi: 10.1002/hep.30988. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, et al. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol. 2017;32:1754–1762. doi: 10.1111/jgh.13771. [DOI] [PubMed] [Google Scholar]
- 37.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology. 2018;154:989–997. doi: 10.1053/j.gastro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Yeh ML, Huang CI, Huang CF, Hsieh MH, Liu TW, Lin YH, et al. Pretreatment hepatitis B viral load predicts long-term hepatitis b response after anti-hepatitis C therapy in hepatitis B/C dual-infected patients. J Infect Dis. 2019;219:1224–1233. doi: 10.1093/infdis/jiy648. [DOI] [PubMed] [Google Scholar]
- 39.Mücke MM, Backus LI, Mücke VT, Coppola N, Preda CM, Yeh ML, et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:172–180. doi: 10.1016/S2468-1253(18)30002-5. [DOI] [PubMed] [Google Scholar]
- 40.Kanda T, Lau GKK, Wei L, Moriyama M, Yu ML, Chuang WL, et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol Int. 2019;13:649–661. doi: 10.1007/s12072-019-09988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh ML, Huang CF, Huang CI, Holmes JA, Hsieh MH, Tsai YS, et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. 2020 Feb 13; doi: 10.1016/j.jhep.2020.01.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–994. [PubMed] [Google Scholar]
- 43.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 Sep 5; doi: 10.1016/j.jhep.2017.08.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421. doi: 10.1053/j.gastro.2018.04.008. e4. [DOI] [PubMed] [Google Scholar]
- 46.Yu ML, Huang CF, Yeh ML, Tsai PC, Huang CI, Hsieh MH, et al. Time-degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatitis C virus patients: a model for prioritization of treatment. Clin Cancer Res. 2017;23:1690–1697. doi: 10.1158/1078-0432.CCR-16-0921. [DOI] [PubMed] [Google Scholar]
- 47.Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, et al. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086–1097. doi: 10.1002/hep.21363. [DOI] [PubMed] [Google Scholar]
- 48.Huang CF, Yeh ML, Huang CY, Tsai PC, Ko YM, Chen KY, et al. Pretreatment glucose status determines HCC development in HCV patients with mild liver disease after curative antiviral therapy. Medicine (Baltimore) 2016;95:e4157. doi: 10.1097/MD.0000000000004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang CF, Yeh ML, Tsai PC, Hsieh MH, Yang HL, Hsieh MY, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Lee K, Sinn DH, Gwak GY, Cho HC, Jung SH, Paik YH, et al. Prediction of the risk of hepatocellular carcinoma in chronic hepatitis C Patients after sustained virological response by aspartate aminotransferase to platelet ratio index. Gut Liver. 2016;10:796–802. doi: 10.5009/gnl15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69:1088–1098. doi: 10.1016/j.jhep.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun HS, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, et al. Design and validation of risk prediction model for hepatocellular carcinoma development after sustained virological response in patients with chronic hepatitis C. Eur J Gastroenterol Hepatol. 2020;32:378–385. doi: 10.1097/MEG.0000000000001512. [DOI] [PubMed] [Google Scholar]
- 53.Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology. 2019;157:1264–1278. doi: 10.1053/j.gastro.2019.07.033. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A, Meirson T, et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019;15:e1008181. doi: 10.1371/journal.pgen.1008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Na SK, Song BC. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C. Clin Mol Hepatol. 2019;25:234–244. doi: 10.3350/cmh.2018.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Serag HB, Christie IC, Puenpatom A, Castillo D, Kanwal F, Kramer JR. The effects of sustained virological response to direct-acting anti-viral therapy on the risk of extrahepatic manifestations of hepatitis C infection. Aliment Pharmacol Ther. 2019;49:1442–1447. doi: 10.1111/apt.15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butt AA, Yan P, Shuaib A, Abou-Samra AB, Shaikh OS, Freiberg MS. Direct-acting antiviral therapy for HCV infection is associated with a reduced risk of cardiovascular disease events. Gastroenterology. 2019;156:987–996. doi: 10.1053/j.gastro.2018.11.022. e8. [DOI] [PubMed] [Google Scholar]
- 58.Mahale P, Engels EA, Li R, Torres HA, Hwang LY, Brown EL, et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67:553–561. doi: 10.1136/gutjnl-2017-313983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drazilova S, Gazda J, Janicko M. Chronic hepatitis C association with diabetes mellitus and cardiovascular risk in the era of DAA therapy. Can J Gastroenterol Hepatol. 2018;2018:6150861. doi: 10.1155/2018/6150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Narita S, Toda S, et al. Carotid intima-media thickness and small dense low-density lipoprotein cholesterol increase after one year of treatment with direct-acting antivirals in patients with hepatitis C virus infection. Intern Med. 2019;58:1209–1215. doi: 10.2169/internalmedicine.1514-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis c virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ingiliz P, Rockstroh JK. Hepatitis C virus reinfection-more to come? Lancet Gastroenterol Hepatol. 2017;2:150–151. doi: 10.1016/S2468-1253(16)30223-0. [DOI] [PubMed] [Google Scholar]
- 63.Berenguer J, Gil-Martin Á, Jarrin I, Montes ML, Domínguez L, Aldámiz-Echevarria T, et al. Reinfection by hepatitis C virus following effective all-oral direct-acting antiviral drug therapy in HIV/hepatitis C virus coinfected individuals. AIDS. 2019;33:685–689. doi: 10.1097/QAD.0000000000002103. [DOI] [PubMed] [Google Scholar]
- 64.Razavi H. Mathematical modelling: treatment-as-prevention. The 4th International Symposium on Hepatitis in Substance Users (INHSU 2015); 2015 Oct 7-9; Sydney, Australia. [Google Scholar]
- 65.World Health Organization . Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. Geneva: WHO Document Production Services; 2016. [Google Scholar]
- 66.Mendizabal M, Alonso C, Silva MO. Overcoming barriers to hepatitis C elimination. Frontline Gastroenterol. 2019;10:207–209. doi: 10.1136/flgastro-2018-101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–1329. doi: 10.1016/S0140-6736(18)32277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu ML, Huang CI, Liang PC, Wei YW, Hsu PY, Hsu CT, et al. A comprehensive people‐centered outreach health‐care system targeting HCV micro‐elimination in hyperendemic areas of taiwan (compact)-establishment of a model toward HCV elimination: interim report. Hepatology. 2019;70(S1):378A–379A. [Google Scholar]
- 69.Huang CF, Chiu YW, Yu ML. Patient-centered outreach treatment toward micro-elimination of hepatitis C virus infection in hemodialysis patients. Kidney Int. 2020;97:421. doi: 10.1016/j.kint.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Lawitz E, Poordad F, Gutierrez JA, Wells JT, Landaverde CE, Evans B, et al. Short-duration treatment with elbasvir/grazoprevir and sofosbuvir for hepatitis C: a randomized trial. Hepatology. 2017;65:439–450. doi: 10.1002/hep.28877. [DOI] [PubMed] [Google Scholar]
- 71.Kumada H, Watanabe T, Suzuki F, Ikeda K, Sato K, Toyoda H, et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 2018;53:566–575. doi: 10.1007/s00535-017-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson E, Covert E, Hoffmann J, Comstock E, Emmanuel B, Tang L, et al. A pilot study of safety and efficacy of HCV retreatment with sofosbuvir/velpatasvir/voxilaprevir in patients with or without HIV (RESOLVE STUDY) J Hepatol. 2019;71:498–504. doi: 10.1016/j.jhep.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lok AS, Sulkowski MS, Kort JJ, Willner I, Reddy KR, Shiffman ML, et al. Efficacy of glecaprevir and pibrentasvir in patients with genotype 1 hepatitis C virus infection with treatment failure after NS5A inhibitor plus sofosbuvir therapy. Gastroenterology. 2019;157:1506–1517. doi: 10.1053/j.gastro.2019.08.008. e1. [DOI] [PubMed] [Google Scholar]


